- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Oxidative stress (OS) impact on a single neuron’s function _in vivo_ remains obscure. Using _C. elegans_ as a model organism, we report the effect of paraquat (PQ)-induced OS on
wild type worms on the function of the ASH polymodal neuron. By calcium (Ca2+) imaging, we quantified ASH activation upon stimulus delivery. PQ-treated worms displayed higher maximum
depolarization (peak of the Ca2+ transients) compared to untreated animals. PQ had a similar effect on the ASH neuron response time (rising slope of the Ca2+ transients), except in very
young worms. OS effect on ASH was partially abolished in vitamin C-treated worms. We performed octanol and osmotic avoidance tests, to investigate the OS effect on ASH-dependent behaviors.
PQ-treated worms have enhanced avoidance behavior compared to untreated ones, suggesting that elevated ASH Ca2+ transients result in enhanced ASH-mediated behavior. The above findings
suggest a possible hormetic effect of PQ, as a factor inducing mild oxidative stress. We also quantified locomotion parameters (velocity, bending amplitude), which are not mediated by ASH
activation. Bending amplitude did not differ significantly between treated and untreated worms; velocity in older adults decreased. The differential effect of OS on behavioral patterns may
mirror a selective impact on the organism’s neurons. SIMILAR CONTENT BEING VIEWED BY OTHERS _C. ELEGANS_ ELECTROTAXIS BEHAVIOR IS MODULATED BY HEAT SHOCK RESPONSE AND UNFOLDED PROTEIN
RESPONSE SIGNALING PATHWAYS Article Open access 04 February 2021 GLYCOLATE COMBATS MASSIVE OXIDATIVE STRESS BY RESTORING REDOX POTENTIAL IN _CAENORHABDITIS ELEGANS_ Article Open access 01
February 2021 A REVERSIBLE MITOCHONDRIAL COMPLEX I THIOL SWITCH MEDIATES HYPOXIC AVOIDANCE BEHAVIOR IN _C. ELEGANS_ Article Open access 03 May 2022 INTRODUCTION Oxidative stress (OS) is one
of the most significant types of stress an organism experiences throughout its life. It is the result of exposure to numerous environmental factors, including chemical compounds1. The effect
of OS on the nervous system is of special interest, since it has been associated with neurodegenerative diseases2,3, such as Alzheimer’s4 and Parkinson’s5. Moreover, there is evidence that
exposure to certain herbicides, namely paraquat (PQ), known to induce OS6, is strongly associated with higher frequency of neurodegenerative diseases in humans7. However, the effect of OS on
sensory neurons physiology remains obscure. Altered function of sensory neurons could lead to misperception of the organism’s environment, resulting in a modified behavior, not uncommon in
neurodegenerative diseases. A strong correlation between hyperexcitability of sensory neurons and characteristic symptoms (e.g. photophobia in migraine) has been shown8, as well as a strong
connection between OS and behavioral changes9,10. Monitoring _in vivo_ neuronal function under OS could deepen our understanding on the impact of OS on an organism’s nervous system as well
as on the engendered behaviors. Nevertheless, there is a lack of studies exploring _in vivo_ the effects of OS on single neuron functionality. In parallel, _C. elegans_ has been broadly used
in numerous studies which explore the phenomenon of hormesis, caused, among other harmful conditions, by mild OS11. Beneficial effects of low doses of oxidative factors have been reported
to result in increased lifespan and increased resistance to other types of stress12,13. However, the possibility of OS-driven hormetic effects on neuronal Ca2+ transients has not been
investigated yet. Here, we investigate the effects of chemically induced OS in the nematode _Caenorhabditis elegans_, using state-of-the-art microfluidic technology and _in vivo_ calcium
(Ca2+) imaging. _C. elegans_ has been widely used to investigate both OS and neurodegenerative diseases14,15 and is therefore an ideal model organism to examine the interplay between OS and
neuronal functional physiology16,17. We exposed wild type worms of different ages to PQ and to vitamin C (VitC), a well-established antioxidant18,19. We chose PQ concentrations that are
known to cause changes in the worms’ physiology due to oxidative damage16,20, since PQ is known to interfere with electron transfer and catalyze the production of reactive oxygen species
(ROS). Using a microfluidic chip21, we delivered a hyperosmotic stimulus (glycerol) to the worm’s nose and recorded Ca2+ transients from the ASH sensory neuron. We chose ASH, the
well-studied worms’ main nociceptor21,22,23,24,25,26,27,28, because it can be activated by a wide range of repellents25,29,30, thus being especially significant for _C. elegans_ survival and
environmental perception. With the exception of very young worms, stimulus-evoked calcium transients from the ASH neuron are elevated in OS-treated worms, when compared to non-treated
animals. This effect is partially abolished when the worms are simultaneously treated with PQ and VitC. Octanol avoidance test and glycerol drop assay confirmed that behaviors directly
mediated by ASH are enhanced by OS, and this effect is also partially abolished in the presence of VitC. In younger worms, VitC does not reverse the enhancement of the avoidance to glycerol,
whereas it reverses the effect on the response to octanol. In contrast, we found that OS-exposed worms did not display significant differences in their locomotive parameters (velocity and
bending amplitude) compared to untreated ones, with the exception of the average velocity in older worms. We conclude that exposure to chemically induced OS significantly affects the
function of ASH neuron, as well as avoidance behaviors directly mediated by ASH. The observation that behaviors independent of ASH are not correspondingly affected, could strongly suggest
that neurons are not uniformly affected by chemically induced OS. This could further imply a differential susceptibility to OS among neurons, neuronal circuits and subsequently, behavioral
patterns. Our results can pave the way for further experiments on the interplay between OS and neuronal function. Last, we suggest that calcium imaging and microfluidic platforms can be a
powerful tool for revealing OS-dependent changes in neuronal function in _C. elegans_, and potentially in other model organisms as well. RESULTS PQ TREATMENT INCREASES THE MAGNITUDE OF ASH
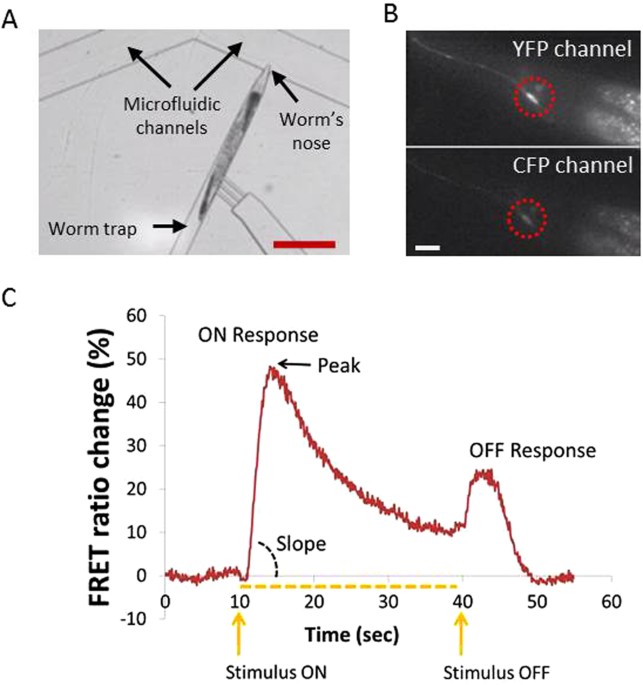
CA2+ TRANSIENTS We used the microfluidic platform to quantify the effect of rearing on 0.1 mM PQ to the function of the ASH neuron (Fig. 1). We monitored the activity of ASH using the TNXL
indicator in response to a 30 sec stimulus pulse (1 M glycerol). We examined four groups of age-synchronous worm populations: treated with 0.1 mM PQ, treated with 1 mM VitC, treated with 0.1
mM PQ + 1 mMVitC and untreated (control) ones (Fig. 2A). We imaged a total of 256 worms, aged L4 + 1 day (marked here as “Day 1” worms), L4 + 4 days (referred to as “Day 4” worms) and L4 +
5 days (mentioned here as “Day 5” worms). The average peak and the slope of the ON response (Fig. 1) in animals previously treated with PQ increased in all days studied, compared to the
control animals, with the exception of Day 1 rising slope (Fig. 2B and Table 1). Comparing the peak and the slope of the rising phase between the PQ-treated animals and worms treated with PQ
+ VitC, we find that in Days 4 and 5 simultaneous treatment with PQ + VitC results in bringing calcium (Ca2+) transients back to the level of control animals of the same age. However, this
is not the case in Day 1 worms, where concurrent treatment with PQ and VitC has the same effect as PQ alone. Interestingly, VitC has no effect on the peak of Ca2+ transients, compared to Day
4 and Day 5 untreated worms (Fig. 2B). However, in Day 1 worms, VitC treatment increases both the peak and the slope of the FRET % change, whereas the slope is reduced in Day 4 and Day 5
worms. When delivering PQ instead of glycerol to the nose of untreated animals, no ASH activation was observed (suppl. Fig. 1). PQ TREATMENT ENHANCES ASH MEDIATED BEHAVIOR OSMOTIC AVOIDANCE
We examined whether the observed OS-related changes in ASH function result in modified ASH-mediated worm behavior. To this end, as a direct read out of ASH activation, we assayed osmotic
avoidance, by running the glycerol drop test. Our results show (Fig. 3A and Table 1) that treatment with PQ increases the avoidance index (a.i.) in all days studied (56% for Day 1, 25% for
Day 4 and 15% for Day 5). Simultaneous exposure of the worms to PQ and VitC results in reversing the PQ effect during Days 4 and 5, but not on Day 1. The VitC rescuing effect on the osmotic
avoidance is aligned with the effect of VitC on ASH Ca2+ transients, where the amplitude of the ion influx after concurrent exposure to VitC and PQ hovers around the control levels during
Days 4 and 5, but not on Day 1 (Figs 3A and 2B, top). OCTANOL ASSAY We tested worms’ reaction time to the repellent odor of octanol, a behavior known to be mediated by ASH. The aversive
response to 30% octanol is found to be significantly faster in worms exposed to PQ during their life (Fig. 3B and Table 1) in all days studied. Indeed, the time needed for a worm to initiate
a reversal is decreased by 52% in Days 1 and 4, and by 47% in Day 5, showing almost twice as fast a response in all days examined (Fig. 3B). Simultaneously administering VitC and PQ,
appears to restore response time by 88% at Day 1, by 96% at Day 4 and by 85% at Day 5, showing that the presence of the antioxidant has indeed a rescuing effect on PQ-induced impact. Vitamin
C alone does not have any effect on the octanol avoidance response. EXPOSURE TO PQ REDUCES OLDER WORMS MEAN VELOCITY, BUT DOES NOT AFFECT OTHER LOCOMOTION PARAMETERS By using a custom-made
tracking system31,32,33, we analyzed worms’ locomotion parameters (mean velocity, bending amplitude). It is shown that treatment with PQ results in decreased velocity (μm/sec) in animals of
Day 5, by 38% (control animals mean velocity = 77 μm/sec, PQ-treated animals mean velocity = 48 μm/sec) (Fig. 4A and Table 1). No significant change was detected, however, for Days 1 and 4.
It is also shown that, in Day 5, simultaneous treatment with PQ and VitC results in reduced velocity, as well (PQ/VitC-treated animals mean velocity = 52 μm/sec; 32% decrease, compared to
control animals) (Fig. 3A). As far as it concerns treatment with VitC alone, no statistically significant change was detected in any of the examined days. When analyzing the bending
amplitude (Fig. 4B and Table 1), the only effect observed is a significant drop between untreated and VitC-treated Day 4 animals on Days 4 and 5 (VitC-treated animals bending amplitude =
0.10 mm compared to control Day 4 animals bending amplitude = 0.12 mm, and VitC-treated Day 5 animals bending amplitude = 0.09 mm _versus_ control Day 5 animals bending amplitude = 0.12 mm).
However, this difference is of borderline statistical significance, since the _p_-value is exactly 0.05. DISCUSSION Paraquat (PQ) is well-known to induce OS in living organisms34, including
_C. elegans_16,35. Additionally, the hormetic effect of low intensity PQ-induced OS has been well articulated in _C. elegans_36,37, as it has been reported that mildly elevated ROS levels
through treatment with PQ can actually promote longevity16,36,38. Here we show for the first time that previous exposure to chemically induced OS affects both the magnitude and the time rate
of the Ca2+ influx which takes place upon stimulus delivery, at ASH sensory neuron. Specifically, our results reveal that in young worms (Days 1, 4 and 5 of adult life), treatment with a
sublethal concentration of PQ leads to elevated ASH Ca2+ transients, compared to untreated animals, when stimulated by hyperosmotic solution. The observed ASH hypersensitivity (as quantified
by the maximum peak and rising slope of the Ca+ transients upon delivery of the stimulus) increases the longer the worms are exposed to OS (Fig. 2). This could be attributed to the effect
of accumulated ROS as a result of sustained exposure to PQ39,40 or to age-related deterioration of the antioxidant mechanisms41,42. Moreover, it is known that Ca2+ influx in ASH depends on
transient receptor potential channels (TRPV) and voltage-gated ion channels (VGCC)27,43, the physiology of which is age-dependent44,45. Therefore, a potential explanation for age-related,
OS-induced Ca2+ transients modifications21 could be that the abovementioned channels are affected by chemically induced OS more as the worms age. Interestingly, alterations in Ca2+ transient
rising slope do not always follow the changes in the transients’ maximum peak, as is the case of Day 1 worms, where PQ raises the maximum peak value but has no effect on the slope.
Possibly, the molecular components of the Ca2+ channels46, responsible for the rate and the magnitude of the influx, do not get uniformly affected by OS. However, the opposite is observed
when administering vitamin C alone, since in worms of Days 4 and 5 the latter does not affect the maximum peak of the transient, but it results in smaller slope. This finding could be
indicative of the opposite ways PQ and vitamin C act on the Ca2+ influx mechanism. The increase in the amplitude and rate of stimulus-evoked ASH Ca2+ transients could mark an OS-caused
enhanced reaction to environmental cues. Indeed, PQ-exposed worms have a stronger avoidance response against the hyperosmotic solution and they respond faster to octanol in all days
examined, compared to control animals (Fig. 3). These findings could be interpreted in the context of mild OS hormetic effect47, resulting in a potentially advantageous enhancement of
physiological mechanisms and behaviors. Moreover, since substances with hormetic effects typically prolong the worm’s lifespan11,12,13, it is possible that treatment with PQ results in ASH
Ca2+ transients comparable to the ones of untreated, younger animals. Furthermore, since PQ has been shown to increase lifespan16,36,38, we can speculate that other life-prolonging agents
might have a similar effect on ASH Ca2+ transients. Further experiments would be necessary to clarify whether PQ acts through ASH-specific or global mechanisms. Findings regarding both
glycerol drop test and octanol avoidance seem to be lined up more with changes in the magnitude than the rate of the Ca2+ influx response, especially in Day 1 worms. This could mean that the
behaviors examined depend more on the total amount of Ca2+ ions than on the rate of the influx, at least for younger adults. Intracellular ascorbic acid (vitamin C) serves several
protective functions, as it scavenges radical species before they can damage DNA, proteins, or lipids48, and it contributes in recycling other antioxidants48. The established antioxidant
role of vitamin C18,49, has not been really proven when it comes to _C. elegans_50. This is partially due to conflicting reports about its impact on _C. elegans_ lifespan37,51,52. Our
results contribute to this discussion, showing for the first time that when offered concurrently with the oxidative factor, it can lead to abolishing OS implications. Importantly, the
reversing effect of vitamin C in PQ-exposed worms applies to both the ASH Ca2+ transients and to ASH-depended behaviors. High ascorbate concentration in neurons is thought to be generated
and maintained due to specific transporters53, the function of which, in relation to aging, is under investigation54. The existence of a similar transporter in _C. elegans_ could offer a
possible explanation as to why the rescuing effects of vitamin C are more intense in older worms. Interestingly, vitamin C alone increases Ca2+ influx in the ASH neurons of Day 1 worms, and
also affects ASH controlled behaviors (Figs 2 and 3). It has been found, in human embryonic cells, that the number of TRPV1 channels increases due to vitamin C impact55. Since calcium influx
in ASH depends on both TRPV and VGCC channels44,45, we can assume that the vitamin C effect might possibly occur because of changes in the TRPV channels abundance on ASH membrane. It is
noteworthy that Vitamin C alone decreases slope in days 4 and 5 (Fig. 2B, bottom); however, it does not decrease the peak of % FRET change (Fig. 2B, top), which accounts for the magnitude of
the response. As the slope represents the rate by which the Ca2+ enter the cell, we can assume that Vitamin C has an effect exactly on this part of the Ca2+ influx controlling molecular
mechanism. The fact that Vitamin C reduces that rate whereas PQ increases it and the two combined have an intermediate effect, could mean that the two compounds act antagonistically. If this
is true, then Vitamin C does not “undo” whatever effect PQ has; however, it still results in abolishing this effect. Future experiments that will shed light on the exact mechanism by which
Vitamin C acts would be of special interest. The possibility of Vitamin C itself having a hormetic effect could be further examined, as well. It has been reported that mutants with increased
oxidative stress show reduced motor activity56 and that exposure to peroxide stress causes loss in mobility39. These findings are in agreement with our results, which unveil a decrease in
average velocity in PQ-treated worms on Day 5. This decrease is not reversed by vitamin C, suggesting that the amount of vitamin C used may not be enough to invert the effect. More
importantly, velocity depends on a number of neurons, not including ASH, which may be affected differently by PQ-induced OS. The actual finding that avoidance behaviors and locomotion
parameters are affected differently by chemically-induced OS, could suggest that this also applies to the neurons involved in the respective behavioral expressions. Neuron-specific induction
of OS has been reported in the past57,58, and cell specific susceptibility to OS has been observed59,60. Possibly, the developmental stage of both PQ and vitamin C administration, the type
of OS (chemically induced), and importantly, the dose of PQ used, might not affect in the same extent all neurons in the worms’ nervous system. What is more, vitamin C transmembrane
transporters seem to display themselves a type of cell-specificity, supporting further the above assumption61. Hence, we claim that our results can be considered as a potent indication
towards a type of neuron-specific sensitivity when it comes to chemically induced oxidative stress. METHODS _C. ELEGANS_ STRAINS AND TREATMENT We used an integrated line that expressed the
TN-XL calcium indicator in the ASH neuron, under the _sra-6_ promoter [NKC2311 micIs211(p_sra-6_::TNXL)]. We integrated the line AQ20801jEx211[p_sra-6_::TN-XL] by using a standard UV
exposure protocol, as described previously21. In all of our experiments, we used age-synchronized hermaphrodite worms62 that were grown at 20 °C on OP50-seeded agar plates. For treating the
worms with PQ, VitC and PQ + VitC, synchronized populations were grown on seeded NGM plates containing 0.1 mM PQ, 1 mM VitC and 0.1 mM PQ/1 mM VitC16 respectively. When conducting the Ca2+
imaging experiments, animals were imaged within 10 min after removal from the treatment plates. MICROFLUIDIC CHIP AND FLUIDIC SETUP Soft lithography was used to fabricate the PDMS/glass
microfluidic chip. The fluidic setup was calibrated and controlled through a custom-made LabView algorithm as described previously21. In all calcium imaging experiments, the stimulus was
delivered on the 10th sec of the recording period and was withdrawn on the 40th sec of the recording period (Fig. 1) (the presence of the stimulus is marked on the plots with a dotted line).
The length of the stimulus was chosen based on previous experiments of our group (see also Chokshi _et al_., 2010). FRET ANALYSIS, PEAK AND SLOPE CALCULATIONS IMAGE AND DATA ANALYSIS We
used a custom made LabVIEW program to extract the mean FRET ratio from each fluorescent image we acquired (Chokshi _et al_., 2010). Briefly, each fluorescent image was split by a dual imager
into two separate images, one representing the YFP (yellow fluorescent protein) and one the CFP (cyan fluorescent protein) channels. The LabVIEW program required four pieces of initial
information, specified by the user for each examined worm: (1) the region of interest, defining the boundary of the neuronal soma, in the YFP channel, (2) the region within which the neuron
moves while the animal is immobilized in the microfluidic channel, in the YFP channel (3) the region defining the background fluorescence of the worm, in the YFP channel and (4) the initial
coordinates of the neuron in both YFP and CFP channels. The LabVIEW program first calculates the difference between the initial coordinates of the neuron’s center in each channel. It uses
this difference to correct for any misalignment between the YFP and CFP images. It then tracks the neuron of interest in each channel and extracts the corresponding background-subtracted,
mean fluorescence intensity. The FRET ratio is calculated as the ratio of mean fluorescence intensity of the neuron in the two channels. FRET RATIO % CHANGE, PEAK AND SLOPE CALCULATIONS For
the FRET ratio % change, the average fluorescence ratio in a 3 second window (t = 1–4 s) was set as the baseline. The % change in fluorescence intensity for the region of interest was
plotted for all image stacks (Chalasani _et al_., 2007). The “peak” refers to the maximum value of the FRET ratio % change as observed during the ON response of the ASH neuron (Fig. 1). The
“slope” refers to the rising slope of the ON response, and is calculated as the maximum value of the FRET ratio change observed (% peak FRET ratio change), over the time needed to reach this
maximum, counting from the stimulus onset. We have selected the peak and rising slope of the ON response since this peak is indicative of the total amount of calcium entering the cell and
consequently accounts for the amplitude of the cell’s response, whereas the rising slope corresponds to the calcium ions influx. BEHAVIORAL ASSAYS OCTANOL AVOIDANCE ASSAY We used 30% (v/v)
1-octanol solution (freshly prepared daily, diluted in 100% ethanol) to assay the octanol avoidance behavior of OS-exposed worms. Octanol avoidance in this concentration range is mediated
solely by the ASH neurons63,64. Fresh NGM plates, used as intermediate and assay plates were prepared on the day of the experiment. Worms were picked from their OP50 growing plates and
transferred to new (intermediate) plates, left for 1 min, and then were transferred into the assay plates, where they were tested after 5 min64. Intermediate and assay plates did not contain
PQ or VitC and they were not seeded with bacteria. The blunt end of an eyelash hair was dipped in the octanol solution and was placed in front of the nose of a forward moving animal. The
time required by the animal to initiate backward movement was recorded using a timer63. Octanol avoidance assays were conducted on well-fed worms, as feeding status may affect the response
of animals to octanol63. Animals were tested on different days, at least 5 worms on each day. In total, ~20 animals were examined for each experimental condition. GLYCEROL DROP TEST The drop
assay was performed following a previously described procedure65. A drop of 0.5 M glycerol (freshly prepared daily, dissolved in 30 mM Tris, 100 mM NaCl, 10 mM KCl; M13 buffer) was placed
on the agar surface, near the tail of a forward moving animal. The drop quickly spread out surrounding the animal and finally reached the anterior amphid sensory neurons. Animals’ response
to the osmotic stimulus resulting in initiating a backward motion was scored as a positive response, when the worm responded within 5 sec. Drop tests were conducted on unseeded NGM plates
and drops were delivered using custom-made glass capillaries (pulled by hand on a flame to make them narrower). On the other end of the capillary, a piece of polyethylene tubing was mounted
and connected to a 5 ml syringe through a lauer. The avoidance index (a.i.) was calculated by dividing the number of positive responses to the total number of trials65 and the mean a.i. was
calculated at the end of each experiment. All groups were tested using single worm assays, where each worm’s response was tested by applying 5 drops, with a 5 min interval between successive
trials. 8–10 individual, well-fed animals per group were tested each day, for 2–3 days, giving a total of ~20 worms for each group of interest. WORM TRACKING Worms were tracked to analyze
their locomotion behavior on NGM plates using an automated worm tracking system developed in Xu Lab, Life Sciences Institute, University of Michigan, as previously described31,32,33.
Briefly, NGM plates were seeded with a thin layer of fresh bacterial lawn (OP50) 10 min prior to tracking. Tracking was performed on freely moving worms, at ~20 °C and at a relative humidity
of ∼40%, with the petri dish lid off. The tracking system includes a stereomicroscope (Zeiss Stemi 2000 C) mounted with a digital camera (Cohu 7800) and a digital motion system (Parker
Automation) that follows worm movement, as well as a custom made software package. To record locomotion, worm images were captured at 2 Hz for 2 min and the mean velocity (centroid
displacement per second) of each worm was quantified using laboratory-developed software. The motion data were also compressed, integrated and stored as a multimedia file format (AVI). The
extent of body bending (bending angle) was calculated as described previously33,66 using binary worm images, thinned to obtain the “skeleton image” of the worm and broken into equal-length
segments. STATISTICAL ANALYSES Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA) and Excel (Microsoft, Redmont, WA). Results are
presented as the mean ± standard error. Imaging data, octanol avoidance and glycerol drop test results as well as worm tracker derived data were tested by unpaired two-tailed Student’s
_t_-test and all differences were considered statistically significant at _p_-value < 0.05 (* indicates p < 0.05, ** indicate p < 0.01, *** indicate p < 0.001). Grubb’s outlier’s
test was used in the processing of the behavioral assays results. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Gourgou, E. and Chronis, N. Chemically induced oxidative stress affects
ASH neuronal function and behavior in _C. elegans. Sci. Rep._ 6, 38147; doi: 10.1038/srep38147 (2016). PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. REFERENCES * Schröder, P. & Krutmann, J. In Reactions, Processes Vol. 2O The Handbook of Environmental Chemistry (ed. Tilman
Grune ) Ch. 2, 19–31 (Springer Berlin Heidelberg, 2005). * Simonian, N. A. a. & Coyle, J. T. Oxidative Stress in Neurodegenerative Diseases. Annual Review of Pharmacology and Toxicology
36, 83–106, doi: 10.1146/annurev.pa.36.040196.000503 (1996). Article CAS PubMed Google Scholar * Uttara, B., Singh, A. V., Zamboni, P. & Mahajan, R. T. Oxidative Stress and
Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Current Neuropharmacology 7, 65–74, doi: 10.2174/157015909787602823 (2009). Article CAS
PubMed PubMed Central Google Scholar * Smith, M. A., Rottkamp, C. A., Nunomura, A., Raina, A. K. & Perry, G. Oxidative stress in Alzheimer’s disease. Biochimica et Biophysica Acta
(BBA) - Molecular Basis of Disease 1502, 139–144, doi: 10.1016/S0925-4439(00)00040-5 (2000). Article CAS Google Scholar * Hwang, O. Role of Oxidative Stress in Parkinson’s Disease.
Experimental Neurobiology 22, 11–17, doi: 10.5607/en.2013.22.1.11 (2013). Article PubMed PubMed Central Google Scholar * McCarthy, S., Somayajulu, M., Sikorska, M., Borowy-Borowski, H.
& Pandey, S. Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicology and applied pharmacology 201, 21–31, doi:
10.1016/j.taap.2004.04.019 (2004). Article CAS PubMed Google Scholar * Landrigan, P. J. et al. Early Environmental Origins of Neurodegenerative Disease in Later Life. Environmental
Health Perspectives 113, 1230–1233, doi: 10.1289/ehp.7571 (2005). Article CAS PubMed PubMed Central Google Scholar * Boulloche, N. et al. Photophobia in migraine: an interictal PET
study of cortical hyperexcitability and its modulation by pain. Journal of neurology, neurosurgery, and psychiatry 81, 978–984, doi: 10.1136/jnnp.2009.190223 (2010). Article PubMed Google
Scholar * Truong, T. et al. Oxidative Stress in _Caenorhabditis elegans_: Protective Effects of Spartin. PLoS One 10, e0130455, doi: 10.1371/journal.pone.0130455 (2015). Article CAS
PubMed PubMed Central Google Scholar * Sakashita, T. et al. Radiation Biology of Caenorhabditis elegans: Germ Cell Response, Aging and Behavior. Journal of Radiation Research 51, 107–121,
doi: 10.1269/jrr.09100 (2010). Article CAS ADS PubMed Google Scholar * Ristow, M. & Zarse, K. How increased oxidative stress promotes longevity and metabolic health: The concept of
mitochondrial hormesis (mitohormesis). Exp Gerontol 45, 410–418, doi: 10.1016/j.exger.2010.03.014 (2010). Article CAS PubMed Google Scholar * Zhou, K. I., Pincus, Z. & Slack, F. J.
Longevity and stress in C. elegans. Aging 3, 1–21 (2011). Article Google Scholar * Cypser, J. R. & Johnson, T. E. Multiple Stressors in Caenorhabditis elegans Induce Stress Hormesis
and Extended Longevity. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 57, B109–B114, doi: 10.1093/gerona/57.3.B109 (2002). Article Google Scholar *
Knoefler, D. et al. Quantitative _in vivo_ redox sensors uncover oxidative stress as an early event in life. Mol Cell 47, 767–776, doi: 10.1016/j.molcel.2012.06.016 (2012). Article CAS
PubMed PubMed Central Google Scholar * Dimitriadi, M. & Hart, A. C. Neurodegenerative disorders: insights from the nematode Caenorhabditis elegans. Neurobiol Dis 40, 4–11, doi:
10.1016/j.nbd.2010.05.012 (2010). Article CAS PubMed PubMed Central Google Scholar * Yang, W. & Hekimi, S. A mitochondrial superoxide signal triggers increased longevity in
Caenorhabditis elegans. PLoS Biol 8, e1000556, doi: 10.1371/journal.pbio.1000556 (2010). Article CAS PubMed PubMed Central Google Scholar * Honda, Y. & Honda, S. Oxidative stress
and life span determination in the nematode Caenorhabditis elegans. Annals of the New York Academy of Sciences 959, 466–474 (2002). Article CAS ADS PubMed Google Scholar * Padayatty, S.
J. et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. Journal of the American College of Nutrition 22, 18–35 (2003). Article CAS PubMed Google Scholar *
Bendich, A., Machlin, L. J., Scandurra, O., Burton, G. W. & Wayner, D. D. M. The antioxidant role of vitamin C. Advances in Free Radical Biology & Medicine 2, 419–444, doi:
10.1016/S8755-9668(86)80021-7 (1986). Article CAS Google Scholar * Khare, S., Gomez, T. & Clarke, S. G. Defective responses to oxidative stress in protein L-isoaspartyl
repair-deficient Caenorhabditis elegans. Mechanisms of ageing and development 130, 670–680, doi: 10.1016/j.mad.2009.08.002 (2009). Article CAS PubMed PubMed Central Google Scholar *
Chokshi, T. V., Bazopoulou, D. & Chronis, N. An automated microfluidic platform for calcium imaging of chemosensory neurons in Caenorhabditis elegans. Lab Chip 10, 2758–2763, doi:
10.1039/c004658b (2010). Article CAS PubMed Google Scholar * Chokshi, T. V., Bazopoulou, D. & Chronis, N. Probing the physiology of ASH neuron in Caenorhabditis elegans using
electric current stimulation. Appl Phys Lett 99, 53702–537023, doi: 10.1063/1.3615821 (2011). Article CAS ADS PubMed Google Scholar * Guo, M. et al. Reciprocal inhibition between
sensory ASH and ASI neurons modulates nociception and avoidance in Caenorhabditis elegans. Nat Commun 6, 5655, doi: 10.1038/ncomms6655 (2015). Article CAS ADS PubMed Google Scholar *
Hilliard M. A., Bergamasco C., Arbucci S., Plasterk R. H. & Bazzicalupo, P. Worms taste bitter ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans.
The EMBO Journal 23, 1101–1111, doi: 10.1038/ (2004). Article CAS PubMed PubMed Central Google Scholar * Hilliard, M. A., Apicella, A.J., Kerr, R., Suzuki, H., Bazzicalupo, P. &
Schafer, W. R. _In vivo_ imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. The EMBO Journal 24, 63–72, doi: 10.1038/ (2005). * Kaplan, J. M. &
Horvitz, H. R. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc Natl Acad Sci USA 90, 2227–2231 (1993). Article CAS ADS PubMed PubMed Central Google Scholar
* Wang, W. et al. Off-response in ASH neurons evoked by CuSO4 requires the TRP channel OSM-9 in Caenorhabditis elegans. Biochem Biophys Res Commun 461, 463–468, doi:
10.1016/j.bbrc.2015.04.017 (2015). Article CAS PubMed Google Scholar * Chalasani, S. H. et al. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450, 63–70,
doi: 10.1038/nature06292 (2007). Article CAS ADS PubMed Google Scholar * Ezcurra, M., Tanizawa, Y., Swoboda, P. & Schafer, W. R. Food sensitizes C. elegans avoidance behaviours
through acute dopamine signalling. EMBO J 30, 1110–1122, doi: 10.1038/emboj.2011.22 (2011). Article CAS PubMed PubMed Central Google Scholar * Zahratka, J. A., Williams, P. D. E.,
Summers, P. J., Komuniecki, R. W. & Bamber, B. A. Serotonin differentially modulates Ca2+ transients and depolarization in a C. elegans nociceptor. Journal of Neurophysiology 113,
1041–1050, doi: 10.1152/jn.00665.2014 (2015). Article CAS PubMed Google Scholar * Feng, Z. et al. A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels.
Cell 127, 621–633, doi: 10.1016/j.cell.2006.09.035 (2006). Article CAS PubMed PubMed Central Google Scholar * Hsu, A. L., Feng, Z., Hsieh, M. Y. & Xu, X. Z. Identification by
machine vision of the rate of motor activity decline as a lifespan predictor in C. elegans. Neurobiol Aging 30, 1498–1503, doi: 10.1016/j.neurobiolaging.2007.12.007 (2009). Article PubMed
Google Scholar * Li, W., Feng, Z., Sternberg, P. W. & Xu, X. Z. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature 440, 684–687, doi:
10.1038/nature04538 (2006). Article CAS ADS PubMed PubMed Central Google Scholar * Hassan, H. M. & Fridovich, I. Intracellular production of superoxide radical and of hydrogen
peroxide by redox active compounds. Archives of Biochemistry and Biophysics 196, 385–395, doi: 10.1016/0003-9861(79)90289-3 (1979). Article CAS PubMed Google Scholar * Schaar, C. E. et
al. Mitochondrial and cytoplasmic ROS have opposing effects on lifespan. PLoS Genet 11, e1004972, doi: 10.1371/journal.pgen.1004972 (2015). Article CAS PubMed PubMed Central Google
Scholar * Lee, S.-J., Hwang, A. B. & Kenyon, C. Inhibition of Respiration Extends C. elegans Life Span via Reactive Oxygen Species that Increase HIF-1 Activity. Current Biology 20,
2131–2136, doi: 10.1016/j.cub.2010.10.057 (2010). Article CAS PubMed Google Scholar * Schulz, T. J. et al. Glucose restriction extends Caenorhabditis elegans life span by inducing
mitochondrial respiration and increasing oxidative stress. Cell Metab 6, 280–293, doi: 10.1016/j.cmet.2007.08.011 (2007). Article CAS PubMed Google Scholar * Van Raamsdonk, J. M. &
Hekimi, S. Superoxide dismutase is dispensable for normal animal lifespan. Proceedings of the National Academy of Sciences 109, 5785–5790, doi: 10.1073/pnas.1116158109 (2012). Article ADS
Google Scholar * Kumsta, C., Thamsen, M. & Jakob, U. Effects of Oxidative Stress on Behavior, Physiology, and the Redox Thiol Proteome of Caenorhabditis elegans. Antioxidants &
redox signaling 14, 1023–1037, doi: 10.1089/ars.2010.3203 (2011). Article CAS Google Scholar * Kregel, K. C. & Zhang, H. J. An integrated view of oxidative stress in aging: basic
mechanisms, functional effects, and pathological considerations. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology 292, R18–R36, doi:
10.1152/ajpregu.00327.2006 (2007). Article CAS PubMed Google Scholar * Miller, C. J. et al. Disruption of Nrf2/ARE signaling impairs antioxidant mechanisms and promotes cell degradation
pathways in aged skeletal muscle. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1822, 1038–1050, doi: 10.1016/j.bbadis.2012.02.007 (2012). Article CAS Google Scholar *
Bishop, N. A., Lu, T. & Yankner, B. A. Neural mechanisms of ageing and cognitive decline. Nature 464, 529–535 (2010). Article CAS ADS PubMed PubMed Central Google Scholar * Kato,
S., Xu, Y., Cho, C. E., Abbott, L. F. & Bargmann, C. I. Temporal responses of C. elegans chemosensory neurons are preserved in behavioral dynamics. Neuron 81, 616–628, doi:
10.1016/j.neuron.2013.11.020 (2014). Article CAS PubMed PubMed Central Google Scholar * Bargmann, C. I. Comparative chemosensation from receptors to ecology. Nature 444, 295–301 (2006).
Article CAS ADS PubMed Google Scholar * Foster, T. C. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging cell 6, 319–325, doi:
10.1111/j.1474-9726.2007.00283.x (2007). Article CAS ADS PubMed Google Scholar * Catterall, W. A. Voltage-Gated Calcium Channels. Cold Spring Harbor Perspectives in Biology 3, doi:
10.1101/cshperspect.a003947 (2011). * Ristow, M. & Schmeisser, S. Extending life span by increasing oxidative stress. Free Radical Biology and Medicine 51, 327–336, doi:
10.1016/j.freeradbiomed.2011.05.010 (2011). Article CAS PubMed Google Scholar * May, J. M. Vitamin C transport and its role in the central nervous system. Sub-cellular biochemistry 56,
85–103, doi: 10.1007/978-94-007-2199-9_6 (2012). Article CAS PubMed PubMed Central Google Scholar * Meister, A. Glutathione-ascorbic acid antioxidant system in animals. Journal of
Biological Chemistry 269, 9397–9400 (1994). CAS PubMed Google Scholar * Gruber, J., Ng, L. F., Poovathingal, S. K. & Halliwell, B. Deceptively simple but simply deceptive –
Caenorhabditis elegans lifespan studies: Considerations for aging and antioxidant effects. FEBS letters 583, 3377–3387, doi: 10.1016/j.febslet.2009.09.051 (2009). Article CAS PubMed
Google Scholar * Shibamura, A., Ikeda, T. & Nishikawa, Y. A method for oral administration of hydrophilic substances to Caenorhabditis elegans: Effects of oral supplementation with
antioxidants on the nematode lifespan. Mechanisms of ageing and development 130, 652–655, doi: 10.1016/j.mad.2009.06.008 (2009). Article CAS PubMed Google Scholar * Dallaire, A., Proulx,
S., Simard, M. J. & Lebel, M. Expression profile of Caenorhabditis elegans mutant for the Werner syndrome gene ortholog reveals the impact of vitamin C on development to increase life
span. BMC Genomics 15, 1–11, doi: 10.1186/1471-2164-15-940 (2014). Article CAS Google Scholar * Tsukaguchi, H. et al. A family of mammalian Na+-dependent L-ascorbic acid transporters.
