- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Cholestasis is a clinically significant symptom and widely associated with liver diseases, however, there are very few effective therapies for cholestasis. Danning tablet (DNT, a
Chinese patent medicine preparation) has been clinically used to treat human liver and gallbladder diseases for more than 20 years in China. However, which ingredients of DNT contributed to
this beneficial effect and their mechanistic underpinnings have been largely unknown. In the present study, we discovered that DNT not only demonstrated greater benefits for cholecystitis
patients after cholecystectomy surgery in clinic but also showed protective effect against alpha-naphthylisothiocyanate (ANIT)-induced cholestasis model in rodent. Curcumin, one major
compound derived from DNT, exerted the protective effect against cholestasis through farnesoid X receptor (FXR), which has been focused as potential therapeutic targets for treating
cholestasis. The underlying mechanism of curcumin against cholestasis was restoring bile acid homeostasis and antagonizing inflammatory responses in a FXR-dependent manner and in turn
contributed to overall cholestasis attenuation. Collectively, curcumin can be served as a potential treatment option for liver injury with cholestasis. SIMILAR CONTENT BEING VIEWED BY OTHERS
EMODIN ALLEVIATES CHOLESTATIC LIVER INJURY BY MODULATING SIRT1/FXR SIGNALING PATHWAYS Article Open access 20 July 2024 CAFFEIC ACID PHENETHYL ESTER SUPPRESSES INTESTINAL FXR SIGNALING AND
AMELIORATES NONALCOHOLIC FATTY LIVER DISEASE BY INHIBITING BACTERIAL BILE SALT HYDROLASE ACTIVITY Article 02 June 2022 DEHYDROTRAMETENOLIC ACID METHYL ESTER, A TRITERPENOID OF _PORIA COCOS_,
ALLEVIATES NON-ALCOHOLIC STEATOHEPATITIS BY SUPPRESSING NLRP3 INFLAMMASOME ACTIVATION VIA TARGETING CASPASE-1 IN MICE Article 06 May 2025 INTRODUCTION Cholestasis is a common symptom of
liver injuries and characterized as the interruption of bile flow from hepatocytes to intestine, which leads to bile acid accumulation in the liver, resulting in oxidative stress,
inflammation, apoptosis and fibrosis. Cholestasis is highly associated with a wide spectrum of diseases, such as obstructive jaundice1, biliary atresia2, gallstones3, acute hepatitis4,
cystic fibrosis5, primary sclerosing cholangitis (PSC)6 and primary biliary cirrhosis (PBC)7. So far, there are very few effective therapies for cholestasis, with ursodeoxycholic acid being
the only approved drug by Food and Drug Administration8. Therefore, it is extremely important to develop new therapeutic medicines. In China, Danning tablet (DNT, a Chinese patented medicine
preparation, Shanghai Hutchison Pharmaceuticals) has been clinically used to treat human liver and gallbladder disease for more than 20 years. We have previously discovered that DNT exerted
anti-cholestatic effects in rodent liver injury models. However, the translational potential and mechanistic underpinnings are largely unknown. The pathogenesis of cholestasis is intimately
related to dysregulated bile acid homeostasis. Farnesoid X receptor (FXR) belongs to a nuclear receptor superfamily and functions as a key bile acid sensor. As a result, FXR may serve as a
potential therapeutic target in cholestasis treatment. Indeed, previous studies have demonstrated that FXR agonist (GW4064) could improve cholestasis symptoms induced by
alpha-naphthylisothiocyanate (ANIT) or bile duct ligation (BDL) in rodent models9. In addition, emerging evidence have implicated the pronounced activation of inflammatory responses in
hepatic cell apoptosis and necrosis during cholestasis. However, whether bile acids overload triggers the inflammatory cascade in hepatocytes has not been characterized. In this study, we
showed that DNT exerted anti-cholestatic effects not only in rodents, but also in cholecystitis patients after cholecystectomy surgery. Mechanistically, curcumin derived from DNT was
identified to play a predominant role in alleviating cholestasis-associated bile acid deregulation and consequent inflammation. Our data also unambiguously proved that FXR mediated the
therapeutic effects of DNT and curcumin, thus supporting to further investigate FXR as a molecular target in cholestasis management. RESULTS DNT REDUCED PATHOLOGICAL BILE ACID ACCUMULATION
AND INFLAMMATION IN CHOLECYSTITIS PATIENTS AND RODENTS Since our previous work has revealed anti-cholestatic functions of Danning tablet (DNT) in rodent models10,11, we sought to validate
these findings in clinic. To this end, we recruited 20 patients with cholecystitis based on the diagnostic results by biochemistry and ultrasonography imaging. All the patients underwent
cholecystectomy surgery and randomized into two groups. Ten patients were treated with DNT, and the other ten patients received normal antibiotics. To determine the impact of DNT on bile
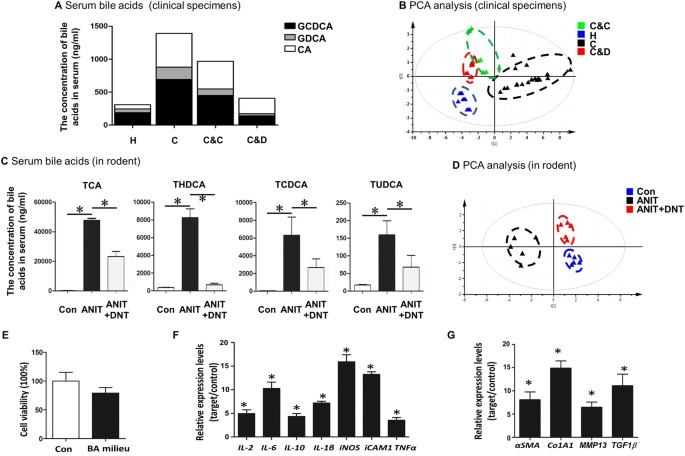
acid homeostasis, the concentrations of serum bile acids including GCDCA, GCA and CA were analyzed by liquid chromatograph-mass spectrometer (LC-MS) (Fig. 1A). We found that serum levels of
GCDCA, GCA and CA were abnormally higher in cholecystitis patients compared to the healthy people. Cholecystectomy operation followed by DNT therapy significantly reduced these bile acids
compared to surgery followed by normal antibiotics. We also determined the serum inflammatory cytokine signatures using protein array (Fig. 1B, Supplementary Table S1). Intriguingly, PCA
analysis clearly distinguished the four groups, and patients treated with DNT are more closely related to the healthy subjects (Fig. 1B). Our data indicated that DNT supplement following
cholecystectomy operation alleviated the cholestasis symptoms and inflammatory responses of the cholecystitis patients. Similar findings were recapitulated in a rodent cholestasis model
induced by ANIT compound. Our data revealed that elevated serum levels of various bile acids, including TCA, THDCA, TCDCA and TUDCA, were significantly reduced by DNT treatment in
ANIT-induced cholestatic mice (Fig. 1C). In addition, we examined the expression levels of inflammation-related genes. DNT treatment resulted in the downregulation of inflammatory genes
evoked by ANIT exposure including IL-4, IL-6, IL-10, IL-1β, TGF-β1/2/3 and TNF-α (Supplementary Table S2). The PCA plot based on inflammatory gene expressions demonstrated that DNT treated
mice formed a distinct cluster and more correlated with control group (Fig. 1D). Taken together, DNT reduced pathological bile acid accumulation and inflammation in cholecystitis patients
and rodents. Since imbalanced bile acid homeostasis and sustained inflammatory responses are both associated with cholestasis12,13, we further investigated the potential interaction between
bile acids and inflammation. To address this point, mouse primary hepatocytes were treated with reconstituted bile acids (BA) milieu composed of TCA, βMCA, TαMCA and TβMCA, which is similar
composition in the serum of ANIT treated mice. The bile acids pool did not show significant difference in cell viability (Fig. 1E). However, it substantially stimulated inflammatory
responses, as indicated by the dramatic induction of _IL-2_, _IL-6_, _IL-10_, _IL-1β_, _iNOS_, _iCAM1_ and _TNFα_ gene expressions (Fig. 1F). Additionally, it is noteworthy that the markers
for fibrosis, including _αSMA, Co1A1, MMP13_ and _TGF1β_ were remarkably increased upon bile acid exposure (Fig. 1G), suggesting that bile acid overload in hepatocytes had a profound effect
on the development of fibrosis. These data imply that bile acids may, at least partially, contribute to unfavorable chronic inflammation often observed during cholestasis. DNT AND CURCUMIN
ACTIVATED FXR AND MITIGATED CHOLESTASIS Since FXR plays a vital role in regulating bile acids and inflammation pathway14,15, we tested whether DNT exerted protective effects against
cholestasis through modulating FXR activities. Interestingly, DNT exposure resulted in a dose-dependent activation of FXR (Fig. 2A), as indicated by the FXR reporter assay. To elucidate the
candidate functional ingredient from DNT, herbal compounds were isolated and screened using FXR reporter assay (Fig. 2B). Two compounds, curcumin and resveratrol, induced FXR activity. As
curcumin exhibited the most prominent impact on FXR activity, we focus on curcumin and continued the further study. The results showed FXR activity increased at a dose-dependent manner
without cell toxicity (Fig. 2C,D). Molecular docking experiment showed that curcumin could theoretically bind with FXR protein at the same site as GW4064 (Arr331, His447), which is a
synthesized FXR agonist, with an energy score of −29.5630. Consistently, we found that the majority of FXR target genes were regulated by curcumin (Fig. 2F,G). These data collectively
suggested that curcumin was the main FXR activator in the ingredient of DNT _in vitro_. Since curcumin activated FXR _in vitro_, we further investigated whether curcumin mitigated
cholestasis symptoms through FXR _in vivo_. To this end, we evaluated the protective effect of curcumin against ANIT by biochemical and histological studies. Although the serum levels of
biochemical markers of cholestasis, including ALT, ALP, TBA, DBIL and TBIL, were profoundly elevated after ANIT administration in WT mice, curcumin or 6ECDCA, which is a FXR agonist,
improved the elevation of these markers (Fig. 3A). Surprisingly, neither curcumin nor 6ECDCA showed the inhibitory effect of the increased biochemical markers of cholestasis in FXRKO mice.
Histologically, ANIT-induced liver injuries, including inflammatory infiltration and parenchymal necrosis, were attenuated by curcumin or 6ECDCA pretreatment in WT mice (Fig. 3B upper and
Supplementary Table S3). FXRKO mice demonstrated more obvious liver injuries in comparison to WT mice after ANIT administration, whereas curcumin or 6ECDCA did not ameliorate the injuries
(Fig. 3B lower and Supplementary Table S3). Taken together, these observations implicated that curcumin ameliorated ANIT-induced cholestasis through FXR activation. CURCUMIN REDUCED HEPATIC
BILE ACIDS ACCUMULATION AFTER ANIT EXPOSURE IN THE PRESENCE OF FXR Subsequently, we quantitated profiling of bile acids in a cholestasis mice model by LC-MS (Supplementary Figure S1). In WT
mice, PCA analysis directly distinguishes the four groups. Either the ANIT+curcumin group or the ANIT+6ECDCA group is more closely related to the control group (Fig. 4A, left). Surprisingly,
in FXRKO mice, the control group distributes furthest from the ANIT group, while the ANIT+curcumin and the ANIT+6ECDCA groups are scattered in a similar pattern to the ANIT group without
noticeable discrepancy (Fig. 4A, right). These findings suggested that curcumin and 6ECDCA re-corrected the imbalance of bile acid homeostasis caused by ANIT in the presence of FXR.
Furthermore, each hepatic bile acid was visualized in heatmap (Fig. 4B). In both WT and FXRKO mice, hepatic levels of CA, TCA, βMCA, ωMCA, TαMCA, TβMCA and TωMCA increased significantly
after ANIT administration. As expected, these bile acids were dramatically recovered with curcumin or 6ECDCA treatment in ANIT-WT mice but not in ANIT-FXRKO mice. As a further proof, our
data showed that curcumin protected from ANIT-induced cholestasis through reducing FXR-regulated bile acid overload in the liver. CURCUMIN NORMALIZED THE IMBALANCE OF BILE ACID HOMEOSTASIS
CAUSED BY ANIT THROUGH FXR-REGULATED BILE ACID PATHWAY To deeply examine the protective effect of curcumin against ANIT-induced cholestasis, some representative genes related to the bile
acid signaling pathway were detected. First of all, we focused on the expressions of bile acid excretory transporters, including _Bsep_, _Mrp4_ and _Ostβ_ (Fig. 5A). ANIT treatment resulted
in pronounced repression of _Bsep_, and induction of _Mrp4_ and _Ostβ_ expressions in WT mice (Fig. 5A). With curcumin or 6ECDCA treatment, the expression levels of these genes were
correspondingly restored. Although these genes showed similar expression patterns in ANIT-treated FXRKO mice, neither curcumin nor 6ECDCA showed the rescue. Secondly, the genes in bile acid
synthesis and uptake pathway, including _Cyp7a1_, _Cyp8b1_ and _Oatp1a1_, were examined (Fig. 5B). ANIT treatment significantly downregulated these gene expression levels in WT mice, and
curcumin or 6ECDCA treatment canceled these downregulation. While ANIT treatment suppressed these gene expressions in FXRKO mice, curcumin and 6ECDCA were unable to restore them. Finally,
considering the importance of bile acid enterohepatic circulation on liver injury restoration, we therefore investigated the expressions of _Ibat_ and _Ostβ_ in ileums of all groups (Fig.
5C). ANIT treatment resulted in marked induction of _Ibat_ expression as well as repression of _Ostβ_ in the ileums of both types of mice. Similar as Fig. 5A,B, curcumin and 6ECDCA treatment
restored these expressions in WT mice, not in FXRKO mice. These data highlighted the dominate impact of curcumin on bile acid signaling pathway through FXR regulation, resulting in
inhibition of bile acid synthesis, acceleration of the bile acid efflux system and the normalization of the bile acid pool. CURCUMIN REDUCED INFLAMMATORY RESPONSES CAUSED BY ANIT IN THE
PRESENCE OF FXR Since imbalanced bile acid homeostasis and sustained inflammatory responses are both associated with cholestasis, both of which are driven by FXR, thereby we investigated
inflammatory signaling genes in both liver and ileum tissues. Inflammatory cytokines including _IL-2_, _IL-6_, _IL-10_ and _TNFα_ were over-expressed in both liver and ileum of ANIT-WT or
-FXRKO mice (Fig. 6A). Except for _IL-10_, curcumin or 6ECDCA treatment reduced the induction of these genes in ANIT-WT, not in ANIT-FXRKO mice. Similarly, _iNOS_ and _ICAM-1_ induced by
ANIT were restored by curcumin or 6ECDCA treatment in ANIT-WT mice, but not in ANIT-FXRKO mice. All the results suggested that curcumin alleviated liver injury by reducing the FXR-regulated
inflammatory responses. DISCUSSION Our data highlighted that DNT exerted its beneficial effect in both clinical and animal studies. Mechanistic insights first revealed that curcumin, as
important ingredient of DNT, had a profound role in cholestasis treatment, and the underlying basis was through signaling pathway of FXR-regulated bile acid and inflammation. Cholecystitis
is commonly considered as a complication of gallstone diseases and highly companied with cholestasis symptoms. Nowadays, laparoscopic cholecystectomy represents the gold standard treatment
of acute cholecystitis to avoid recurrent symptoms and the operation should be performed as early as possible16. Normally, the surgery accompanied with a high possibility of infection and
operative complications, which affected the recovery of cholecystitis. Up to date, except for antibiotics, there are no effective medicines for the conservative therapy or the complementary
therapy for improving symptoms after surgery. Hence, we studied the wildly used formula for cholecystitis in China and discovered DNT as the most effective one. Notably, we employed the
translational medicine strategy by shifting from the clinical investigations to understand the molecular basis in rodent study. Our data pointed out that the beneficial effect of DNT
performance was highly related to FXR regulation. FXR emerged as a therapeutic target in treating gallbladder diseases (such as cholestasis) has become increasingly evident17. GW4064, and
OCA, which are synthesized FXR agonists showed notable FXR activation and protected cholestasis in animal models9,18. In the present study, we screened the most prominent and highly
concentrated compounds derived from DNT such as, emodin, chrysophanol, physcion, alo-emodin, rhein, curcumin, polydatin, tangeratin, nobiletin, resveratrol, and hesperidin. It was apparent
that curcumin was the most promising compound that could trigger FXR, and the effect was further confirmed by different dose experiments and FXR downstream target expressions. Except of
curcumin, resveratrol exhibited the modest FXR-activated effect as well, and published evidences showed the protective effect of resveratrol on cholestasis and inflammation was related to
the regulation of bile acid metabolism19,20, implying the underlying mechanism was relied on FXR activation, which indeed warrants further investigation. Current study showed that curcumin
attenuated ethanol-induced hepatic steatosis through modulating Nrf2/FXR signaling in hepatocytes21. Although they used alcoholic model disease model, their findings support our data. In
addition, accumulative evidences regarding curcumin-activated PPARγ have been reported22,23, and some of them showed curcumin exerted anti-inflammatory and anti-fibrotic effect through PPARγ
activation24, However, Narala VR _et al_. showed curcumin does not bind to PPARγ and not induced its activation directly25, suggesting FXR, not PPARγ may mainly contributes to the above
effects because these two molecules have been reported the deep crosstalk. We proved that the activation of FXR by curcumin _in vitro_ study, and exhibited its anti-cholestatic effect _in
vivo_ by using FXRKO mice, thus verified the beneficial effect of curcumin dependent on FXR. Importantly, for the first time, we showed the FXR was the predominate mediator in curcumin
attenuating cholestasis study. Bile acids are mainly regulated by FXR, and are the major metabolites of cholesterol. Not only do they facilitate in the absorption of hydrophobic materials
and the metabolism of lipids and carbohydrates, they also play a critical role in liver injury and cholestasis protection26. Bile acid homeostasis depends on an intact enterohepatic
circulation, which is disrupted by cholestasis. Abnormal increases in the bile acid pool due to cholestasis could in turn damage hepatocytes27. There are very few evidences regarding the
direct effect of curcumin on bile acids. In our study, FXR deficiency abrogated the protective effect of curcumin on cholestasis, where hepatic bile acid overload persisted. A substantial
number of bile acids in the liver increased a hundred times after ANIT treatment, and with FXR presence, curcumin was able to decrease bile acid levels back to normal. This comprehensive
effect was helpful to avoid the second hit of accumulative bile acids on hepatocytes. On the other hand, FXR also controls the inflammation pathway14. Cholestatic liver injury can further
lead to severely chronic liver diseases if pro-inflammatory and pro-fibrogenic cytokines production are not controlled well. Beyond its role in bile acid regulation, curcumin is well known
to exert to a potent anti-inflammatory activity28,29,30,31. The above results identified curcumin protecting liver injury with cholestasis through FXR-regulated inflammatory signaling
pathway. Our results were consistent with many previous studies that FXR inhibited the inflammation mediator genes, such as _TNFα_, _IL-1β_, _IL-6_, and _iNOS_32,33. Moreover, we found that
curcumin had a greater impact on the inflammatory genes expressed in the ileum than in the liver. One plausible explanation is that curcumin is poorly absorbed following oral administration,
thus the majority of the ingested curcumin is exposed to intestinal tract, where it can exert its anti-inflammatory effect. This interpretation, however, required for the further
investigation. The above findings raised the question of how interactions between bile acid and inflammatory cytokines, and whether overload hepatic bile acids can directly induce the
hepatocytes necrosis and trigger the inflammatory responses. To address this point, bile acids exposure in primary mouse hepatocyte were conducted and resulted in a slight hepatocyte
toxicity. In contrast to histological observations, necrosis was observed in a large area after ANIT treatment (Fig. 3B) whereas there were only a small portion of hepatocyte deaths _in
vitro_ (Fig. 1E), which may lead to the conclusion that abnormal bile acids accumulation was not the primary cause of liver injury. If so, bile acid release should be considered as an
outcome of liver injury triggered by endogenous stimulus. Our results were consistent with other observations by using single bile acid or different bile acid composition exposure to cells,
such as GCDCA, TCA, CDCA, DCA, CA and UDCA34,35,36,37. Consistent reports showed these bile acids did not cause apoptosis or necrosis but induced the inflammatory genes or affected the rate
of DNA repair or proliferation37,38. Another report challenged the cell death by using bile acids in rat hepatocytes, LCA and CDCA at dose of 100 μM caused significant decrease of cell
viability, while GCDCA, TCDCA and TUDCA, at more than 250 μM showed cell death39,40. Importantly, the above literatures illustrated the combination of individual bile acids would enhance the
cell death. In these cases, whether bile acid directly induced cell apoptosis or necrosis still remained debated, especially dependent on the concentrations and different kinds of bile
acids used in each studies. Our data parallels the recent findings revealing that overload of bile acids is proposed as a mechanism for the inflammatory responses in the cholestasis mouse
model12,36, and the constant inflammation responses are the important factors to induce HSC activation, pro-fibrotic responses, and eventually fibrosis41. In present study, we observed the
fibrosis-related genes were markedly induced by bile acid mixture exposure, indicating bile acids being as the important signals or stimulus to induce inflammation and fibrosis. Similar as
ANIT-induced cholestasis model, bile duct ligation can develop hepatic fibrosis by 2–3 weeks, accompanied by marked increase in various pro-fibrotic mediators and elevation of bile
acids42,43. However, we reasoned that the inflammatory responses were not only due to bile acid overload in the liver but also ANIT itself. Bile acids and inflammation-signaling pathway are
complementary in ANIT-induced cholestasis, and allowed curcumin to exert its protective effect through FXR activation. Taken together, the strategies for removal of the injury stimulus, the
reduction of inflammatory burden and hepatic fibrosis should be focused on the regulation of bile acid homeostasis. Besides, in order to discover other signaling pathways which contributed
to cholestasis, we did the microarray analysis in different treatment groups. Initially, a wide spectrum of genes were obtained by comparing the ANIT group and Con group in WT mice, and then
the selected genes were performed a functional annotation by KEGG and GO pathway analysis. As shown in Supplementary Tables S4 and S5, bile secretion, steroid hormone biosynthesis, acute
inflammatory response, were the important pathways which had a profound impact on the cholestasis and were thoroughly investigated in the present study. Besides, such as metabolism of
xenobiotics by cytochrome P450, retinol metabolism, PI3K-Akt signaling and p53 signaling pathway were also responsible for the cholestasis, which provided a comprehensive picture of the
function of cholestasis and still warrants further investigation. By using these genes, we observed the protective effect of curcumin against ANIT in WT mice by cluster analysis
(Supplementary Figure S2). In conclusion, our data highlighted the dominate role of DNT in benefiting the outcome of cholecystits in clinic and cholestasis in rodents. Several complementary
lines of evidences supported that curcumin was the most important ingredient of DNT for cholestasis treatment, and offered an alternative treatment approach particularly in FXR-regulated
bile acid and inflammation pathway. MATERIALS AND METHODS CHEMICALS AND REAGENTS Alpha-naphthylisothiocyanate (ANIT) and curcumin (CUR) were obtained from Sigma-Aldrich (Sigma-Aldrich, USA).
All the bile acid standards were purchased from Sigma-Aldrich as well. Danning tablet used in this study were produced by Shanghai Huchison Pharmaceuticals Co. Ltd (batch No. Z10910040).
Ammonium acetate, formic acid, acetonitrile and methanol (HPLC grade) were purchased from Fisher Scientific (Nepean, Ont, Canada). Ultrapure water was prepared by a Milli-Q50 SP Reagent
Water System (Millipore Corporation, MA, USA) for the preparation of samples and buffer solutions. CLINICAL SPECIMENS Plasma samples were collected from patients in Shuguang Hospital
Affiliated with Shanghai University of Traditional Chinese Medicine. The symptom of the patients like unremitting right upper quadrant pain, anorexia, nausea, vomiting or fever lasted for
several weeks. Ultrasonography imaging indicated thickening and rough gallbladder wall, distortional gallbladder with gallstones. They have surgical indication of laparoscopic
cholecystectomy, open cholecystectomy or minilaparoscopic surgery. A detailed medical questionnaire was completed to ensure that subjects were eligible for the study and 20 patients
participated in this study in the end. They are double-blind randomized into two groups after cholecystectomy operation. The treatment groups received Danning tablet regimen (15 tablets per
day) for 12 weeks since 5 days after operation relative to the normal antibiotics therapy which control groups received. Their blood were collected at 13 weeks after the operation. All
samples were prepared for detection of inflammatory cytokines and bile acid concentrations. The methods were carried out in accordance with the Ethical Guidelines for Medical and Health
Research Involving Human Subjects. All subjects provided written informed consent and the study was approved by Institutional Ethical Committee of the Shuguang Hospital Affiliated with
Shanghai University of Traditional Chinese Medicine (Approval Notice No. 2011LC3Y033). CELL CULTURE All the reagents used for cell culture were obtained from Gibco-BRL (Carlsbad, CA, USA)
unless otherwise noted. Huh7 (Japanese Collection of Research Bioresources Cell Bank) and HEK293T cell lines (American Type Culture Collection) were cultured in Dulbecco’s modified Eagle’s
medium supplemented with 10% fetal bovine serum. Primary mouse hepatocyte cells (Research Institute for Liver Disease (Shanghai)) was cultured in RPMI 1640 medium supplemented with 10% fetal
bovine serum. LUCIFERASE ASSAY HEK293T cells were transfected with βRE (FXR downstream gene promoter region), expressed FXR and RXRα plasmids for 6 h using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA). Then, the medium was replenished with fresh medium containing Danning tablet, compounds derived from DNT for 24 h. After treatment, cells were collected to measure firefly and
renilla luciferase activity using the Dual-luciferase Reporter system (Promega, Madison, WI). Renilla luciferase activity was standardized to the Firefly luciferase activities.
QUANTIFICATION OF MRNA Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA), and reverse transcribed into cDNA with a High Capacity RNA-to-cDNA Kit (Applied Biosystems,
CA), according to manufacturer instructions. The mRNA levels were quantified by real-time PCR on an ABI ViiA 7 Real time PCR system (Applied Biosystems, CA, USA) using Power SYBR Green PCR
Master Mix (Applied Biosystems, CA, USA). _Gapdh_ were used as internal controls to normalize the detected mRNAs. Primers were designed using Primer3 Input software and the primers sequences
are available upon request. ANIMALS Wild type C57/BL mice were purchased from the Laboratory Animal Center of Shanghai University of Traditional Chinese Medicine (SHUTCM, Shanghai). FXRKO
mice were transferred from UC Davis medical center, and reproduced in SHUTCM animal room. The mice were housed at 20 (±2)°C with relative humidity at 60–70%. The animal welfare strictly
complied with the Guide for the Care and Use of Laboratory Animals, and the protocols for the animal experiments were approved by the Institutional Animal Committee of Shanghai University of
Traditional Chinese Medicine (Permit number: SCXK (Hu) 2012-0002). Wild-type and FXRKO mice were randomly assigned into four groups (Control, ANIT, ANIT+curcumin, ANIT+6ECDCA),
respectively. ANIT+curcumin and ANIT+6ECDCA were treated with curcumin (120 mg/kg) or 6ECDCA (20 mg/kg), respectively, for 5 days while Control and ANIT were given normal saline. (Curcumin
at dose of 120 mg/kg or 6ECDCA at dose of 20 mg/kg was selected based on our previous study and published findings44,45,46). Four hours after curcumin or 6ECDCA treatment on day 2, ANIT
(dissolved in oil, 60 mg/kg) were administered intragastrically in the ANIT, ANIT+curcumin, and ANIT+6ECDCA groups. In contrast, the control group were administered olive oil
intragastrically. After 48 h ANIT administration, mice were anesthetized with isoflurane and euthanized. Another sets of WT mice were assigned into Control, ANIT and ANIT+DNT (Danning
tablet) groups. The treatment were the same as described above. Blood samples were coagulated in 1.5 h followed by centrifugation to obtain serum at 4 °C (3000× g). Livers and ileums were
frozen in liquid nitrogen immediately after collection and stored in −80 °C freezer for further assays. BIOCHEMICAL ASSAY AND HISTOLOGICAL STUDY Alkaline phosphatase (ALP), total bilirubin
(TBIL), aspartate aminotransferase (ALT) and the concentration of total bile acid (TBA) were measured using an automated biochemistry analyzer (Olympus 2700, Japan). Livers were stained with
a standard hematoxylin and eosin (H&E) procedure to reveal patterns of cellular labeling under the light-microscope for necrosis and other structural changes. Serum inflammatory
cytokines (CD14, CD27, CD30, CD40, CD40L, CRP, CXCL16, IFNγ, IL-10, IL-18 Rb, IL-1α, IL-4, IL-6, IL-8, MCP-1, TGFβ RIII, TGF-β1, TGF-β2, TGF-β3 and TNFα) were detected by Wayen Biotechnology
Company using Human Antibody Array (QAH-CYT-SW). QUANTIFICATION OF BILE ACIDS IN MOUSE LIVERS An ultra-performance liquid chromatography instrument coupled with a quatropole mass
spectrometry (UPLC-MS, Waters Co., MA, USA) was used to detect hepatic bile acids. Livers were homogenized in acetonitrile (100 mg tissue/500 μl acetonitrile) followed by centrifugation at
14,300 rpm for 10 min. The supernatant was blown to dryness under nitrogen stream, then re-dissolved in methanol-water solution (methanol:water:formic acid = 50:50:0.01) followed by
centrifugation at 14,300 rpm for 10 min. Samples of 5 μl of the supernatants were injected into the UPLC-MS instrument. The instrument parameters were applied based on previous study47.
MOLECULAR DOCKING To evaluate the activity of curcumin, the docking program Molegro Virtual Docker (MVD) was used to dock the structure of FXR (PDB code: 3DCT, FXR with GW4064). Before
docking analysis, original structure of all crystal water molecules should be removed and added hydrogen in the DS CDOCKER module. The compound should start with the lowest energy state to
reach an optimal starting conformation before docking. Based on reported docking sites in GW4064 docking analysis, such as Arg331, Trp469, and His447 were bond with GW4064 by hydrogen,
thereby the conformation of curcumin was evaluated based on those bond status. In the end, there is an energy score output to evaluate curcumin being as FXR agonist or not. _IN VITRO_ STUDY
Mouse primary hepatocytes derived from male C57/BL mice were purchased from Shanghai Research Institute Liver Disease Co. Ltd (originally from Celsis _in vitro_ technologies). Mouse primary
hepatocytes was cultured with DMEM medium supplemented with 10% fetal bovine serum. Cells were treated with reconstituted BA milieu, composition of which comes from the high concentration
and most elevated individual bile acids after ANIT administration in _in vivo_ study. (TCA 10 μg/ml, TDCA 18 μg/ml, βMCA 18 μg/ml, TαMCA 18 μg/ml and TβMCA 120 μg/ml). Cell viability was
studied using CCK-8 assay kit according to manufacturer instructions (Shanghai Yeasen Biotech, Shanghai, China). Inflammation-related and fibrosis-related gene expressions were detected by
Real-time PCR. DATA ANALYSIS One-way ANOVA statistic and PCA algorithm were performed by SPSS 18.0 (SPSS Inc. Chicago, IL, USA). PCA plot was performed by SIMCAP (11.5 version, Umetrics,
Ume, Sweden) and heatmap was imaged by The R Programming Language. All graphs were generated with the Graphpad software (GraphPad Inc, San Diego, CA). ADDITIONAL INFORMATION HOW TO CITE THIS
ARTICLE: Yang, F. _et al_. Curcumin protects ANIT-induced cholestasis through signaling pathway of FXR-regulated bile acid and inflammation. _Sci. Rep._ 6, 33052; doi: 10.1038/srep33052
(2016). REFERENCES * Yoshidome, H. et al. Obstructive jaundice impairs hepatic sinusoidal endothelial cell function and renders liver susceptible to hepatic ischemia/reperfusion. J. Hepatol.
33, 59–67 (2000). CAS PubMed Google Scholar * Mieli-Vergani, G. & Vergani, D. Biliary atresia. Semin Immunopathol. 31, 371–381 (2009). PubMed Google Scholar * Moghadamrad, S.,
Montani, M., Weimann, R. & De Gottardi, A. Cholestasis in a patient with gallstones and a normal gamma-glutamyl transferase. Hepatology 57, 2539–2541 (2013). PubMed Google Scholar *
Tekin, R. et al. Evaluation of adults with acute viral hepatitis a and review of the literature. Clin Ter. 164, 537–541 (2013). ADS CAS PubMed Google Scholar * Flora, K. D. & Benner,
K. G. Liver disease in cystic fibrosis. Clin Liver Dis. 2, 51–61 (1998). CAS PubMed Google Scholar * Eaton, J. E., Talwalkar, J. A., Lazaridis, K. N., Gores, G. J. & Lindor, K. D.
Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology 145, 521–536 (2013). CAS PubMed Google Scholar * Hirschfield, G. M. et al. The
genetics of complex cholestatic disorders. Gastroenterology 144, 1357–1374 (2013). CAS PubMed PubMed Central Google Scholar * Fickert, P. et al. Differential effects of norUDCA and UDCA
in obstructive cholestasis in mice. J. Hepatol. 58, 1201–1208 (2013). CAS PubMed PubMed Central Google Scholar * Liu, Y. et al. Hepatoprotection by the farnesoid X receptor agonist
GW4064 in rat models of intra- and extrahepatic cholestasis. J. Clin Invest. 112, 1678–1687 (2003). CAS PubMed PubMed Central Google Scholar * Ding, L., Zhang, B., Zhan, C., Yang, L.
