- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Combination of anti-retroviral therapy, high-dose chemotherapy (HCT) and autologous stem cell transplantation (ASCT) has led to an improved survival of HIV+ non-Hodgkin lymphoma
(NHL) patients. We compared T- and B-cell subset recovery and related capability to respond to _in-vitro_ stimulation, as well as T-cell repertoire modifications of HIV+ and HIV− NHL
patients undergoing HCT and ASCT as first-line consolidation or salvage treatment, using sequential blood samples obtained before and at 3, 6, 12 and 24 months after ASCT. B lymphocyte
recovery occurred earlier, reaching higher levels in HIV+ patients as compared to HIV− patients and healthy controls; in particular, immature and naïve B cells were significantly higher in
HIV+ patients who had received rituximab in the pre-ASCT period. These lymphocytes equally responded to _in-vitro_ stimulation. Newly produced T cells similarly increased in HIV+ and HIV−
NHL patients, but their levels remained constantly lower than in healthy controls. T lymphocytes showed a reduced proliferative capacity, but their repertoire was reassorted by the
treatment. The functional and numeric B-cell recovery and the qualitative modifications of T-cell receptor repertoire may explain, at least in part, the success of this aggressive
therapeutic approach in HIV+ patients. SIMILAR CONTENT BEING VIEWED BY OTHERS T-CELL DIVERSITY AND EXCLUSION OF BLOOD-DERIVED T-CELLS IN THE TUMOR MICROENVIRONMENT OF CLASSICAL HODGKIN
LYMPHOMA Article Open access 17 December 2024 T-CELL TRACKING, SAFETY, AND EFFECT OF LOW-DOSE DONOR MEMORY T-CELL INFUSIONS AFTER ΑΒ T CELL-DEPLETED HEMATOPOIETIC STEM CELL TRANSPLANTATION
Article 17 November 2020 DURABLE RESPONSE TO CAR T IS ASSOCIATED WITH ELEVATED ACTIVATION AND CLONOTYPIC EXPANSION OF THE CYTOTOXIC NATIVE T CELL REPERTOIRE Article Open access 23 May 2025
INTRODUCTION The introduction of combination anti-retroviral therapy (cART) has modified the natural history of HIV infection, reducing HIV-related morbidity and mortality, a significant
portion of which, however, is still accounted for by HIV-associated lymphoma1. Moreover, immune preservation with cART has changed the therapeutic approach to HIV-associated lymphoma,
allowing the use of aggressive treatment strategies, including high-dose chemotherapy (HDC) with autologous stem cell transplantation (ASCT). This approach has been explored at several
Institutions in patients with refractory or relapsed HIV-associated lymphomas, showing high clinical efficacy with low toxicity and lack of significant increase in opportunistic
infections2,3,4,5,6,7,8,9. ASCT has also been used with encouraging results as early consolidation treatment after first-line therapy in HIV+ patients with lymphoma at high risk of
relapse2,10. The effects of ASCT, used as salvage treatment, were similar between HIV+ and HIV− subjects, and a trend towards a lower probability of relapse after ASCT was observed in HIV+
patients10,11,12. The initial concerns related to the possibility that HDC could exacerbate the immune depression already present in HIV+ patients, leading to infection progression, were
ruled out by the demonstration that ASCT does not enhance viral replication or the peripheral HIV reservoir in the long term and does not worsen the T-cell impairment13. Rather, a T-cell
recovery has been described, probably related to the maintained thymus capability of transplanted patients to generate new T cells, as demonstrated by the peripheral increase of lymphocytes
containing T-cell receptor excision circles (TRECs)+ cells13,14. The post-ASCT immune recovery appears not to be different in HIV+ versus HIV− patients because total and naïve
CD4+-lymphocytes, as well as TRECs, are similarly increased in both groups of patients15. This suggests that conditioning regimens may create an identically appropriate lymphoid niche that
can be equally replenished by the transferred cells in both groups of patients15,16. While it has been reported that the lymphocyte recovery also involves CD8+ and CD19+ cells, which undergo
a rapid expansion in both HIV+ and HIV− groups after the period of aplasia15, whether the kinetics of the recovery of CD4+, CD8+ and CD19+ cells and their subsets differ among HIV+ and HIV−
patients remain not fully answered. Moreover, it is not known whether lymphocytes that replenish the immune system in the post-ASCT period are functional. Finally, whether the T-cell
receptor (TCR) repertoire undergoes similar modifications in HIV+ and HIV− patients has not been explored yet. RESULTS PATIENTS’ CHARACTERISTICS AND TREATMENT Of the 32 enrolled patients (17
HIV+ and 15 HIV−), 20 (11 HIV+ and 9 HIV−) were included in the analysis. Twelve patients were not analyzed for immune recovery either because they relapsed early after ASCT (4 in each
group) or because we included in the study only patients whose samples were collected at least at four different time points. At study entry, all HIV+ patients were receiving cART; median
time from HIV diagnosis to cART initiation and from HIV diagnosis to ASCT was 30 (range: 5–192) and 46 (6–336) months, respectively. The main characteristics of the patients and clinical
data are shown in Table 1. The prevalence of men in the HIV+ group reflects the epidemiology of HIV infection in Italy17, while the difference in the lymphoma histology reflects the
different epidemiology of NHL in HIV+ and HIV− populations18. This translates into a different percentage of patients treated in the pre-ASCT period, with the anti CD20 monoclonal antibody
rituximab, which was administered only in patients with CD20+ lymphoma. Induction regimens for HIV+ patients included cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) +/−
rituximab (n: 8); high dose methotrexate-containing regimens (n: 2); and doxorubicin, cyclophosphamide, vincristine, bleomycin, prednisone, and etoposide (VACOP-B; n: 1). Platinum-based
regimens were administered to 4 of these patients as salvage therapy. Induction regimens for HIV− patients included CHOP and rituximab (n: 7); intensified CHOP and rituximab (n: 2). As for
HIV+, also two HIV− patients received platinum-based regimens as salvage therapy. The number of mobilized CD34+ cells were significantly lower in HIV+ compared to HIV− patients, but similar
in rituximab versus non-rituximab-treated HIV+ patients. The observed differences in the number of mobilized CD34+ cells could be explained on the basis of: (1) heterogeneity in the therapy
used for stem cell mobilization between HIV+ and HIV− patients; (2) different CD34+ mobilization goal19 (3) the depletion of hematopoietic reservoir in HIV+ patients20,21. However, the
number of infused CD34+ cells was comparable and not statistically different between the two groups of patients. Patients received antibacterial, antifungal and antiviral prophylaxis until
stable engraftment, while trimethoprim/sulfamethoxazole prophylaxis was suspended after stem cell infusion. None of the HIV+ patients interrupted cART due to oral mucositis or other
toxicities, nor modified the cART due to viral response failure. A single intravenous immunoglobulin injection has been administered to one HIV+ and 3 HIV− patients. Of note, this was given
before engraftment, few days after transplant. A patient with detectable HIV viremia at ASCT became negative early afterwards, while 4 had a short-lasting detectable viremia after ASCT.
Before engraftment, 4 HIV+ and 2 HIV− patients had a bacterial documented infection, and 2 HIV+ patients had CMV reactivation. During the 2 years observation period, 2 herpes zoster
infections and 2 CMV reactivations were seen in the HIV+ group, and 1 episode of pulmonary infection happened in both groups of patients. QUANTITATIVE POST-ASCT IMMUNE RECOVERY Immediately
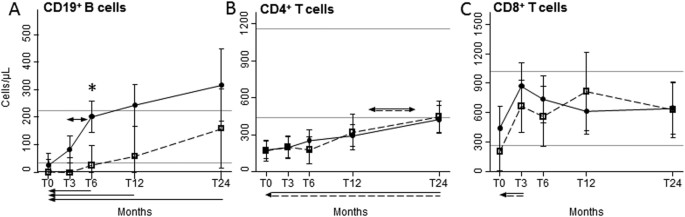
after ASCT, total CD19+ B lymphocytes of HIV+ and HIV− patients were below the lower values observed in healthy controls (HC), but differentially increased after ASCT, being significantly
and generally higher in HIV+ patients, going beyond the highest value found in HC after 12 and 24 months (Fig. 1A). Specifically, B-cell number started to increase at 3 months post-ASCT and
doubled in the following 3 months only in HIV+ patients, leading to significant differences between the two groups after 6 months. The mean values of B cells of HIV− patients returned within
the normal range at 12 and 24 months after ASCT. Post-ASCT median values of serum IgG increased preferentially in HIV+ patients (from 654 [range: 338–1680] mg/dL at 3 months since ASCT to
983 [623–1670] mg/dL at 24 months) that in HIV− subjects (from 621 [160–1312] mg/dL to 691 [224–1313] mg/dL). Before ASCT, the number of CD4+ T lymphocytes of HIV+ and HIV− patients were
significantly lower as compared to HC, but significantly and comparably increased from 12 to 24 months after ASCT (Fig. 1B). The mean CD8+ cell counts were below the lowest values of HC at
the pre-ASCT time point in HIV+ individuals only, significantly increased after 3 months in both groups, and always remained within the reference interval (Fig. 1C). QUANTITATIVE POST-ASCT
B-CELL SUBSET RECOVERY The better recovery of total B cells of HIV+ individuals involved immature CD19+CD10+ cells, which increase was significantly higher but not at a specific time point
(Fig. 2A), and mature CD19+CD10− B cells (Fig. 2B), that grew faster in HIV+ patients starting from 3 months, and becoming significantly higher than in HIV− patients at 12 months after ASCT.
The most represented mature B-cell subset, namely the IgD+CD27− naïve population, showed a significant expansion in HIV+ patients compared to HIV− patients at 6 and 12 months after
transplantation (Fig. 2C). The early and preferential expansion of these B-lymphocyte subsets in HIV+ patients was confirmed by the analysis of K-deleting recombination excision circles
(KRECs), which are the products of B-cell receptor rearrangements and therefore markers of B-cell neo-production. In HIV+ patients, KRECs were significantly higher already at baseline and
increased as soon as 3 months after ASCT, while in HIV− patients the increase was slightly delayed. At 24 months after ASCT, KRECs become similar in the two groups, and also those of HIV−
patients finally moved into the normal range (Fig. 2D). No significant differences were detected between the two groups of patients for both CD19+ CD10−IgD+CD27+ un-switched and
CD19+CD10−IgD−CD27+ switched memory subsets (Fig. 2E and F). B-cell subset values of rituximab-treated HIV+ and HIV− patients with diffuse large B-cell lymphoma (DLBCL), were compared with
those of rituximab-untrated HIV+ patients with other lymphoma histologic types. We found that rituximab strongly influenced the levels of B-cell subpopulations and KREC production in HIV+
patients, both before and after ASCT (Fig. 3). In addition, we found that complete or partial remission status did not exert any effect on B-cell recovery in HIV+ patients (data not shown).
FUNCTIONAL POST-ASCT B-CELL RECOVERY _In-vivo_ replication history of B lymphocytes, evaluated by measuring the average number of their divisions, was within the normal range in both HIV+
and HIV− patients (Fig. 4A). The only exception was documented within HIV− patient samples collected at 3 months post ASCT, where the number of B-cell divisions fell below the minimum value
of HC, thus resulting significantly lower than that of HIV+ individuals, likely reflecting the lack of B cells early after ASCT. B-cell functional response was evaluated by CFSE assay,
measuring the ability of patient cells to proliferate after combined stimulation with anti-Ig for B-cell receptor cross-linking and CpG, which targets toll-like receptor 9, in presence of
interleukin-10, known to increases mRNA expression of toll-like receptor 922, and interleukin-2, which sustains B-cell proliferation23. The fraction of B cells that divided at least once
(Fig. 4B) and the average number of divisions performed by responding cells (Fig. 4C) did not differ significantly between HIV+ and HIV− patients and HC, indicating that B lymphocytes
displayed an _in-vitro_ capability to proliferate after stimulation. QUANTITATIVE POST-ASCT T-CELL SUBSET RECOVERY Naïve CD4 (Fig. 5A), recent thymic emigrants (RTE; Fig. 5C) and TRECs+
(Fig. 5D) lymphocytes similarly and significantly increased starting from 6 months after ASCT in HIV+ and HIV− patients. Of note, naïve CD4 cells never reached the lower limit of the
reference range found in HC. In contrast, the overall level of naïve CD8+ cells was significantly higher in HIV+ patients, where we observed that cells went above the lowest value obtained
in HC after 12 months since ASCT (Fig. 5B). In both groups of patients, CD4+ TCM significantly increased in respect to the baseline, and especially starting from 12 months of therapy, but
the values were always under those of HC (Supplementary Figure 1A). On the contrary, CD4+ TEM increased in the first 12 months of therapy (Supplementary Figure 1B). The number of CD8+ TCM
was significantly higher in HIV+ patients, with values always close or higher than the top values found in HC (Supplementary Figure 1C), while CD8+ TCM similarly increased only in the months
immediately after ASCT (Supplementary Figure 1D). FUNCTIONAL POST-ASCT T-CELL RECOVERY The capability of T cells to respond to _in-vitro_ stimulation was assessed by CFSE assay after
stimulation with PHA. The percentage of CD4+ and CD8+ cells that divided at least once after 4 days of culture was significantly lower in HIV+ and HIV− patients compared to HC (Fig. 6A and
B). The average number of CD4+ cell divisions upon stimulation was similarly modulated in both patients and HC (Fig. 6C), while CD8+ cell divisions were reduced compared to HC (Fig. 6D). TCR
REPERTOIRE ANALYSIS TCR diversity was evaluated before and 24 months after ASCT in 14 (7 HIV+ and 7 HIV−) patients. No correlation was found between the extent of therapy-induced
lymphopenia and the degree of repertoire perturbation observed at the pre-ASCT period (data not shown). The mean proportion of TCRBV elements with normal, shifted, restricted and
mono/oligoclonal profiles was significantly higher in samples obtained from the two groups of patients both in pre- and post-ASCT periods in comparison to samples of HC (Fig. 7A).
Accordingly, the mean percentages of TCRBV perturbations were significantly higher in HIV+ and HIV− patients at both time points (Fig. 7B). However, if TCRBV perturbations were evaluated at
single-TCRBV chain and single-patient level, the types of TCR repertoire restrictions appeared different because certain perturbations of TCRBV families found in the pre-ASCT period were
lost 24 months after ASCT. In parallel, for other TCRBV families, certain over-perturbations became evident only after the treatment (Fig. 7C and Supplementary Figure 2A). Finally, an
unsupervised hierarchical clustering of the average perturbation changes, calculated as fold-change between post- and pre-ASCT periods, could group 6 out of the 7 HIV+ patients and 6 out of
the 7 HIV− patients (Supplementary Figure 2B). DISCUSSION We found that the profound depletion of the B-cell population observed in HIV+ and HIV− NHL transplanted patients at the pre-ASCT
time point was followed by a differential and long lasting expansion of naïve and immature B cells that progressively and significantly increased in HIV+ patients to overcome the values
observed in HC. While there were no differences in B-cell functionality between the two groups, HIV+ patients also showed a better trend towards an IgG increase after ASCT in comparison to
HIV− patients. The observed differences in the B-cell compartment between HIV+ and HIV− patients, treated or not with rituximab before ASCT, are intriguing. Previous results obtained in HIV−
subjects with NHL demonstrated that an anti-CD20-based treatment induces a complete depletion of B lymphocytes, followed by a delayed recovery of memory B cells with abnormal function,
which leads to a significantly increased incidence of hypogammaglobulinemia, lasting several months after ASCT24,25. However, we found that non-rituximab-treated non-DLBCL HIV+ patients
showed a pattern of B-cell subset increase closer to that of HIV− rituximab treated DLBCL patients, while the highest number of B-cell increase was preferentially observed in HIV+ rituximab
treated DLBCL patients. Possibly, the diverse B-cell increase in the two groups of HIV+ and HIV− patients could be related to the different molecular characteristics, cell origin, and
prognosis of DLBCL, which are known to be related to a different incidence of EBV infection26. Indeed, in the pre-cART and pre-rituximab era, this infection was detected in 63% of HIV+, but
only in 3% of HIV− patients. Nevertheless, similar studies have not been performed in patients treated with cART; moreover, EBV was measured only in a minority of our patients, so we cannot
reach any conclusion on the role of EBV in mediating differential B-cell recovery in our HIV+ patients. Previous data on T-cell recovery obtained by measuring TRECs content in HIV+ patients
have been criticized because the results may be altered by the influence of ongoing HIV replication on the rates of cell division, apoptosis, and life span of CD4 cells27,28. This was not
the case of our patients because they had a good control of HIV infection as result of the cART responsible for a reduced cell activation, proliferation, and apoptosis29,30. In addition,
both approaches used to quantify thymic output, namely TREC quantification and RTE phenotyping, confirmed that the kinetics of increase of newly produced CD4+ lymphocytes is not different in
our HIV+ and HIV− patients. However, although increased, these cells always remained at low levels, and the percentage of CD4+ lymphocytes that responded to stimulation was lower than in HC
in both groups. While CD4+ T-cell recovery following ASCT has been previously characterized, at least partially, in HIV+ patients, potential fluctuations of CD8+ cells are less defined. It
has been shown that HIV infection does not influence naïve CD8+ T-cell levels in lymphoma patients with and without HIV infection candidates for ASCT31, but the kinetic measurements and a
phenotypic characterization of CD8 subsets were not previously reported. Phenotypic characterization of CD8+ T-cell-recovery among HIV− lymphoma patients undergoing ASCT has suggested a
preferential expansion of antigen-primed CD8+ cells rather than CD8+ naïve cells32. Our findings show that immune recovery preferentially involved CD8 TCM, as well as naïve CD8+ cells.
Moreover, naïve CD8+ cells were more expanded in HIV+ rather than in HIV− patients, across all the evaluated time points, starting from the pre-conditioning (T0), going to the 24 months of
therapy (T24). The percentage of CD8+ lymphocytes that proliferated upon stimulation was significantly lower in all patients as compared to HC. Therefore, CD8+ cells are functionally
different from those of HC, and may represent a population of “exhausted” clonal T cells, similar to those found during physiological aging33,34,35,36. Accordingly, as observed in the
elderly, and despite the observed increase in newly produced diversified T cells in both groups of patients, TCR repertoires at 24 months after ASCT are as restricted as in the
pre-transplant period. However, we could also demonstrate relevant therapy-induced modifications of TCRBV chain profiles, including enlargement in TCR heterogeneity of T cells expressing
certain TCRBV, and contraction in diversity of cells bearing other TCRBV, as well as disappearance of some clonal expansions and appearance of others. Therefore, our data differ from those
reporting that patients undergoing ASCT after HDC regenerate clonal expansions consistent with those found in the pre-treatment period37. Furthermore, these modifications could be different
in HIV+ and HIV− patients as shown by unsupervised hierarchical clustering discriminating between the two groups. Finally, TCRBV modifications are not merely due to a physiologic repertoire
“drift” over time because TCR repertoire of HC is extremely stable38. Taken together these findings indicate that T- and B-cell recovery following HDC and ASCT is similar or even better in
HIV+ than in HIV− patients. Thus, the functional and numeric B-cell recovery and the qualitative modifications of T-cell repertoire may explain, at least in part, the success of this
aggressive therapeutic approach in HIV+ patients, both considering the low number of infectious complications commonly seen in these patients, and, more intriguing, the high anti-lymphoma
efficacy and the very low relapse rate observed after ASCT. METHODS PATIENTS From October 2009 to February 2012, all consecutive HIV+ patients with NHL who received ASCT as first-line
consolidation or as salvage therapy at our Institution were enrolled in this prospective study. Patients signed an informed consent and the project was carried out in agreement with
Declaration of Helsinki principles. Approval for these studies was obtained by our Institutional Board of Ethics Committee (Institution: ASST Spedali Civili di Brescia, Brescia, Italy;
approved protocol no. NP 2352). Blood samples were obtained from patients at different time points: the day before starting the conditioning regimen (T0), and at 3 (T3), 6 (T6), 12 (T12),
and 24 (T24) months after ASCT. Results obtained in HIV+ patients were compared with those of HIV− patients and those of age-matched HC. Peripheral blood mononuclear cells (PBMC) were
prepared by Ficoll-Hypaque gradient centrifugation, and frozen in liquid nitrogen until use. QUANTIFICATION OF LYMPHOCYTE SUBPOPULATIONS Newly produced T and B lymphocytes were quantified by
measuring TRECs and KRECs in PBMC using a duplex quantitative real-time PCR performed as previously reported39. Results were expressed as copies/mL of blood. For B-cell subpopulation
identification, one million PBMCs from HIV+, HIV− and HC were phenotyped after staining with peridin-clorophyll protein-Cy5.5 anti-CD19, phycoerythrin-Cy7 anti-CD10, fluorescein
isothiocyanate anti-IgD, and phycoerythrin anti-CD27 mAbs. The cells were first gated for CD19 expression on lymphocytes and then analyzed for the expression of CD10 marker to identify
CD19+CD10+ immature B cells and CD19+CD10− mature B cells. This last subset was examined for IgD and CD27 molecule expression in order to recognize IgD+CD27− naïve B cells, IgD+CD27+
unswitched memory B cells, and IgD−CD27+ switched memory B cells. For T-cell subpopulation characterization, one million PBMCs were stained with phycoerythrin anti-CD3, allophycocyanin-H7
anti-CD4, phycoerythrin-Cy7 anti-CD8, fluorescein isothiocyanate anti-CD45RA, peridin-clorophyll protein-Cy5.5 anti-CCR7, and allophycocyanin anti-CD31 mAbs. PBMCs were first gated on the
basis of CD3 expression, analyzed for CD4 and CD8 markers, and then for the expression of CD45RA and CCR7 in order to identify: CD45RA+CCR7+CD4+ and CD45RA+CCR7+CD8+ naïve T lymphocytes;
CD45RA−CCR7+ central memory (TCM) and CD45RA−CCR7− effector memory (TEM). RTE were naïve CD4+ T lymphocytes expressing the CD31 molecule. mAbs were purchased from BD Pharmingen, eBioscience,
BioLegend (San Diego, CA) and Miltenyi Biotec (Bergisch Gladbach, Germany). Data were analysed with the FACS Diva software (BD Bioscience, San Diego, CA), and reported as absolute counts
per μL of blood. AVERAGE NUMBER OF _IN-VIVO_ B-CELL DIVISIONS AND _IN-VITRO_ T- AND B-CELL ACTIVATION The replication history of B lymphocytes was evaluated calculating the differences
between the cycle threshold numbers (ΔCt) obtained by real-time PCR of the signal joint and the coding joint, which are generated during the rearrangement of IGK genes40. For proliferation
assay, PBMC (3–5 × 106/mL) prepared using samples obtained at 24 months since ASCT were labelled with 0.2 μmol/L of carboxyfluorescein succinimidyl ester (CFSE; Invitrogen, Eugene, OR) for
20 minutes. Cells were plated in 96-well U-bottom culture plates and stimulated at 37 °C with 6.25 μg/mL phytohemagglutinin or 10 μg/mL CpG ODN 2006 (InvivoGen, San Diego, CA), 5 μg/mL of
F(ab)2 anti-human IgM/IgG/IgA (Jackson Immunoresearch, West Grove, PA), 40 U/mL interleukin-2 and 50 ng/mL interleukin-10 (Sigma-Aldrich, St Louis, MO), as described elsewhere23,41. T- and
B-cell proliferation was measured by flow cytometry after 5 days of culture. Data were analysed as previously reported42. TCR REPERTOIRE ANALYSIS BY COMPLEMENTARITY-DETERMINING REGION 3
(CDR3) SPECTRATYPING The diversity of TCR beta variable (TCRBV) families was studied by spectratyping after performing multiplex PCR43. The length distributions of PCR products were analyzed
on an ABI 3500 Genetic Analyzer; distribution of fragment lengths, number of detectable peaks per TCRBV element, and area under the curve were calculated by Gene Mapper (Applied
Biosystems). The CDR3 size distribution of TCRBV families of each subject was classified into four categories: normal (Gaussian distribution and >7 peaks), shifted (deviation from
Gaussian distribution and >7 peaks), restricted (prominent deviation from Gaussian distribution), and mono/oligoclonal (1 or 2 dominant peaks)43. Furthermore, the distribution of TCRBV
perturbations was also calculated using the generalized Hamming distance method44, in which the CDR3 length distribution of each TCRBV of a patient was subtracted from the average
Gaussian-like CDR3 length distribution obtained by analysing a “reference group” composed of age-matched HC. When a TCRBV family was not represented (no detectable peaks), the condition of
maximal perturbation was reached, and its value was arbitrarily set to 100%. STATISTICAL ANALYSIS Comparisons between the mean of quantitative variables measured in HIV+ and HIV− subjects at
several time points during the follow-up were performed by repeated measures ANOVA using linear mixed-models with a random slope. In particular, comparisons between the mean values of
lymphocyte subpopulation counts, including log-KRECs and log-TRECs, measured in HIV+ and HIV− subjects at more than one time point during the follow-up were performed by repeated measures
ANOVA using linear mixed-models with a random slope. In these models, HIV serostatus was considered as a covariate and part of an interaction term with time. A similar model was employed to
compare immune reconstitution in HIV+ patients, using a binary covariate to compare partial vs complete remission. The same technique was used to compare average perturbations (after arcsine
data transformation). In this case, the covariate included the HC group. When this interaction was significant, post-hoc comparisons between HIV+ and HIV− subjects at the different time
points were performed by linear contrasts, and Bonferroni corrected p-values were calculated. On the contrary, if the interaction was not significant, but a main effect with more than two
levels was significant, Bonferroni corrected post-hoc comparisons were used to compare the differences between the levels. Alternatively, in case the significant main effect included only
two levels, only its p-value was reported (e.g. HIV+ vs HIV−). In the case of rituximab treatment, only planned contrasts were used to compare the means of the cell population counts between
the three subgroups at the different time points. _In-vitro_ patient cell proliferations were compared to those of HC by one-way ANOVA followed by the Dunnett’s test. Comparisons between
categorical variables were performed by the Fisher’s exact test. Differences were considered significant when P < 0.05. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Bertoli, D. _et
al_. B- and T-lymphocyte number and function in HIV+/HIV− lymphoma patients treated with high-dose chemotherapy and autologous bone marrow transplantation. _Sci. Rep._ 6, 37995; doi:
10.1038/srep37995 (2016). PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. REFERENCES * Bonnet,
F. et al. Changes in cancer mortality among HIV-infected patients: the Mortalite 2005 Survey. Clin Infect Dis 48, 633–9 (2009). Article PubMed Google Scholar * Diez-Martin, J. L. et al.
Comparable survival between HIV+ and HIV- non-Hodgkin and Hodgkin lymphoma patients undergoing autologous peripheral blood stem cell transplantation. Blood 113, 6011–4 (2009). Article CAS
PubMed Google Scholar * Gabarre, J., Azar, N., Autran, B., Katlama, C. & Leblond, V. High-dose therapy and autologous haematopoietic stem-cell transplantation for HIV-1-associated
lymphoma. Lancet 355, 1071–2 (2000). Article CAS PubMed Google Scholar * Levine, A. M. Acquired immunodeficiency syndrome-related lymphoma: clinical aspects. Semin Oncol 27, 442–53
(2000). CAS PubMed Google Scholar * Palella, F. J. Jr. et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study
Investigators. N Engl J Med 338, 853–60 (1998). Article PubMed Google Scholar * Re, A. et al. High-dose therapy and autologous peripheral-blood stem-cell transplantation as salvage
treatment for HIV-associated lymphoma in patients receiving highly active antiretroviral therapy. J Clin Oncol 21, 4423–7 (2003). Article CAS PubMed Google Scholar * Re, A. et al.
High-dose therapy and autologous peripheral blood stem cell transplantation as salvage treatment for AIDS-related lymphoma: long-term results of the Italian Cooperative Group on AIDS and
Tumors (GICAT) study with analysis of prognostic factors. Blood 114, 1306–13 (2009). Article CAS PubMed Google Scholar * Serrano, D. et al. HIV-associated lymphoma successfully treated
with peripheral blood stem cell transplantation. Exp Hematol 33, 487–94 (2005). Article CAS PubMed Google Scholar * Zanet, E. et al. Postautologous stem cell transplantation long-term
outcomes in 26 HIV-positive patients affected by relapsed/refractory lymphoma. AIDS 29, 2303–8 (2015). Article PubMed Google Scholar * Krishnan, A. et al. Durable remissions with
autologous stem cell transplantation for high-risk HIV-associated lymphomas. Blood 105, 874–8 (2005). Article CAS PubMed Google Scholar * Krishnan, A. et al. HIV status does not affect
the outcome of autologous stem cell transplantation (ASCT) for non-Hodgkin lymphoma (NHL). Biol Blood Marrow Transplant 16, 1302–8 (2010). Article PubMed PubMed Central Google Scholar *
Re, A. et al. Early Consolidation with High Dose Therapy and Autologous Stem Cell Transplantation in HIV-Associated Non Hodgkin Lymphoma at High Risk (aa-IPI 2–3). Mature Results of a
Multicenter Prospective Phase II Trial. Blood 124, 2528 (2014). Google Scholar * Simonelli, C. et al. Immune recovery after autologous stem cell transplantation is not different for
HIV-infected versus HIV-uninfected patients with relapsed or refractory lymphoma. Clin Infect Dis 50, 1672–9 (2010). Article CAS PubMed Google Scholar * Benicchi, T. et al. T-cell immune
reconstitution after hematopoietic stem cell transplantation for HIV-associated lymphoma. Transplantation 80, 673–82 (2005). Article PubMed Google Scholar * Klebanoff, C. A., Khong, H.
T., Antony, P. A., Palmer, D. C. & Restifo, N. P. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol 26, 111–7
(2005). Article CAS PubMed PubMed Central Google Scholar * Muranski, P. et al. Increased intensity lymphodepletion and adoptive immunotherapy–how far can we go? Nat Clin Pract Oncol 3,
668–81 (2006). Article CAS PubMed PubMed Central Google Scholar * Torti, C. et al. Cohort Profile: Standardized Management of Antiretroviral Therapy Cohort (MASTER Cohort). Int J
Epidemiol (2015). * Kaplan, L. D. HIV-associated lymphoma. Best Pract Res Clin Haematol 25, 101–17 (2012). Article CAS PubMed Google Scholar * Re, A. et al. Stem cell mobilization in HIV
seropositive patients with lymphoma. Haematologica 98, 1762–8 (2013). Article PubMed PubMed Central Google Scholar * Sloand, E. M. et al. Secondary colony formation after long-term bone
marrow culture using peripheral blood and bone marrow of HIV-infected patients. AIDS 11, 1547–53 (1997). Article CAS PubMed Google Scholar * Zauli, G. & Capitani, S. HIV-1-related
mechanisms of suppression of CD34+ hematopoietic progenitors. Pathobiology 64, 53–8 (1996). Article CAS PubMed Google Scholar * He, B., Qiao, X. & Cerutti, A. CpG DNA induces IgG
class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol 173, 4479–91 (2004). Article CAS PubMed Google
Scholar * Huggins, J. et al. CpG DNA activation and plasma-cell differentiation of CD27- naive human B cells. Blood 109, 1611–9 (2007). Article CAS PubMed PubMed Central Google Scholar
* Nishio, M. et al. Delayed redistribution of CD27, CD40 and CD80 positive B cells and the impaired _in vitro_ immunoglobulin production in patients with non-Hodgkin lymphoma after
rituximab treatment as an adjuvant to autologous stem cell transplantation. Br J Haematol 137, 349–54 (2007). Article CAS PubMed Google Scholar * Shortt, J. & Spencer, A. Adjuvant
rituximab causes prolonged hypogammaglobulinaemia following autologous stem cell transplant for non-Hodgkin’s lymphoma. Bone Marrow Transplant 38, 433–6 (2006). Article CAS PubMed Google
Scholar * Morton, L. M. et al. Molecular characteristics of diffuse large B-cell lymphoma in human immunodeficiency virus-infected and -uninfected patients in the pre-highly active
antiretroviral therapy and pre-rituximab era. Leuk Lymphoma 55, 551–7 (2014). Article CAS PubMed Google Scholar * Harris, J. M. et al. Multiparameter evaluation of human thymic function:
interpretations and caveats. Clin Immunol 115, 138–46 (2005). Article CAS PubMed Google Scholar * Ho Tsong Fang, R., Colantonio, A. D. & Uittenbogaart, C. H. The role of the thymus
in HIV infection: a 10 year perspective. AIDS 22, 171–84 (2008). Article PubMed Google Scholar * Franco, J. M. et al. T-cell repopulation and thymic volume in HIV-1-infected adult
patients after highly active antiretroviral therapy. Blood 99, 3702–6 (2002). Article CAS PubMed Google Scholar * Kolte, L. et al. Association between larger thymic size and higher
thymic output in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy. J Infect Dis 185, 1578–85 (2002). Article PubMed Google Scholar * Pratesi,
C. et al. Recent thymic emigrants in lymphoma patients with and without human immunodeficiency virus infection candidates for autologous peripheral stem cell transplantation. Clin Exp
Immunol 151, 101–9 (2008). Article CAS PubMed PubMed Central Google Scholar * Geddes, M. & Storek, J. Immune reconstitution following hematopoietic stem-cell transplantation. Best
Pract Res Clin Haematol 20, 329–48 (2007). Article CAS PubMed Google Scholar * Buchholz, V. R., Neuenhahn, M. & Busch, D. H. CD8+ T cell differentiation in the aging immune system:
until the last clone standing. Curr Opin Immunol 23, 549–54 (2011). Article CAS PubMed Google Scholar * Papagno, L. et al. Immune activation and CD8+ T-cell differentiation towards
senescence in HIV-1 infection. PLoS Biol 2, E20 (2004). Article CAS PubMed PubMed Central Google Scholar * Pawelec, G. & Larbi, A. Immunity and ageing in man: Annual Review
2006/2007. Exp Gerontol 43, 34–8 (2008). CAS PubMed Google Scholar * Appay, V., Almeida, J. R., Sauce, D., Autran, B. & Papagno, L. Accelerated immune senescence and HIV-1 infection.
Exp Gerontol 42, 432–7 (2007). Article CAS PubMed Google Scholar * Protheroe, A. S. et al. Persistence of clonal T-cell expansions following high-dose chemotherapy and autologous
peripheral blood progenitor cell rescue. Br J Haematol 111, 766–73 (2000). CAS PubMed Google Scholar * Muraro, P. A. et al. T cell repertoire following autologous stem cell
transplantation for multiple sclerosis. J Clin Invest 124, 1168–72 (2014). Article CAS PubMed PubMed Central Google Scholar * Sottini, A. et al. Simultaneous quantification of recent
thymic T-cell and bone marrow B-cell emigrants in patients with primary immunodeficiency undergone to stem cell transplantation. Clin Immunol 136, 217–27 (2010). Article CAS PubMed Google
Scholar * van Zelm, M. C., Szczepanski, T., van der Burg, M. & van Dongen, J. J. Replication history of B lymphocytes reveals homeostatic proliferation and extensive antigen-induced B
cell expansion. J Exp Med 204, 645–55 (2007). Article CAS PubMed PubMed Central Google Scholar * Bernasconi, N. L., Onai, N. & Lanzavecchia, A. A role for Toll-like receptors in
acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood 101, 4500–4 (2003). Article CAS PubMed Google Scholar *
Roederer, M. Interpretation of cellular proliferation data: avoid the panglossian. Cytometry A 79, 95–101 (2011). Article PubMed Google Scholar * Chiarini, M. et al. Newly produced T and
B lymphocytes and T-cell receptor repertoire diversity are reduced in peripheral blood of fingolimod-treated multiple sclerosis patients. Mult Scler 21, 726–34 (2015). Article CAS PubMed
Google Scholar * Gorochov, G. et al. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat Med 4,
215–21 (1998). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS The study was supported by Regione Lombardia [Piano Regionale Sangue 2009]. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Centro di Ricerca Emato-oncologica AIL (CREA), ASST Spedali Civili, Brescia, Italy Diego Bertoli, Marco Chiarini, Alessandra Sottini, Federico Serana, Viviana
Giustini, Aldo M. Roccaro, Luigi Caimi & Luisa Imberti * Hematology, ASST Spedali Civili, Brescia, Italy Alessandro Re, Chiara Cattaneo & Giuseppe Rossi Authors * Diego Bertoli View
author publications You can also search for this author inPubMed Google Scholar * Alessandro Re View author publications You can also search for this author inPubMed Google Scholar * Marco
Chiarini View author publications You can also search for this author inPubMed Google Scholar * Alessandra Sottini View author publications You can also search for this author inPubMed
Google Scholar * Federico Serana View author publications You can also search for this author inPubMed Google Scholar * Viviana Giustini View author publications You can also search for this
author inPubMed Google Scholar * Aldo M. Roccaro View author publications You can also search for this author inPubMed Google Scholar * Chiara Cattaneo View author publications You can also
search for this author inPubMed Google Scholar * Luigi Caimi View author publications You can also search for this author inPubMed Google Scholar * Giuseppe Rossi View author publications
You can also search for this author inPubMed Google Scholar * Luisa Imberti View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS D.B., M.C.,
A.S., V.G.: perfomed the experimental procedures. F.S.: performed statistical analysis and revised the paper. A.R., C.C., G.R.: provided clinical assistance to patients and provided related
samples. L.C.: helped with discussion. A.R., G.R., A.M.R.: helped with discussion and revised the paper. L.I.: conceived, supervised the studies and wrote the paper. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY DATA RIGHTS AND PERMISSIONS This work is licensed under a Creative
Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in
the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of
this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Bertoli, D., Re, A., Chiarini, M. _et al._ B- and T-lymphocyte
number and function in HIV+/HIV− lymphoma patients treated with high-dose chemotherapy and autologous bone marrow transplantation. _Sci Rep_ 6, 37995 (2016).
https://doi.org/10.1038/srep37995 Download citation * Received: 25 August 2016 * Accepted: 02 November 2016 * Published: 01 December 2016 * DOI: https://doi.org/10.1038/srep37995 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative