- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Metal oxychlorides are proved to be new cathode materials for chloride ion batteries. However, this kind of cathode materials is still in a very early stage of research and
development. The obtained reversible capacity is low and the electrochemical reaction mechanism concerning chloride ion transfer is not clear. Herein, we report FeOCl/carbon composites
prepared by mechanical milling of the as-prepared FeOCl with carbon nanotube, carbon black or graphene nanoplatelets as cathode materials for chloride ion batteries. The electrochemical
performance of the FeOCl electrode is evidently improved by the incorporation of graphene into the cathode. FeOCl/graphene cathode shows a high reversible capacity of 184 mAh g−1 based on
the phase transformation between FeOCl and FeO. Two stages of this phase transformation are observed for the FeOCl cathode. New insight into the reaction mechanism of chloride ion
dissociation of FeOCl is investigated by DFT + U + D2 calculations. SIMILAR CONTENT BEING VIEWED BY OTHERS FACILE FORMULATION AND FABRICATION OF THE CATHODE USING A SELF-LITHIATED CARBON FOR
ALL-SOLID-STATE BATTERIES Article Open access 16 July 2020 FE3O4-DOPED MESOPOROUS CARBON CATHODE WITH A PLUMBER’S NIGHTMARE STRUCTURE FOR HIGH-PERFORMANCE LI-S BATTERIES Article Open access
27 June 2024 GRAPHENE COLLAGE ON NI-RICH LAYERED OXIDE CATHODES FOR ADVANCED LITHIUM-ION BATTERIES Article Open access 09 April 2021 INTRODUCTION The research in rechargeable batteries is
mainly focused on the electrochemical systems based on cation transfer such as Li+, Na+, Mg2+ 1,2,3,4,5,6. In spite of the cation batteries, new kinds of batteries using the O2−, F− or Cl−
anion for the electrochemical mass transfer have been reported recently7,8,9,10,11. Chloride ion battery shows high theoretical energy density, abundant material resources, high safety and
environmentally friendly features10,11, which fit the requirements for the development of new rechargeable battery systems. Moreover, Cl− anion was found to have high mobility and high
reversibility in the magnesium battery using metal chloride as cathode12, although it has higher ionic radius than Li+, Na+, Mg2+, O2− or F−. A key challenge of chloride ion batteries is to
develop cathode materials which are stable in the electrolytes. Metal oxychlorides, which show higher stabilty and lower volumetric change than metal chlorides during cycling, have been
proposed to be new cathode materials for chloride ion batteries11,12. The feasibility of the metal oxychloride cathodes has been proved in these first studys. However, the experimental
discharge capacity is far from the corresponding theoretical discharge capacity. For instance, only about 30% of the theoretical discharge capacity (249.7 mAh g−1) of the FeOCl cathode was
obtained at the fifth cycle11. FeOCl is a low-dimensional Mott insulator and has a high electrical resistivity of 107 Ω⋅cm at ambient conditions13,14. Moreover, a large volume contraction
(−58.6%) or expansion (141.7%) can occur during the loss or return of chloride ion in FeOCl. The aforementioned low discharge capacity should be caused by the sluggish charge transfer and
mass transfer of the FeOCl cathode during cycling. Therefore, the improvement on the conductivity and structural stability of the FeOCl cathode is necessary. Carbon material such as
graphene15, carbon nanotube16, porous carbon17, carbon black18, activated carbon19 or their hybrid20 has been incorporated into the electrodes of rechargeable batteries. It could provide a
conductive matrix and buffer volume change in the electrodes. Herein, we report the FeOCl/carbon composite cathodes, which were prepared by mechanical milling the as-prepared pure FeOCl with
graphene nanoplatelets (GN), carbon nanotube (CN) or carbon black (CB). The electrochemical performance and reaction mechanism of the FeOCl/carbon composite cathodes were investigated. Part
of FeOCl was decomposed into FeCl3 and Fe2O3 when milling pure FeOCl or the FeOCl with carbon black, while this decomposition was restrained when the carbon material of graphene
nanoplatelets or carbon nanotube was used during milling. The FeOCl/GN composite cathode shows a high reversible capacity of 184 mAh g−1 by the phase transformation between FeOCl and FeO.
Two stages of this phase transformation were observed from the charge and discharge curves and the corresponding differential capacity plot. The DFT + U + D2 calculations have been performed
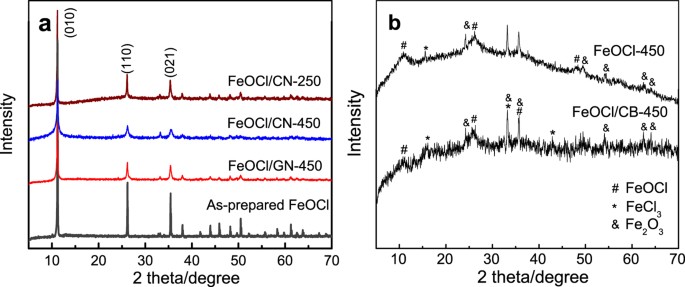
to get an insight into the chloride ion dissociation process of FeOCl. RESULTS AND DISCUSSION Figure 1 shows the XRD patterns of the as-prepared FeOCl, the FeOCl/carbon composites and the
mechanically milled FeOCl. All reflections of the as-prepared FeOCl, FeOCl/CN and FeOCl/GN can be indexed and assigned to the orthorhombic layered FeOCl phase (PDF card no. 72–619) with
three characteristic peaks corresponding to (010), (110) and (021) planes. The crystal structure of FeOCl was maintained during mechanical milling (MM) when GN or CN was used. MM results in
the broadening and drastic decrease in the intensity of the diffraction peaks, refining the grains of the FeOCl phase. For instance, the FeOCl/CN composite milled at 450 rpm (FeOCl/CN-450)
has an average grain size of 22 nm calculated according to the aforementioned three characteristic planes using the Scherrer equation, which is much smaller than 92 nm of the FeOCl/CN-250
sample. Furthermore, the large intensity of (010) plane indicates an orientation perpendicular to _b_-axis, facilitating the formation of a flake-like morphology during synthesis (Fig. 2a).
The intensity ratio of the aforementioned three peaks is listed in Supplementary Table S1. MM reduced the intensity ratio; however, the FeOCl/CN and FeOCl/GN samples still shows high
I(010)/I(110) and I(010)/I(021) values more than 2.5 and 2.2, respectively. Therefore, flake-like morphology was kept and nanosheets were formed after MM of FeOCl and CN or GN, as shown in
Fig. 2b,c,e. The sample milled at a high speed possesses a smaller size. The EDS result for the FeOCl/GN sample in Fig. 2f demonstrates that the obtained FeOCl phase has a fine elemental
composition. For the FeOCl/CB-450 sample, very fine grains of FeOCl phase were obtained after MM according to the weak and broad diffraction peaks (Fig. 1b). But the decomposition of FeOCl
phase occurred because of the formation of α-Fe2O3 and FeCl3 phases. Similar decomposition occurred when the as-prepared pure FeOCl was milled at the same condition. Carbon black cannot
limit this decomposition. Note that the FeOCl/CB-450 sample shows a very low I(010)/I(110) or I(010)/I(021) value of about 0.8 and accordingly a granular morphology. Maybe this is the caused
by the decomposition of FeOCl, which prefers to have a stable structure with an orientation along (010) plane. CN or GN contributed to a dominant fracture along the (010) plane during
milling and thus the decomposition of FeOCl was restrained. Figure 3 shows the discharge and charge curves of the FeOCl/Li electrode systems using different cathodes. The FeOCl/CN-250
cathode shows a discharge capacity of 103 mAh g−1, which is 41% of the theoretical capacity (249.7 mAh g−1) and a sloping discharge profile after activation (Fig. 3a). This low discharge
capacity may be attributed to that part of FeOCl powders did not contribute to the electrochemical reaction. Some large FeOCl flakes without contact with CN could be found in Fig. 2b.
Therefore, electrical contact would be blocked by these flakes and the utilization of the active material was limited. The FeOCl/CN sample prepared at a higher milling speed of 450 rpm
possesses a better distribution between FeOCl and CN (Fig. 2c). Then the FeOCl/CN-450 cathode has a higher discharge capacity of 165 mAh g−1. Moreover, this cathode exhibits the discharge
and charge profiles with different stages instead of only sloping profiles for the FeOCl/CN-250 cathode. For the FeOCl/CB-450 cathode, the sloping discharge and charge profiles are similar
to those of the FeOCl/CB cathode prepared by a soft milling11. However, a maximum discharge capacity of only about 73 mAh g−1 and a low Coulombic efficiency of 46% were received at the first
cycle. This may be caused by the decomposition of the FeOCl phase during the milling of FeOCl and CB (Fig. 1b) and thus the formation of Lewis acid FeCl3, which could be dissolved into the
electrolyte by the formation of the soluble complex ion FeCl4−. As a result, the amount of the active material was decreased and the structural stability of the electrode would be weakened,
leading to a depressed electrochemical performance. Figure 3d shows the discharge and charge curves of the FeOCl/GN-450/Li electrode system. The FeOCl/GN-450 cathode shows a maximum
discharge capacity of 184 mAh g−1 (73% of the theoretical discharge capacity) and a high Coulombic efficiency of 99% at the first cycle. Slight capacity decay was observed in the initial
cycles. The electrochemical performance of the FeOCl electrode was evidently improved by the incorporation of graphene into the cathode by MM. Electrochemical impedance spectroscopy (EIS)
measurement was carried out for the FeOCl/Li electrode systems using different FeOCl cathodes before cycling. The FeOCl cathode was served as the working electrode. The electrochemical
processes of all the electrode systems show a controlling-step of a mixed rate-determining process containing charge transfer and chloride ion diffusion steps. For instance, the Nyquist
plots (Supplementary Fig. S1a) of the FeOCl/CN-250 electrode consist of three parts, a semicircle at high frequency, followed by a further semicircle and a straight line at low frequency.
This is consistent with the corresponding Bode-phase plots (Supplementary Fig. S1b) with three time constants. The former semicircle is considered to be the contact resistance, the later
semicircle may be related to the charge transfer process and the straight line should be associated with the Warburg impedance by chloride ion diffusion. It is evident that the FeOCl/CB
electrode shows the highest electrochemical resistance, which was reduced when CN or GN was used. The FeOCl/GN-450 electrode possesses the best electrochemical kinetic performance,
contributing to superior discharge and charge properties (Fig. 3d). A more distinct two-step electrochemical process could be found in both the discharge and charge profiles of the
FeOCl/GN-450 cathode as compared with the FeOCl/CN-450 cathode. The capacity was almost equally divided into two parts for the two steps, respectively. Figure 4 shows the corresponding
differential capacity curves of the discharge and charge curves in Fig. 3d. Two pairs of the redox peaks could be observed in first six cycles. The first reduction peak appears at 2.77 V and
the corresponding oxidation peak is located at 2.94 V. The other redox couple shows an oxidation peak at 2.22 V, followed by a small reduction peak at 2.16 V and a large reduction peak at
2.05 V. Generally, a single reduction peak should be formed. This might be due to the difference in the particle size and also the contact with graphene. Probably some FeOCl flakes have
smaller particle size and better contact with graphene after MM, resulting in a higher discharge voltage plateau at 2.16 V. This small discharge plateau was weakened during cycling and
almost disappeared at the 6th cycle. It means the distribution of the FeOCl/graphene regions tends to be uniform during cycling and thus a single reduction peak was formed. Similar
phenomenon has been reported in the FeF2/carbon cathode for lithium ion battery21. The reversible chloride ion transfer at metal oxychloride cathode (BiOCl or FeOCl) side has been proved by
STEM/EDS/EELS in our previous work11. However, the product after discharge of FeOCl or VOCl was not detected by XRD11,12. This may be due to the formation of amorphous and/or nanosized
particles. XPS was used to verify the structural evolution in the FeOCl/GN-450 electrode before and after cycling. Fe 2p3/2 and Fe 2P1/2 main peaks corresponding to the as-prepared pure
FeOCl (Supplementary Fig. S2) and FeOCl/GN-450 (Fig. 5 and Supplementary Fig. S2) materials are located at 711.1 and 724.9 eV, respectively. When the FeOCl/GN-450/Li electrode system was
fully discharged, the peaks of the FeOCl/GN-450 cathode at 709.6 and 723.0 eV were observed and can be assigned to FeO (Fe2+) phase22. For the charged electrode, the same Fe 2p signal as
that of the as-prepared FeOCl/GN-450 material is received, indicating the formation of FeOCl by the reaction between FeO and Cl− during charge and also the reversible reactions in the
chloride ion battery. FeOCl/Li electrode system shows a one-electron electrochemical reaction. However, a two-step electrochemical process in both discharge and charge profiles (Fig. 3b,d)
was obtained. Herein we employ DFT + U + D2 calculations to get an insight into the two-step chloride ion dissociation of FeOCl. The calculated lattice parameters of FeOCl have been improved
in our previous work and agree well with the experimental data12. Each Fe3+ is coordinated with four oxygen ions and two chloride ions and two neighboring ions are coupled
anti-ferromagneticly to form a ground state without magnetism. A 2 × 1 × 2 super cell Fe8O8Clx (x = 0–8) was chosen to simulate the chloride ion dissociation process (Fig. 6). We start with
the removal of chloride ion 1 (Cl1) from Fe8O8Cl8 (perfect FeOCl crystal structure) in Fig. 6. The charges of two iron ions which connect to Cl1 are decreased by 0.17e− according to the
Bader charge analysis23,24,25. The reduced iron ions then move inward to the oxygen row, resulting in an increase in the lattice parameter a by about 0.2 Å, while the other two lattice
parameters are barely changed. Another consequence is the interaction between the reduced iron ions and the neighboring chloride ions, Cl5 for example, is weakened. The calculated
electrostatic potential energy based on equation (1) of Cl5 is −5.86 eV, which is much higher than −8.46, −8.76, −8.01, −7.39, −7.34 and −7.83 eV of Cl2, Cl3, Cl4, Cl6, Cl7 and Cl8,
respectively. This indicates that Cl5 is less stable and thus the next dissociative chloride ion prefers to be Cl5. The chloride ions in the whole c-row (Cl1-Cl5) would dissociate
subsequently (25% chloride ion dissociation). Then the neighboring Fe-row would be reduced and move towards to the adjacent oxygen row. The whole framework of FeOCl (Fe8O8Cl6), however, is
almost unchanged; the lattice parameters b and c stay the same. We then remove another c-row of chloride ions from our model system (Fe8O8Cl4, 50% chloride ion dissociation). The b-parameter
drops dramatically by about 2 Å (Fig. 7); a and c parameters are slightly changed. -Fe-O- planes tend to be formed, since the reduced iron ions keep moving to oxygen rows. For the Fe8O8Cl2
(75% chloride ion dissociation) model, the transformation from FeOCl to FeO is almost finished. The b-parameter is decreased further and a,c-parameters are evidently increased to form
Fe2+O2+ rows. In the end, a rock salt type FeO structure is obtained after fully dechlorination. Each iron ion connects to six oxygen ions, occupying an octahedral site. The optimized
lattice parameter of FeO is 4.35 Å, which agrees well with the experimental data 4.30 Å26. This two-step chloride ion dissociation process is in accordance with the result of the
electrochemical test. CONCLUSION In summary, FeOCl/carbon composites have been prepared by mechanical milling the as-prepared FeOCl with carbon nanotube, carbon black or graphene
nanoplatelets. The decomposition of part of FeOCl into Fe2O3 and FeCl3 during MM was restrained when carbon nanotube or graphene nanoplatelets was used. Graphene incorporated into the FeOCl
cathode contributed to an improvement in the electrochemical kinetic performance and the obtained FeOCl/GN-450 cathode shows a high reversible capacity of 184 mAh g−1. This reversible
reaction was proved to be according to the phase transformation between FeOCl and FeO by XPS. Moreover, the incorporation of carbon nanotube or graphene into the FeOCl cathode could result
in a change in the discharge and charge profiles. The FeOCl/GN-450 cathode exhibits a distinct two-step discharge and charge process. New insight into the reaction mechanism of the chloride
ion dissociation of FeOCl was investigated by DFT + U + D2 calculations, which show that one of the chlorine layers in FeOCl dissociates first, leading to a drastic decrease in the lattice
parameter b of FeOCl. Then the loss of the other chlorine layer results in the formation of FeO. METHODS SYNTHESIS OF MATERIALS The preparation of FeOCl using a chemical vapor transport
method has been reported in our previous work11. FeOCl/carbon composite materials were fabricated by mechanical milling 1 g of the as-prepared FeOCl and 20 mass % graphene nanoplatelets
aggregates (GN, Alfa Aesar), carbon nanotube (CN, Shanghai ANT) or carbon black (CB, Alfa Aesar) using a 50 ml agate vial with agate balls (10 and 6 mm in diameter) under an argon
atmosphere. The ball to powder ratio was 40:1. The milling was performed in a planetary mill with a rotation speed of 250 or 450 rpm and the milling time was 10 h. The anhydrous ionic
liquids of 1-Butyl-1-methylpiperidinium bis(trifluoromethylsulfonyl)imide (PP14TFSI, 99%, IoLiTech) and 1-Butyl-1-methylpiperidinium chloride (PP14Cl, 99%, IoLiTech) were all dried at 358 K
for 72 h under vacuum. STRUCTURAL ANALYSIS AND ELECTROCHEMICAL TESTS X-ray Powder diffraction (XRD) was performed using a Rigaku SmartLab diffractometer with Cu-Kα radiation. The morphology
and composition of the sample were characterized by a field-emission scanning electron microscopy (Ultra55 FE-SEM) incorporated with an energy-dispersive X-ray spectroscopy (EDS). X-ray
photoelectron spectroscopy (XPS) measurements were conducted using a K-Alpha (Thermo Scientific) spectrometer with Al Ka radiation as the X-ray source. The vacuum in the analyzer chamber was
kept at about 1 × 10−9 mbar during the measurements. The C 1s line with a binding energy of 284.8 eV was used as a standard. Electrochemical measurements were conducted using coin cells
(CR2032) with lithium metal (Alfa Aesar) as anode. The cathode electrodes were fabricated by mixing as-synthesized material, PVDF and carbon black in the mass ratio of 80:10:10.
N-methyl-2-pyrrolidinone (NMP) was used as the solvent for PVDF to get homogeneous slurry, which was spread on a stainless steel (SS) foil and dried on hot plate at 373 K for 20 h. A mixture
of 0.5 M PP14Cl in PP14TFSI was used as electrolyte. Celgard 2400 film was used as separator. Discharge and charge tests were carried out galvanostatically at 10 mA g−1 over a voltage range
between 1.6 and 3.5 V by using Arbin BT2000 multi-channel battery testing system at 298 K. The specific capacities were calculated according to the corresponding active material of the
cathode electrode. Electrochemical impedance spectroscopy (EIS) data were collected in the frequency range from 100 kHz to 10 mHz with an ac amplitude of 10 mV under a CHI660D
electrochemical workstation. DFT CALCULATIONS We follow our previous work27, where we obtained the calculated lattice parameters (a = 3.851 Å, b = 8.050 Å and c = 3.298 Å) of FeOCl, which
agree well with the experimental data. All of the spin-polarized density-functional theory (DFT) calculations were performed with the Vienna ab initio Simulation Package (VASP)28,29,30. The
generalized gradient approximation with the functional described by Perdew _et al._ (GGA-PBE) was used for all calculations31. The projector-augmented wave (PAW) method is applied to
describe the wavefunctions in the core regions32,33, while the valence wavefunctions are expanded as linear combination of plane-waves with a cutoff energy of 550 eV. The on-site Coulomb
interactions were included only for strongly correlated Fe 3d electrons34,35, with an effective U value of 4.636. DFT-D2 approaches were employed for the long-range dispersion
corrections37,38,39. Our unit cell for simulating the dissociation process is restrained to Fe8O8Clx (x = 0–8), the Brillouin zone was sampled with a (3 × 3 × 3) Monkhorst-Pack mesh of
k-points40, which have been tested with respect to total energy of the system. In the optimization of the lattice constants, we always fix two lattice constants and optimize the third one;
this was done iteratively until the changes were less than 0.02 Å. Electrostatic potential energy is calculated upon the formula as follow, where _k__e_ represents the Coulombic constant,
_j_ is the index for the point charges within a point charge field, which has been extended from an optimized unit cell consisting of 81000 point charges. ADDITIONAL INFORMATION HOW TO CITE
THIS ARTICLE: Zhao, X. _et al._ Carbon incorporation effects and reaction mechanism of FeOCl cathode materials for chloride ion batteries. _Sci. Rep._ 6, 19448; doi: 10.1038/srep19448
(2016). REFERENCES * Wang, C. et al. Fabrication and shell optimization of synergistic TiO2-MoO3 core-shell nanowire array anode for high energy and power density lithium-ion batteries. Adv.
Funct. Mater. 25, 3524–3533 (2015). Article CAS Google Scholar * Chen, R. Y. et al. Disordered lithium-rich oxyfluoride as a stable host for enhanced Li+ intercalation storage. Adv.
Energy Mater. 5, 1401814 (2015). Article Google Scholar * Yabuuchi, N., Kubota, K., Dahbi, M. & Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 114, 11636–11682
(2014). Article CAS PubMed Google Scholar * Liu, H. D., Xu, J., Ma, C. Z. & Meng, Y. S. A new O3-type layered oxide cathode with high energy/power density for rechargeable Na
batteries. Chem. Commun. 51, 4693–4696 (2015). Article CAS Google Scholar * Zhao-Karger, Z. et al. Performance improvement of magnesium sulfur batteries with modified non-nucleophilic
electrolytes. Adv. Energy Mater. 5, 1401155 (2015). Article Google Scholar * Okamoto, S. et al. Intercalation and push-out process with spinel-to-rocksalt transition on Mg insertion into
spinel oxides in magnesium batteries. Adv. Sci. 2, 1500072 (2015). Article Google Scholar * Hibino, M., Kimura, T., Suga, Y., Kudo, T. & Mizuno, N. Oxygen rocking aqueous batteries
utilizing reversible topotactic oxygen insertion/extraction in iron-based perovskite oxides Ca1–xLaxFeO3-δ . Sci. Rep. 2, 601 (2012). Article ADS PubMed PubMed Central Google Scholar *
Rongeat, C., Reddy, M. A., Witter, R. & Fichtner, M. Solid electrolytes for fluoride ion batteries: ionic conductivity in polycrystalline tysonite-type fluorides. ACS Appl. Mater.
Interfaces 6, 2103–2110 (2015). Article Google Scholar * Rongeat, C., Reddy, M. A., Diemant, T., Behm, R. J. & Fichtner, M. Development of new anode composite materials for fluoride
ion batteries. J. Mater. Chem. A 2, 20861–20872 (2014). Article CAS Google Scholar * Zhao, X. Y., Ren, S., Bruns, M. & Fichtner, M. Chloride ion battery: a new member in the
rechargeable battery family. J. Power Sources 245, 706–711 (2014). Article ADS CAS Google Scholar * Zhao, X. Y., Zhao-Karger, Z., Wang, D. & Fichtner, M. Metal oxychlorides as
cathode materials for chloride ion batteries. Angew. Chem., Int. Ed. 52, 13621–13624 (2013). Article CAS Google Scholar * Zhao, X. Y. et al. Vanadium oxychloride/magnesium electrode
systems for chloride ion batteries. ACS Appl. Mater. Interfaces 6, 22430–22435 (2014). Article PubMed Google Scholar * Kim, S. H., Kang, J. K., Hwang, S. & Kim, H. A theoretical study
on the electronic structures of MOCl (M=Ti, V and Fe) and their relationship with physical properties. Bull. Korean Chem. Soc. 16, 299–304 (1995). CAS Google Scholar * Bykov, M. et al.
High-pressure behavior of FeOCl. Phys.Rev. B 88, 014110 (2013). Article ADS Google Scholar * Raccichini, R., Varzi, A., Passerini, S. & Scrosati, B. The role of graphene for
electrochemical energy storage. Nature Mater. 14, 271–279 (2015). Article ADS CAS Google Scholar * Li, R. Z. et al. Carbon-stabilized high-capacity ferroferric oxide nanorod array for
flexible solid-State alkaline battery-supercapacitor hybrid device with high environmental suitability, Adv. Funct. Mater. 25, 5384–5394 (2015). Article CAS Google Scholar * Jung, D. S.
et al. Hierarchical porous carbon by ultrasonic spray pyrolysis yields stable cycling in lithium–sulfur battery. Nano Lett. 14, 4418–4425 (2014). Article ADS CAS PubMed Google Scholar *
Park, C. M. & Sohn, H. J. Black phosphorus and its composite for lithium rechargeable batteries. Adv. Mater. 19, 2465–2468 (2007). Article CAS Google Scholar * Elazari, R., Salitra,
G., Garsuch, A., Panchenko, A. & Aurbach, D. Sulfur-impregnated activated carbon fiber cloth as a binder-Free Cathode for rechargeable Li-S batteries. Adv. Mater. 23, 5641–5644 (2011).
Article CAS PubMed Google Scholar * Tang, C. et al. Nitrogen-Doped Aligned Carbon Nanotube/graphene sandwiches: Facile catalytic growth on bifunctional natural catalysts and their
applications as scaffolds for high-Rate lithium-sulfur batteries. Adv. Mater. 26, 6100–6105 (2014). Article CAS PubMed Google Scholar * Reddy, M. A. et al. CFx Derived carbon–FeF2
nanocomposites for reversible lithium storage. Adv. Energy Mater. 3, 308–313 (2013). Article CAS Google Scholar * Grosvenor, A. P., Kobe, B. A., Biesinger, M. C. & McIntyre, N. S.
Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 36, 1564–1574 (2004). Article CAS Google Scholar * Tang, W., Sanville, E.
& Henkelman, G. A grid-based bader analysis algorithm without lattice bias. J. Phys. Condens. Matter. 21, 084204 (2009). Article ADS CAS PubMed Google Scholar * Sanville, E., Kenny,
S. D., Smith, R. & Henkelman, G. Improved grid-based algorithm for bader charge allocation J. Comput. Chem. 28, 899–908 (2007). Article CAS PubMed Google Scholar * Henkelman, G.,
Arnaldsson, A. & Jónsson, H. A fast and robust algorithm for bader decomposition of charge density. Comput. Mater. Sci. 36, 354–360 (2006). Article Google Scholar * Yamamoto, A.
Modulated structure of wustite (Fe1–_x_O) (three-dimensional modulation). Acta Cryst. B 38, 1451–1456 (1982). Article Google Scholar * Zhao, X. Y. et al. Magnesium anode for chloride ion
batteries. ACS Appl. Mater. Interfaces 6, 10997–11000 (2014). Article CAS PubMed Google Scholar * Kresse, G. & Hafner, J. Ab Initio Molecular dynamics for open-shell transition
metals. Phys.Rev. B 48, 13115–13118 (1993). Article ADS CAS Google Scholar * Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and
semiconductors. Comput. Mater. Sci. 6, 15–50 (1996). Article CAS Google Scholar * Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations
using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996). Article ADS CAS Google Scholar * Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made
simple. Phys. Rev. Lett. 77, 3865–3868 (1996). Article ADS CAS Google Scholar * Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys.
Rev. B 59, 1758–1775 (1999). Article ADS CAS Google Scholar * Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994). Article ADS Google Scholar *
Rohrbach, A., Hafner, J. & Kresse, G. Electronic correlation effects in transition-metal sulfides. J. Phys.: Condens. Matter 15, 979–996 (2003). ADS CAS Google Scholar * Dudarev, S.
L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509
(1998). Article ADS CAS Google Scholar * Liao, P. & Carter, E. A. Ab initio DFT + U predictions of tensile properties of iron oxides. J. Mater. Chem. 20, 6703–6719 (2010). Article
CAS Google Scholar * Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A Consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94
Elements H-Pu. J. Chem. Phys. 132, 154104 (2010). Article ADS Google Scholar * Grimme, S. Semiempirical GGA-Type Density functional constructed with a long-range dispersion correction. J.
Comput. Chem. 27, 1787–1799 (2006). Article CAS PubMed Google Scholar * Bučko, T., Hafner, J., Lebègue, S. & Ángyán, J. G. Improved description of the structure of molecular and
layered crystals: ab initio DFT calculations with van der Waals corrections. J. Phys. Chem. A 114, 11814–11824 (2010). Article PubMed Google Scholar * Monkhorst, H. J. & Pack, J. D.
Special points for brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976). Article ADS MathSciNet Google Scholar Download references ACKNOWLEDGEMENTS The project supported by the
Joint Funds of the National Natural Science Foundation of China (Grant No. U1407106) and the support from the Priority Academic Program Development of Jiangsu Higher Education Institutions
(PAPD) are gratefully acknowledged. The computational resource bwUniCluster funded by the Ministry of Science, Research and the Arts Baden-Württemberg and the Universities of the State of
Baden-Württemberg, Germany, within the framework program bwHPC is also acknowledged. Q. L. thanks the financial support from the Helmholtz Research School “Energy-related catalysis”. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * College of Materials Science and Engineering, Nanjing Tech University, Nanjing, 210009, China Xiangyu Zhao, Tingting Yu, Meng Yang & Xiaodong Shen
* Institute of Nanotechnology, Karlsruhe Institute of Technology (KIT), Postfach 3640, Karlsruhe, 76021, Germany Qiang Li & Karin Fink * State Key Laboratory of Materials-Oriented
Chemical Engineering, Nanjing Tech University, Nanjing, 210009, China Xiaodong Shen Authors * Xiangyu Zhao View author publications You can also search for this author inPubMed Google
Scholar * Qiang Li View author publications You can also search for this author inPubMed Google Scholar * Tingting Yu View author publications You can also search for this author inPubMed
Google Scholar * Meng Yang View author publications You can also search for this author inPubMed Google Scholar * Karin Fink View author publications You can also search for this author
inPubMed Google Scholar * Xiaodong Shen View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS X.Y.Z. conceived the idea and performed the study.
T.T.Y., M.Y. and X.D.S. assisted the experiments and the corresponding analyses. Q.L. and K.F. performed DFT calculations. The manuscript was written through contributions of all authors.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS This work is
licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license,
unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce
the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhao, X., Li, Q., Yu, T. _et
al._ Carbon incorporation effects and reaction mechanism of FeOCl cathode materials for chloride ion batteries. _Sci Rep_ 6, 19448 (2016). https://doi.org/10.1038/srep19448 Download citation
* Received: 20 November 2015 * Accepted: 14 December 2015 * Published: 18 January 2016 * DOI: https://doi.org/10.1038/srep19448 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative









