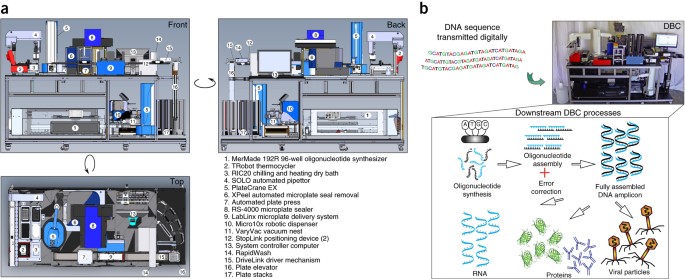
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Colon cancers arising in most patients with hereditary nonpolyposis colorectal cancer (HNPCC) show microsatellite instability (MSI). _BAT26_, a quasimonomorphic polyA stretch
located just 3′ of _MSH2_ exon 5, is considered the most sensitive and specific marker of MSI. A total of 10 HNPCC families with large intragenic _MSH2_ deletions, encompassing exon 5 and
intron 5, identified by multiplex ligation-dependent probe amplification (MLPA) were included in this study. The deletions under study were del1-16, del1-8, del1-7, del1-6, and del3-6,
detected in 3, 1, 2, 3, and 1 families, respectively. Although all patients examined from these 10 families developed unstable tumours, 13/19 MSI-H tumours (68 %) surprisingly showed
stability of _BAT26_. By MLPA and _MSH2_ sequence analyses of the _BAT26_-stable tumours, we demonstrated that the wild-type _MSH2_ allele was somatically inactivated by an identical large
deletion, with complete loss of intron 5/_BAT26_ sequences at the tumour DNA level. We could infer that the apparent stability of _BAT26_ was due to the complete absence of target _BAT26_
sequences in the tumour sample, which results in exclusive amplification of contaminant normal DNA, containing a single copy of a wild-type stable _BAT26_ sequence. Identification of a
subset of _MSH2_-related unstable tumours that are not recognized by analysis of _BAT26_ instability indicates that this marker should never be used alone for rapid MSI screening of HNPCC
tumours. Moreover, our findings indicate that _BAT26_ stability in the context of MSI is strongly suggestive of the presence of a large intragenic _MSH2_ deletion. SIMILAR CONTENT BEING
VIEWED BY OTHERS THE ATOM-SEQ SEQUENCE CAPTURE PANEL CAN ACCURATELY PREDICT MICROSATELLITE INSTABILITY STATUS IN FORMALIN-FIXED TUMOUR SAMPLES, ALONGSIDE ROUTINE GENE MUTATION TESTING
Article Open access 19 September 2024 COPY NEUTRAL LOSS OF HETEROZYGOSITY (CNLOH) PATTERNS IN SYNCHRONOUS COLORECTAL CANCER Article 02 December 2020 HIGH PREVALENCE OF SOMATIC _PIK3CA_ AND
_TP53_ PATHOGENIC VARIANTS IN THE NORMAL MAMMARY GLAND TISSUE OF SPORADIC BREAST CANCER PATIENTS REVEALED BY DUPLEX SEQUENCING Article Open access 29 June 2022 INTRODUCTION Hereditary
nonpolyposis colorectal cancer (HNPCC), the most common form of hereditary colon cancer, is an autosomal dominant syndrome characterized by a high incidence of early onset colorectal cancer
and an excess of cancers of extracolonic sites, such as endometrium, small intestine, urinary and hepatobiliary tracts, ovary, and stomach. 1, 2 HNPCC predisposition is caused by germline
mutations in one of the known human mismatch repair (_MMR_) genes, namely _MLH1_, _MSH2_, _MSH6_, and _PMS2_ (InSiGHT Mutation Database: http://www.insight-group.org/). Their inactivation
may result from point mutations, identifiable in approximately 50–80% of the HNPCC families, or from genomic rearrangements, which can be detected in up to 20% of cases.3, 4, 5, 6, 7, 8, 9
Large deletions are especially frequent in _MSH2_, and they account for up to one-third of _MSH2_ mutations in some populations.10 Colon cancer arising in patients with a defective MMR
system usually shows microsatellite instability (MSI), determined by the occurrence of frameshift mutations in simple sequence repeats. This molecular hallmark is useful for selecting
patients and families eligible for _MMR_ mutation analysis. To this aim, a standard panel of microsatellite markers for MSI testing of tumour tissues was proposed at the 1997 NCI
international consensus meeting.11 This panel is comprised of five markers, including two mono- (_BAT26_ and _BAT25_) and three dinucleotide repeats (_D2S123, D5S346_ and _D17S250_).