Nature 399, 70–75, doi: 10.1038/19986 (1999). Article CAS ADS PubMed Google Scholar * Harrison, F. E., Bowman, G. L. & Polidori, M. C. Ascorbic Acid and the Brain: Rationale for the
Use against Cognitive Decline. Nutrients 6, 1752–1781, doi: 10.3390/nu6041752 (2014). Article CAS PubMed PubMed Central Google Scholar * Kim, K. S. et al. Differential effects of acute
hypoxia on the activation of TRPV1 by capsaicin and acidic pH. The Journal of Physiological Sciences 62, 93–103, doi: 10.1007/s12576-011-0185-4 (2012). Article CAS PubMed Google Scholar
* Murakami, S. & Murakami, H. The effects of aging and oxidative stress on learning behavior in C. elegans. Neurobiol Aging 26, 899–905, doi: 10.1016/j.neurobiolaging.2004.08.007
(2005). Article CAS PubMed Google Scholar * Miyazaki, I. & Asanuma, M. Dopaminergic neuron-specific oxidative stress caused by dopamine itself. Acta medica Okayama 62, 141–150
(2008). CAS PubMed Google Scholar * Asanuma, M., Miyazaki, I., Diaz-Corrales, F. J. & Ogawa, N. Quinone formation as dopaminergic neuron-specific oxidative stress in the pathogenesis
of sporadic Parkinson’s disease and neurotoxin-induced parkinsonism. Acta medica Okayama 58, 221–233 (2004). CAS PubMed Google Scholar * Dargusch, R. & Schubert, D. Specificity of
resistance to oxidative stress. Journal of neurochemistry 81, 1394–1400 (2002). Article CAS PubMed Google Scholar * D’Autreaux, B. & Toledano, M. B. ROS as signalling molecules:
mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8, 813–824 (2007). Article PubMed Google Scholar * Rivas, C. I. et al. Vitamin C transporters. Journal of
physiology and biochemistry 64, 357–375 (2008). Article CAS PubMed Google Scholar * Stiernagle, T. Wormbook (ed. The _C. elegans_ Research Community) (2006). * Chao, M. Y., Komatsu, H.,
Fukuto, H. S., Dionne, H. M. & Hart, A. C. Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc Natl Acad Sci USA 101,
15512–15517, doi: 10.1073/pnas.0403369101 (2004). Article CAS ADS PubMed PubMed Central Google Scholar * Mills, H. et al. Monoamines and neuropeptides interact to inhibit aversive
behaviour in Caenorhabditis elegans. EMBO J 31, 667–678, doi: 10.1038/emboj.2011.422 (2012). Article CAS PubMed Google Scholar * Hilliard, M., Bargmann, C. & Bazzicalupo, P. C.
elegans Responds to Chemical Repellents by Integrating Sensory Inputs from the Head and the Tail. Current Biology 12, 730–734 (2002). Article CAS PubMed Google Scholar * Cronin, C. J. et
al. An automated system for measuring parameters of nematode sinusoidal movement. BMC Genet 6, 5, doi: 10.1186/1471-2156-6-5 (2005). Article CAS PubMed PubMed Central Google Scholar
Download references ACKNOWLEDGEMENTS This work is supported by the National Institutes of Health (NIH) Director’s New Innovator Award #DP2OD006458 and #G013990 University of Michigan
Internal Grant. We thank Trushal Chokshi, Amrita Ray Chaudhury and Daphne Bazopoulou for their help in microfluidic fabrication, building the fluidic platform and for providing the strains,
and the Lurie Nanofabrication Facility personnel at the University of Michigan, where all the microfluidic devices were fabricated. We are grateful to Shawn Xu, Guang Li and Bi Zhang for
helping us with their worm tracking system and for useful discussions, to Sreekanth Chalasani and Aolin Hsu for valuable feedback and to Richard Komuniecki and Mitchel Oakes for helpful
discussions about the octanol assay. AUTHOR INFORMATION Author notes * Present address: Department of Mechanical Engineering, University of Michigan, 2350 Hayward Str., Ann Arbor, MI, 48109,
USA. AUTHORS AND AFFILIATIONS * Department of Mechanical Engineering, University of Michigan, 2350 Hayward Str., Ann Arbor, 48109, MI, USA Eleni Gourgou & Nikos Chronis * Department of
Internal Medicine, Division of Geriatric Medicine, Medical School, University of Michigan, 109 Zina Pitcher Place, Ann Arbor, 48109, MI, USA Eleni Gourgou * Department of Biomedical
Engineering, University of Michigan, 2200 Bonisteel Blvd., Ann Arbor, 48109, MI, USA Nikos Chronis Authors * Eleni Gourgou View author publications You can also search for this author
inPubMed Google Scholar * Nikos Chronis View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS E.G. conceived the idea and run the experiments,
E.G. and N.C. designed the experiments, analyzed the data and wrote the manuscript. All authors reviewed and approved the manuscript. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0
International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the
material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gourgou, E., Chronis, N. Chemically induced oxidative stress affects ASH neuronal
function and behavior in _C. elegans_. _Sci Rep_ 6, 38147 (2016). https://doi.org/10.1038/srep38147 Download citation * Received: 19 April 2016 * Accepted: 27 October 2016 * Published: 06
December 2016 * DOI: https://doi.org/10.1038/srep38147 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
:max_bytes(150000):strip_icc():focal(216x0:218x2)/benedict-cumberbatch-1-435-4-20cc736017b24435a3498a49d7c22b0e.jpg)