- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Pyogenic liver abscess (PLA) is a common intra-abdominal infection in adults. In this study, we aim to explore demographic and clinical characteristics of PLA focusing on
_Klebsiella pneumoniae (K. pneumoniae_) induced PLA (KP-PLA) in mainland China. A retrospective review of medical records from all patients with KP-PLA admitted to a tertiary teaching
hospital over a 21-year period (1994–2015) was performed. Among 296 PLA cases with confirmed culture-positive data, _K. pneumoniae_ was revealed as the predominant pathogen (_n_ = 189,
63.9%), followed by _Escherichia coli (n_ = 39, 13.2%). Strikingly, KP-PLA patients had a higher incidence of metabolic disorders, such as diabetes mellitus (49.7% vs. 36.4%, _P_ = 0.027;
odds ratio (OR): 1.725; 95% confidence interval (CI): 1.061–2.805), hypertension (38.1% vs. 19.6%, _P_ = 0.001; OR: 2.520; 95% CI: 1.439–4.413), and fatty liver (32.3% vs. 14.0%, _P_ =
0.001; OR: 2.923; 95% CI: 1.564–5.462) than those with non-_K. pneumoniae_ induced PLA (non-KP-PLA). Moreover, patients with KP-PLA had higher susceptibility to septic metastatic infection
at distant sites compared to those with non-KP-PLA (10.6% vs. 3.7%, _p_ = 0.038). Our results indicate that _K. pneumoniae_ is the predominant pathogen of PLA in mainland China. KP-PLA is
frequently diagnosed in patients with metabolic diseases and has a higher risk for septic metastatic infection. SIMILAR CONTENT BEING VIEWED BY OTHERS CLINICAL MANAGEMENT, PATHOGEN SPECTRUM
AND OUTCOMES IN PATIENTS WITH PYOGENIC LIVER ABSCESS IN A GERMAN TERTIARY-CARE HOSPITAL Article Open access 05 June 2024 THE IMPACT OF ASPIRIN ON _KLEBSIELLA PNEUMONIAE_ LIVER ABSCESS IN
DIABETIC PATIENTS Article Open access 07 December 2020 CLINICAL CHARACTERISTICS AND OUTCOME OF PATIENTS WITH ENTEROCOCCAL LIVER ABSCESS Article Open access 15 November 2021 INTRODUCTION
Pyogenic liver abscess (PLA) is a common infectious disease worldwide. A recent striking finding disclosed a much higher incidence of PLA in Taiwan (17.6 per 100,000 population) than that in
the United States, Denmark and Canada (1.1–3.6 per 100,000 population)1,2,3,4. _Klebsiella pneumoniae (K. pneumoniae_) is a gram-negative bacterium. In the late 1980’s, _K. pneumoniae_
induced PLA (KP-PLA) was first described in Taiwan5, and highlighted with subsequent case reports and clinical studies in the Asia-Pacific region6,7,8,9,10. Up to now, _K. pneumoniae_ is
considered to surpass _Escherichia coli (E. coli_) to become the predominant cause of PLA over the past three decades11,12. A definition of invasive liver abscess syndrome was proposed based
on a systematic review, referring to KP-PLA with characteristic extrahepatic metastatic infection13. Recently, KP-PLA has been reported from North America, Europe and
Oceania14,15,16,17,18,19,20, and was regarded as an emerging public health problem worldwide. Although KP-PLA occurs predominantly in Asian people or Asian descent, there are few reports
about this disease in mainland China in spite of its large population. The aim of this study was to investigate the clinical and pathogenic characteristics of PLA in mainland China with a
focus on KP-PLA by a retrospective review of 21 years’ medical records in a tertiary teaching hospital since its opening in May 1994, to better understand the clinical features of this
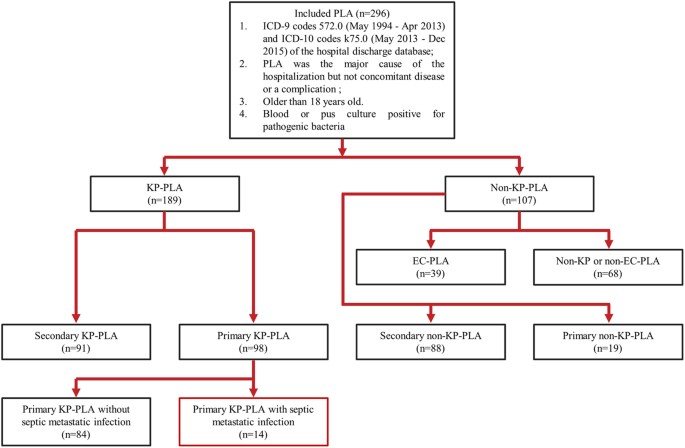
disease and to increase awareness about KP-PLA with metastatic infections among clinicians. RESULTS CHARACTERISTICS OF THE STUDY POPULATION In total, 802 patients were identified by
accessing the hospital discharge database, wherein 220 patients were excluded due to the absence of blood or pus culture results. Among the remaining 582 patients whose blood or pus was
assessed by microbiology-culture, 286 were culture-negative and excluded from our analyses. Eventually, a total of 296 patients with blood or pus culture-confirmed PLA were enrolled in this
single-center retrospective study (Fig. 1). Demographic characteristics and clinical features of patients with PLA are summarized in Table 1. They were male-predominant (n = 181, 61.1%) and
had a mean age of 59.1 ± 12.7 years. The majority of PLA were solitary, large (size >5 cm) and localized in right hepatic lobe. Among the 296 PLA patients who had identifiable
microorganisms on blood or pus culture, _K. pneumoniae_ was the most commonly isolated pathogenic bacteria and was found in 63.8% (n = 189) of the culture-positive PLA patients, followed by
_E. coli_ (n = 39, 13.2%), _Enterococcus_ (n = 20, 6.8%), _Staphylococcus_ (n = 17, 5.7%), _Streptococcus_ (n = 16, 5.4%), _Pseudomonas aeruginosa_ (n = 15, 5.1%), Acinetobacter baumannii (n
= 6, 2.0%), Corynebacterium (n = 3, 1.0%), Proteus mirabilis (n = 2, 0.7%), Klebsiella oxytoca (n = 2, 0.7%), Morganella morganii (n = 2, 0.7%), Enterobacter cloacae (n = 2, 0.7%),
Citrobacter freundii complex (n = 2, 0.7%), Enterobacter aerogenes (n = 2, 0.7%), Klebsiella ozaenae (n = 1, 0.3%), Shewanella putrefaciens (n = 1, 0.3%), Pasteurella multocida (n = 1,
0.3%), Yersinia pseudotuberculosis (n = 1, 0.3%), Proteus penneri (n = 1, 0.3%) and Edwardsiella tarda (n = 1, 0.3%). Although all PLA patients were given empirical antibiotics, 256 patients
(86.5%) undertook abscess drainage intervention, 36 patients (12.2%) developed septic shock, 65 patients (22.0%) were admitted to Intensive Care Unit (ICU), and 17 patients (5.7%) died
eventually. As for the method of abscess drainage, 5 patients shifted to surgical drainage after failure of percutaneous drainage, and 6 patients received another CT-guided percutaneous
drainage in case of dislodgement or blockage of catheters placed by previous ultrasound-guided percutaneous drainage. There were 172 patients (58.1%) who had a medical history of
hepatobiliary disease, 127 patients (42.9%) who previously had intra-abdominal trauma or surgery, and 45 patients (15.2%) who had been previously diagnosed with malignancy, especially
gastrointestinal (GI) cancers, such as gastric cancer (n = 1), duodenal cancer (n = 3), colorectal cancer (n = 5), biliary tract cancer (n = 28) and pancreatic cancer (n = 4). On the whole,
179 (60.5%) cases of PLA enrolled in our study were secondary to above underlying disease, and the rest of the cases (n = 117, 39.5%) were considered as primary PLA and cryptogenic in
origin. _K. pneumoniae_ isolated was susceptible to most antimicrobial agents except for ampicillin. Only 2 strains of _K. pneumoniae_ showed ESBL production, which were both blood-isolated.
These 2 KP-PLA patients were >70 years of age, diabetic and had a history of hypertension. During hospitalization, they developed septic shock, required ICU admission and eventually
died. CHANGES IN THE CLINICAL PRESENTATION OF PLA DURING THE PAST 21 YEARS In this study, the clinical features of patients with PLA, predominant pathogens causing PLA and therapeutic
interventions for liver abscess drainage have changed over the past two decades (Table 2). We observed a trend towards an increased proportion of KP-PLA in all the PLA cases from 2004 to
2015 as compared to the first 10 years (64.6% vs. 58.3%), but the difference did not reach statistical significance. Furthermore, the crude annual incidences of PLA and KP-PLA were raised
from 0.266% and 0.155% during the initial 10 years to 0.448% and 0.290% in next 11 years, respectively, with significant differences (_p_ = 0.003 and _p_ = 0.006). Although the incidence of
PLA or KP-PLA had been increasing, in-hospital mortality remained stable (5.6% vs. 5.8%, _p_ = 1.000), in contrast, Length of hospital stay (LOHS) reduced from 25.7 ± 19.8 days to 17.6 ±
11.3 days (_p_ = 0.004). Moreover, in recent years (2004–2015) the mean size of abscess was increased (7.0 ± 2.6 cm vs. 6.0 ± 2.3 cm, p = 0.028), and more patients received percutaneous
drainage (93.8% vs. 60.0%, p < 0.001), but less patients underwent surgical drainage (7.5% vs. 46.7%, p < 0.001), compared to that in 1994–2004. Compared to the initial 10 years,
CT-guided percutaneous drainage (63.7% vs. 5.6%, p < 0.001) had been increasingly replaced ultrasound-guided percutaneous drainage (38.7% vs. 100.0%, p < 0.001) in the latter 10 years
and was considered as the first choice intervention for PLA drainage currently. COMPARISON OF PATIENTS WITH KP-PLA AND NON-KP-PLA Given that _K. pneumoniae_ was found to be the leading
causative pathogen in PLA, we further explored the clinical characteristics of KP-PLA (Table 3). Comparison of patients with KP-PLA and non-KP-PLA revealed no significant differences in age
or gender. KP-PLA was found to be preferentially located in the right hepatic lobe (71.7% vs. 57.9%, _p_ < 0.05) and predominately cryptogenic in origin (51.9% vs. 17.8%, p < 0.001)
compared with non-KP-PLA, which commonly developed secondary to underlying hepatobiliary or colorectal diseases, malignancy and intra-abdominal trauma or surgery. Although it seemed more
patients with KP-PLA were subjected to blood-culture (81.0% vs. 70.1%, _p_ = 0.033), we observed a lower positive rate as compared with non-KP-PLA (33.3% vs. 56.0%, _p_ < 0.001). On the
contrary, KP-PLA showed a higher positive rate in liver abscess aspirate culture (97.6% vs. 90.1%, _p_ = 0.020). As for treatment strategies, more KP-PLA patients underwent radiologic-guided
percutaneous drainage comparing to patients with non-KP-PLA (92.8% vs. 84.4%, _p_ = 0.035). Strikingly, medical chart review of KP-PLA patients revealed significant higher incidence of
underlying metabolic disorders, such as diabetes mellitus (49.7% vs. 36.4%, _p_ = 0.027; OR, 1.725; 95% CI, 1.061–2.805), hypertension (38.1% vs. 19.6%, _p_ = 0.001; OR, 2.520; 95% CI,
1.439–4.413), and fatty liver (32.3% vs. 14.0%, _p_ = 0.001; OR, 2.923; 95% CI, 1.564–5.462), as compared with patients with non-KP-PLA. In contrast, concomitant hepatobiliary disease (46.6%
vs. 78.5%, _p_ < 0.001; OR, 0.239; 95% CI, 0.139–0.410), malignancy (7.9% vs. 28.0%, _p_ < 0.001; OR, 0.221; 95% CI, 0.113–0.435), history of hepatobiliary surgery (25.4% vs. 64.5%,
_p_ < 0.001; OR, 0.187; 95% CI, 0.112–0.313), and history of intra-abdominal trauma or surgery (28.6% vs. 68.2%, _p_ < 0.001; OR, 0.186; CI, 0.111–0.312) were less prevalent among
patients with KP-PLA. We next compared the clinical outcomes of KP-PLA and non-KP-PLA. More patients with KP-PLA developed septic shock (14.8% vs. 7.5%) and required ICU admission (24.9% vs.
16.8%). Patients with KP-PLA also tended to spend a longer time in ICU (9.0 ± 12.5 days vs. 4.0 ± 2.7 days) and hospital (18.7 ± 13.1 days vs. 18.5 ± 12.5 days). But above findings did not
reach statistical significance. Notably, patients with KP-PLA frequently developed metastatic infection at distant sites (10.6% vs. 3.7%, _p_ = 0.038), leading to poor prognosis (Tables 3
and 4). Comparing the difference between metastatic KP-PLA and non-metastatic KP-PLA, we observed that metastatic KP-PLA had greater requirement for ICU support (45.0% vs. 22.5%, _p_ =
0.028), longer length of ICU stay (LOIS) (11.7 ± 10.3 days vs. 8.4 ± 13.1 days, _p_ = 0.040) and higher in-hospital mortality (20.0% vs, 4.1%, _p_ = 0.019) (Tables 4 and 5). We also found
that underlying hepatobiliary disease was less common in patients with metastatic KP-PLA as compared to non-metastatic KP-PLA (25.0% vs. 49.1%, p = 0.041) (Table 5). COMPARISON OF PATIENTS
WITH KP-PLA AND _E. COLI_ INDUCED PLA (EC-PLA) There were 39 cases of EC-PLA, which accounted for 36.3% of non-KP-PLA. Patients with either KP-PLA or EC-PLA had almost the same age and a
similar gender ratio. Unlike KP-PLA, of which almost half were primary and cryptogenic in origin, all EC-PLA cases were secondary to intra-abdominal trauma or surgery, and hepatobiliary
diseases. As listed in Table 6, EC-PLA patients more often had a history of hepatobiliary surgery (76.9% vs. 25.4%, _p_ < 0.001) and underlying hepatobiliary diseases (97.4% vs. 46.6%,
_p_ < 0.001) as compared with KP-PLA, implying an association between EC-PLA and concomitant hepatobiliary pathology abnormalities. Moreover, we also observed that more KP-PLA patients
had underlying metabolic diseases, such as hypertension (38.1% vs. 17.9%, _p_ = 0.016) and fatty liver (32.3% vs. 10.3%, _p_ = 0.006) compared with EC-PLA. Overall, the prognosis appeared to
be better in patients with KP-PLA than patients with EC-PLA as far as in-hospital mortality (5.9% vs. 12.8% is concerned, but this did not reach statistical significance. PRIMARY KP-PLA AND
INVASIVE LIVER ABSCESS SYNDROME CAUSED BY _K. PNEUMONIAE_ To further explore the clinical features of KP-PLA, we analyzed the subgroup primary KP-PLA and highlighted the emergence of
invasive liver abscess syndrome associated with _K. pneumoniae_. As shown in Table 4, among primary KP-PLA reviewed in present study, 14 cases (14.3%) developed metastatic infection at
distant sites and considered as invasive liver abscess syndrome caused by _K. pneumoniae_. Of those patients, 2 cases (14.3%) developed meningitis, 1 case (7.1%) had brain abscess, 3 cases
(21.4%) grew lung abscess, 1 case (7.1%) had kidney abscess, 4 cases (28.6%) developed septic shock, 6 cases (42.9%) required ICU support, and 3 cases (21.4%) died eventually. We also
noticed that 4 (4.1%) out of 98 cases of primary KP-PLA had a history of PLA, so these 4 cases were considered as recurrence of primary KP-PLA, and the range of interval of recurrence was
from 7 to 96 months. DISCUSSION To the best of our knowledge, this single center retrospective study on PLA, in particular KP-PLA, covered the longest time span of all the studies reported
thus far (May 1994 to Dec 2015), and provided new insights into the demographics and clinical features of patients with KP-PLA in mainland China. At our institution, 1 out of 2400 and 3700
hospital admissions were due to PLA and KP-PLA, respectively. We observed that the crude annual incidence of PLA and KP-PLA increased significantly from 1994–2004 to 2004–2015, which could
be due to larger number of inpatients and improved early diagnostic techniques. In short, PLA and KP-PLA are common health problems in mainland China, which consistent with the recent
epidemiological trends observed in other countries and regions6,12,13,19,20,21,22,23,24,25. _K. pneumoniae_ was considered as the main pathogen of PLA in last three decades. Most clinical
studies on KP-PLA were from the Asia-Pacific region such as Taiwan, Hong Kong, Singapore and Korea6,7,8,9,11,12,25,26. To date, few study cohorts were reported from mainland China. This
retrospective review of all cases of PLA at our hospital confirmed _K. pneumoniae_ as the predominant pathogen of PLA followed by _E. coli_. Among KP-PLA cases, approximately half were
primary PLA and cryptogenic in origin, implying that _K. pneumoniae_ can invade a previously healthy liver. Whilst a report from Taiwan only discovered 1 from 160 cases of KP-PLA that was
secondary to intrahepatic duct stones11, in our cohort a substantial number of KP-PLA cases were reported in patients with a history of intra-abdominal surgery or underlying hepatobiliary
diseases. Therefore, there are considerable region differences with respect to causes that underlie development of KP-PLA. Variations in demographic composition of the study population may
also contribute to different findings. Non-KP-PLA mainly occurred as secondary to intra-abdominal surgery or hepatobiliary diseases involving _E. coli_, Enterococcus, Staphylococcus,
Streptococcus, _Pseudomonas aeruginosa_ infection etc., consistent with previous reports2. Next, we explored the patient characteristics that were associated with PLA and KP-PLA. Our data
supported the views that KP-PLA mainly occurred in middle-aged men, mostly located in right hepatic lobe and had the tendency to develop distant metastasis infection13. More importantly, our
study demonstrated that KP-PLA was significantly associated with metabolic disorders including hypertension, diabetes mellitus and fatty liver. We also showed that metastatic infection was
more frequently in KP-PLA patients with hypertension, diabetes mellitus or fatty liver. Diabetes mellitus is a known predisposing factor of KP-PLA, but the incidences of diabetes mellitus in
KP-PLA are different due to demographic variance, such as 15.2–25.0% in United States15,16, 27.3–39.9% in Korea6,7, 40.0% in Europe17,24, and 61.0–78.4% in Taiwan11,26,27. In our study,
49.7% of KP-PLA patients were diabetic. Impaired neutrophil activity and phagocytic function may contribute to relatively high frequency of KP-PLA in diabetes mellitus11,26,28. Up to date,
limited studies observed the potential association between KP-PLA and hypertension or fatty liver, but the hidden reason still required further clarification6,15,29. Apart from that, quite a
few PLA patients had a history of gastrointestinal cancer (41/296, 13.9%), implying a correlation between PLA and gastrointestinal cancer. Consistent with our data, Sung _et al_.30 showed
that gastrointestinal cancer had a 4-fold higher incidence among PLA patients as compared to controls. Hence, further evaluation to rule out potential malignancies in PLA patients should be
recommended in clinical practice. Extrahepatic metastatic infection is a devastating complication for KP-PLA patients. Metastatic KP-PLA patients were in relatively severe conditions and
more often admitted to ICU, but even so, about 20% patients still died eventually. To eliminate the effect of variation in demographic composition of KP-PLA study population, we traced
distant metastatic infection both in cases of KP-PLA and primary KP-PLA. In this study, the incidences of extrahepatic metastatic infection in KP-PLA and primary KP-PLA were 10.6% and 14.3%,
respectively, consistent with reports from other countries and regions (8–28%)11,13,14,31. A small number of primary KP-PLA cases (n = 2) developed metastatic meningitis, a rare but
life-threatening complication, whose rate of death in present study was as high as 100%. Septic endophthalmitis, a major complication reported in studies from Taiwan and other
regions14,26,27,31,32, was not observed in current study. Such discrepancy may be caused by geographic differences and inadequate follow-up of patients. In total, the in-hospital mortality
in KP-PLA and primary KP-PLA with metastatic infection were 20% (4/20) and 21.4% (3/14). Given the potentially catastrophic metastatic complications associated with KP-PLA, more attention
should be paid to its detection and management among clinicians. Given the prevalence of PLA and its severe complications, particularly KP-PLA, there is a need for early detection and
appropriate treatment strategy for this disease. In our study, pus culture was found to be more sensitive in detecting _K. pneumoniae_ than blood culture. Thus, it is preferable to obtain
pus samples from either fine needle aspiration or abscess drainage for bacteria identification prior to the use of empirical antibiotics. For those that needed abscess drainage,
radiology-guided percutaneous drainage was the preferred method. As an alternative, surgical drainage was often performed in PLA patients secondary to hapatobiliary diseases or as salvage
therapy in case percutaneous drainage failed. The advances in less invasive interventional radiology and endoscopic techniques33 will further improve the treatment of PLA. In this study,
almost all of the _K. pneumoniae_ isolates from patients with KP-PLA were susceptible to all the antibiotics tested except for ampicillin despite the large amount of antibiotics consumption
in mainland China, which may lead to the change of gut microbiota. Considering the poor prognosis of metastatic infection complication of KP-PLA, it is of great importance for clinicians to
detect the extrahepatic infection earlier and choose appropriate antibiotics with higher tissue concentration. In present study, we observed that the blood culture positive rate (33.3%) in
KP-PLA was lower than previously reported 46–61.1% by other studies12,20,31. We speculated that the collection time, the frequency of sampling, the amount of blood drawn for culture and
number of bottles within 24 hours all might contribute to the relatively low positive rate. Furthermore, empirical antibiotics usage prior to the collection of blood culture might be another
factor contributing to the low culture positive rate. We have retrospectively reviewed these patients’ medical charts and found some patients have visited local clinics or hospitals before
being referred to our hospital. Thus, we cannot exclude the possibility that some of these patients were already on antibiotics prior to blood collection. This study has several limitations.
First, this study is a single center, retrospective analysis that might cause selection bias in terms of patient population admitted to this hospital and recall bias related to medical
history. Second, strain specificities were not determined due to limited awareness of _K. pneumoniae_ in mainland China, whilst virulence factors such as aerobactin rmpA genes, and capsular
type K1 or K2 antigen have been shown to contribute to metastatic infection and severe disease7,8,13,26,27. Third, high percentage of monomicrobial liver abscess in Non-KP-PLA may be
attributed to the presence of predominant bacteria such as _E. coli_, low positive rate of anaerobes, and may reflect inappropriate culture techniques for microorganism identification.
Finally, quite a number of patients involved in present study developed septic shock and required ICU support, some predictor tools such as APACHE II score, _Glasgow_ Coma Scale (GCS) and
Sequential Organ Failure Assessment (SOFA) can be applied to further explore the severity and prognosis of this infectious disease. In summary, PLA is a common infectious disease that
requires hospitalization in mainland China. _K. pneumoniae_ is the leading pathogen of PLA, and KP-PLA patients are at higher risk of developing metastatic complications and in-hospital
mortality. Metabolic disorders, including hypertension, diabetes mellitus and fatty liver are common underlying conditions in patients with KP-PLA. Invasive liver abscess syndrome caused by
_K. pneumoniae_ is an emerging clinical syndrome with distant metastasis infection in mainland China and clinicians should be highly alert of its clinical characteristics to optimize patient
management. METHODS STUDY POPULATION We performed a single-center retrospective study by compiling the inpatient medical records of all consecutive cases of PLA at the Sir Run Run Shaw
Hospital, School of Medicine, Zhejiang University, Hangzhou, China, from May 1994 to December 2015. This hospital is a 2400-bed tertiary teaching hospital, which was opened in May 1994 and
has an approximate annual adult admission of 46,000 during 21 years. It was the first public hospital in mainland China accredited by the Joint Commission International, a US-based, World
Health Organization-authorized organization for medical quality evaluation. The list of patients with liver abscess was generated by accessing the ICD-9 (International Classification of
Diseases, 9th Revision) code (572.0) of the hospital discharge database from May 1994 to April 2013 and ICD-10 (International Classification of Diseases, 10th Revision) code (K75.0) from May
2013 to December 2015. The initial date of discharge with a diagnosis of liver abscess was defined as the index date. DATA COLLECTION Patient data were retrieved from paper medical records
(from May 1994 to May 2010) and electronic medical charts (from June 2010 to December 2015). The patients with PLA included in our study met the following criteria: 1) older than 18 years;
2) PLA was the major cause of the hospitalization. 3) had identifiable bacteria on blood or pus culture; We excluded patients who were 1) hospitalized due to concomitant diseases, 2)
developed PLA only as a complication, 3) diagnosed with amoebic liver abscess, infected liver cyst or hepatobiliary cancer diagnosed by pathological results after discharge, and 4)
previously diagnosed with PLA 30 days prior to their admission date to include only patients with newly-onset PLA. ETHICS STATEMENT The institutional review board of Sir Run Run Shaw
Hospital, School of Medicine, Zhejiang University approved this retrospective study protocol and waived from the need for written informed consents from these patients. PATIENT
CHARACTERISTICS The patients’ demographics, clinical presentations, laboratory values, radiographic findings, microbiological characteristics, treatment strategies and outcomes were
collected and analyzed. LOHS, admission to ICU, LOIS (if applicable), and invasive procedures performed were documented and summarized. DEFINITIONS PLA was defined as the presence of liver
abscess detected by imaging technologies, together with typical clinical manifestations of infection, such as fever and right upper abdominal pain. Primary PLA was defined as PLA that occurs
in the absence of a history of intra-abdominal trauma or surgery, including medical interventions such as transarterial chemoembolization (TACE) and radiofrequency ablation for treatment of
hepatocellular carcinoma (HCC), underlying hepatobiliary diseases such as cholecystitis, gallstones, cholangiolithiasis, and malignant obstruction of bile ducts, underlying colorectal
diseases such as polyps, adenoma and cancer, and immunosuppression due to cancer chemotherapy. On the contrary, secondary PLA was defined as PLA with above comorbidities. According to the
identified pathogenic bacteria isolated from blood or liver abscess aspirate, PLA was separated into KP-PLA and non-KP-PLA, with the latter was further classified as EC-PLA and those that
was neither KP-PLA nor EC-PLA. Septic metastatic infection was defined as extrahepatic infectious complications including Central Nervous System (CNS), eye, lung, muscular and skeletal
system and urinary system infection, such as meningitis, brain abscess, endophthalmitis, lung abscess, necrotising fasciitis, kidney abscess and other type infection. Invasive liver abscess
syndrome was defined as primary KP-PLA with septic metastatic infection at distant sites13. The definition of septic shock was updated by the Sepsis Definitions Task Force in 2016 and
patients with septic shock were identified with a clinical construct of sepsis with persisting hypotension requiring vasopressors to maintain MAP (mean arterial pressure) ≥65 mmHg and having
a serum lactate level >2 mmol/L despite adequate fluid resuscitation34. Diabetes mellitus was defined as a 2 h-plasma glucose ≥200 mg/dl, a fasting plasma glucose ≥126 mg/dl or a random
plasma glucose ≥200 mg/dl combined with classic symptoms of hyperglycemia35. Hypertension was defined as a systolic blood pressure ≥140 mmHg or a diastolic blood pressure ≥90 mmHg36. Fatty
liver was defined as the presence of hepatic steatosis based on an ultrasound or CT scan37. In-hospital mortality was defined as death from any cause during hospitalization. Size of abscess
was defined as the maximal cavity diameter of abscess or largest abscess when there were multiple abscesses. MICROBIOLOGY LABORATORY PROCEDURES All microbiology samples including blood and
pus were processed in a central laboratory for culture. The Dade Microscan Walkaway (Dade Behring, US) (from May 1994 to Dec 2002) and the VITEK 2 system (bioMe ́rieux, Marcy l’Etoile,
France) (from Jan 2003 to Dec 2015) were used to identify the bacterial isolates and perform susceptibility testing. Anti-microbial agents tested included ampicillin, ampicillin-sulbactam,
amikacin, aztreonam, ciprofloxacin, cefuroxime, cefepime, cefotaxime, ceftazidime, piperacillin, piperacillin-tazobactam, cefoperazone-sulbactam, tetracycline, sulphamethoxazole-trimethoprim
(SMZ-TMP) and imipenem. The extended-spectrum beta-lactamases (ESBLs) phenotype for all the collected isolates was confirmed by both a double-disk synergy test (DDST) and phenotypic
confirmatory disc diffusion test (PCDDT) according to the manual of Clinical and Laboratory Standards Institute (CLSI). STATISTICAL ANALYSIS SPSS 20.0 software (SPSS Inc, Chicago, IL) was
used for data analysis. Continuous variables were presented as mean ± SD and categorical data were described as frequency and percentage. Normality of the data was evaluated with
Kolmogorov-Smirnov test. Independent Sample t-test was used for normally distributed data and Mann–Whitney U test was performed for non-normally distributed data. Differences were considered
significant with P < 0.05. Categorical variables were compared using Pearson chi-square test or Fisher’s exact test. The presence of underlying diseases was indicated by the OR with a
95% CI. P < 0.05/n (n = comparisons number) was considered statistically significant based on the Bonferroni correction for multiple comparisons. ADDITIONAL INFORMATION HOW TO CITE THIS
ARTICLE: Qian, Y. _et al_. A retrospective study of pyogenic liver abscess focusing on _Klebsiella pneumoniae_ as a primary pathogen in China from 1994 to 2015. _Sci. Rep._ 6, 38587; doi:
10.1038/srep38587 (2016). PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. REFERENCES * Tsai, F. C.,
Huang, Y. T., Chang, L. Y. & Wang, J. T. Pyogenic liver abscess as endemic disease, Taiwan. Emerg. Infect. Dis. 14, 1592–1600 (2008). Article Google Scholar * Meddings, L. et al. A
Population-Based Study of Pyogenic Liver Abscesses in the United States: Incidence, Mortality, and Temporal Trends. Am. J. Gastroenterol. 105, 117–124 (2010). Article Google Scholar *
Jepsen, P., Vilstrup, H., Schonheyder, H. C. & Sorensen, H. T. A nationwide study of the incidence and 30-day mortality rate of pyogenic liver abscess in Denmark, 1977–2002. Aliment.
Pharmacol. Ther. 21, 1185–1188 (2005). Article CAS Google Scholar * Kaplan, G. G., Gregson, D. B. & Laupland, K. B. Population-based study of the epidemiology of and the risk factors
for pyogenic liver abscess. Clin. Gastroenterol. Hepatol. 2, 1032–1038 (2004). Article Google Scholar * Liu, Y. C., Cheng, D. L. & Lin, C. L. Klebsiella pneumoniae liver abscess
associated with septic endophthalmitis. Arch. Intern. Med. 146, 1913–1916 (1986). Article CAS Google Scholar * Yoon, J. H. et al. Liver abscess due to Klebsiella pneumoniae: Risk factors
for metastatic infection. Scand. J. Infect. Dis. 46, 21–26 (2014). Article Google Scholar * Chung, D. R. et al. Emerging invasive liver abscess caused by K1 serotype Klebsiella pneumoniae
in Korea. J. Infect. 54, 578–583 (2007). Article CAS Google Scholar * Yeh, K. M. et al. Capsular serotype K1 or K2, rather than magA and rmpA, is a major virulence determinant for
Klebsiella pneumoniae liver abscess in Singapore and Taiwan. J. Clin. Microbiol. 45, 466–471 (2007). Article CAS Google Scholar * Seetoh, T., Lye, D., Archuleta, S. & Fisher, D.
Klebsiella pneumoniae liver abscesses. Lancet Infect. Dis. 13, 391–392 (2013). Article Google Scholar * Maruno, T. et al. A liver abscess deprived a healthy adult of eyesight: endogenous
endophthalmitis associated with a pyogenic liver abscess caused by serotype K1 Klebsiella pneumonia. Intern. Med. 52, 919–922 (2013). Article Google Scholar * Wang, J. H. et al. Primary
liver abscess due to Klebsiella pneumoniae in Taiwan. Clin. Infect. Dis. 26, 1434–1438 (1998). Article CAS Google Scholar * Chang, F. Y. & Chou, M. Y. Comparison of pyogenic liver
abscesses caused by Klebsiella pneumoniae and non-K. pneumoniae pathogens. J. Formos. Med. Assoc. 94, 232–237 (1995). CAS PubMed Google Scholar * Siu, L. K., Yeh, K. M., Lin, J. C., Fung,
C. P. & Chang, F. Y. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect. Dis. 12, 881–887 (2012). Article Google Scholar * Lederman, E. R. & Crum, N. F.
Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am. J. Gastroenterol. 100, 322–331 (2005).
Article Google Scholar * Pastagia, M. & Arumugam, V. Klebsiella pneumoniae liver abscesses in a public hospital in Queens, New York. Travel Med. Infect. Dis. 6, 228–233 (2008). Article
Google Scholar * Rahimian, J., Wilson, T., Oram, V. & Holzman, R. S. Pyogenic liver abscess: recent trends in etiology and mortality. Clin. Infect. Dis. 39, 1654–1659 (2004). Article
Google Scholar * Moore, R., O’Shea, D., Geoghegan, T., Mallon, P. W. G. & Sheehan, G. Community-acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and
Europe. Infection 41, 681–686 (2013). Article CAS Google Scholar * Melot, B., Colot, J. & Guerrier, G. Bacteremic community-acquired infections due to Klebsiella pneumoniae: clinical
and microbiological presentation in New Caledonia, 2008–2013. Int. J. Infect. Dis. 41, 29–31 (2015). Article Google Scholar * Chavada, R., Ng, J., Maley, M. & Descallar, J. Emergence
of Klebsiella pneumoniae liver abscesses in South-western Sydney. Infection 42, 595–596 (2014). Article CAS Google Scholar * Fazili, T. et al. Klebsiella pneumoniae Liver Abscess: An
Emerging Disease. Am. J. Med. Sci. 351, 297–304 (2016). Article Google Scholar * Chang, E. K. et al. Occult Klebsiella pneumoniae bacteremia at emergency department: A single center
experience. J. Microbiol. Immunol. 48, 684–691 (2015). Google Scholar * Shelat, V. G. et al. Pyogenic Liver Abscess: Does Escherichia Coli Cause more Adverse Outcomes than Klebsiella
Pneumoniae? World J. Surg. 39, 2535–2542 (2015). Article Google Scholar * Wu, P. F. et al. Clinical characteristics and economic consequence of Klebsiella pneumoniae liver abscess in
Taiwan. J. Microbiol. Immunol. Infect. 48, 190–197 (2015). Article Google Scholar * Moore, L. S. P., Clarke, I. L., Donaldson, H. & Azadian, B. Community-acquired Klebsiella pneumoniae
liver abscess: the London experience. Infection 42, 219–221 (2014). Article CAS Google Scholar * Lok, K. H., Li, K. F., Li, K. K. & Szeto, M. L. Pyogenic liver abscess: clinical
profile, microbiological characteristics, and management in a Hong Kong hospital. J. Microbiol. Immunol. Infect. 41, 483–490 (2008). PubMed Google Scholar * Fung, C. P. et al. A global
emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut 50, 420–424 (2002). Article Google Scholar * Fang, C. T. et
al. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin. Infect. Dis. 45, 284–293
(2007). Article CAS Google Scholar * Lin, J. C. et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic
control. J. Clin. Endocrinol. Metab. 91, 3084–3087 (2006). Article CAS Google Scholar * Li, J. et al. Early diagnosis and therapeutic choice of Klebsiella pneumoniae liver abscess. Front.
Med. China 4, 308–316 (2010). Article Google Scholar * Lai, H. C. et al. Increased incidence of gastrointestinal cancers among patients with pyogenic liver abscess: a population-based
cohort study. Gastroenterology 146, 129–137 (2014). Article ADS Google Scholar * Lee, S. S. et al. Predictors of septic metastatic infection and mortality among patients with Klebsiella
pneumoniae liver abscess. Clin. Infect. Dis. 47, 642–650 (2008). Article Google Scholar * Lee, J. Y. & Kim, K. H. Endogenous Endophthalmitis Complicated by Pyogenic Liver Abscess: A
Review of 17 Years’ Experience at a Single Center. Digestion 90, 116–121 (2014). Article Google Scholar * Ogura, T. et al. Clinical Outcome of Endoscopic Ultrasound-Guided Liver Abscess
Drainage Using Self-Expandable Covered Metallic Stent (with Video). Dig. Dis. Sci. 61, 303–308 (2016). Article Google Scholar * Singer, M. et al. The Third International Consensus
Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810 (2016). Article CAS Google Scholar * Organization., W. H. Global report on diabetes. (2016). * Liao, Y., Gilmour, S.
& Shibuya, K. Health Insurance Coverage and Hypertension Control in China: Results from the China Health and Nutrition Survey. PloS One 11, e0152091 (2016). Article Google Scholar *
Li, Z. et al. Prevalence of nonalcoholic fatty liver disease in mainland of China: a meta-analysis of published studies. J. Gastroenterol. Hepatol. 29, 42–51, (2014). Article CAS Google
Scholar Download references ACKNOWLEDGEMENTS The study was funded by Zhejiang Province Key Science and Technology Innovation Team, No. 2013TD13 and National Natural Science Foundation of
China (NSFC) (81401708 and 81502064). We gratefully acknowledge the help of Professor Yi Shen, Associate Professor, Department of Epidemiological and Health Statistics, School of Medicine,
Zhejiang University, Hangzhou, China, for guidance in data analysis. AUTHOR INFORMATION Author notes * Qian Yun and Wong Chi Chun contributed equally to this work. AUTHORS AND AFFILIATIONS *
Department of Gastroenterology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China; Institute of Gastroenterology, Zhejiang University, Hangzhou, China Yun
Qian, Sanchuan Lai, Xingkang He, Leimin Sun, Jiaguo Wu, Weili Liu & Jianmin Si * Institute of Digestive Disease and Department of Medicine and Therapeutics, State Key Laboratory of
Digestive Disease, Li Ka Shing Institute of Health Sciences, CUHK Shenzhen Research Institute, The Chinese University of Hong Kong, Hong Kong, China Chi Chun Wong, Huarong Chen & Jun Yu
* Department of Critical Care Medicine, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China Jiancang Zhou * Emergency Department, Sir Run Run Shaw Hospital,
School of Medicine, Zhejiang University, Hangzhou, China Daoyang Zhou Authors * Yun Qian View author publications You can also search for this author inPubMed Google Scholar * Chi Chun Wong
View author publications You can also search for this author inPubMed Google Scholar * Sanchuan Lai View author publications You can also search for this author inPubMed Google Scholar *
Huarong Chen View author publications You can also search for this author inPubMed Google Scholar * Xingkang He View author publications You can also search for this author inPubMed Google
Scholar * Leimin Sun View author publications You can also search for this author inPubMed Google Scholar * Jiaguo Wu View author publications You can also search for this author inPubMed
Google Scholar * Jiancang Zhou View author publications You can also search for this author inPubMed Google Scholar * Jun Yu View author publications You can also search for this author
inPubMed Google Scholar * Weili Liu View author publications You can also search for this author inPubMed Google Scholar * Daoyang Zhou View author publications You can also search for this
author inPubMed Google Scholar * Jianmin Si View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.Q. collected and analyzed the data, and
drafted the manuscript. C.C.W. analyzed the data and wrote the manuscript. S.L., X.H., L.S., J.W., W.L. and J.Z. all assisted in data collection. H.C. performed statistical analysis and
commented on the manuscript. J.Y. commented on the manuscript. D.Z. and J.S. designed the study, supervised the study and commented on the manuscript. All authors have reviewed the
manuscript. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution
4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if
the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Qian, Y., Wong, C., Lai, S. _et al._ A retrospective study of pyogenic liver
abscess focusing on _Klebsiella pneumoniae_ as a primary pathogen in China from 1994 to 2015. _Sci Rep_ 6, 38587 (2016). https://doi.org/10.1038/srep38587 Download citation * Received: 19
May 2016 * Accepted: 11 November 2016 * Published: 08 December 2016 * DOI: https://doi.org/10.1038/srep38587 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative

:max_bytes(150000):strip_icc():focal(319x0:321x2)/people_social_image-60e0c8af9eb14624a5b55f2c29dbe25b.png)