Conventionally, all samples showing altered length in ⩾2/5 markers are classified as MSI-H (high degree of instability), whereas samples with 1/5 (or less than 30%, if additional markers are
tested) unstable markers and no altered markers are defined as MSI-L (low level of instability) and MSS (microsatellite stable), respectively. More recently, an alternative panel of five
mononucleotide repeats has been suggested.12, 13 This still includes _BAT26_ and _BAT25_, the most widely used markers, which are deemed to be the most sensitive and specific markers for the
detection of MSI-H tumours.14, 15, 16, 17, 18 _BAT26_ is a quasimonomorphic marker formed by a poly-A tract located immediately downstream of _MSH2_ exon 5. For its high sensitivity,
comprised between 93 and 100%, it has been proposed that it could be used as a single marker for MSI testing.14, 15, 18, 19, 20 Here we show that HNPCC tumours from a subset of patients who
are heterozygous for _MSH2_ deletions are characterized by frequent stability of _BAT26_. MATERIALS AND METHODS PATIENTS AND TUMOURS A total of 10 HNPCC families were selected from a group
of 16 families in which we previously identified large intragenic _MSH2_ deletions by multiplex ligation-dependent probe amplification (MLPA) (Table 1). Overall, 18 deletions carriers from
these 10 families, including the probands, were characterized by the inclusion of exon and intron 5 in the constitutionally deleted tracts. Lack of immunohistochemical MSH2 expression in
tumours from nine out of nine tested families was consistent with the underlying _MSH2_ genetic defect (data not shown). Some patients developed more than one tumour, and MSI status could be
investigated on a total of 22 tumour DNA samples. In the remaining six families with large _MSH2_ deletions, exon 5/intron 5 were retained in the mutant allele. A total of nine tumours from
six patients belonging to this subset were available for MSI analysis. The research was approved by the local Ethical Committee and written consent was obtained from patients. MSI ANALYSIS
Genomic DNA was obtained from blood and from paraffin-embedded or frozen tissues. Tumour DNA was extracted by the DNAeasy tissue kit (Qiagen GmbH, Hilden, Germany) following the
manufacturer's instructions. Standard MSI analysis was performed on paired tumour–normal tissue DNA samples using the NCI panel of microsatellite markers (_BAT26, BAT25, D2S123_,
_D5S346_, _D17S250_).11 Where possible, additional markers were investigated (_NR21_, _NR22_, and _NR24_).12 The fluorescently labelled PCR products were mixed with a size standard (GeneScan
400HD ROX, Applera, Foster City, CA, USA), run on an automatic ABI3100 DNA analyzer, and evaluated with the GeneScan software (Applera). A variation in number and size of peaks of a marker
in tumour DNA compared with normal DNA was interpreted as instability for that marker. Tumours were scored as MSI-H, MSI-L, or MSS, according to the international criteria.11 MLPA ANALYSIS
MLPA was performed with 200 ng of normal and tumour DNAs using the MRC-Holland (Amsterdam, Holland) HNPCC probe kit, according to the supplier's protocol. Then, 1 _μ_l of the
FAM-labelled PCR product was mixed with 1 _μ_l of fluorescent GeneScan 500 LIZ size standard (Applera) in 15 _μ_l of HiDi Formamide, run on an automatic ABI3100 DNA analyser, and evaluated
with GeneScan software (Applera). The electropherograms showed specific peaks corresponding to each exon of _MSH2_ and _MLH1_, as well as additional peaks corresponding to control sequences
mapping on different chromosomes. Dosage analyses based on comparison between deleted and reference wild-type DNA samples were performed on an Excel file following the manufacturer's
protocol (www.mrc-holland.com). Briefly, all _MSH2_ peak areas were normalized by dividing each peak area by the combined area of all other peaks of the electropherogram. Then, these
normalized peak areas were divided by the corresponding wild-type normalized peak area (average of three independent DNA samples) of that probe amplification product, in order to obtain a
series of 16 _a__ex_ values. In constitutional and tumour DNA samples, a 40–55% decrease of the area of an _MSH2_ exon peak compared to the wild-type control samples was considered as
indicative of a heterozygous deletion of that exon. In tumor DNAs, a 60–90% decrease was considered as indicative of a homozygous deletion of that exon. To quantitatively evaluate deletions
in tumour DNAs, the following values were also calculated for each electropherogram of all tumour/normal DNA pairs: _Adel_, the mean value of normalized deleted _MSH2_ peak areas; and
_Aret_, the mean value of normalized retained _MSH2_ peak areas, where present. POLYMORPHISM ANALYSIS Four sequence polymorphisms of the _MSH2_ locus were analysed: IVS1(+9)C/G, IVS9(−9)A/T,
IVS10(+12)G/A, and IVS12(−6)A/G. Analysis was first performed by direct sequencing of PCR products on normal DNA. In heterozygous patients, the same polymorphism was evaluated on tumour DNA
to check for the presence of loss of heterozygosity (LOH). RESULTS MSI status was investigated on a total of 22 tumour DNA samples, including 15 colorectal carcinomas, two colorectal
adenomas, two kidney tumours, and one tumour each of the stomach, bladder, and skin, from the 18 selected individuals with deleted exon 5/intron 5, and a total of nine tumours, including
five colorectal carcinomas, two colorectal adenomas, and one tumour each of the uterus and skin, from the six patients with retained exon 5/intron 5. With the exception of the kidney tumour
of patient CFS185 (family A-AV62), of the bladder and skin tumours of patient CFS366 (family A-VA25) and of the adenoma and skin tumour of patient CFS316 (family A-VA16), which were MSS
(data not shown), all other tumours were clearly MSI-H. In particular, they all displayed instability at the _BAT25_ marker. However, 13/19 tumours (68%) in the first group surprisingly
showed stability of _BAT26._ This result was observed in tumours from nine different families, and was absent in four out of five tumours from family A-AV62 (del1-16), in one out of three
tumours from family A-VA25 (del1-6), and in the only tumour of family A-PD21 (del1-6) (Table 1). _BAT26_ was unstable in the seven MSI-H tumours from patients carrying _MSH2_ deletions not
including exon 5 (Table 1). In order to gain some insight into the mechanisms underlying this unexpected phenomenon, we carried out additional molecular tests on tumour DNAs. As a first
step, we used MLPA analysis to determine the copy number of each _MSH2_ exon in tumour DNA samples. Owing to the poor yield and/or quality of some samples, only 13 tumour samples gave
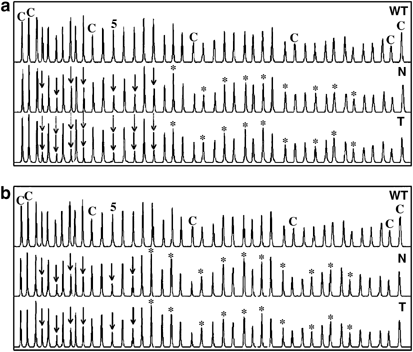
reliable results and could be used for tumour/constitutional comparisons. Careful visual inspection of the tumour electropherograms from the 10 _BAT26_-stable samples showed a further
reduction in size of the same peaks deleted in matched normal DNA, without significant changes in the peaks that were not constitutionally deleted (Figure 1a). On the other hand, the
electropherograms of the three _BAT26_-unstable tumours were undistinguishable from those of the corresponding constitutional DNAs (Figure 1b). Precise qualitative and quantitative
definition of these exonic deletions was carried out by dosage analysis of all single peaks. The exact extension of deletions in the different tumour/normal pairs was confirmed by
calculating the _a__ex_ values, which approximated ∼1 for the retained peaks, but were ∼0.5 or lower for the deleted peaks (Figure 2a and b). Moreover, to better define the borders of the
somatically deleted regions, we verified that the flanking peak (ie the first retained peak immediately adjacent to the deleted region) had a normalized area of at least 85% of the expected
value in each tumor DNA (data not shown). _Adel_ ranged from 0.45±0.08 to 0.58±0.09 in constitutional DNAs and from 0.18±0.04 to 0.58±0.13 in tumor DNAs (Figure 2c). However, in the
_BAT26_-stable tumours, these values were in the range of 0.18±0.04–0.37±0.08 and, in each couple of tumor/normal DNA, the tumour _Adel_ value was always significantly lower (_P_<0.001)
than the matched constitutional _Adel_ value. On the contrary, _Adel_ of the _BAT26_-unstable tumours had values between 0.51±0.05 and 0.58±0.13, overlapping their corresponding
constitutional _Adel_ values (0.47±0.08 and 0.56±0.10). _Aret_ varied from 0.82±0.12 to 1.08±0.04 and from 0.80±0.04 to 1.20±0.04 in constitutional and tumour DNAs, respectively. The _Aret_
values of the eight _BAT26_-stable tumours with retained exons were not dissimilar from the _Aret_ value calculated for CFS356, the only _BAT26_-unstable sample retaining some _MSH2_ exons
(data not shown). Overall, these data indicate that tumour somatic inactivation of the wild-type _MSH2_ allele was determined by a second (intra)genic deletion, which was limited to the
exons already deleted in constitutional DNA, in the _BAT26_-stable, but not in the _BAT26-_unstable tumour samples (Table 2). Polymorphism analyses of some samples were not informative, due
to constitutional homozygosity or to PCR difficulty in some paraffin-embedded samples. Only five tumours, four _BAT26_-stable and one unstable, could be evaluated for at least one of the
four _MSH2_ polymorphisms analysed. Heterozygosity was retained in the _BAT26_-unstable, but not in the four _BAT26_-stable tumour DNAs, which displayed total or partial LOH of _MSH2_
sequence polymorphisms mapping outside the deleted region (Table 2). DISCUSSION This is the first study investigating the correlations between _BAT26_ status and mutation type in _MSH2_. We
observed an association between stability of the _BAT26_ microsatellite marker and presence of large intragenic _MSH2_ deletions encompassing exon and intron 5. Since _BAT26_ stability was
present in 13 out of 19 MSI-H tumours with such deletions, it appears to be a very frequent (68%) and specific phenomenon. In fact, _BAT26_ was unstable in seven out of seven tumour samples
from subjects who were heterozygous for _MSH2_ deletions not encompassing exon and intron 5, and in our 10 year-laboratory experience, _BAT26_ stability was very rarely observed in tumour
DNAs from patients with _MMR_ gene point mutations and never observed in patients with large intragenic _MLH1_ deletions. Only recently it has become evident that large rearrangements of
_MMR_ genes cause Lynch syndrome in about 20% of cases. MLPA, a new high-resolution, robust and quick assay for detecting copy number variations in genomic sequences, has provided an
important contribution for the detection of several new _MSH2_ and _MLH1_ deletions in some cohorts of families.4, 5, 8 Owing to its high sensitivity and specificity, _BAT26_ was initially
proposed as an unambiguous marker of MSI,14 and therefore it has been included in both microsatellite panels proposed at the first11 and second13 Bethesda consensus meetings. Recently, it
has been reported that _BAT26_, together with _BAT25_, accurately detects MSI-H tumours and can also be used to predict tumour content (the percentage of tumour cells) in DNA samples.18
_BAT26_ is a quasimonomorphic poly-A stretch located just 3′ of _MSH2_ exon 5. We hypothesized that _BAT26_ stability could be due to somatic deletions, with complete loss of intron
5/_BAT26_ sequences in tumour cells, and, consequently, amplification of a wild-type allele present in infiltrating leucocytes and/or stromal cells. By MLPA analysis, we demonstrated that
the wild-type _MSH2_ allele in _BAT26_-stable tumours, but not in the _BAT26_-unstable samples, is somatically inactivated by another large deletion encompassing intron 5/_BAT26_ sequences,
thus confirming our hypothesis. We found that MLPA is a very easy and efficient test to detect large _MSH2_ rearrangements also in the majority of DNA samples extracted from
paraffin-embedded tumours. Visual and quantitative evaluation of the MLPA peaks areas showed that normal and tumour DNAs had similar patterns in the _BAT26_ unstable cases, with _Adel_
values ≈0.5, as expected. On the other hand, the MLPA patterns of the _BAT26_-stable tumours were different from those of the corresponding normal tissues, showing on average _Adel_ <0.5
and _Aret_ ≈1. This suggests that somatic inactivation of the wild-type _MSH2_ allele was determined by a second identical deletion, which leads to homozygosity of the constitutional
mutation. However, only DNA sequencing of breakpoints on both alleles could definitively confirm this hypothesis. Hemizygosity, as alternative to homozygosity, was excluded because the
height of retained _MSH2_ peaks relative to the other internal peaks did not significantly vary between normal and tumour DNAs. However, reduction to homozygosity was never complete, showing
smaller deleted peaks of variable height, likely due to the presence of contaminating normal DNA. We then investigated the status of _MSH2_ intragenic polymorphic markers in
tumour/constitutional DNA pairs. Data from these polymorphisms were concordant with those obtained with MLPA analysis, showing the presence of two different alleles in the only informative
_BAT26_-unstable tumour DNA sample (CFS356), and LOH in all four _BAT26_-stable samples, possibly due to the presence of two identical _MSH2_ alleles. Two mechanisms can account for the
reduction to homozygosity for _MSH2_ deletions observed in the majority of the samples with a stable _BAT26_ marker: loss of the wild-type chromosome with duplication of the whole chromosome
2 containing the mutant allele or, alternatively, a somatic recombination, possibly associated with DNA repair failure, with copy/duplication of a DNA tract restricted to a region of the
short arm surrounding the _MSH2_ locus. Further analyses will be necessary to discriminate between these two hypotheses. Our findings indicate that _BAT26_ stability in a context of MSI-H is
strongly suggestive of the presence of a large intragenic _MSH2_ deletion, especially when supported by absence of MSH2 immunoistochemical expression and positive family history. In fact,
68% of MSI-H tumours developed by patients with different _MSH2_ deletions encompassing exon/intron 5 were _BAT26_ stable, because of a second hit leading the constitutional mutation to
homozygosity. Only two deletions escaped this rule: del1-16 (_BAT26_ instability in 4/7 MSI-H tumours) and del1-6 (two of the five samples examined were unstable). Although literature data
on the types of somatic mutations in _MSH2_-related tumours are scanty, point mutations have also been reported in addition to allelic loss/LOH mutations.21, 22, 23, 24 Our data demonstrate
that in a small subgroup of patients with specific _MSH2_ constitutional mutations, LOH associated with homozygosity of the mutant allele is the preferred mechanism of somatic inactivation,
and suggest that the first _MSH2_ hit can drive the molecular events associated with the second hit. Finally, our findings bear important consequences for the clinical setting. Assessment of
MSI status is used for the diagnosis of HNPCC and may also be relevant for disease prognosis25 and for predicting response to chemotherapy.26 As a consequence of an increasing demand for
this molecular test, it is very important to arrange rapid and cost-effective tests with panels of microsatellite markers able to satisfy both clinical requirements. Identification of a
subset of _MSH2_-related MSI-H tumours that are not recognized by _BAT26_ indicates that this marker should never be used alone for the evaluation of instability in a rapid MSI screening of
HNPCC tumours. REFERENCES * Lynch HT, de la Chapelle A : Hereditary colorectal cancer. _N Engl J Med_ 2003; 348: 919–932. Article CAS Google Scholar * Lucci-Cordisco E, Zito I, Gensini F,
Genuardi M : Hereditary nonpolyposis colorectal cancer and related conditions. _Am J Med Genet_ 2003; 122A: 325–334. Article Google Scholar * Viel A, Petronzelli F, Della Puppa L _et al_:
Different molecular mechanisms underlie genomic deletions in the _MLH1_ gene. _Hum Mutat_ 2002; 20: 368–374. Article CAS Google Scholar * Gille JJP, Hogervorst FBL, Pals G _et al_:
Genomic deletions of _MSH2_ and _MLH1_ in colorectal cancer families detected by a novel mutation detection approach. _Br J Cancer_ 2002; 87: 892–897. Article CAS Google Scholar *
Nakagawa H, Hampel H, de la Chapelle A : Identification and characterization of genomic rearrangements of _MSH2_ and _MLH1_ in Lynch syndrome (HNPCC) by novel techniques. _Hum Mutat_ 2003;
22: 258. Article Google Scholar * Taylor CF, Charlton RS, Burn J, Sheridan E, Taylor GR : Genomic deletions in _MSH2_ or _MLH1_ are a frequent cause of hereditary non-polyposis colorectal
cancer: identification of novel and recurrent deletions by MLPA. _Hum Mutat_ 2003; 22: 428–433. Article CAS Google Scholar * Wagner A, Barrows A, Wijnen JT _et al_: Molecular analysis of
hereditary nonpolyposis colorectal cancer in the United States: high mutation detection rate among clinically selected families and characterization of an American founder genomic deletion
of the MSH2 gene. _Am J Hum Genet_ 2003; 72: 1088–1100. Article CAS Google Scholar * Bunyan DJ, Eccles DM, Sillibourne J _et al_: Dosage analysis of cancer predisposition genes by
multiplex ligation-dependent probe amplification. _Br J Cancer_ 2004; 91: 1155–1159. Article CAS Google Scholar * Di Fiore F, Charbonnier F, Martin C _et al_: Screening for genomic
rearrangements of the _MMR_ genes must be included in the routine diagnosis of HNPCC. _J Med Genet_ 2004; 41: 18–20. Article CAS Google Scholar * Wijnen J, van der Klift H, Vasen H _et
al_: _MSH2_ genomic deletions are a frequent cause of HNPCC. _Nat Genet_ 1998; 20: 326–328. Article CAS Google Scholar * Boland CR, Thibodeau SN, Hamilton SR _et al_: A national cancer
institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability
in colorectal cancer. _Cancer Res_ 1998; 58: 5248–5257. CAS PubMed Google Scholar * Suraweera N, Duval A, Reperant M _et al_: Evaluation of tumor microsatellite instability using five
quasimonomorphic mononucleotide repeats and pentaplex PCR. _Gastroenterology_ 2002; 123: 1804–1811. Article CAS Google Scholar * Umar A, Boland CR, Terdiman JP _et al_: Revised Bethesda
Guidelines for Hereditary Nonpolyposis Colorectal Cancer (Lynch Syndrome) and Microsatellite Instability. _J Natl Cancer Inst_ 2004; 96: 261–268. Article CAS Google Scholar * Hoang JM,
Cottu PH, Thuille B, Salmon RJ, Thomas G, Hamelin R : BAT-26, an indicator of the replication error phenotype in colorectal cancers and cell lines. _Cancer Res_ 1997; 57: 300–303. CAS
PubMed Google Scholar * Dietmaier W, Wallinger S, Bocker T, Kullmann F, Fishel R, Rüschoff J : Diagnostic microsatellite instability: definition and correlation with mismatch repair
protein expression. _Cancer Res_ 1997; 57: 4749–4756. CAS PubMed Google Scholar * Bacher JW, Flanagan LA, Smalley RL _et al_: Development of a fluorescent multiplex assay for detection of
MSI-high tumors. _Dis Markers_ 2004; 20: 237–250. Article Google Scholar * Buhard O, Suraweera N, Lectard A, Duval A, Hamelin R : Quasimonomorphic mononucleotide repeats for high-level
microsatellite instability analysis. _Dis markers_ 2004; 20: 251–257. Article Google Scholar * Brennetot C, Buhard O, Jourdan F, Flejou JF, Duval A, Hamelin R : Mononucleotide repeats
BAT-26 and BAT-25 accurately detect MSI-H tumours and predict tumor content: implications for population screening. _Int J Cancer_ 2005; 113: 446–450. Article CAS Google Scholar * de la
Chapelle A : Testing tumors for microsatellite instability. _Eur J Hum Genet_ 1999; 7: 407–408. Article CAS Google Scholar * Laghi L, Bianchi P, Roncalli M, Malesci A : Re: Revised
Bethesda Guidelines for Hereditary Nonpolyposis Colorectal Cancer (Lynch Syndrome) and Microsatellite Instability. _J Natl Cancer Inst_ 2004; 96: 1402–1403. Article Google Scholar *
Wheeler JMD, Beck NE, Kim HC, Tomlinson IPM, McC Mortensen NJ, Bodmer WF : Mechanisms of inactivation of mismatch repair genes in human colorectal cancer cell lines: the predominant role of
hMLH1. _Proc Natl Acad Sci USA_ 1999; 96: 10296–10301. Article CAS Google Scholar * Kruse R, Rutten A, Hosseiny-Malayeri HR _et al_: ‘Second hit’ in sebaceous tumors from Muir-Torre
patients with germline mutations in MSH2: allele loss is not the preferred mode of inactivation. _J Invest Dermatol_ 2001; 116: 463–465. Article CAS Google Scholar * Yuen ST, Chan TL, Ho
JWC _et al_: Germline, somatic and epigenetic events underlying mismatch repair deficiency in colorectal and HNPCC-related cancers. _Oncogene_ 2002; 21: 7585–7592. Article CAS Google
Scholar * Miyaki M, Iijima T, Yamaguchi T _et al_: Novel germline _hMSH2_ genomic deletion and somatic _hMSH2_ mutations in a hereditary nonplyposis colorectal cancer family. _Mutat Res_
2004; 548: 19–25. Article CAS Google Scholar * Gryfe R, Kim H, Hsieh ET _et al_: Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. _N Engl J
Med_ 2000; 342: 69–77. Article CAS Google Scholar * Ribic CM, Sargent DJ, Moore MJ _et al_: Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based
adjuvant chemotherapy for colon cancer. _N Engl J Med_ 2003; 349: 247–257. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The study was supported by Alleanza Contro il
Cancro (to AV) and by a grant from the Italian Ministry for Education, University and Research (MIUR to MG). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Preclinical Research
and Epidemiology, Experimental Oncology 1, Centro Riferimento Oncologico-IRCCS, Aviano, PN, Italy Chiara Pastrello & Alessandra Viel * Department of Clinical Pathophysiology, Section of
Medical Genetics, University of Florence School of Medicine, Firenze, Italy Silvana Baglioni, Laura Papi & Maurizio Genuardi * Pathology Unit, Insubria University, Varese, Italy Maria
Grazia Tibiletti * Department of Medical Oncology, Gastroenterology Unit, Centro Riferimento Oncologico-IRCCS, Aviano, PN, Italy Mara Fornasarig * Oncology Unit, ULSS No. 5, Hospital,
Cittadella, PD, Italy Alberto Morabito * Department of Oncological and Surgical Sciences, Clinica Chirurgica II, Padua University, Padova, Italy Marco Agostini Authors * Chiara Pastrello
View author publications You can also search for this author inPubMed Google Scholar * Silvana Baglioni View author publications You can also search for this author inPubMed Google Scholar *
Maria Grazia Tibiletti View author publications You can also search for this author inPubMed Google Scholar * Laura Papi View author publications You can also search for this author
inPubMed Google Scholar * Mara Fornasarig View author publications You can also search for this author inPubMed Google Scholar * Alberto Morabito View author publications You can also search
for this author inPubMed Google Scholar * Marco Agostini View author publications You can also search for this author inPubMed Google Scholar * Maurizio Genuardi View author publications
You can also search for this author inPubMed Google Scholar * Alessandra Viel View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR
Correspondence to Alessandra Viel. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Pastrello, C., Baglioni, S., Tibiletti, M. _et al._ Stability of
_BAT26_ in tumours of hereditary nonpolyposis colorectal cancer patients with _MSH2_ intragenic deletion. _Eur J Hum Genet_ 14, 63–68 (2006). https://doi.org/10.1038/sj.ejhg.5201517 Download
citation * Received: 31 May 2005 * Revised: 05 August 2005 * Accepted: 20 September 2005 * Published: 26 October 2005 * Issue Date: January 2006 * DOI:
https://doi.org/10.1038/sj.ejhg.5201517 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * hereditary nonpolyposis colorectal cancer *
microsatellite instability * _BAT26_ * multiplex ligation-dependent probe amplification