& Wang, Z. Danning tablets attenuates alpha-naphthylisothiocyanate-induced cholestasis by modulating the expression of transporters and metabolic enzymes. BMC. Complement Altern Med. 14,
249 (2014). PubMed PubMed Central Google Scholar * Ding, L. L. et al. Protective effect of Danning tablet on acute livery injury with cholestasis induced by alpha-naphthylisothiocyanate
in rats. J. Ethnopharmacol. 140, 222–229 (2012). PubMed Google Scholar * Woolbright, B. L. & Jaeschke, H. Novel insight into mechanisms of cholestatic liver injury. World J.
Gastroenterol. 18, 4985–4993 (2012). CAS PubMed PubMed Central Google Scholar * Herr, K. J. et al. Loss of alpha-catenin elicits a cholestatic response and impairs liver regeneration.
Sci. Rep. 4, 6835 (2014). CAS PubMed PubMed Central Google Scholar * Shaik, F. B., Prasad, D. V. & Narala, V. R. Role of farnesoid X receptor in inflammation and resolution. Inflamm
Res. 64, 9–20 (2015). CAS PubMed Google Scholar * Wang, Y. D., Chen, W. D., Moore, D. D. & Huang, W. FXR: a metabolic regulator and cell protector. Cell Res. 18, 1087–1095 (2008). CAS
PubMed Google Scholar * Knab, L. M., Boller, A. M. & Mahvi, D. M. Cholecystitis. Surg Clin North Am. 94, 455–470 (2014). PubMed Google Scholar * Jonkera, J. W., Liddle, C. &
Downes, M. FXR and PXR: Potential therapeutic targets in cholestasis. J. Steroid Biochem Mol Biol. 130, 147–158 (2011). Google Scholar * Verbeke, L. et al. The FXR agonist obeticholic acid
prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. Am J. Pathol. 185, 409–419 (2015). CAS PubMed Google Scholar * Wang, T. et al. Resveratrol effectively
attenuates alpha-naphthyl-isothiocyanate-induced acute cholestasis and liver injury through choleretic and anti-inflammatory mechanisms. Acta Pharmacol Sin. 35, 1527–1536 (2014). PubMed
PubMed Central Google Scholar * Chen, M. L. et al. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via
Remodeling of the Gut Microbiota. MBio. 7, e02210–e02215 (2016). CAS PubMed PubMed Central Google Scholar * Lu, C. et al. Curcumin attenuates ethanol-induced hepatic steatosis through
modulating Nrf2/FXR signaling in hepatocytes. IUBMB Life 67, 645–658 (2015). CAS PubMed Google Scholar * Liu, Z. J. et al. Curcumin protects neurons against oxygen-glucose
deprivation/reoxygenation-induced injury through activation of peroxisome proliferator-activated receptor-gamma function. J. Neurosci Res. 92, 1549–1559 (2014). CAS PubMed Google Scholar
* Meng, Z., Yu, X. H., Chen, J., Li, L. & Li, S. Curcumin attenuates cardiac fibrosis in spontaneously hypertensive rats through PPAR-gamma activation. Acta Pharmacol Sin. 35, 1247–1256
(2014). CAS PubMed PubMed Central Google Scholar * Baghdasaryan, A. et al. Curcumin improves sclerosing cholangitis in Mdr2−/− mice by inhibition of cholangiocyte inflammatory response
and portal myofibroblast proliferation. Gut. 59, 521–530 (2010). CAS PubMed PubMed Central Google Scholar * Narala, V. R. et al. Curcumin is not a ligand for peroxisome
proliferator-activated receptor-gamma. Gene Ther Mol Biol. 13, 20–25 (2009). CAS PubMed PubMed Central Google Scholar * Hirschfield, G. M., Heathcote, E. J. & Gershwin, M. E.
Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology 139, 1481–1496 (2010). CAS PubMed Google Scholar * Hofmann, A. F. The continuing importance of bile
acids in liver and intestinal disease. Arch Intern Med. 159, 2647–2658 (1999). CAS PubMed Google Scholar * Matsuda, H., Ninomiya, K., Morikawa, T. & Yoshikawa, M. Inhibitory effect
and action mechanism of sesquiterpenes from Zedoariae Rhizoma on D-galactosamine/lipopolysaccharide-induced liver injury. Bioorg Med Chem Lett. 8, 339–344 (1998). CAS PubMed Google Scholar
* Morikawa, T., Matsuda, H., Ninomiya, K. & Yoshikawa, M. Medicinal foodstuffs. XXIX. Potent protective effects of sesquiterpenes and curcumin from Zedoariae Rhizoma on liver injury
induced by D-galactosamine/lipopolysaccharide or tumor necrosis factor-alpha. Biol Pharm Bull 25, 627–631 (2002). CAS PubMed Google Scholar * Kaur, G. et al. Inhibition of oxidative
stress and cytokine activity by curcumin in amelioration of endotoxin-induced experimental hepatoxicity in rodents. Clin Exp Immunol. 145, 313–321 (2006). CAS PubMed PubMed Central Google
Scholar * Yun, S. S., Kim, S. P., Kang, M. Y. & Nam, S. H. Inhibitory effect of curcumin on liver injury in a murine model of endotoxemic shock. Biotechnol Lett. 32, 209–214 (2010).
CAS PubMed Google Scholar * Xu, Z. et al. FXR ligands protect against hepatocellular inflammation via SOCS3 induction. Cell Signal 24, 1658–1664 (2012). CAS PubMed Google Scholar *
Vavassori, P., Mencarelli, A., Renga, B., Distrutti, E. & Fiorucci, S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 183, 6251–6261 (2009). CAS
PubMed Google Scholar * Woolbright, B. L. et al. Bile acid-induced necrosis in primary human hepatocytes and in patients with obstructive cholestasis. Toxicol Appl Pharmacol. 283, 168–177
(2015). CAS PubMed PubMed Central Google Scholar * Woolbright, B. L., McGill, M. R., Yan, H. & Jaeschke, H. Bile Acid-Induced Toxicity in HepaRG Cells Recapitulates the Response in
Primary Human Hepatocytes. Basic Clin Pharmacol Toxicol. 118, 160–167 (2016). CAS PubMed Google Scholar * Allen, K., Jaeschke, H. & Copple, B. L. Bile acids induce inflammatory genes
in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 178, 175–186 (2011). CAS PubMed PubMed Central Google Scholar * Martinez-Diez, M. C.,
Serrano, M. A., Monte, M. J. & Marin, J. J. Comparison of the effects of bile acids on cell viability and DNA synthesis by rat hepatocytes in primary culture. Biochim Biophys Acta 1500,
153–160 (2000). CAS PubMed Google Scholar * Zhang, Y. et al. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int. 32, 58–69 (2012). PubMed Google
Scholar * Rolo, A. P., Palmeira, C. M. & Wallace, K. B. Interactions of combined bile acids on hepatocyte viability: cytoprotection or synergism. Toxicol Lett. 126, 197–203 (2002). CAS
PubMed Google Scholar * Perreault, M. et al. Role of glucuronidation for hepatic detoxification and urinary elimination of toxic bile acids during biliary obstruction. PLoS One 8, e80994
(2013). ADS PubMed PubMed Central Google Scholar * Hartupee, J. & Mann, D. L. Role of inflammatory cells in fibroblast activation. _J. Mol Cell Cardiol._ In Press (2015). * Park, K.
C. et al. A new histone deacetylase inhibitor improves liver fibrosis in BDL rats through suppression of hepatic stellate cells. Br. J Pharmacol. 171, 4820–4830 (2014). CAS PubMed PubMed
Central Google Scholar * Mukhopadhyay, P. et al. Poly (ADP-ribose) polymerase-1 is a key mediator of liver inflammation and fibrosis. Hepatology 59, 1998–2009 (2014). CAS PubMed PubMed
Central Google Scholar * Deters, M., Klabunde, T., Meyer, H., Resch, K. & Kaever, V. Effects of curcumin on cyclosporine-induced cholestasis and hypercholesterolemia and on
cyclosporine metabolism in the rat. Planta Med. 69, 337–343 (2003). CAS PubMed Google Scholar * Reyes-Gordillo, K. et al. Curcumin prevents and reverses cirrhosis induced by bile duct
obstruction or CCl4 in rats: role of TGF-beta modulation and oxidative stress. Fundam Clin Pharmacol. 22, 417–427 (2008). CAS PubMed Google Scholar * Fiorucci, S. et al. Protective
effects of 6-ethyl chenodeoxycholic acid, a farnesoid X receptor ligand, in estrogen-induced cholestasis. J. Pharmacol Exp Ther. 313, 604–612 (2005). CAS PubMed Google Scholar * Yang, L.
et al. Bile acids metabonomic study on the CCl4- and alpha-naphthylisothiocyanate-induced animal models: quantitative analysis of 22 bile acids by ultraperformance liquid chromatography-mass
spectrometry. Chem Res Toxicol. 21, 2280–2288 (2008). CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This work is financially supported by the National Natural Science
Foundations of China (81222053, 81303186 and 81573581), the Program for New Century Excellent Talents in University (NCET-12-1056) and the National S&T Major Special Projects
(2014ZX09301306-007). AUTHOR INFORMATION Author notes * Yang Fan and Tang Xiaowen contributed equally to this work. AUTHORS AND AFFILIATIONS * The MOE Key Laboratory for Standardization of
Chinese Medicines and the Shanghai Key Laboratory of Compound Chinese Medicines Institute of Chinese Materia Medica, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203,
China Fan Yang, Xiaowen Tang, Lili Ding, Yue zhou, Qiaoling Yang, Junting Gong, Guangyun Wang, Zhengtao Wang & Li Yang * Center for Chinese Medical Therapy and Systems Biology, Shanghai
University of Traditional Chinese Medicine, Shanghai, 201203, China Li Yang Authors * Fan Yang View author publications You can also search for this author inPubMed Google Scholar * Xiaowen
Tang View author publications You can also search for this author inPubMed Google Scholar * Lili Ding View author publications You can also search for this author inPubMed Google Scholar *
Yue zhou View author publications You can also search for this author inPubMed Google Scholar * Qiaoling Yang View author publications You can also search for this author inPubMed Google
Scholar * Junting Gong View author publications You can also search for this author inPubMed Google Scholar * Guangyun Wang View author publications You can also search for this author
inPubMed Google Scholar * Zhengtao Wang View author publications You can also search for this author inPubMed Google Scholar * Li Yang View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS Participated in research design: L.Y. and Z.W. Conducted experiments: F.Y., X.T., Q.Y., G.J.T. and G.W. Performed data analysis: L.D. and Y.Z.
Wrote or contributed to the writing of the manuscript: F.Y., X.T. and Y.Z. Revised the manuscript: F.Y., X.T., L.D., Y.Z., L.Y. and Z.W. All authors read and approved the final manuscript.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS This work is
licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license,
unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce
the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yang, F., Tang, X., Ding, L. _et
al._ Curcumin protects ANIT-induced cholestasis through signaling pathway of FXR-regulated bile acid and inflammation. _Sci Rep_ 6, 33052 (2016). https://doi.org/10.1038/srep33052 Download
citation * Received: 25 April 2016 * Accepted: 15 August 2016 * Published: 14 September 2016 * DOI: https://doi.org/10.1038/srep33052 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative