- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
KEY POINTS * Cell adhesions represent the interaction interfaces between cells and the extracellular matrix. Their study provides exciting insights into the interplay between physical forces
and molecular signalling in cell regulation. * Cells use transmembrane actin–integrin adhesion complexes as mechanosensors to probe the rigidity of the extracellular environment, mediate
adhesion, trigger signalling, and remodel the extracellular matrix (ECM). * Local physical forces induce transitions in the types and functions of these cell–matrix adhesions: Focal
complexes transform into focal adhesions, which serve as the source of fibrillar adhesions. * Integrin translocation appears to stretch fibronectin molecules, exposing cryptic sites that
mediate matrix assembly into extracellular fibrils. * A key mechanism in these transitions appears to be conformational changes induced by force or a local reorganization of scaffold or
signalling molecules to promote multimolecular assembly. * Both intracellular molecular-complex formation at adhesion sites and ECM assembly are regulated by Rho-family GTPases. * The main
challenges for the future include: The identification of the full repertoire of adhesion-associated molecules. Comparison of the various 'focal complex'-like structures.
Characterization of the molecular and cellular nature of the mechanosensors involved in cell–matrix adhesion. Characterization of the regulation and functional integration of the various
forms of adhesions, which change depending on the state of differentiation, tissue location, and application of local forces. Exploring the structure and function of cell–matrix adhesions
in three-dimensional microenvironments _in vivo_ and explaining the roles of complex carbohydrates in cell–matrix interactions. ABSTRACT Integrin-mediated cell adhesions provide dynamic,
bidirectional links between the extracellular matrix and the cytoskeleton. Besides having central roles in cell migration and morphogenesis, focal adhesions and related structures convey
information across the cell membrane, to regulate extracellular-matrix assembly, cell proliferation, differentiation, and death. This review describes integrin functions, mechanosensors,
molecular switches and signal-transduction pathways activated and integrated by adhesion, with a unifying theme being the importance of local physical forces. Access through your institution
Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and
online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes
which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY
OTHERS CANONICAL AND NON-CANONICAL INTEGRIN-BASED ADHESIONS DYNAMICALLY INTERCONVERT Article Open access 07 March 2024 ORGANIZATION, DYNAMICS AND MECHANOREGULATION OF INTEGRIN-MEDIATED
CELL–ECM ADHESIONS Article 27 September 2022 THE ROLE AND REGULATION OF INTEGRINS IN CELL MIGRATION AND INVASION Article 30 September 2024 REFERENCES * Gumbiner, B. M. Cell adhesion: the
molecular basis of tissue architecture and morphogenesis. _Cell_ 84, 345–357 (1996). CAS PubMed Google Scholar * Giancotti, F. G. & Ruoslahti, E. Integrin signalling. _Science_ 285,
1028–1032 (1999). Article CAS PubMed Google Scholar * Curtis, A. & Wilkinson, C. New depths in cell behaviour: reactions of cells to nanotopography. _Biochem. Soc. Symp._ 65, 15–26
(1999). CAS PubMed Google Scholar * Huang, S. & Ingber, D. E. The structural and mechanical complexity of cell-growth control. _Nature Cell Biol._ 1, E131–E138 (1999). CAS PubMed
Google Scholar * Katz, B. Z. et al. Physical state of the extracellular matrix regulates the structure and molecular composition of cell–matrix adhesions. _Mol. Biol. Cell_ 11, 1047–1060
(2000). CAS PubMed PubMed Central Google Scholar * Lo, C. M., Wang, H. B., Dembo, M. & Wang, Y. L. Cell movement is guided by the rigidity of the substrate. _Biophys. J._ 79, 144–152
(2000). CAS PubMed PubMed Central Google Scholar * Abercrombie, M. & Dunn, G. A. Adhesions of fibroblasts to substratum during contact inhibition observed by interference reflection
microscopy. _Exp. Cell Res._ 92, 57–62 (1975). CAS PubMed Google Scholar * Izzard, C. S. & Lochner, L. R. Cell-to-substrate contacts in living fibroblasts: an interference reflexion
study with an evaluation of the technique. _J. Cell. Sci._ 21, 129–159 (1976). CAS PubMed Google Scholar * Sastry, S. K. & Burridge, K. Focal adhesions: A nexus for intracellular
signaling and cytoskeletal dynamics. _Exp. Cell. Res._ 261, 25–36 (2000). CAS PubMed Google Scholar * Zamir, E. & Geiger, B. Molecular diversity of actin–integrin adhesion complexes.
_J. Cell Sci._ (in the press). * Kano, Y., Katoh, K., Masuda, M. & Fujiwara, K. Macromolecular composition of stress fiber-plasma membrane attachment sites in endothelial cells _in
situ_. _Circ. Res._ 79, 1000–1006 (1996). CAS PubMed Google Scholar * Turner, C. E., Kramarcy, N., Sealock, R. & Burridge, K. Localization of paxillin, a focal adhesion protein, to
smooth muscle dense plaques, and the myotendinous and neuromuscular junctions of skeletal muscle. _Exp. Cell Res._ 192, 651–655 (1991). CAS PubMed Google Scholar * Chen, W. T. &
Singer, S. J. Immunoelectron microscopic studies of the sites of cell-substratum and cell-cell contacts in cultured fibroblasts. _J. Cell Biol._ 95, 205–222 (1982). CAS PubMed Google
Scholar * Zamir, E. et al. Molecular diversity of cell-matrix adhesions. _J. Cell Sci._ 112, 1655–1669 (1999). CAS PubMed Google Scholar * Zamir, E. et al. Dynamics and segregation of
cell-matrix adhesions in cultured fibroblasts. _Nature Cell Biol._ 2, 191–196 (2000).THE DYNAMICS OF FOCAL ADHESIONS AND FIBRILLAR ADHESIONS AND FORMATION OF THE LATTER FROM THE FORMER WERE
SHOWN USING TIME-LAPSE MICROSCOPY OF GFP–PAXILLIN AND GFP–TENSIN. CAS PubMed Google Scholar * Bershadsky, A. D., Tint, I. S., Neyfakh, A. A. Jr, & Vasiliev, J. M. Focal contacts of
normal and RSV-transformed quail cells. Hypothesis of the transformation-induced deficient maturation of focal contacts. _Exp. Cell Res._ 158, 433–444 (1985). CAS PubMed Google Scholar *
Nobes, C. D. & Hall, A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. _Cell_ 81,
53–62 (1995). CAS PubMed Google Scholar * Rottner, K., Hall, A. & Small, J. V. Interplay between rac and rho in the control of substrate contact dynamics. _Curr. Biol._ 9, 640–648
(1999).IN THIS STUDY AND IN REFERENCE 17 , DIFFERENTIAL SIGNALLING REQUIREMENTS FOR FOCAL ADHESIONS AND FOCAL COMPLEXES WERE ESTABLISHED. FOCAL COMPLEX FORMATION DEPENDS ON RAC ACTIVITY,
WHEREAS FOCAL ADHESIONS REQUIRE RHO AND RHO-KINASE ACTIVITY. CAS PubMed Google Scholar * Tarone, G., Cirillo, D., Giancotti, F. G., Comoglio, P. M. & Marchisio, P. C. Rous sarcoma
virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. _Exp. Cell Res._ 159, 141–157 (1985). CAS PubMed Google Scholar *
Zambonin-Zallone, A. et al. Immunocytochemical distribution of extracellular matrix receptors in human osteoclasts: a β 3 integrin is colocalized with vinculin and talin in the podosomes of
osteoclastoma giant cells. _Exp. Cell Res._ 182, 645–652 (1989). CAS PubMed Google Scholar * Gaidano, G. et al. Integrin distribution and cytoskeleton organization in normal and malignant
monocytes. _Leukemia_ 4, 682–687 (1990). CAS PubMed Google Scholar * Chellaiah, M. et al. Gelsolin deficiency blocks podosome assembly and produces increased bone mass and strength. _J.
Cell Biol._ 148, 665–678 (2000). CAS PubMed PubMed Central Google Scholar * Ochoa, G. C. et al. A functional link between dynamin and the actin cytoskeleton at podosomes. _J. Cell Biol._
150, 377–389 (2000).AN INTERESTING DISCOVERY OF DYNAMIN LOCALIZATION AT THE TUBULAR INVAGINATIONS OF THE PLASMA MEMBRANE IN PODOSOMES AND CHARACTERIZATION OF ITS INVOLVEMENT IN PODOSOME
FORMATION. CAS PubMed PubMed Central Google Scholar * Xu, W., Baribault, H. & Adamson, E. D. Vinculin knockout results in heart and brain defects during embryonic development.
_Development_ 125, 327–337 (1998). CAS PubMed Google Scholar * Sheppard, D. _In vivo_ functions of integrins: lessons from null mutations in mice. _Matrix Biol._ 19, 203–209 (2000). CAS
PubMed Google Scholar * Liu, S., Calderwood, D. A. & Ginsberg, M. H. Integrin cytoplasmic domain-binding proteins. _J. Cell Sci._ 113, 3563–3571 (2000). CAS PubMed Google Scholar *
Echtermeyer, F., Baciu, P. C., Saoncella, S., Ge, Y. & Goetinck, P. F. Syndecan-4 core protein is sufficient for the assembly of focal adhesions and actin stress fibers. _J. Cell Sci._
112, 3433–3441 (1999). CAS PubMed Google Scholar * Bono, P., Rubin, K., Higgins, J. M. & Hynes, R. O. Layilin, a novel integral membrane protein, is a hyaluronan receptor. _Mol. Biol.
Cell_ 12, 891–900 (2001). CAS PubMed PubMed Central Google Scholar * Wei, Y., Yang, X., Liu, Q., Wilkins, J. A. & Chapman, H. A. A role for caveolin and the urokinase receptor in
integrin-mediated adhesion and signaling. _J. Cell Biol._ 144, 1285–1294 (1999). CAS PubMed PubMed Central Google Scholar * Prehoda, K. E., Scott, J. A., Mullins, R. D. & Lim, W. A.
Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. _Science_ 290, 801–806 (2000). CAS PubMed Google Scholar * Raucher, D. et al.
Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. _Cell_ 100, 221–228 (2000).THIS STUDY DIRECTLY SHOWED PTDINS(4,5)P
2 -MEDIATED CONTROL OF PLASMA MEMBRANE INTERACTION WITH THE UNDERLYING CYTOSKELETON. CAS PubMed Google Scholar * Fukami, K., Endo, T., Imamura, M. & Takenawa, T. α-actinin and
vinculin are PIP2-binding proteins involved in signaling by tyrosine kinase. _J. Biol. Chem._ 269, 1518–1522 (1994). CAS PubMed Google Scholar * Tall, E. G., Spector, I., Pentyala, S. N.,
Bitter, I. & Rebecchi, M. J. Dynamics of phosphatidylinositol 4,5-bisphosphate in actin-rich structures. _Curr. Biol._ 10, 743–746 (2000). CAS PubMed Google Scholar * Serra-Pages, C.
et al. The LAR transmembrane protein tyrosine phosphatase and a coiled–coil LAR-interacting protein co-localize at focal adhesions. _EMBO J._ 14, 2827–2838 (1995). CAS PubMed PubMed
Central Google Scholar * Inagaki, K. et al. SHPS-1 regulates integrin-mediated cytoskeletal reorganization and cell motility. _EMBO J._ 19, 6721–6731 (2000). CAS PubMed PubMed Central
Google Scholar * Lin, S. & Lin, D. Mapping of actin, vinculin, and integrin binding domains suggests a direct role of tensin in actin–membrane association. _Mol. Biol. Cell_ 7, (suppl.)
389a (1996). Google Scholar * Han, D. C., Rodriguez, L. G. & Guan, J. L. Identification of a novel interaction between integrin β1 and 14-3-3β. _Oncogene_ 20, 346–357 (2001). CAS
PubMed Google Scholar * Liu, S. & Ginsberg, M. H. Paxillin binding to a conserved sequence motif in the α4 integrin cytoplasmic domain. _J. Biol. Chem._ 275, 22736–22742 (2000). CAS
PubMed Google Scholar * Liu, S., Slepak, M. & Ginsberg, M. H. Direct binding of paxillin to the α9 integrin cytoplasmic domain inhibits cell spreading. _J. Biol. Chem._ 27, 37086–37092
(2001). Google Scholar * Prasad, N., Topping, R. S. & Decker, S. J. SH2-containing inositol 5′-phosphatase SHIP2 associates with the p130(Cas) adapter protein and regulates cellular
adhesion and spreading. _Mol. Cell. Biol._ 21, 1416–1428 (2001). CAS PubMed PubMed Central Google Scholar * Miyamoto, S. et al. Integrin function: molecular hierarchies of cytoskeletal
and signaling molecules. _J. Cell Biol._ 131, 791–805 (1995). CAS PubMed Google Scholar * Miyamoto, S., Akiyama, S. K. & Yamada, K. M. Synergistic roles for receptor occupancy and
aggregation in integrin transmembrane function. _Science_ 267, 883–885 (1995).THE LOCAL REACTION THAT IS INDUCED BY INTERACTIONS OF INTEGRINS WITH MATRIX PROTEINS WAS ANALYSED BY APPLICATION
OF MICRON-SIZED BEADS THAT WERE COVERED WITH A PROTEIN OF INTEREST TO THE CELL SURFACE. BOTH THE CLUSTERING OF INTEGRIN MOLECULES AND THEIR DIRECT INTERACTION WITH MATRIX PROTEINS THROUGH
THE RGD MOTIF (OCCUPANCY) WERE SHOWN TO BE NECESSARY FOR INDUCING RECRUITMENT OF THE APPROPRIATE CYTOPLASMIC PROTEINS TO THE ADHESION SITE. CAS PubMed Google Scholar * Laukaitis, C. M.,
Webb, D. J., Donais, K. & Horwitz, A. F. Differential dynamics of α 5 integrin, paxillin, and α-actinin during formation and disassembly of adhesions in migrating cells. _J. Cell Biol._
153, 1427–1440 (2001). CAS PubMed PubMed Central Google Scholar * Chrzanowska-Wodnicka, M. & Burridge, K. Rho-stimulated contractility drives the formation of stress fibers and focal
adhesions. _J. Cell Biol._ 133, 1403–1415 (1996).THIS STUDY FIRST SHOWED THE ROLE OF MYOSIN II DOWNSTREAM OF RHO IN THE FORMATION OF FOCAL ADHESIONS. CAS PubMed Google Scholar *
Bershadsky, A., Chausovsky, A., Becker, E., Lyubimova, A. & Geiger, B. Involvement of microtubules in the control of adhesion-dependent signal transduction. _Curr. Biol._ 6, 1279–1289
(1996).THIS STUDY SHOWED THAT MICROTUBULE DISRUPTION INDUCES FORMATION OF FOCAL ADHESIONS THROUGH THE ACTIVATION OF MYOSIN II-DRIVEN CONTRACTILITY. CAS PubMed Google Scholar * Riveline,
D. et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. _J. Cell Biol._
153, 1175–1186 (2001).THIS STUDY SHOWED THE INDUCTION OF FOCAL-ADHESION GROWTH BY LOCALLY APPLIED TENSION FORCE. APPLICATION OF EXTERNAL FORCE BYPASSES THE REQUIREMENTS FOR RHO KINASE (ROCK)
AND MYOSIN II-DEPENDENT CELL CONTRACTILITY FOR THE FORMATION OF FOCAL ADHESIONS, PROVIDED THAT ANOTHER TARGET OF RHO, MDIA1, IS ACTIVE. CAS PubMed PubMed Central Google Scholar *
Volberg, T., Geiger, B., Citi, S. & Bershadsky, A. D. Effect of protein kinase inhibitor H-7 on the contractility, integrity, and membrane anchorage of the microfilament system. _Cell
Motil. Cytoskel._ 29, 321–338 (1994). CAS Google Scholar * Uehata, M. et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. _Nature_
389, 990–994 (1997). CAS PubMed Google Scholar * Helfman, D. M. et al. Caldesmon inhibits nonmuscle cell contractility and interferes with the formation of focal adhesions. _Mol. Biol.
Cell_ 10, 3097–3112 (1999). CAS PubMed PubMed Central Google Scholar * Geiger, B. & Bershadsky, A. Assembly and mechanosensory function of focal contacts. _Curr. Opin. Cell Biol._
13, 584–592 (2001). CAS PubMed Google Scholar * Watanabe, N., Kato, T., Fujita, A., Ishizaki, T. & Narumiya, S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization.
_Nature Cell Biol._ 1, 136–143 (1999). CAS PubMed Google Scholar * Choquet, D., Felsenfeld, D. P. & Sheetz, M. P. Extracellular matrix rigidity causes strengthening of
integrin-cytoskeleton linkages. _Cell_ 88, 39–48 (1997).THE SIMULATION OF ECM RIGIDITY BY TRAPPING ECM-COATED BEADS BY LASER TWEEZERS LEADS TO A LOCAL INCREASE IN ACTOMYOSIN-MEDIATED FORCE
APPLIED TO THE TRAPPED BEAD BY THE CELL. THIS INDICATES THAT ISOMETRIC FORCE APPLIED TO AN INTEGRIN-MEDIATED ADHESION SITE INDUCES LOCAL REINFORCEMENT OF ASSOCIATION OF THIS SITE WITH THE
ACTIN CYTOSKELETON. CAS PubMed Google Scholar * Beningo, K. A., Dembo, M., Kaverina, I., Small, J. V. & Wang, Y. L. Nascent focal adhesions are responsible for the generation of
strong propulsive forces in migrating fibroblasts. _J. Cell Biol._ 153, 881–888 (2001).THIS STUDY SHOWED THAT LOCOMOTING CELLS APPLY THE STRONGEST FORCES TO SMALL ZYXIN-CONTAINING FOCAL
COMPLEXES AT THE LEADING EDGE. CAS PubMed PubMed Central Google Scholar * Harris, A. K., Wild, P. & Stopak, D. Silicone rubber substrata: a new wrinkle in the study of cell
locomotion. _Science_ 208, 177–179 (1980).THIS STUDY SHOWED FOR THE FIRST TIME THAT THE CELL APPLIES MECHANICAL FORCE TO THE SUBSTRATE TO WHICH IT ADHERES. CAS PubMed Google Scholar *
Burton, K., Park, J. H. & Taylor, D. L. Keratocytes generate traction forces in two phases. _Mol. Biol. Cell_ 10, 3745–3769 (1999). CAS PubMed PubMed Central Google Scholar *
Galbraith, C. G. & Sheetz, M. P. A micromachined device provides a new bend on fibroblast traction forces. _Proc. Natl Acad. Sci. USA_ 94, 9114–9118 (1997). CAS PubMed PubMed Central
Google Scholar * Dembo, M. & Wang, Y. L. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. _Biophys. J._ 76, 2307–2316 (1999). CAS PubMed PubMed Central
Google Scholar * Munevar, S., Wang, Y. & Dembo, M. Traction force microscopy of migrating normal and h-ras transformed 3t3 fibroblasts. _Biophys. J._ 80, 1744–1757 (2001). CAS PubMed
PubMed Central Google Scholar * Balaban, N. Q. et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. _Nature Cell Biol._ 3,
466–472 (2001).THIS STUDY USED MICROPATTERNED ELASTOMER SUBSTRATE AND FLUORESCENCE IMAGING OF FOCAL ADHESIONS IN LIVE CELLS, WHICH SHOWED A STRONG CORRELATION BETWEEN FOCAL-ADHESION SIZE AND
LOCAL FORCES THAT ARE APPLIED BY THE CELL AT THESE SITES. THE SIZE OF THE FOCAL CONTACTS DECREASED RAPIDLY IN PROPORTION TO A DECREASE OF THESE FORCES THAT WERE INDUCED BY BDM TREATMENT.
CAS PubMed Google Scholar * Kornberg, L., Earp, H. S., Parsons, J. T., Schaller, M. & Juliano, R. L. Cell adhesion or integrin clustering increases phosphorylation of a focal
adhesion-associated tyrosine kinase. _J. Biol. Chem._ 267, 23439–23442 (1992). CAS PubMed Google Scholar * Burridge, K., Turner, C. E. & Romer, L. H. Tyrosine phosphorylation of
paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. _J. Cell Biol._ 119, 893–903 (1992).THIS AND THE PREVIOUS STUDY INITIATED A CASCADE
OF PUBLICATIONS SHOWING THAT THE FORMATION OF FOCAL ADHESIONS IS ACCOMPANIED BY TYROSINE PHOSPHORYLATION OF THEIR MAIN COMPONENTS. CAS PubMed Google Scholar * Rankin, S. & Rozengurt,
E. Platelet-derived growth factor modulation of focal adhesion kinase (p125FAK) and paxillin tyrosine phosphorylation in Swiss 3T3 cells. Bell-shaped dose response and cross-talk with
bombesin. _J. Biol. Chem._ 269, 704–710 (1994). CAS PubMed Google Scholar * Casamassima, A. & Rozengurt, E. Insulin-like growth factor I stimulates tyrosine phosphorylation of
p130(Cas), focal adhesion kinase, and paxillin. Role of phosphatidylinositol 3'-kinase and formation of a p130(Cas). Crk complex. _J. Biol. Chem._ 273, 26149–26156 (1998). CAS PubMed
Google Scholar * Li, S. et al. Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. _J. Biol. Chem._ 272, 30455–30462 (1997). CAS PubMed
Google Scholar * Yano, Y., Geibel, J. & Sumpio, B. E. Tyrosine phosphorylation of pp125FAK and paxillin in aortic endothelial cells induced by mechanical strain. _Am. J. Physiol._ 271,
C635–C649 (1996). * Sai, X., Naruse, K. & Sokabe, M. Activation of pp60(src) is critical for stretch-induced orienting response in fibroblasts. _J. Cell Sci._ 112, 1365–1373 (1999). CAS
PubMed Google Scholar * Parsons, J. T., Martin, K. H., Slack, J. K., Taylor, J. M. & Weed, S. A. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement.
_Oncogene_ 19, 5606–5613 (2000). CAS PubMed Google Scholar * Kaplan, K. B. et al. Association of the amino-terminal half of c-Src with focal adhesions alters their properties and is
regulated by phosphorylation of tyrosine 527. _EMBO J._ 13, 4745–4756 (1994). CAS PubMed PubMed Central Google Scholar * Kaplan, K. B., Swedlow, J. R., Morgan, D. O. & Varmus, H. E.
c-Src enhances the spreading of _src_−/− fibroblasts on fibronectin by a kinase-independent mechanism. _Genes Dev._ 9, 1505–1517 (1995).THIS STUDY FIRST SHOWED THE INVOLVMENT OF PP60C-SRC IN
THE REGULATION OF CELL–MATRIX ADHESION. CAS PubMed Google Scholar * Felsenfeld, D. P., Schwartzberg, P. L., Venegas, A., Tse, R. & Sheetz, M. P. Selective regulation of
integrin–cytoskeleton interactions by the tyrosine kinase Src. _Nature Cell Biol._ 1, 200–206 (1999). CAS PubMed Google Scholar * Volberg, T., Romer, L., Zamir, E. & Geiger, B.
pp60(c-src) and related tyrosine kinases: a role in the assembly and reorganization of matrix adhesions. _J. Cell Sci._ 114, 2279–2289 (2001). CAS PubMed Google Scholar * Ilic, D. et al.
Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. _Nature_ 377, 539–544 (1995). CAS PubMed Google Scholar * Schwartzberg, P. L. et al.
Rescue of osteoclast function by transgenic expression of kinase-deficient Src in _src_−/− mutant mice. _Genes Dev._ 11, 2835–2844 (1997). CAS PubMed PubMed Central Google Scholar *
Arthur, W. T., Petch, L. A. & Burridge, K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. _Curr. Biol._ 10, 719–722 (2000). CAS PubMed Google Scholar *
Ren, X. D. et al. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. _J. Cell Sci._ 113, 3673–3678 (2000). CAS PubMed Google Scholar * Stephens, L. E. et
al. Deletion of β1 integrins in mice results in inner cell mass failure and peri-implantation lethality. _Genes Dev._ 9, 1883–1895 (1995). CAS PubMed Google Scholar * Henry, M. D. &
Campbell, K. P. A role for dystroglycan in basement membrane assembly. _Cell_ 95, 859–870 (1998). CAS PubMed Google Scholar * Colognato, H., Winkelmann, D. A. & Yurchenco, P. D.
Laminin polymerization induces a receptor-cytoskeleton network. _J. Cell Biol._ 145, 619–631 (1999). CAS PubMed PubMed Central Google Scholar * Avnur, Z. & Geiger, B. The removal of
extracellular fibronectin from areas of cell-substrate contact. _Cell_ 25, 121–132 (1981). CAS PubMed Google Scholar * Pankov, R. et al. Integrin dynamics and matrix assembly:
tensin-dependent translocation of α5β1 integrins promotes early fibronectin fibrillogenesis. _J. Cell Biol._ 148, 1075–1090 (2000).DIRECT DEMONSTRATION OF THE SPECIFIC TRANSLOCATION OF Α 5 Β
1 INTEGRIN FROM FOCAL ADHESIONS TO, AND ALONG, FIBRILLAR ADHESIONS THAT DRIVE THE FORMATION OF FIBRONECTIN MATRIX. CAS PubMed PubMed Central Google Scholar * McDonald, J. A., Kelley, D.
G. & Broekelmann, T. J. Role of fibronectin in collagen deposition: Fab′ to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast
extracellular matrix. _J. Cell Biol._ 92, 485–492 (1982). CAS PubMed Google Scholar * Akiyama, S. K., Yamada, S. S., Chen, W. T. & Yamada, K. M. Analysis of fibronectin receptor
function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. _J. Cell Biol._ 109, 863–875 (1989). CAS PubMed Google Scholar *
Harris, A. K., Stopak, D. & Wild, P. Fibroblast traction as a mechanism for collagen morphogenesis. _Nature_ 290, 249–251 (1981). CAS PubMed Google Scholar * Mosher, D. F. (ed.) in
_Fibronectin_. (Academic, San Diego, 1989). Google Scholar * Hynes, R. O. in _Fibronectins_. (Springer, New York, 1990). Google Scholar * Yamada, K. M. & Clark, R. A. F. in _The
Molecular and Cellular Biology of Wound Repair_ (ed. Clark, R. A. F.) 51–93 (Plenum, New York, 1996). Google Scholar * Wennerberg, K. et al. β1 integrin-dependent and-independent
polymerization of fibronectin. _J. Cell Biol._ 132, 227–238 (1996). CAS PubMed Google Scholar * Yang, J. T. & Hynes, R. O. Fibronectin receptor functions in embryonic cells deficient
in α5 β1 integrin can be replaced by αV integrins. _Mol. Biol. Cell_ 7, 1737–1748 (1996). CAS PubMed PubMed Central Google Scholar * Sechler, J. L., Cumiskey, A. M., Gazzola, D. M. &
Schwarzbauer, J. E. A novel RGD-independent fibronectin assembly pathway initiated by α4β1 integrin binding to the alternatively spliced V region. _J. Cell Sci._ 113, 1491–1498 (2000). CAS
PubMed Google Scholar * Zhang, Q. & Mosher, D. F. Cross-linking of the NH2-terminal region of fibronectin to molecules of large apparent molecular mass. Characterization of
fibronectin assembly sites induced by the treatment of fibroblasts with lysophosphatidic acid. _J. Biol. Chem._ 271, 33284–33292 (1996). CAS PubMed Google Scholar * Schwarzbauer, J. E.
& Sechler, J. L. Fibronectin fibrillogenesis: a paradigm for extracellular matrix assembly. _Curr. Opin. Cell Biol._ 11, 622–627 (1999). CAS PubMed Google Scholar * Erickson, H. P.
Reversible unfolding of fibronectin type III and immunoglobulin domains provides the structural basis for stretch and elasticity of titin and fibronectin. _Proc. Natl Acad. Sci. USA_ 91,
10114–10118 (1994). CAS PubMed PubMed Central Google Scholar * Hocking, D. C., Smith, R. K. & McKeown-Longo, P. J. A novel role for the integrin-binding III-10 module in fibronectin
matrix assembly. _J. Cell Biol._ 133, 431–444 (1996). CAS PubMed Google Scholar * Wu, C., Keivens, V. M., O'Toole, T. E., McDonald, J. A. & Ginsberg, M. H. Integrin activation
and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. _Cell_ 83, 715–724 (1995). CAS PubMed Google Scholar * Halliday, N. L. & Tomasek, J. J. Mechanical
properties of the extracellular matrix influence fibronectin fibril assembly _in vitro_. _Exp. Cell Res._ 217, 109–117 (1995). CAS PubMed Google Scholar * Zhong, C. et al. Rho-mediated
contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. _J. Cell Biol._ 141, 539–551 (1998).THIS PAPER LINKS CELL CONTRACTILITY REGULATED BY RHO AND
STRETCH-INDUCED EXPOSURE OF CRYPTIC ASSEMBLY SITES IN FIBRONECTIN TO PROVIDE MECHANISTIC INSIGHT INTO MATRIX ASSEMBLY. CAS PubMed PubMed Central Google Scholar * Sechler, M. S. et al. A
novel fibronectin binding site required for fibronectin fibril growth during matrix assembly. _J. Cell Biol._ 154, 1081–1088 (2001).THE LATEST PUBLICATION IN ONGOING STUDIES TO IDENTIFY THE
KEY BINDING SITES IN FIBRONECTIN, WHICH POINTS TO REPEATING UNIT III 2 AS PARTICULARLY IMPORTANT FOR MATRIX ASSEMBLY. CAS PubMed PubMed Central Google Scholar * Ohashi, T., Kiehart, D.
P. & Erickson, H. P. Dynamics and elasticity of the fibronectin matrix in living cell culture visualized by fibronectin-green fluorescent protein. _Proc. Natl Acad. Sci. USA_ 96,
2153–2158 (1999).THE FIRST PAPER TO DOCUMENT STRETCHING OF FIBRONECTIN IN LIVING CELLS USING GREEN FLUORESCENT PROTEIN (GFP). CAS PubMed PubMed Central Google Scholar * Finer, J. T.,
Simmons, R. M. & Spudich, J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. _Nature_ 368, 113–119 (1994). CAS PubMed Google Scholar * Smilenov, L. B.,
Mikhailov, A., Pelham, R. J., Marcantonio, E. E. & Gundersen, G. G. Focal adhesion motility revealed in stationary fibroblasts. _Science_ 286, 1172–1174 (1999). CAS PubMed Google
Scholar * Selve, N. & Wegner, A. Rate of treadmilling of actin filaments _in vitro_. _J. Mol. Biol._ 187, 627–631 (1986). CAS PubMed Google Scholar * Waterman-Storer, C. M., Desai,
A., Bulinski, J. C. & Salmon, E. D. Fluorescent speckle microscopy, a method to visualize the dynamics of protein assemblies in living cells. _Curr. Biol._ 8, 1227–1230 (1998). CAS
PubMed Google Scholar * Small, J. V., Rottner, K., Kaverina, I. & Anderson, K. I. Assembling an actin cytoskeleton for cell attachment and movement. _Biochim. Biophys. Acta_ 1404,
271–281 (1998). CAS PubMed Google Scholar * Lo, S. H., Janmey, P. A., Hartwig, J. H. & Chen, L. B. Interactions of tensin with actin and identification of its three distinct
actin-binding domains. _J. Cell Biol._ 125, 1067–1075 (1994). CAS PubMed Google Scholar * Craig, D., Krammer, A., Schulten, K. & Vogel, V. Comparison of the early stages of forced
unfolding for fibronectin type III modules. _Proc. Natl Acad. Sci. USA_ 98, 5590–5595 (2001). CAS PubMed PubMed Central Google Scholar * Krammer, A., Lu, H., Isralewitz, B., Schulten, K.
& Vogel, V. Forced unfolding of the fibronectin type III module reveals a tensile molecular recognition switch. _Proc. Natl Acad. Sci. USA_ 96, 1351–1356 (1999). CAS PubMed PubMed
Central Google Scholar * McKeown-Longo, P. J. & Mosher, D. F. Binding of plasma fibronectin to cell layers of human skin fibroblasts. _J. Cell Biol._ 97, 466–472 (1983). CAS PubMed
Google Scholar * Langenbach, K. J. & Sottile, J. Identification of protein-disulfide isomerase activity in fibronectin. _J. Biol. Chem._ 274, 7032–7038 (1999). CAS PubMed Google
Scholar * Teti, A., Marchisio, P. C. & Zallone, A. Z. Clear zone in osteoclast function: role of podosomes in regulation of bone-resorbing activity. _Am. J. Physiol._ 261, C1–C7 (1991).
CAS PubMed Google Scholar * Danen, E. H., Sonneveld, P., Sonnenberg, A. & Yamada, K. M. Dual stimulation of Ras/mitogen-activated protein kinase and RhoA by cell adhesion to
fibronectin supports growth factor–stimulated cell cycle progression. _J. Cell Biol._ 151, 1413–1422 (2000). CAS PubMed PubMed Central Google Scholar * Davies, P. F., Robotewskyj, A.
& Griem, M. L. Quantitative studies of endothelial cell adhesion. Directional remodeling of focal adhesion sites in response to flow forces. _J. Clin. Invest._ 93, 2031–2038 (1994). CAS
PubMed PubMed Central Google Scholar * Chen, K. D. et al. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. _J. Biol. Chem._ 274,
18393–18400 (1999). CAS PubMed Google Scholar * Jalali, S. et al. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands.
_Proc. Natl Acad. Sci. USA_ 98, 1042–1046 (2001). CAS PubMed PubMed Central Google Scholar * Hamill, O. P. & Martinac, B. Molecular basis of mechanotransduction in living cells.
_Physiol. Rev._ 81, 685–740 (2001). CAS PubMed Google Scholar * Muller, U. & Littlewood-Evans, A. Mechanisms that regulate mechanosensory hair cell differentiation. _Trends Cell
Biol._ 11, 334–342 (2001). CAS PubMed Google Scholar * Nicklas, R. B., Campbell, M. S., Ward, S. C. & Gorbsky, G. J. Tension-sensitive kinetochore phosphorylation _in vitro_. _J. Cell
Sci._ 111, 3189–3196 (1998). CAS PubMed Google Scholar * Bjorge, J. D., Jakymiw, A. & Fujita, D. J. Selected glimpses into the activation and function of Src kinase. _Oncogene_ 19,
5620–5635 (2000). CAS PubMed Google Scholar * Ishikawa, H. et al. Structural conversion between open and closed forms of radixin: low-angle shadowing electron microscopy. _J. Mol. Biol._
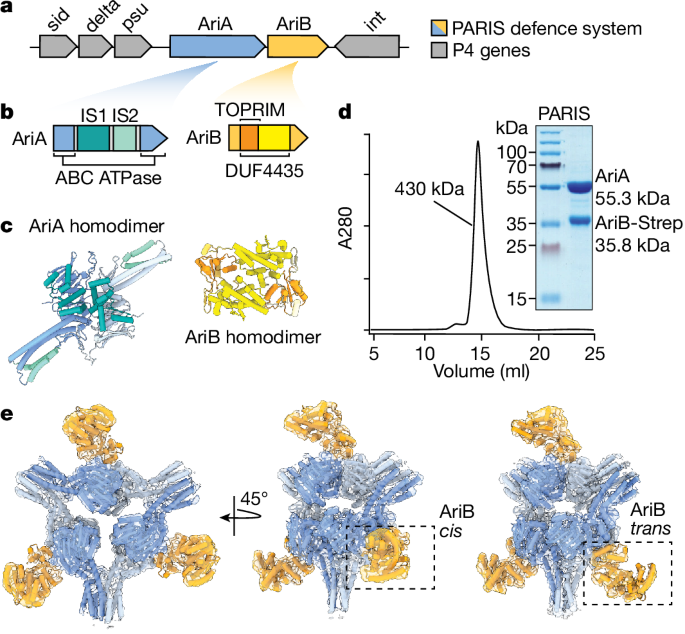
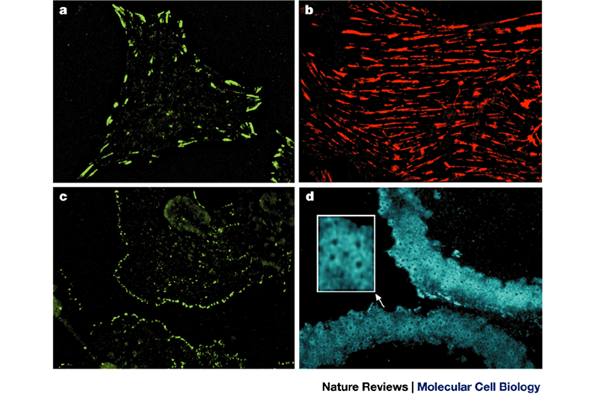
310, 973–978 (2001). CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS Figure 1a and b were kindly provided by E. Zamir, figure 1c by I. Grosheva and Figure 1d by J. Blair.
B.G. is the incumbent of the E. Neter Chair in Cell and Tumor Biology, A.B. holds the J. Moss Chair of Biomedical Research. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Molecular Cell Biology, The Weizmann Institute of Science, Rehovot, 76100, Israel Benjamin Geiger & Alexander Bershadsky * Craniofacial Developmental Biology and Regeneration Branch,
National Institute of Craniofacial and Dental Research, National Institutes of Health, Bethesda, 20892, MD, USA Roumen Pankov & Kenneth M. Yamada Authors * Benjamin Geiger View author
publications You can also search for this author inPubMed Google Scholar * Alexander Bershadsky View author publications You can also search for this author inPubMed Google Scholar * Roumen
Pankov View author publications You can also search for this author inPubMed Google Scholar * Kenneth M. Yamada View author publications You can also search for this author inPubMed Google
Scholar CORRESPONDING AUTHOR Correspondence to Benjamin Geiger. RELATED LINKS RELATED LINKS DATABASES INTERPRO: SH2 domain LOCUSLINK: 14-3-3-β α-actinin ASAP1 calpain II caveolin dynamin
fibronectin filamin ILK α4 integrin α9 integrin LAR mDia1 PAK PI3K ponsin ROCK tensin uPAR VASP/Ena SWISS-PROT: caldesmon DOCK180 DRAL dystroglycan gelsolin GRAF α3 integrin α5 integrin α6
integrin αv integrin β1 integrin β3 integrin layilin paxillin SHIP-2 SHPS-1 SHP-2 syndecan-4 talin vinculin vinexin vitronectin GLOSSARY * INTEGRINS A group of heterodimeric transmembrane
adhesion receptors for extracellular-matrix proteins such as fibronectin and vitronectin. * BASEMENT MEMBRANE A dense, sheet-like, laminated extracellular matrix that separates epithelia,
muscle, or other tissues from connective tissue. * LAMELLIPODIUM A thin, flat extension at the cell periphery, which is filled with a branching meshwork of actin filaments. * RHO-FAMILY
GTPASES A family of monomeric G proteins — comprising Rho, Rac and Cdc42 — that are homologous to Ras. These are important molecular switches, which control cytoskeletal assembly and
contraction. * MACROPHAGE A white blood cell that is specialized for phagocytosis. * OSTEOCLAST A specialized cell that is involved in active bone resorption. * FIBRONECTIN MODULES Subunits
of fibronectin are comprised of repeating structural modules of three types (I, II, III). Each module is encoded by one or two exons with introns that precisely separate repeats. There are
12 type I modules, each around 45 amino-acids long and clustered into three groups; two type 2 modules, each 60 amino acids-long; and 15–17 type III repeats, each about 90 amino-acids long
(see Fig. 6). * BDM (2,3-Butanedione monoxime) An inhibitor of myosin ATPase. * ML-7 (1-(5-iodonaphthalene-1-sulphonyl)-1-H-hexahydro-1, 4-diazepine) A kinase inhibitor thought to be
relatively specific for myosin light-chain kinase. * H-7 (1-(5-isoquinolinylsulphonyl)-2-methylpiperazine) A broad-spectrum serine–threonine kinase inhibitor that blocks myosin light-chain
kinase, Rho kinase and certain other kinases. * LASER TWEEZERS Microscope-based device that traps micron-sized particles in a focused laser beam. Can be used to move or to stop such
particles. * LEADING EDGE The leading region of the advancing lamellipodium in a motile cell. * CELL-INDUCED SUBSTRATE WRINKLING An approach for visualizing cellular contractility that is
based on wrinkling of a thin and flexible silicone rubber film on which the cell is cultured. * MICRO-CANTILEVER TILTING DEVICE A microscopic device in which cells are attached to a surface
that consists of arrays of cantilevers. Local forces that are applied to this surface induce tilting of these cantilevers, which can be measured. * DEFORMATION OF ELASTIC GELS Polymeric
elastic gels that either contain impregnated beads or are surface micro-patterned are used as substrates for cultured cells. Local forces that are applied to these substrates can be
measured, based on the distortion of these patterns. * CONNECTIVE TISSUES Tissues that form the architectural framework of the vertebrate body. In these tissues, the extracellular matrix is
plentiful and cells are sparsely distributed within it. * GRANULATION TISSUE A contractile, myofibroblast-containing tissue formed in wounds. * ISOMETRIC TENSION A condition in which
contraction of muscle, non-muscle cells or the actomyosin network is opposed by an equal load that prevents net shortening, even though tension increases. * LATRUNCULIN-A A macrolide that is
derived from the Red Sea sponge _Latrunculia magnifica_, which binds and sequesters actin molecules, and thereby prevents the assembly of actin filaments. * TREADMILLING A special state in
polymer dynamics, when monomer addition at one end occurs at the same rate as monomer dissociation at the other end, which keeps the polymer length unchanged. * BARBED END The
fast-polymerizing end of actin filaments (defined by the arrowhead-shaped decoration of actin filaments with myosin fragments). * STEERED MOLECULAR DYNAMICS SIMULATION A computer simulation
method for studying force-induced reactions in biopolymers. * RGD ADHESION SEQUENCE The primary adhesive motif in many extracellular matrix molecules, which contains the amino-acid triplet,
Arg–Gly–Asp. * TRANSGLUTAMINASE An enzyme (such as factor XIIIa) that helps to crosslink fibronectin and other molecules through isopeptide linkages. RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Geiger, B., Bershadsky, A., Pankov, R. _et al._ Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. _Nat Rev Mol
Cell Biol_ 2, 793–805 (2001). https://doi.org/10.1038/35099066 Download citation * Issue Date: 01 November 2001 * DOI: https://doi.org/10.1038/35099066 SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative