- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Identifying the genetic _cis_ associations between DNA variants (single-nucleotide polymorphisms (SNPs)) and gene expression in brain tissue may be a promising approach to find
functionally relevant pathways that contribute to the etiology of psychiatric disorders. In this study, we examined the association between genetic variations and gene expression in
prefrontal cortex, hippocampus, temporal cortex, thalamus and cerebellum in subjects with psychiatric disorders and in normal controls. We identified _cis_ associations between 648
transcripts and 6725 SNPs in the various brain regions. Several SNPs showed brain regional-specific associations. The expression level of only one gene, _PDE4DIP_, was associated with a SNP,
rs12124527, in all the brain regions tested here. From our data, we generated a list of brain _cis_ expression quantitative trait loci (eQTL) genes that we compared with a list of
schizophrenia candidate genes downloaded from the Schizophrenia Forum (SZgene) database (http://www.szgene.org/). Of the SZgene candidate genes, we found that the expression levels of four
genes, _HTR2A_, _PLXNA2_, _SRR_ and _TCF4_, were significantly associated with _cis_ SNPs in at least one brain region tested. One gene, _SRR_, was also involved in a coexpression module
that we found to be associated with disease status. In addition, a substantial number of _cis_ eQTL genes were also involved in the module, suggesting eQTL analysis of brain tissue may
identify more reliable susceptibility genes for schizophrenia than case–control genetic association analyses. In an attempt to facilitate the identification of genetic variations that may
underlie the etiology of major psychiatric disorders, we have integrated the brain eQTL results into a public and online database, Stanley Neuropathology Consortium Integrative Database
(SNCID; http://sncid.stanleyresearch.org). SIMILAR CONTENT BEING VIEWED BY OTHERS CELL-TYPE-SPECIFIC _CIS_-EQTLS IN EIGHT HUMAN BRAIN CELL TYPES IDENTIFY NOVEL RISK GENES FOR PSYCHIATRIC AND
NEUROLOGICAL DISORDERS Article 01 August 2022 ASSOCIATED TRANSCRIPTIONAL, BRAIN AND CLINICAL VARIATIONS IN SCHIZOPHRENIA Article 09 September 2024 MULTI-ANCESTRY EQTL META-ANALYSIS OF HUMAN
BRAIN IDENTIFIES CANDIDATE CAUSAL VARIANTS FOR BRAIN-RELATED TRAITS Article 20 January 2022 INTRODUCTION Schizophrenia, bipolar disorder and severe depression are common and highly
disabling brain diseases caused by an interaction of genetic and environmental factors.1, 2 However, despite enormous efforts, the genetic variations that contribute to these diseases and
their environmental risk factors remain elusive. Genome-wide association studies have frequently been employed to identify susceptibility genes and single-nucleotide polymorphisms (SNPs)
that may be associated with these mental disorders.3, 4, 5 A number of candidate genes for the disorders have been reported. For instance, a web resource for schizophrenia, the Schizophrenia
Forum (SZgene) database (http://www.szgene.org/), includes results from 1727 genetic association studies and reports 1008 candidate genes and 8788 polymorphisms in the update on 15 April
2011.6 Despite the numerous candidate genes reported for schizophrenia, the effect size of each variant is small or moderate and most associated SNPs have failed to be replicated. The need
for independent and systematic validation to prioritize further examination of possible candidate genes for mental disease is widely acknowledged. Identification of DNA sequence variants
that regulate gene expression levels in a relevant tissue is one of the most promising approaches used to initially scan for candidate genes as well as to prioritize previously identified
candidate genes that are associated with complex disease such as psychiatric disorders.7, 8, 9 The identification of a _cis_ association of a SNP with gene expression levels has been
previously used to validate candidate genes for complex traits mapped to the same chromosomal locations.10 Our recent study using an integrative approach that combined results from
genome-wide SNP scans for the cytoarchitectural traits and _cis_ expression quantitative trait loci (eQTL) analysis in the brain tissue revealed two novel candidate genes associated with
cellular abnormalities in the prefrontal cortex of major psychiatric disorders.11 Limited availability of human post-mortem brain tissues is a major obstacle to obtaining detailed brain
expression complex trait loci (eQTL) mapping. Utilization of publicly available resources is an effective alternative strategy that may overcome such limitation. The Stanley Neuropathology
Consortium Integrative Database (SNCID; http://sncid.stanleyresearch.org) is a publicly available and web-based tool that integrates expression microarray data sets from five brain regions
including frontal cortex, temporal cortex, thalamus, cerebellum and hippocampus and genome-wide SNP genotype data sets of subjects in the Stanley Neuropathology Consortium (SNC) and the
Array Collection (AC).12 A total of 1749 neuropathology data sets using the SNC are integrated into the database, which thereby enables one to further explore the correlations between gene
expression levels and quantitative measures of neuropathological markers in the various brain regions. The specific aims of this study are twofold. First, we explore the candidate genes that
may be functionally relevant for major psychiatric disorders by identifying _cis_ associations between SNPs and gene expression in various brain tissues. Second, we examine the possible
functional role of schizophrenia candidate genes that were previously identified in genetic association studies. Thus, we explored _cis_ eQTLs in the four brain regions, frontal cortex,
temporal cortex, thalamus and cerebellum, of SNC subjects and in hippocampus of AC subjects. We also repeated the analysis in frontal cortex data from the AC as a replication study to
examine the overall consensus of _cis_ eQTLs between the two frontal data sets. We then examined whether the expression levels of any candidate genes from the SZgene database meta-analysis
(http://www.szgene.org/) were regulated by _cis_ expressed SNPs (eSNPs) in brain tissues, in order to determine if there were any functional effects on gene expression of the previously
identified schizophrenia susceptibility genes. Finally, we performed a coexpression network analysis between the genes in the frontal cortex that were differentially expressed between
schizophrenia and normal controls and the _cis_ eQTL genes in an attempt to identify the potential role of these genes in a disease-specific coexpression module. MATERIALS AND METHODS DATA
USED IN THIS STUDY Gene expression microarray data from frontal cortex,13 cerebellum, thalamus and temporal cortex14were generated by multiple independent groups using samples from the SNC
(_N_=60), which contains 15 well-matched cases in each of four groups: schizophrenia, bipolar disorder, major depression and unaffected controls.15 Other sets of microarray data from frontal
cortex16, 17and hippocampus were generated using samples from the AC (_N_=105). The AC is an independent tissue collection containing 35 cases in each of three groups: schizophrenia,
bipolar disorder and unaffected controls. The groups from both tissue collections are matched for descriptive variables such as age, gender, race, post-mortem interval, mRNA quality, brain
pH and hemisphere. Outlier chip data were excluded in this analysis based on previous quality-control analyses for chip-level parameters such as scaling factor, gene call and average
correlation.18 Information for the microarray studies such as tissue collection, brain region and number of outlier chips is listed in the Supplementary Table S1 online. The confounding
effects on the Frozen Robust Multiarray Analysis (fRMA)-normalized microarray gene expression data were identified using Surrogate Variable Analysis (SVA).19 To adjust disease effect on the
gene expression data, we randomly assign 0 or 1 for the primary variable in the SVA. All covariates from SVA were used in the linear regression to adjust the confounding effects on the gene
expression data. The standardized residuals from the linear regression were used to evaluate the effectiveness of this method on removing confounding variables on two microarray data sets
from both the SNC and AC. Transcripts correlated with potential confounding variables were identified using nonparametric analysis. The continuous variables such as age, brain pH,
post-mortem interval and lifetime exposure to antipsychotics were examined by correlation analysis using R (open source program from Comprehensive R Archive Network (CRAN)). Two categorical
variables such as microarray batch and sex were tested using variance analysis. Adjusted _P_-values, based on the Hochberg method that were <0.05, were considered significant. Although
all cases and controls were included in the analysis, only the disorder cases were used for the correlation analysis for the effect of lifetime exposure to antipsychotics. SNP genotyping
data using DNA samples from the SNC and the AC were generated by Dr Chun-Yu Liu and colleagues (University of Chicago, IL, USA) using the Human SNP Array 5.0 chips (Affymetrix, Santa Clara,
CA, USA).20 EQTL ANALYSIS Raw image files from SNP chips, quality-control analysis and identification of ethnic outliers were performed as previously described.11 Briefly, genotypes were
called using the BRLMM algorithm (Affymetrix). SNPs with a call rate of <90%, minor allele frequency <5% or extreme deviation from Hardy–Weinberg equilibrium test (_P_<0.05) were
filtered out for further eQTL analyses. A total of 309 531 SNPs passed this filter. For examination of population stratification, clustering was initially performed using the pairwise
identity-by-state (IBS) calculator in the PLINK.21 IBS pairwise distances were then plotted and examined by multidimensional scaling analysis and Z statistical analysis. Samples of >3
s.d. compared with the group mean were considered outliers. Four ethnic outliers from the SNC and three outliers from the AC were excluded in the eQTL analysis. One additional sample from AC
was excluded because of a final diagnosis of CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy). We only used genotyped SNP data from chips
for our association analysis rather than imputing genotypes because SNP imputation can often result in errors in genotyping and cause false-positive associations.22 The standardized
residuals from the linear regression were used as traits in PLINK for eQTL analyses. We defined _cis_ eSNPs as those that were localized within 1 Mb of either the 5′ or the 3′ end of the
gene. The _trans_ eSNPs were defined as all SNPs that reached genome-wide significance level, except those in a _cis_ position. We employed a conservative Bonferroni method to correct
multiple testing for controlling false positives.7 Adjusted _P_-values of <0.05 (unadjusted _P_-value; 1.6E−07 =0.05/309 531) were considered genome-wide significant for eQTL analyses.
COEXPRESSION NETWORK ANALYSIS Unsupervised and supervised coexpression network analyses were performed using the Weighted Correlation Network Analysis (WGCNA) in R.23 The coexpression
network was generated using expression values of all genes in the frontal cortex of schizophrenia and normal controls from the AC (unsupervised WGCNA). A second coexpression network was
generated using significant _cis_ eQTL genes and genes that were differentially expressed in the frontal cortex between schizophrenia and normal controls from the AC samples (supervised
WGCNA).24 A total of four microarray data sets (at www.stanleygenomics.org; study no. 1, 3, 5 and 7) were generated from prefrontal cortex. Three of these (study no. 1, 3 and 7)16, 17 were
generated using the same platform, Affymetrix 133a, and hence to avoid variations between platforms we pooled the data from these three data sets. The pooled data were then subjected to
median normalization with the biometric research branch (BRB)-array tools (http://linus.nci.nih.gov/BRB-ArrayTools.html) to remove systematic variations. After median normalization,
confounding effects were adjusted using SVA and a linear regression method as described in the previous section. However, disease effect was not removed. Standardized residuals that were
significantly associated with disease (nominal _P_-value <0.05) and standardized residuals of _cis_ eQTL genes were then used as input for the WGCNA.23 The minimum module size and the
minimum height for merging modules were set at 30 and 0.25, respectively. The coexpression module was visualized using VisANT.25 FUNCTIONAL ANNOTATION The _cis_ eQTL genes and genes that
were involved in the coexpression module were functionally annotated using the Database for Annotation, Visualization and Integrated Discovery (DAVID) database
(http://david.abcc.ncifcrf.gov/home.jsp) and by the over-representational analysis method.26 The biological processes of Gene Ontology Consortium (http://www.geneontology.org) were used for
functional annotations. The _P_-values of <0.05 were considered significant. RESULTS EQTL ANALYSIS IN VARIOUS HUMAN POST-MORTEM BRAIN TISSUES Gene expression microarray data derived from
post-mortem brain tissue are often confounded by uncontrolled biological, clinical and technical variables.27 Batch effect is particularly problematic and has been shown to significantly
affect gene expression levels in microarray data.28, 29 To remove the effect of batch and other confounding variables in our gene expression microarray data, we normalized the data using the
newly developed method, fRMA, followed by the SVA.19, 30 We evaluated how effective this method was at removing confounding variables using two microarray data sets from both the SNC and AC
(Supplementary Table S1 online). Using the data set from SNC temporal cortex (study 18) we found that microarray batch was the most significant confounding variable in both the RMA and
fRMA-normalized data sets, with 947 and 1031 transcripts significantly correlated with batch, respectively (Supplementary Table S2 online). Using the data set from AC frontal cortex (study
1) we found that microarray batch and brain pH were both major confounding variables (Supplementary Table S3 online). The SVA successfully adjusted the effects of the confounding variables
on both microarray data sets (Supplementary Table S2 and S3 online). Using the SVA we obtained the standardized residuals from the linear regression with covariates and conducted a
genome-wide eQTL analysis of various brain tissues. We used the standardized residuals as traits. We initially analyzed gene expression microarray data from frontal cortex, temporal cortex,
thalamus and cerebellum from the SNC (Supplementary Table S1 online). Expression levels of a total of 53, 11, 84 and 27 genes were correlated with _cis_ SNPs in the frontal cortex, temporal
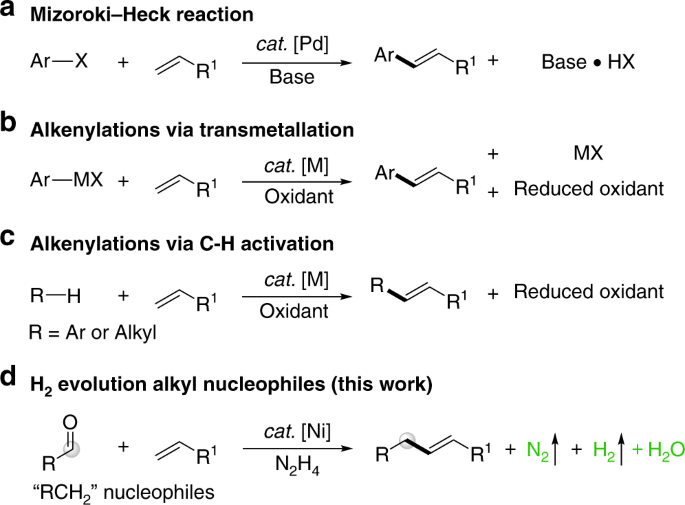
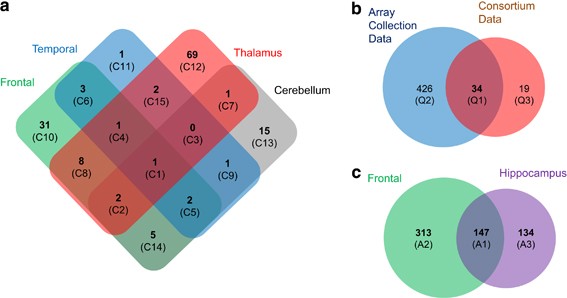
cortex, thalamus and cerebellum at genome-wide significance level, respectively (nominal _P_<1.6E−07; Figure 1a and Supplementary Table S4 online). Among the _cis_ eQTL genes, expression
levels of 16, 0, 20 and 5 genes were also significantly associated with _trans_ SNPs in the frontal cortex, temporal cortex, thalamus and cerebellum, respectively (Supplementary Table S5
online). In addition, correlations between the expression levels of 31, 1, 69 and 15 genes and _cis_ SNPs were unique in the frontal cortex, temporal cortex, thalamus and cerebellum,
respectively (Figure 1a). The expression level of only one gene, _phosphodiesterase 4D interacting protein_ (_PDE4DIP_), was associated with a SNP, rs12124527, in all the brain regions
tested here. We then replicated the _cis_ eQTLs of the frontal cortex using the larger AC collection. The replication study revealed associations between _cis_ SNPs and expression levels of
460 genes and replicated 34 _cis_ eQTL genes out of 53 (64%) that were identified in the SNC study (Figure 1b and Supplementary Table S6 online). Moreover, 281 _cis_ eQTL genes were
identified in the AC hippocampus data and 147 _cis_ eQTLs were common to both the frontal cortex and hippocampus (Figure 1c and Supplementary Table S7 online). Among the _cis_ eQTL genes,
expression levels of 43 and 46 genes were also significantly associated with _trans_ SNPs in the frontal cortex and hippocampus, respectively (Supplementary Table S5 online). The association
between _PDE4DIP_ expression and the rs12124527 SNP was replicated in the AC frontal cortex and hippocampal data. Next, we performed a functional annotation analysis to identify biological
processes that were overrepresented in the brain _cis_ eQTL genes. Whereas several processes such as cell adhesion, visual perception and glutamatergic transmission were overrepresented in
the genes with _cis_ eSNPs in the SNC frontal cortex (Table 1), metabolic processes such as glutamine metabolic process and protein transport and targeting and antigen processing were
overrepresented in the AC frontal cortex (Table 2). Amino acid metabolic process, nucleotide biosynthesis and enzyme-linked receptor protein signaling pathways were significantly
overrepresented in _cis_ eQTL genes in the AC hippocampus (Supplementary Table S8 online). COMPARISON BETWEEN SCHIZOPHRENIA SUSCEPTIBILITY CANDIDATE GENES AND BRAIN _CIS_ EQTL GENES Genetic
association studies have yielded numerous candidate genes that may increase the risk for schizophrenia. However, most candidate genes have not been replicated nor functionally validated. To
examine the possible functional role of schizophrenia candidate genes, we compared the list of the candidate genes in the SZgene database meta-analysis (updated 12/1/2010) to our list of
_cis_ eQTL genes. The SZgene meta-analysis identified 45 genetic variants and 42 linked genes. After excluding the non-SNP variants from their data set, we were left with 39 SNPs and 39
linked candidate genes. Because only 6 SNP markers out of the 39 SNPs were included in our Affymetrix SNP 5.0 data set, we conducted a gene-level comparison instead of SNP-level comparison.
We determined whether there were _cis_ associations between the expression levels of the 39 candidate genes and SNPs within 1 Mb of the genes. Among the 39 candidate genes, we found that the
expression levels of four genes, _HTR2A_, _PLXNA2_, _SRR_ and _TCF4_, were significantly associated with _cis_ SNPs in at least one brain region tested (Table 3). The expression levels of
_HR2A_ and PLXNA2 were associated with _cis_ SNPs in the frontal cortex, whereas the expression levels of _SRR_ (_serine racemase_) and _TCF4_ were associated with _cis_ SNPs in two brain
regions. The _cis_ eSNPs of these genes are located at least 25 kb from the SNPs that were significantly associated with schizophrenia in the SZgene meta-analysis. Thus the SZgene
case–control genetic association analyses for schizophrenia may not have identified the most functionally relevant genetic variations that contribute to the etiology of psychiatric
disorders. COEXPRESSION NETWORK ANALYSIS IN THE FRONTAL CORTEX To further examine whether or not the four schizophrenia candidate genes (_HTR2A_, _PLXNA2_, _SRR_ and _TCF4_) and genes of
which expression levels were regulated by _cis_ SNPs may be involved in the etiology of schizophrenia, we performed both unsupervised and supervised gene coexpression network analyses using
the AC frontal cortex data. We were unable to construct a coexpression module that was significantly associated with schizophrenia disease status using the unsupervised analysis. One module
was associated with disease (_P_=0.05); however, it was also associated with post-mortem interval (_P_=0.01). We then constructed a supervised coexpression network using genes differentially
expressed between schizophrenia and normal controls (Supplementary Table S9 online) and the _cis_ eQTL genes obtained from the pooled data of three Affymetrix 133a microarray data sets that
measured gene expression in the frontal cortex. We constructed one coexpression module that was significantly associated with schizophrenia disease status (_P_=2E−08; Figure 2a). Age, sex,
post-mortem interval, brain pH and lifetime antipsychotic treatment were not significantly associated with this module (all _P_>0.05). Genes associated with apoptosis, chromatin
organization, RNA splicing, cell cycle, regulation of nucleic acid metabolism and endocytosis were overrepresented in this module (Figure 2b and Supplementary Table 10 online). A previous
coexpression network analysis that used gene expression microarray data from prefrontal cortex from schizophrenia subjects and controls31 also identified a module (module 16) with similar
overrepresentation of biological processes such as chromatin organization, cell cycle, endocytosis and regulation of nucleic acid metabolism. Apoptosis and endocytosis have previously been
associated with the pathophysiology of the frontal cortex in schizophrenia,32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 and recent studies have also indicated that aberrant RNA
splicing and epigenetic alterations may be involved in the pathophysiology of schizophrenia.35, 36 Several genes associated with GABAergic neurons, including γ-aminobutyric acid (GABA) A
receptor, δ (_GABRD_) and _parvalbumin_ (_PVALB_), were found in the coexpression module. However, out of the four schizophrenia candidate genes common to both the SZgene meta-analysis and
our _cis_ eQTL gene list, only one, _SRR_, was found in the module. The biological process, response to drug, was overrepresented in the module and was enriched with 11 genes including
_SRR_. A substantial number of _cis_ eQTL genes were involved in the coexpression module that was significantly associated with schizophrenia disease status and were also associated with the
biological processes. This result indicates that _cis_ eQTL analysis in brain tissue may more reliably identify susceptibility genes for schizophrenia as compared with the current
case–control genetic association studies. DISCUSSION Identifying genetic variations that affect gene expression in the brain may be a promising approach for finding molecular pathways that
are functionally relevant to the etiology and/or treatment of mental disease. In this study, we conducted an eQTL analysis of 315 440 transcripts in 5 different brain regions from two
different tissue collections and identified _cis_ associations between 648 transcripts and 6725 SNPs. The expression of one gene, _PDE4DIP_, was associated with one SNP, rs12124527, in all
brain regions examined. This association was also previously described in the frontal cortex.20 The protein encoded by PDE4DIP serves to anchor phosphodiesterase 4D to the Golgi/centrosome
region of the cell. A number of abnormalities in the phosphodiesterase signaling system have been described in the brains of subjects with schizophrenia, bipolar disorder and depression,37,
38, 39, 40, 41, 42 indicating that molecules within this system could be potential targets for therapeutic intervention.38, 43 Approximately 14% of _cis_ eQTL genes were also correlated with
_trans_ SNPs in various brain regions, suggesting that the expression levels of a subset of _cis_ eQTL genes may be regulated by multiple variants. However, when we examined whether or not
the expression levels of candidate genes from the SZgene database meta-analysis were significantly associated with _cis_ SNPs, we found only 4 genes that overlapped between the SZgene
database and our eQTL gene list. Furthermore, only one candidate gene, _SRR_, was involved in a coexpression module that was associated with schizophrenia. _SRR_ maps to chromosome 17p13 and
encodes an enzyme that synthesizes D-serine from L-serine.44 The D-serine is an endogenous co-agonist of the _N_-methyl-D-aspartate (NMDA) receptor.45 Hypofunction of the NMDA receptor is
potentially a major underlying pathophysiology of schizophrenia.46, 47 Our results support this hypothesis and suggest that abnormal NMDA receptor-mediated signaling may be influenced by
genetic variations. A SNP, rs16952025, localized in an intron of the neighboring gene, _SMG6_, was significantly associated with the expression level of _SRR_. However, there was no
significant association between this SNP and the expression level of _SMG6_. Several post-mortem studies have examined levels of _SRR_ mRNA and serine racemase protein in schizophrenia48 and
found abnormalities in schizophrenia, although the results have been inconsistent. Although _SRR_ mRNA levels appear to be unchanged in frontal cortex of schizophrenia,41 the protein levels
have been reported to be either decreased,49 increased50 or unchanged.51 The inconsistent results are most likely because of different methodologies, different cohorts (often with small
numbers) and the different brain areas used. Consequently further study will be required in the future when larger cohorts become available to confirm changes in _SRR_ levels in the brain of
subjects with mental illness. Our comprehensive brain eQTL analysis functionally validated only 4 genes out of 39 candidate genes positively identified in the SZgene meta-analysis. We were
unable to identify any significant associations between the expression levels of the remaining genes and _cis_ SNPs in any of the brain regions we tested. In fact, the 39 candidate genes
were derived from 1008 candidate genes that were obtained from 1727 original genetic association studies. Such a low functional validation rate raises the possibility that the current
case–control genetic association studies may not effectively identify genetic variations that underlie the etiology of schizophrenia. However, there are other reasons that may contribute to
a low functional validation rate. For example, the probes on the microarray platforms used to analyze gene expression in this study mainly bind to sequences in the 3′-untranslated regions
and do not distinguish between various alternative splicing isoforms. Indeed, tissue-specific alternative RNA-splicing is very predominant in the brain.52 Furthermore, intronic SNPs can be
associated with the altered expression of specific alternative splicing isoforms of certain schizophrenia candidate genes, for example, _ErbB4_ and _GRM3_.53, 54 Therefore, comprehensive
expression profiling that includes various alternative splicing isoforms using deep mRNA-sequencing technology may aid in the identification of novel _cis_ eQTL genes in human post-mortem
brain tissues in the future. The frontal cortex is one of the most thoroughly examined brain regions in post-mortem studies and many neuropathology abnormalities have been identified in this
region in schizophrenia.55, 56 Previous gene expression microarray studies in the frontal cortex identified several biological processes that were overrepresented in the genes
differentially expressed between schizophrenia and normal controls; for example, decreased presynaptic function, abnormal mitochondrial function and altered expression of apoptosis-related
genes are all major findings from microarray studies of frontal cortex in schizophrenia.33, 34, 57 However, glutamatergic transmission, amino acid metabolism, proteolysis and protein
targeting were all overrepresented in the eQTL genes in the frontal cortex in our current study. Thus, the abnormalities described in the biological pathways from the eQTL study may be more
directly related to genetic variation, whereas the pathways identified by gene expression studies are likely to be influenced by factors in addition to genetic variation, including
epigenetics and environmental factors. Although our study reveals a number of associations between _cis_ SNPs and gene expression in multiple brain regions, the results should be interpreted
with caution. First, the SNC, which we used for the initial eQTL analyses, contains a relatively small sample size (_N_=56). Small sample size is known to generate higher false-positive
associations as well as to be a cause of low detection power in genome-wide association analysis. Thus, the _cis_ eQTL results from frontal cortex, cerebellum, thalamus, and temporal cortex
using SNC samples should be viewed as exploratory. However, we subsequently performed a second analysis using an independent collection (AC) with a larger sample size (_N_=101). Our previous
power analysis using AC as well as a previous eSNP association study indicated that a relatively small sample size (_N_=100) has >80% power to detect an association of gene expression
traits with moderate effect size (_R_2=0.35).11, 58 We therefore attempted to replicate the results of _cis_ eQTLs in frontal cortex from SNC using the AC data. A total of 34 (64%) of the
_cis_ eQTL genes identified in the SNC frontal cortex data were also found in the AC frontal data. However, we identified 450 additional _cis_ eQTL genes in the AC frontal cortex samples,
which were not identified in the SNC frontal samples, suggesting that some significant _cis_ eQTL genes may have been missed in the SNC analysis. Second, using whole tissues for gene
expression traits may dilute the effect of some genetic variants that may only act on cell type-specific gene expression. Although this phenomena has not been explored in the brain, there
are numerous cell type-specific abnormalities in the brain of subjects with psychiatric disorders.32, 59, 60, 61 Thus, the use of cell type-specific expression traits in future studies may
increase the power to identify _cis_ eQTLs in the brain. In this study, we investigated the associations between SNPs and gene expression in various human brain regions. Although previous
brain eQTL studies focused on cortex,8, 62 we have extended the analysis to include the hippocampus, thalamus and cerebellum. These data can be used to identify genetic variations associated
with psychiatric disorder and can be used to identify genetic variations that affect neuropathological abnormalities and gene expression changes. As we show in this study, the data can be
used to functionally validate candidate genes to determine if they are affecting changes in gene expression in subjects with neuropsychiatric disorders. In order to facilitate further
studies, we have integrated the genome-wide eQTL results from this study into the SNCID, which is a web-based database that also includes 1747 neuropathological markers measured in the same
SNC samples. The update will allow users to investigate associations between SNPs and genes of interests in various brain regions and to further explore associations between SNPs and
neuropathological markers and gene expression traits that are correlated with neuropathological markers in the various brain regions of subject with major psychiatric disorders. REFERENCES *
Mueser KT, McGurk SR . Schizophrenia. _Lancet_ 2004; 363: 2063–2072. Article PubMed Google Scholar * Sullivan PF, Neale MC, Kendler KS . Genetic epidemiology of major depression: review
and meta-analysis. _Am J Psychiatry_ 2000; 157: 1552–1562. Article CAS PubMed Google Scholar * Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF _et al_. Common
polygenic variation contributes to risk of schizophrenia and bipolar disorder. _Nature_ 2009; 460: 748–752. CAS PubMed Google Scholar * Stefansson H, Ophoff RA, Steinberg S, Andreassen
OA, Cichon S, Rujescu D _et al_. Common variants conferring risk of schizophrenia. _Nature_ 2009; 460: 744–747. CAS PubMed PubMed Central Google Scholar * Sklar P, Smoller JW, Fan J,
Ferreira MA, Perlis RH, Chambert K _et al_. Whole-genome association study of bipolar disorder. _Mol Psychiatry_ 2008; 13: 558–569. Article CAS PubMed PubMed Central Google Scholar *
Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ _et al_. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene
database. _Nat Genet_ 2008; 40: 827–834. Article CAS PubMed Google Scholar * Dixon AL, Liang L, Moffatt MF, Chen W, Heath S, Wong KC _et al_. A genome-wide association study of global
gene expression. _Nat Genet_ 2007; 39: 1202–1207. Article CAS PubMed Google Scholar * Myers AJ, Gibbs JR, Webster JA, Rohrer K, Zhao A, Marlowe L _et al_. A survey of genetic human
cortical gene expression. _Nat Genet_ 2007; 39: 1494–1499. Article CAS PubMed Google Scholar * Stranger BE, Nica AC, Forrest MS, Dimas A, Bird CP, Beazley C _et al_. Population genomics
of human gene expression. _Nat Genet_ 2007; 39: 1217–1224. Article CAS PubMed PubMed Central Google Scholar * Emilsson V, Thorleifsson G, Zhang B, Leonardson AS, Zink F, Zhu J _et al_.
Genetics of gene expression and its effect on disease. _Nature_ 2008; 452: 423–428. Article CAS PubMed Google Scholar * Kim S, Webster MJ . Integrative genome-wide association analysis
of cytoarchitectural abnormalities in the prefrontal cortex of psychiatric disorders. _Mol Psychiatry_ 2010; 16: 452–461. Article PubMed Google Scholar * Kim S, Webster MJ . The stanley
neuropathology consortium integrative database: a novel, web-based tool for exploring neuropathological markers in psychiatric disorders and the biological processes associated with
abnormalities of those markers. _Neuropsychopharmacology_ 2010; 35: 473–482. Article CAS PubMed Google Scholar * Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB _et al_.
Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. _Lancet_ 2003; 362: 798–805. Article CAS PubMed Google Scholar * Aston C, Jiang L, Sokolov BP . Microarray analysis of
postmortem temporal cortex from patients with schizophrenia. _J Neurosci Res_ 2004; 77: 858–866. Article CAS PubMed Google Scholar * Torrey EF, Webster M, Knable M, Johnston N, Yolken RH
. The stanley foundation brain collection and neuropathology consortium. _Schizophr Res_ 2000; 44: 151–155. Article CAS PubMed Google Scholar * Iwamoto K, Bundo M, Kato T . Altered
expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large-scale DNA microarray analysis. _Hum Mol Genet_ 2005;
14: 241–253. Article CAS PubMed Google Scholar * Ryan MM, Lockstone HE, Huffaker SJ, Wayland MT, Webster MJ, Bahn S . Gene expression analysis of bipolar disorder reveals downregulation
of the ubiquitin cycle and alterations in synaptic genes. _Mol Psychiatry_ 2006; 11: 965–978. Article CAS PubMed Google Scholar * Higgs BW, Elashoff M, Richman S, Barci B . An online
database for brain disease research. _BMC Genomics_ 2006; 7: 70. Article PubMed PubMed Central Google Scholar * Leek JT, Storey JD . Capturing heterogeneity in gene expression studies by
surrogate variable analysis. _PLoS Genet_ 2007; 3: 1724–1735. Article CAS PubMed Google Scholar * Liu C, Cheng L, Badner JA, Zhang D, Craig DW, Redman M _et al_. Whole-genome
association mapping of gene expression in the human prefrontal cortex. _Mol Psychiatry_ 2010; 15: 779–784. Article CAS PubMed PubMed Central Google Scholar * Purcell S, Neale B,
Todd-Brown K, Thomas L, Ferreira MA, Bender D _et al_. PLINK: a tool set for whole-genome association and population-based linkage analyses. _Am J Hum Genet_ 2007; 81: 559–575. Article CAS
PubMed PubMed Central Google Scholar * Pei YF, Li J, Zhang L, Papasian CJ, Deng HW . Analyses and comparison of accuracy of different genotype imputation methods. _PLoS One_ 2008; 3:
e3551. Article PubMed PubMed Central Google Scholar * Langfelder P, Horvath S . WGCNA: an R package for weighted correlation network analysis. _BMC Bioinformatics_ 2008; 9: 559. Article
PubMed PubMed Central Google Scholar * Gargalovic PS, Imura M, Zhang B, Gharavi NM, Clark MJ, Pagnon J _et al_. Identification of inflammatory gene modules based on variations of human
endothelial cell responses to oxidized lipids. _Proc Natl Acad Sci USA_ 2006; 103: 12741–12746. Article CAS PubMed PubMed Central Google Scholar * Hu Z, Mellor J, Wu J, DeLisi C .
VisANT: an online visualization and analysis tool for biological interaction data. _BMC Bioinformatics_ 2004; 5: 17. Article PubMed PubMed Central Google Scholar * Dennis Jr G, Sherman
BT, Hosack DA, Yang J, Gao W, Lane HC _et al_. DAVID: Database for Annotation, Visualization, and Integrated Discovery. _Genome Biol_ 2003; 4: P3. Article PubMed Google Scholar * Mirnics
K, Levitt P, Lewis DA . DNA microarray analysis of postmortem brain tissue. _Int Rev Neurobiol_ 2004; 60: 153–181. Article CAS PubMed Google Scholar * Fare TL, Coffey EM, Dai H, He YD,
Kessler DA, Kilian KA _et al_. Effects of atmospheric ozone on microarray data quality. _Anal Chem_ 2003; 75: 4672–4675. Article CAS PubMed Google Scholar * Leek JT, Scharpf RB, Bravo
HC, Simcha D, Langmead B, Johnson WE _et al_. Tackling the widespread and critical impact of batch effects in high-throughput data. _Nat Rev Genet_ 2010; 11: 733–739. Article CAS PubMed
Google Scholar * McCall MN, Bolstad BM, Irizarry RA . Frozen robust multiarray analysis (fRMA). _Biostatistics_ 2010; 11: 242–253. Article PubMed PubMed Central Google Scholar *
Torkamani A, Dean B, Schork NJ, Thomas EA . Coexpression network analysis of neural tissue reveals perturbations in developmental processes in schizophrenia. _Genome Res_ 2010; 20: 403–412.
Article CAS PubMed PubMed Central Google Scholar * Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI . Oligodendroglial density in the prefrontal cortex in schizophrenia and mood
disorders: a study from the Stanley Neuropathology Consortium. _Schizophr Res_ 2004; 67: 269–275. Article PubMed Google Scholar * Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P .
Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. _Neuron_ 2000; 28: 53–67. Article CAS PubMed Google Scholar * Kim S,
Webster MJ . Correlation analysis between genome-wide expression profiles and cytoarchitectural abnormalities in the prefrontal cortex of psychiatric disorders. _Mol Psychiatry_ 2010; 15:
326–336. Article CAS PubMed Google Scholar * McInnes LA, Lauriat TL . RNA metabolism and dysmyelination in schizophrenia. _Neurosci Biobehav Rev_ 2006; 30: 551–561. Article CAS PubMed
Google Scholar * Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP _et al_. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone
methylation at GABAergic gene promoters. _J Neurosci_ 2007; 27: 11254–11262. Article CAS PubMed PubMed Central Google Scholar * Millar JK, Pickard BS, Mackie S, James R, Christie S,
Buchanan SR _et al_. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. _Science_ 2005; 310: 1187–1191. Article CAS PubMed Google Scholar *
Wong ML, Whelan F, Deloukas P, Whittaker P, Delgado M, Cantor RM _et al_. Phosphodiesterase genes are associated with susceptibility to major depression and antidepressant treatment
response. _Proc Natl Acad Sci USA_ 2006; 103: 15124–15129. Article CAS PubMed PubMed Central Google Scholar * Fatemi SH, King DP, Reutiman TJ, Folsom TD, Laurence JA, Lee S _et al_.
PDE4B polymorphisms and decreased PDE4B expression are associated with schizophrenia. _Schizophr Res_ 2008; 101: 36–49. Article PubMed Google Scholar * Fatemi SH, Reutiman TJ, Folsom TD,
Lee S . Phosphodiesterase-4A expression is reduced in cerebella of patients with bipolar disorder. _Psychiatr Genet_ 2008; 18: 282–288. Article PubMed Google Scholar * Fatemi SH, Folsom
TD, Reutiman TJ, Vazquez G . Phosphodiesterase signaling system is disrupted in the cerebella of subjects with schizophrenia, bipolar disorder, and major depression. _Schizophr Res_ 2009;
119: 266–267. Article Google Scholar * Numata S, Iga J, Nakataki M, Tayoshi S, Taniguchi K, Sumitani S _et al_. Gene expression and association analyses of the phosphodiesterase 4B (PDE4B)
gene in major depressive disorder in the Japanese population. _Am J Med Genet B Neuropsychiatr Genet_ 2009; 150B: 527–534. Article CAS PubMed Google Scholar * Hennah W, Porteous D . The
DISC1 pathway modulates expression of neurodevelopmental, synaptogenic and sensory perception genes. _PLoS One_ 2009; 4: e4906. Article PubMed PubMed Central Google Scholar * Wolosker
H, Blackshaw S, Snyder SH . Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. _Proc Natl Acad Sci USA_ 1999; 96:
13409–13414. Article CAS PubMed PubMed Central Google Scholar * Leeson PD, Iversen LL . The glycine site on the NMDA receptor: structure-activity relationships and therapeutic
potential. _J Med Chem_ 1994; 37: 4053–4067. Article CAS PubMed Google Scholar * Olney JW, Newcomer JW, Farber NB . NMDA receptor hypofunction model of schizophrenia. _J Psychiatr Res_
1999; 33: 523–533. Article CAS PubMed Google Scholar * Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y _et al_. Postnatal NMDA receptor ablation in corticolimbic interneurons
confers schizophrenia-like phenotypes. _Nat Neurosci_ 2010; 13: 76–83. Article CAS PubMed Google Scholar * Verrall L, Burnet PW, Betts JF, Harrison PJ . The neurobiology of D-amino acid
oxidase and its involvement in schizophrenia. _Mol Psychiatry_ 2010; 15: 122–137. Article CAS PubMed Google Scholar * Bendikov I, Nadri C, Amar S, Panizzutti R, De Miranda J, Wolosker H
_et al_. A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. _Schizophr Res_ 2007; 90: 41–51. Article PubMed Google Scholar * Verrall L, Walker M, Rawlings
N, Benzel I, Kew JN, Harrison PJ _et al_. d-Amino acid oxidase and serine racemase in human brain: normal distribution and altered expression in schizophrenia. _Eur J Neurosci_ 2007; 26:
1657–1669. Article PubMed PubMed Central Google Scholar * Steffek AE, Haroutunian V, Meador-Woodruff JH . Serine racemase protein expression in cortex and hippocampus in schizophrenia.
_NeuroReport_ 2006; 17: 1181–1185. Article CAS PubMed Google Scholar * Grabowski PJ, Black DL . Alternative RNA splicing in the nervous system. _Prog Neurobiol_ 2001; 65: 289–308.
Article CAS PubMed Google Scholar * Sartorius LJ, Weinberger DR, Hyde TM, Harrison PJ, Kleinman JE, Lipska BK . Expression of a GRM3 splice variant is increased in the dorsolateral
prefrontal cortex of individuals carrying a schizophrenia risk SNP. _Neuropsychopharmacology_ 2008; 33: 2626–2634. Article CAS PubMed Google Scholar * Law AJ, Kleinman JE, Weinberger DR,
Weickert CS . Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. _Hum Mol Genet_ 2007; 16: 129–141.
Article CAS PubMed Google Scholar * Knable MB, Barci BM, Bartko JJ, Webster MJ, Torrey EF . Molecular abnormalities in the major psychiatric illnesses: Classification and Regression
Tree (CRT) analysis of post-mortem prefrontal markers. _Mol Psychiatry_ 2002; 7: 392–404. Article CAS PubMed Google Scholar * Knable MB, Torrey EF, Webster MJ, Bartko JJ . Multivariate
analysis of prefrontal cortical data from the Stanley Foundation Neuropathology Consortium. _Brain Res Bull_ 2001; 55: 651–659. Article CAS PubMed Google Scholar * Prabakaran S, Swatton
JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL _et al_. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. _Mol Psychiatry_ 2004; 9:
684–697, 643. Article CAS PubMed Google Scholar * Cheung VG, Spielman RS, Ewens KG, Weber TM, Morley M, Burdick JT . Mapping determinants of human gene expression by regional and
genome-wide association. _Nature_ 2005; 437: 1365–1369. Article CAS PubMed PubMed Central Google Scholar * Beasley CL, Zhang ZJ, Patten I, Reynolds GP . Selective deficits in prefrontal
cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. _Biol Psychiatry_ 2002; 52: 708–715. Article CAS PubMed Google Scholar * Lewis DA,
Hashimoto T, Morris HM . Cell and receptor type-specific alterations in markers of GABA neurotransmission in the prefrontal cortex of subjects with schizophrenia. _Neurotox Res_ 2008; 14:
237–248. Article PubMed PubMed Central Google Scholar * Vostrikov VM, Uranova NA, Orlovskaya DD . Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and
mood disorders. _Schizophr Res_ 2007; 94: 273–280. Article PubMed Google Scholar * Webster JA, Gibbs JR, Clarke J, Ray M, Zhang W, Holmans P _et al_. Genetic control of human brain
transcript expression in Alzheimer disease. _Am J Hum Genet_ 2009; 84: 445–458. Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank all the
investigators who generated the original data in the SNCID, and their many collaborators, who made this database possible. We also thank all the technicians in the SMRI brain laboratory who
prepared the brain tissues and extracted the RNA and DNA from the tissues. We specially thank the Keymind Company for their technical assistance with the database, in particular Marvin Suo.
We thank Dr Horvath for helpful comments on network analysis using the WGCNA. HC and DL were supported by the World Class University program (R32-2008-000-10218-0) of the Ministry of
Education, Science and Technology through the National Research Foundation of Korea. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Stanley Brain Research Laboratory, Stanley Medical Research
Institute, Rockville, MD, USA S Kim & M J Webster * Department of Bio and Brain Engineering, KAIST, Yuseong-gu, Daejeon, Republic of Korea H Cho & D Lee Authors * S Kim View author
publications You can also search for this author inPubMed Google Scholar * H Cho View author publications You can also search for this author inPubMed Google Scholar * D Lee View author
publications You can also search for this author inPubMed Google Scholar * M J Webster View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING
AUTHORS Correspondence to D Lee or M J Webster. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary Information
accompanies the paper on the Translational Psychiatry website SUPPLEMENTARY INFORMATION SUPPLEMENTARY MATERIAL-1 (DOC 276 KB) SUPPLEMENTARY MATERIAL-2 (DOC 2214 KB) RIGHTS AND PERMISSIONS
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit
http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kim, S., Cho, H., Lee, D. _et al._ Association between SNPs and gene
expression in multiple regions of the human brain. _Transl Psychiatry_ 2, e113 (2012). https://doi.org/10.1038/tp.2012.42 Download citation * Received: 12 March 2012 * Accepted: 10 April
2012 * Published: 08 May 2012 * Issue Date: May 2012 * DOI: https://doi.org/10.1038/tp.2012.42 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * _cis_
SNP * eQTL * post-mortem brain * psychiatric disorders * schizophrenia * SNCID