- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Lower socioeconomic position (SEP) has consistently been associated with poorer health. To explore potential biological embedding and the consequences of SEP experiences from early
life to adulthood, we investigate how SEP indicators at different points across the life course may be related to a combination of 28 inflammation markers. Using blood-derived inflammation
profiles measured by a multiplex array in 268 participants from the Italian component of the European Prospective Investigation into Cancer and Nutrition cohort, we evaluate the association
between early life, young adulthood and later adulthood SEP with each inflammatory markers separately, or by combining them into an inflammatory score. We identified an increased
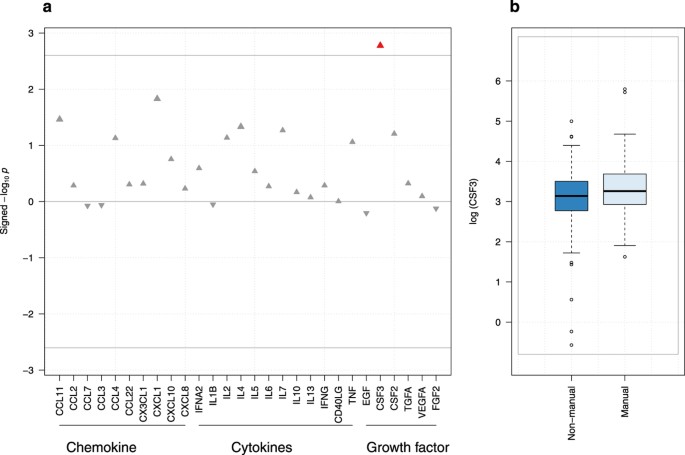
inflammatory burden in participants whose father had a manual occupation, through increased plasma levels of CSF3 (G-CSF; β = 0.29; P = 0.002) and an increased inflammatory score (β = 1.96;
P = 0.029). Social mobility was subsequently modelled by the interaction between father’s occupation and the highest household occupation, revealing a significant difference between “stable
Non-manual” profiles over the life course versus “Manual to Non-manual” profiles (β = 2.38, P = 0.023). Low SEP in childhood is associated with modest increase in adult inflammatory burden;
however, the analysis of social mobility suggests a stronger effect of an upward social mobility over the life course. SIMILAR CONTENT BEING VIEWED BY OTHERS GENE REGULATION CONTRIBUTES TO
EXPLAIN THE IMPACT OF EARLY LIFE SOCIOECONOMIC DISADVANTAGE ON ADULT INFLAMMATORY LEVELS IN TWO COHORT STUDIES Article Open access 04 February 2021 GENETIC PROPENSITY FOR OBESITY,
SOCIOECONOMIC POSITION, AND TRAJECTORIES OF BODY MASS INDEX IN OLDER ADULTS Article Open access 13 October 2021 SOCIOECONOMIC INEQUALITIES IN EARLY ADULTHOOD DISRUPT THE IMMUNE
TRANSCRIPTOMIC LANDSCAPE VIA UPSTREAM REGULATORS Article Open access 13 January 2024 INTRODUCTION It has now been established that health discrepancies exist across socioeconomic groups
worldwide1. In particular, socioeconomic disadvantages over the life course has been related to poor health in adulthood2,3. Several epidemiological studies have shown that health behaviors
and lifestyle factors are important drivers of social inequalities but cannot fully explain the socioeconomic gradient in health4,5. One suspected mechanism linking socioeconomic position
(SEP) and health outcomes over time involves a differential activation of a wide range of physiological and biological reactions6. One important biological mechanism used to adapt to the
environment is the stress response systems7 controlling the release of stress hormones, whose levels alter many biological mechanisms including inflammatory and immune responses8.
Inflammation is a key pathway involved in the development of chronic diseases including cardio-metabolic disorders and multiple cancers9. Several studies have examined the influence of SEP
on inflammatory marker levels and showed that lower education andor income was associated with a greater burden of inflammation10,11,12,13,14,15. Additional studies revealed that both
early-life and adult adverse socioeconomic circumstances have the potential to alter inflammation status16,17. Recent studies used a life course approach and identified inflammation markers
as (partially) explaining social differences in health18,19. A vast majority of these studies used C-reactive protein (CRP) as a general proxy to characterise inflammatory status20 and some
others used alternative inflammatory markers such as interleukin 6 (IL6)10,11,12,13, fibrinogen14,15, or tumour necrosis factor α (TNF-α)11,13. Because previous studies relied only on a
limited number of inflammatory markers, generalisation of the reported biomarker-specific associations with SEP to the inflammatory burden remains uncertain. In order to capture the natural
complexity of inflammatory profiles and their regulation (e.g. pleiotropic effects and partial redundancies across inflammatory markers), we opted here for a more in-depth approach through
the use of a wide range of cytokines, chemokines and proliferation factors21,22,23. Multiplex assay platforms enable simultaneous evaluation of a large number of inflammatory circulating
markers in small amounts of plasma24 and therefore enable a comprehensive evaluation of the role of these markers in our observational context. Our study aims to investigate how SEP over the
life course is likely to be embedded through the inflammatory system. Using proteomic profiles obtained from prospectively collected peripheral blood samples in 268 participants from the
Italian component of the European Prospective Investigation into Cancer and Nutrition (EPIC-Italy), we evaluated the association between early, young and adulthood SEP with 28 markers of
inflammation. These were examined individually and jointly as an inflammatory score. We hypothesize that participants with low SEP have a low-grade inflammation response. In addition, we aim
to determine whether early life SEP interacts with adult SEP to influence inflammation levels. RESULTS As indicated in Supplementary Table S1, key characteristics of the study population
differ from the originating EPIC-Italy population by their age, gender and recruitment centre: they are older, mostly women recruited in Central Italy (43.3% from Florence). Those
differences are all related to the nested case-control design, but no particular differences were observed according their SEP metrics, despite a slightly higher proportion of participants
with lower educational level. Detailed description of the study participants is given in Table 1. Irrespective of the SEP indicator, participants in the low SEP group tend to be older, to
have a higher BMI and to exhibit lower smoking prevalence than those in higher SEP categories. INFLAMMATORY MARKERS OF LIFE COURSE SEP INDICATORS We investigated first associations between
each of the 28 inflammatory markers and our three SEP indicators (father’s occupation, education, highest household occupation) separately. For all indicators, the ‘high’ socioeconomic group
was used as reference and a positive association therefore indicate an increased level of inflammation ‘low’ socioeconomic group (Table 2A). Inflammatory protein concentrations exhibited
general positive associations with father’s occupation and negative associations with education level and highest household occupation. No association remains significant after correction
for multiple testing with both education and highest household occupational position, while one association involving CSF3 levels and father’s occupation survived: lower father’s occupation
being associated with higher levels of CSF3 (β = 0.29, P = 0.002, Fig. 1). Adjusting for education (model B-1) and additionally for adulthood SEP (model C), the association between CSF3 and
early-life SEP, though slightly attenuated, remained significant (P < 0.008). Additional adjustment on the three potential confounders (BMI, smoking, alcohol, see methods) affected only
marginally point estimates (Table 3A). INFLAMMATORY SCORES AND LIFE COURSE SEP INDICATORS We defined a discrete inflammatory score (range: [0–27], see methods) measuring overall inflammatory
status. The score was found significantly associated exclusively to father’s occupation (Table 2B): the inflammatory score was higher in participants whose father had a low occupational
position (β = 1.96, P = 0.029); conversely, the inflammatory score was lower in participants with low educational level or low highest household occupation but these associations were not
significant (β = −1.02, P = 0.26 and β = −1.35, P = 0.15 respectively). The association between inflammatory score and early life SEP (Table 3B) was strengthened by adjusting for education
and highest household occupation. As above, further adjustments for potential confounders did not impact our findings. Participant’s education and participant’s highest household occupation
became significantly associated with the inflammatory score only when controlling for father’s occupation (model B-1 and model B-2, respectively): the inflammatory score was lower in
participants with low educational level or low highest household occupation. Participant’s education and participant’s highest household occupation lost statistical significance when
adjusting simultaneously on both SEP indicators in the model (model C). As a sensitivity analysis, we ran a principal component analysis on the 28 inflammatory markers and the first
principal component (PC1, explaining 35.7% of the variance) was used as an alternative continuous inflammatory score. The use of PC1 as a inflammatory score slightly weakened our results,
but these remained consistent: a significant association between PC1 and father’s occupational position, stable after controlling for early life SEP and a non-significant association with
participant’s education and highest household occupation, in the opposite direction, that became significant after adjustment on father’s occupation (Table 3C). Because of the observed
negative correlation between the inflammatory score and PC1 (ρ = −0.89, P < 0.001), a higher PC1 score indicates lower inflammatory score level and effect size estimates have reversed
signs. INVESTIGATING MARKERS OF SOCIAL MOBILITY To test the potential for an interaction between early life SEP and the participant’s SEP later in life on the inflammatory score, we modelled
the effect of social mobility on the inflammatory score. From the analyses, we identified an increase in the inflammatory score between ‘stable Non-manual’ and ‘Manual to Non-manual’ groups
(β = 2.38, P = 0.02, Table 4A), suggesting a stimulation of the inflammatory system in participants experiencing upward social mobility (Fig. 2). Results using PC1 as inflammatory score
showed a differential inflammatory status between ‘stable Non-manual’ and ‘Manual to Non-manual’ although not significant (β = −0.72, P = 0.17, Table 4B). Sensitivity analyses redefining
social mobility as the interaction between father’s occupation and participant’s own education were conducted and provided similar results, though with stronger associations for the
inflammatory score (Supplementary Table S2). DISCUSSION In the present study, we investigated the association between inflammatory markers and SEP at different time points in life using a
panel of 28 plasma inflammatory protein concentrations considered separately, or combined into an inflammatory score. We hypothesised that SEP may physiologically be embedded at different
time points and subsequently affect the inflammatory burden. Testing all the 28 markers in relation to the three SEP indicators separately, only one significant association emerged: low
father’s occupation was associated with higher CSF3 level. We additionally showed that participants reporting a father with a ‘manual’ occupation had a higher inflammatory score later in
life compared to those whose father had a ‘non-manual’ occupation. No significant associations were found between participant’s education and highest household occupation when examined
separately. Nevertheless, subsequent analysis of the inflammatory score in a life course context (from childhood to adulthood) indicated that participants with upward social mobility had
higher inflammatory scores than those remaining socially advantaged. To test the robustness of our results to the measure of the inflammatory status we also defined, as an unsupervised
alternative, the inflammatory status as the first principal component (PC1) obtained from the 28 inflammatory markers. We identified consistent, though statistically weakened, associations
with father’s occupation in our life course models adjusting for participant’s own education and/or highest household occupation. Social mobility analyses using PC1 also provided similar
associations, but these did not reach statistical significance. This consistency may at least be partially explained by the strong correlation between our inflammatory score and PC1 (ρ =
−0.89). Our estimates and conclusions remained markedly stable upon adjustments for behavioral factors (smoking and alcohol consumption and BMI), hence providing evidence that the
inflammatory signal we report as markers of SEP are independent of the potential inflammatory signatures of these factors. We cannot exclude the possibility that other factors may contribute
to the mechanisms linking SEP and inflammation. This would include anthropometric and obesity variables such as height, weight, waist and hip circumference and waist to hip ratio. Based on
model C, we further adjusted on each of these covariates separately (Supplementary Table S3). We subsequently added physical activity and hormone replacement therapy as potential confounders
in the resulting fully adjusted model (Supplementary Table S4). As indicated in these tables, the association between the inflammatory score and father occupational position was robust
across all these additional models. Our study population remains limited in size, which constrained our methodological choices. First, the available variable categories for father’s
occupation, participant’s education and highest household occupation were all recoded (binary indicators). The reasoning behind this recoding accounted for 1) conceptual typologies of
occupations and educational attainment conferring notions of hierarchy and/or work strain 2) maximising the subgroup samples to achieve sufficient statistical power. Nevertheless,
misclassification error may have occurred where incorrect assumptions were made when recoding. As sensitivity analysis we ran all analysis coding professional in the low education class and
results were only marginally changed (Supplementary Table S5). From the socioeconomic indicators available in the EPIC dataset, we selected our 3 measures based on (i) their unambiguous
definition, (ii) the clear distinction of the critical life stages each corresponded to and (iii) their generalisability to other populations. These indicators offer an individual-based
measure of socioeconomic experience during the life course and as such may provide information about individuals’ access to social and economic resources. They may also capture individual
factors (e.g. material, behavioral or psychosocial factors) or macro-environmental features (e.g. geographical location) driving the link between the social environment and the inflammatory
process. Our results might also be affected by a potential lack of representativeness of our study population which derives from a cancer case control study nested in a cohort. In order to
account for potential (case-driven) population bias, we also ran all analyses by restricting the study population to healthy controls. As summarised in Supplementary Table S6, the smaller
sample size reduced statistical power and measures of association were subsequently weakened. Nevertheless, the sign and estimates of the effect sizes were all consistent and most of the
relevant associations were found nominally significant (P < 0.05). Furthermore, our study population comprises a large proportion of breast cancer prospective cases-control pairs (N = 100
participants). This certainly affects representativeness of the sex ratio within our study population, but may also affect the generalizability of our results, given the higher breast
cancer incidence observed in higher SEP classes25. The latter might potentially explain the counter-intuitive direction of associations found with young and adulthood SEP indicators
separately and inflammation level. Several studies have examined the influence of childhood SEP (as measured by father’s occupation or father’s education) on inflammatory markers levels in
adulthood; most of them have shown that lower early life SEP was associated with a greater level of CRP18,26,27, the fibrinogen18,26,27 and the IL-618,28. However, little is known about the
associations between childhood SEP and other inflammatory markers including other cytokines, chemokines and proliferation factors participating in the inflammatory cascade. Although CSF3 was
only found associated while analysing the inflammatory markers individually, our findings demonstrated that father’s occupational position was inversely associated with the inflammatory
score and thus supported the hypothesis of a global impact on the inflammatory system. To our knowledge, a single study investigated relationship between social mobility and inflammatory
markers concentration. In this work, Loucks _et al._ reported an elevated level of intercellular adhesion molecule 1 (ICAM-1), tumor necrosis factor receptor 2 (TNFR2) and
lipoprotein-associated phospholipase A2(Lp-PLA2) for participants with upward social mobility (low childhood and high adulthood SEP)18. Three models have classically been proposed to explain
the link between life course SEP and adult disease29. The critical period model postulates that adverse socioeconomic circumstances in early life can permanently modify biological systems
with long-term health effects. The accumulation model supposes that accumulation of adversities across the life course has a negative impact on health, while the social mobility framework
assumes that SEP may evolve over tim and consider a differential effect of stable or varying SEP trajectories on health and well-being. Our data support both the critical/sensitive period
hypothesis, since father’s occupational position had a stronger effect on adulthood inflammatory level and also support the social mobility hypothesis where participants whose SEP increased
over time showed higher inflammatory levels. However, our findings do not support the accumulation model. To our knowledge, the present study is the first to provide a comprehensive analysis
of the inflammatory response to socioeconomic experiences in a life course context, using higher resolution profiles. The use of the inflammatory score as a summary measure of the 28
inflammatory markers together with its continuous alternative enabled us to support the hypothesis that early life SEP leads to persistent changes in the inflammation response. Despite the
limitations mentioned above, our study provides evidence supporting the existence of biologically imprinted responses to socioeconomic experiences and trajectories throughout life as
formalised in the concept of embodiment30. It therefore calls for further work involving larger populations and potentially other ‘–omics’ profiles to explore, at several molecular levels,
the mechanisms involved in the biological response to SEP experiences. Although further validation is needed, our results suggest that early SEP and upward social mobility both have a long
term effect on inflammation, that may impact health later in life. METHODS STUDY POPULATION Our study population arises from the EnviroGenoMarkers (EGM) project, which was initially designed
to identify novel biomarkers of non-Hodgkin’s lymphoma and breast cancer risk from multiple ‘–omics’ profiles23. We include in this study 268 EGM participants from the Italian component of
EPIC31, for whom anthropometric, lifestyle, dietary and socioeconomic factors were collected through questionnaires. All participants provided informed consent and the EPIC study protocol
was approved by the review board of the International Agency for Research on Cancer and by all local institutes recruiting participants. The study was conducted in accordance with the
approved guidelines. Incident NHL (N = 84) and breast cancer (N = 50) cases were diagnosed between 2 and 13 years after recruitment in EPIC and were identified through local cancer
registries. For each case identified, one random control was selected among all EPIC Italy participants alive and free of cancer at the time of diagnosis of the index case, matched by centre
(Turin; Varese; Naples; Ragusa and Florence), gender, date of blood collection (+/−6 months) and age at recruitment (+/−2.5 years). Biosamples underwent inflammatory profiling in two
distinct batches including 100 and 34 case/control pairs, respectively. Biosamples were all collected upon inclusion of participants in EPIC Italy. At the time of blood collection, all the
participants were free of cancer. The sample selection strategy is described in Supplementary Figure S1. LIFE COURSE SOCIOECONOMIC POSITION (SEP) To preserve power and interpretability, life
course SEP factors from the EPIC questionnaire were dichotomised. Childhood SEP was measured by father’s occupation and recoded in the two following categories; i) ‘Manual’ (N = 147)
consisting of: unskilled workers (N = 52), skilled workers (N = 59) and farmers (N = 36); and ii) ‘Non-manual’ (N = 87) consisting of: retailers (N = 30), employees (N = 40) and
self-employed (N = 17). Young adulthood SEP was measured through participant’s own education which was dichotomised as i) ‘High’ (above the minimum legal education level, 15 years of age; N
= 120): professional (N = 30), upper secondary school (N = 57) and university (N = 33); and ii) ‘Low’ (below the minimum legal education level; N = 147): none (N = 6), primary school (N =
79), lower secondary school (N = 62). Adulthood SEP was measured using the highest occupational position in the household as defined by either the participant’s own occupation or his/her
partner's. It was classified as ‘Manual’ (N = 79) and ‘Non-manual’ (N = 158), following the same categorisation as for father’s occupation. Characteristics of the 230 participants with
full SEP information are summarised in Table 1. LABORATORY ANALYSES For each participant, a blood sample was collected at enrollment and, within two hours, subsequently processed for the
isolation of buffy coats and other fractions, which were placed in cold storage (liquid nitrogen). A panel of inflammation-related proteins (N = 32) was measured using the MILLIPLEX
HCYTOMAG-60K and HSCYTMAG-60SK kits (Millipore, Billerca, MA), according to the manufacturer’s protocol. As detailed in Supplementary table S7, measurements included 10 chemokines, 12
cytokines and 6 growth factors. Four analytes (IL-12, IL1-RA, sIL2-RA and Flt3ligand) were excluded from further statistical analyses due to a high rate of non-detects (>75%). The lower
limits of detection (LOD) are also reported in Supplementary Table S7. INFLAMMATORY MEASURES Initially, we considered all protein levels separately. As depicted in Supplementary Figure S2,
protein concentrations exhibit a strong pairwise correlation. PCA of these 28 inflammatory markers concentration showed that 20 PCs explained more than 95% of the total variation seen in the
data set (Supplementary Figure S3). The multiple testing corrected significance level accounts for the correlation in the data and is defined as P = 0.05/20 (P = 0.0025). Finally, we
defined an inflammatory score from the 28 protein concentrations. We assumed, as already reported32 and in accordance with the theory of a global wear-and-tear of the organism due to
stressful events33, a global positive association between inflammation and decreasing SEP. For each protein, we defined a dichotomised indicator: ‘high concentration’ = 1 and ‘low
concentration’ = 0 based on the highest quartile of the log-transformed concentrations and summed these across the 28 proteins34. As a continuous and hypothesis-free alternative, we used the
first principal component (explaining 35.7% of the total variance). STATISTICAL MODEL Statistical analyses were performed using R v3.1.235. Protein levels below the LOD were imputed based
on a maximum likelihood estimation method using the correlation structure within the data to draw the missing values36. In all analyses, levels of proteins were log-transformed to normalise
their distributions. As proposed elsewhere37, the per-protein analyses were based on a linear mixed model assuming technically-induced variance across microtiter plates induced a systematic
shift in the concentration measures. We included a random intercept in the model, noted and representing the shift associated to _A__i_, the identifier of the plate on which sample _i_ was
assayed. For sample _i_ the model is defined as follows: where _Y__i_ represents the inflammatory measure in participant i, α is the intercept, ε_i_ is the residual error, _X__i_ is the
binary SEP indicator observed in that same participant (where the highest class is used as the reference category) whose effect is measured by the regression coefficient β1 and _FE__i_ is a
matrix of fixed effect observations and corresponding regression coefficients are compiled in the vector β2. Fixed effect covariates include the case-control matching criteria (age, gender
and centre, recoded in three categories: North, Central and South Italy) and batch. To account for the case-control design of EGM, we also included two binaries indicators of whether a
participant is a prospective breast cancer or lymphoma case. The inflammatory score analysis and PCA were based on ‘de-noised’ protein concentrations as obtained from the above linear mixed
model by subtracting the random effect estimates from the observed levels. As such, these measures are implicitly corrected for technically-induced variation and were analysed using a linear
model corresponding to (1) setting the random intercept term to zero. LIFE COURSE ANALYSES For the different measures of inflammatory status described above, we used the same benchmark
model, and, to mimic life course experiences, we sequentially adjusted for the following chronologically ordered proxies for early life, young adulthood and adulthood SEP indicators;
resulting in four time-sequenced models: (A) Age, gender, case-control status, batch, centre and father’s occupation; (B-1) Model A + education; (B-2) Model A + highest household occupation;
(B) Model B-1 + highest household occupation; A fully adjusted model was subsequently built upon model C including body mass index (BMI, kg/m2), smoking status (categorical: current, former
and never smoker) and alcohol consumption (g/day) as three potential SEP-driven factors. To model social mobility, we generalized the linear model used for our inflammatory score by
introducing a multiplicative interaction term for father’s occupation and highest household occupation, hence defining 4 classes:‘Stable Non-manual’ (reference); ‘Manual to Non- manual’;
‘Non-manual to Manual’; and ‘Stable Manual’. Several sensitivity analyses were conducted. First, to preserve power, we dichotomized SEP indicators, while, especially for education, three
categories would have been more relevant. In particular, based on the minimal legal education level in Italy our choice to include ‘professionals’ in the ‘high’ education category may be
arguable. We ran all the analyses coding professional in the low education class. The data we used arose from a case-control study nested in a prospective cohort. To assess the potential for
reverse causation, we ran all analyses by restricting the study population to healthy controls. Social mobility was modelled by introducing a multiplicative interaction term for father’s
occupation and participant’s education. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Castagné, R. _et al._ A life course approach to explore the biological embedding of socioeconomic
position and social mobility through circulating inflammatory markers. _Sci. Rep._ 6, 25170; doi: 10.1038/srep25170 (2016). REFERENCES * Mackenbach, J. P. et al. Socioeconomic Inequalities
in Health in 22 European Countries. N. Engl. J. Med. 358, 2468–2481 (2008). Article CAS Google Scholar * Mackenbach, J. P. Health inequalities: Europe in profile (Produced by COI for the
Department of Health, 2006). * Marmot, M. et al. Closing the gap in a generation: health equity through action on the social determinants of health. The Lancet 372, 1661–1669 (2008). Article
Google Scholar * Stringhini, S. et al. Health Behaviours, Socioeconomic Status and Mortality: Further Analyses of the British Whitehall II and the French GAZEL Prospective Cohorts. PLos
Med. 8, e1000419 (2011). Article Google Scholar * Gallo, V. et al. Social Inequalities and Mortality in Europe – Results from a Large Multi-National Cohort. PLos One 7, e39013 (2012).
Article CAS ADS Google Scholar * Blane, D., Kelly-Irving, M., d’Errico, A., Bartley, M. & Montgomery, S. Social-biological transitions: how does the social become biological?
Longitud. Life Course Stud. 4, 136–146 (2013). Google Scholar * McEwen, B. S. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 87, 873–904
(2007). Article Google Scholar * Yang, E. V. & Glaser, R. Stress-induced immunomodulation and the implications for health. Int. Immunopharmacol. 2, 315–324 (2002). Article CAS Google
Scholar * Scrivo, R., Vasile, M., Bartosiewicz, I. & Valesini, G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun. Rev. 10, 369–374 (2011). Article Google
Scholar * Ranjit, N. et al. Socioeconomic position, race/ethnicity and inflammation in the multi-ethnic study of atherosclerosis. Circulation 116, 2383–2390 (2007). Article Google Scholar
* Koster, A. et al. Association of inflammatory markers with socioeconomic status. J. Gerontol. A. Biol. Sci. Med. Sci. 61, 284–290 (2006). Article Google Scholar * Gruenewald, T. L.,
Cohen, S., Matthews, K. A., Tracy, R. & Seeman, T. E. Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk
Development in Young Adults (CARDIA) study. Soc. Sci. Med. 69, 451–459 (2009). Article Google Scholar * Fraga, S. et al. Association of socioeconomic status with inflammatory markers: a
two cohort comparison. Prev. Med. 71, 12–19 (2015). Article Google Scholar * Panagiotakos, D. B. et al. The association between educational status and risk factors related to
cardiovascular disease in healthy individuals: The ATTICA study. Ann. Epidemiol. 14, 188–194 (2004). Article Google Scholar * Steinvil, A. et al. Relation of educational level to
inflammation-sensitive biomarker level. Am. J. Cardiol. 102, 1034–1039 (2008). Article CAS Google Scholar * Kelly-Irving, M., Mabile, L., Grosclaude, P., Lang, T. & Delpierre, C. The
embodiment of adverse childhood experiences and cancer development: potential biological mechanisms and pathways across the life course. Int. J. Public Health 58, 3–11 (2013). Article
Google Scholar * Ben-Shlomo, Y. & Kuh, D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int. J.
Epidemiol. 31, 285–293 (2002). Article Google Scholar * Loucks, E. B. et al. Life course socioeconomic position is associated with inflammatory markers: the Framingham Offspring Study.
Soc. Sci. Med. 71, 187–195 (2010). Article Google Scholar * Stringhini, S. et al. Association of lifecourse socioeconomic status with chronic inflammation and type 2 diabetes risk: the
Whitehall II prospective cohort study. PLos Med. 10, e1001479 (2013). Article Google Scholar * Ansar, W. & Ghosh, S. C-reactive protein and the biology of disease. Immunol. Res. 56,
131–142 (2013). Article CAS Google Scholar * Leng, S. X. et al. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J. Gerontol. A. Biol. Sci.
Med. Sci. 63, 879–884 (2008). Article Google Scholar * Moncunill, G., Aponte, J. J., Nhabomba, A. J. & Dobaño, C. Performance of multiplex commercial kits to quantify cytokine and
chemokine responses in culture supernatants from Plasmodium falciparum stimulations. PLos One 8, e52587 (2013). Article CAS ADS Google Scholar * Hebels, D. G. A. J. et al. Performance in
omics analyses of blood samples in long-term storage: opportunities for the exploitation of existing biobanks in environmental health research. Environ. Health Perspect. 121, 480–487
(2013). Article Google Scholar * Chaturvedi, A. K. et al. Evaluation of multiplexed cytokine and inflammation marker measurements: a methodologic study. Cancer Epidemiol. Biomark. Prev.
Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 20, 1902–1911 (2011). Article CAS Google Scholar * Klassen, A. C. & Smith, K. C. The enduring and evolving relationship
between social class and breast cancer burden: a review of the literature. Cancer Epidemiol. 35, 217–234 (2011). Article Google Scholar * Lawlor, D. A., Smith, G. D., Rumley, A., Lowe, G.
D. O. & Ebrahim, S. Associations of fibrinogen and C-reactive protein with prevalent and incident coronary heart disease are attenuated by adjustment for confounding factors. British
Women’s Heart and Health Study. Thromb. Haemost. 93, 955–963 (2005). Article CAS Google Scholar * Pollitt, R. A. et al. Early-life and adult socioeconomic status and inflammatory risk
markers in adulthood. Eur. J. Epidemiol. 22, 55–66 (2007). Article Google Scholar * Mendall, M. A. et al. Relation of serum cytokine concentrations to cardiovascular risk factors and
coronary heart disease. Heart 78, 273–277 (1997). Article CAS Google Scholar * Ben-Shlomo, Y. & Kuh, D. A life course approach to chronic disease epidemiology: conceptual models,
empirical challenges and interdisciplinary perspectives. Int. J. Epidemiol. 31, 285–293 (2002). Article Google Scholar * Krieger, N. Embodiment: a conceptual glossary for epidemiology. J.
Epidemiol. Community Health 59, 350–355 (2005). Article Google Scholar * Palli, D. et al. A molecular epidemiology project on diet and cancer: the EPIC-Italy Prospective Study. Design and
baseline characteristics of participants. Tumori 89, 586–593 (2003). Article Google Scholar * Fraga, S. et al. Association of socioeconomic status with inflammatory markers: a two cohort
comparison. Prev. Med. 71, 12–19 (2015). Article Google Scholar * Seeman, T. E., Singer, B. H., Rowe, J. W., Horwitz, R. I. & McEwen, B. S. Price of adaptation-allostatic load and its
health consequences. MacArthur studies of successful aging. Arch. Intern. Med. 157, 2259–2268 (1997). Article CAS Google Scholar * Juster, R.-P., McEwen, B. S. & Lupien, S. J.
Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 35, 2–16 (2010). Article Google Scholar * Development Core Team, R: A language and
environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0 (2005). * Lubin, J. H. et al. Epidemiologic evaluation of measurement data
in the presence of detection limits. Environ. Health Perspect. 112, 1691–1696 (2004). Article CAS Google Scholar * Chadeau-Hyam, M. et al. Prediagnostic transcriptomic markers of Chronic
lymphocytic leukemia reveal perturbations 10 years before diagnosis. Ann. Oncol. 25, 1065–1072 (2014). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS Protein
measurements were conducted at Institute for Risk Assessment Sciences, Division of Environmental Epidemiology, Utrecht University, Utrecht, the Netherlands. The authors wish to thank all the
centres that took part in the study and the additional members of the EnviroGenoMarkers Consortium. For further information on the EnviroGenoMarkers Consortium please visit
http://www.envirogenomarkers.net/. This work was supported by the Institut National du Cancer [SHSESP14–082 to R.C.]. This work has been carried out as part of the H2020LIFEPATH [633666 to
P.V.] and the 7th European Framework Programme (FP7) Envirogenomarkers [226756 to S.K.] projects. SS is supported by an Ambizione Grant [PZ00P3_147998 to S.S.] from the Swiss National
Science Foundation. MC-H, PV, RV, SK acknowledge the support from the FP7 Exposomics project [308610 to P.V.]. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Epidemiology and
Biostatistics, School of Public Health, Imperial College London, London, UK Raphaële Castagné, Gianluca Campanella, Florence Guida, Roel Vermeulen, Paolo Vineis & Marc Chadeau-Hyam *
INSERM, UMR1027, Université Toulouse III-Paul Sabatier, Toulouse, 31000, France Raphaële Castagné, Cyrille Delpierre, Michelle Kelly-Irving & Thierry Lang * Fondazione IRCCS- Instituto
Nazionale dei Tumori, Milan, Italy Vittorio Krogh * Istituto per lo Studio e la Prevenzione Oncologica (ISPO Toscana), Florence, Italy Domenico Palli * Department of Clinical Medicine and
Surgery, University of Naples Federico II, Naples, Italy Salvatore Panico * Piedmont Reference Centre for Epidemiology and Cancer Prevention (CPO Piemonte), Turin, Italy Carlotta Sacerdote *
Cancer registry and Histopathology Unit, Azienda Ospedaliera ‘Civile –M.P.Arezzo’, Ragusa, Italy Rosario Tumino * National Hellenic Research Foundation, Institute of Biology, Pharmaceutical
Chemistry and Biotechnology, Athens, Greece Soterios Kyrtopoulos * Division of Environmental Epidemiology, Institute for Risk Assessment, Utrecht University, Utrecht, the Netherlands
Fatemeh Saberi Hosnijeh, Roel Vermeulen & Marc Chadeau-Hyam * HuGeF, Human Genetics Foundation, Torino, Italy Paolo Vineis * MRC-PHE Centre for Environment and Health, Imperial College,
London, London, UK Paolo Vineis & Marc Chadeau-Hyam * Institute of Social and Preventive Medicine, Lausanne University Hospital, Lausanne, Switzerland Silvia Stringhini Authors *
Raphaële Castagné View author publications You can also search for this author inPubMed Google Scholar * Cyrille Delpierre View author publications You can also search for this author
inPubMed Google Scholar * Michelle Kelly-Irving View author publications You can also search for this author inPubMed Google Scholar * Gianluca Campanella View author publications You can
also search for this author inPubMed Google Scholar * Florence Guida View author publications You can also search for this author inPubMed Google Scholar * Vittorio Krogh View author
publications You can also search for this author inPubMed Google Scholar * Domenico Palli View author publications You can also search for this author inPubMed Google Scholar * Salvatore
Panico View author publications You can also search for this author inPubMed Google Scholar * Carlotta Sacerdote View author publications You can also search for this author inPubMed Google
Scholar * Rosario Tumino View author publications You can also search for this author inPubMed Google Scholar * Soterios Kyrtopoulos View author publications You can also search for this
author inPubMed Google Scholar * Fatemeh Saberi Hosnijeh View author publications You can also search for this author inPubMed Google Scholar * Thierry Lang View author publications You can
also search for this author inPubMed Google Scholar * Roel Vermeulen View author publications You can also search for this author inPubMed Google Scholar * Paolo Vineis View author
publications You can also search for this author inPubMed Google Scholar * Silvia Stringhini View author publications You can also search for this author inPubMed Google Scholar * Marc
Chadeau-Hyam View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conception, hypothesis delineation and study design: R.C., C.D., M.K.I., P.V.,
S.S. and M.C.-H. Collected the data: V.K., D.P., S.P., C.S., R.T., S.K. and P.V. Analysed the data: R.C., C.D., M.K.I., R.V., P.V., S.S. and M.C.-H. discussed the results. Contributed
reagents/materials/analysis tools: G.C., F.G., S.K., F.S.H., T.L. and R.V. All the other authors reviewed and commented on the manuscript. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0
International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the
material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Castagné, R., Delpierre, C., Kelly-Irving, M. _et al._ A life course approach to
explore the biological embedding of socioeconomic position and social mobility through circulating inflammatory markers. _Sci Rep_ 6, 25170 (2016). https://doi.org/10.1038/srep25170 Download
citation * Received: 25 November 2015 * Accepted: 11 April 2016 * Published: 27 April 2016 * DOI: https://doi.org/10.1038/srep25170 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative









