- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The formation of Aβ is directly controlled by the γ-secretase complex and its activator, γ-secretase activating protein (GSAP). GSAP derives from a C-terminal fragment of a larger
precursor protein via a caspase-3 mediated cleavage. However, the mechanism regulating this process remains unknown. Here we provide _in vitro_ experimental evidence that 5-Lipoxygenase
(5LO) is as an endogenous regulator for GSAP formation, but not for other known γ-secretase modulators, by directly and specifically activating caspase-3. These results were confirmed _in
vivo_ by using transgenic mouse models of Alzheimer’s disease in which 5LO level and activity were modulated genetically or pharmacologically. Taken together, our findings demonstrate that
GSAP cleavage via caspase-3 is regulated and depend upon the availability of 5LO further establishing this protein as an attractive and viable therapeutic target for Alzheimer’s disease.
SIMILAR CONTENT BEING VIEWED BY OTHERS Γ-SECRETASE IN ALZHEIMER’S DISEASE Article Open access 08 April 2022 MECHANISMS OF AMYLOID-Β34 GENERATION INDICATE A PIVOTAL ROLE FOR BACE1 IN AMYLOID
HOMEOSTASIS Article Open access 07 February 2023 EBP1 POTENTIATES AMYLOID Β PATHOLOGY BY REGULATING Γ-SECRETASE Article 08 January 2025 INTRODUCTION Alzheimer’s disease (AD) is the most
prevalent cause of dementia and is associated with accumulation of amyloid-β peptide (Aβ) which is a major characteristic of the AD brain and responsible for some of its clinical
manifestations1. While early-onset AD results from the mutations of genes that are involved in Aβ formation, a combination of environmental risk factors and different genes have been
implicated in its sporadic form2. Among the latter, recent work has highlighted the potential role that the 5-Lipoxygenase (5LO) enzyme plays in AD pathogenesis by showing its involvement in
Aβ formation and deposition3,4,5. Activation of the γ-secretase complex is required for the final formation of Aβ peptides and decreasing Aβ production by blocking this complex as a disease
modifying approach for the treatment of AD has received intense investigation6. However, γ-secretase is known to process multiple substrates in addition to amyloid precursor protein (APP),
most notably Notch and this fact has severely limited the clinical development of inhibitors directly and irreversibly targeting this enzyme7. The recent discovery of a γ-secretase
activating protein (GSAP) which interacts with this protease to facilitate Aβ formation without affecting Notch has established it as a relevant target for a viable and safer anti-Aβ
therapy8,9. GSAP is increased in post-mortem brain tissues of AD patients and its pharmacological or genetic inhibition results in an amelioration of the AD-like amyloidotic phenotype in
transgenic mouse models of the disease10,11. Recently, we identified a caspase-3 processing domain in the GSAP precursor protein sequence and provided experimental evidence that this caspase
is involved in the formation of its active fragment, GSAP 16 kDa and subsequent biogenesis of Aβ peptides12. Thus, while we are learning more about the neurobiology of GSAP, no information
is available about the mechanism regulating its formation. In the current paper, we provide experimental evidence that proteolytic formation of GSAP via caspase-3 is dependent upon the
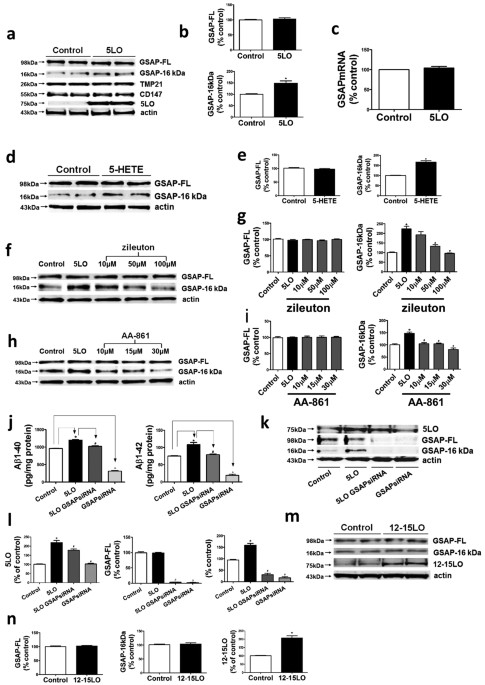
availability of the 5LO. RESULTS _IN VITRO_ STUDIES MODULATION OF GSAP FORMATION BY 5LO IS SPECIFIC Compared with empty vector, neuronal N2A-APPswe cells over-expressing 5LO had a
significant increase in GSAP-16 kDa fragment levels but not its precursor protein, GSAP-FL (Fig. 1a–b). By contrast, no significant changes were detected for two other γ-secretase modulatory
proteins: TMP21 and CD147 (Fig. 1a)13,14. Under this experimental condition, quantitative real time PCR did not show any significant difference in GSAP mRNA levels between the two groups
(Fig. 1c). In the same cells addition of the major 5LO metabolite, 5-HETE, resulted in a significant increase of GSAP-16 kDa levels (Fig. 1d,e), whereas two selective 5LO pharmacological
inhibitors, zileuton and AA-861, lowered GSAP-16 kDa levels in a dose-dependent manner (Fig. 1f–i)15. The Aβ 1–40 and 1–42 levels in supernatants from cells over-expressing 5LO were
significantly increased, however, when the cells were treated with GSAP siRNA these levels were decreased (Fig. 1j), as were both the GSAP-FL and its 16 kDa fragment (Fig. 1k,l). In
contrast, no significant changes for both GSAP-FL and the 16 kDa fragment levels were detected when neuronal cells were over-expressing another LO, the 12–15LO (Fig. 1m,n).
Co-immunoprecipation studies revealed the physical interaction between GSAP and 5LO, but not for GSAP and 12–15LO (Fig. 2a,b). This observation was further substantiated by
immunofluorescence studies showing a significant cellular co-localization between GSAP and 5LO (Fig. 2c,d). CASPASE-3 IS REQUIRED FOR 5LO-DEPENDENT GSAP FORMATION Having shown that 5LO
specifically modulate the formation of GSAP16 kDa and with the knowledge that GSAP precursor is cleaved by caspase-312, next we investigated whether 5LO action on GSAP was dependent or
independent of this very caspase. First, neuronal cells were incubated with increasing doses of a specific and cell permeable caspase-3 inhibitor, z-DEVD-fmk16 and the effect on GSAP 16 kDa
assessed. As shown in Fig. 3, we observed that pharmacological inhibition of caspase-3 activation resulted in a significant decrease in the active form of caspase-3 and GSAP 16 kDa levels,
but no changes were detected for procaspase-3 and GSAP-FL levels (Fig. 3a,b). Incubation of the same inhibitor with 5LO overexpressing cells resulted in a significant reduction in GSAP 16
kDa, active caspase-3 levels and activity and Aβ levels, but no changes were observed for GSAP-FL and procaspase-3 protein levels (Fig. 3c–g). To further confirm this finding, next we used
caspase-3 siRNA to knock-down this protease and evaluated the effect on GSAP fragment formation in cells over-expressing 5LO. As shown in Fig. 4, cells treated with this siRNA showed a
dramatic reduction in pro-caspase-3, active caspase-3 steady state levels, which was accompanied by a decrease of GSAP-16 kDa (Fig. 4a,b). By contrast, no differences in GSAP-FL, TMP21 and
CD147 were detected between 5LO overexpressing cells treated or not treated with caspase-3 siRNA (Fig. 4a,b). Under these experimental conditions, we found that _in vitro_ caspase-3 activity
levels were significantly reduced (Fig. 4c). _IN VIVO_ STUDIES 5LO MODULATES GSAP FORMATION VIA CASPASE-3 Next, we wanted to confirm these findings _in vivo_ by using brain tissues from
3xTg mice over-expressing 5LO (3xTg/H5LO), 3xTg genetically deficient for 5LO mice (3xTg 5LOKO) and 3xTg mice treated with zileuton, a selective 5LO inhibitor (3xTg zileuton), all of which
have been previously described5,17,18. Compared with controls, 3xTg/H5LO mice had a significant increase in GSAP-16 kDa fragment levels, whereas no significant differences were observed
between the two groups for GSAP-FL, TMP21 and CD147 steady state levels (Fig. 5a,b). By contrast, we observed that 3xTg mice genetically deficient for 5LO, or receiving the 5LO inhibitor had
significant decreases in GSAP-16 kDa fragment levels but no changes in GSAP-FL, TMP21 and CD147 levels when compared to their control groups (Fig. 5c–f). Next, we also tested the effect of
the 5LO modulation on GSAP fragment expression in wild-type mice. Compared with controls, WT/H5LO mice had an increase in GSAP-16 kDa fragment levels, which did not reach statistical
significance, whereas no significant differences were observed between the two groups for GSAP-FL, TMP21 and CD147 steady state levels (Fig. 5g,h). By contrast, we observed that WT mice
genetically deficient for 5LO, or receiving the 5LO inhibitor had significant decreases in GSAP-16 kDa fragment levels but no changes in GSAP-FL, TMP21 and CD147 levels when compared to
their control groups (Fig. 5i–l). In addition, we found a significant increase in active caspase-3 but no change in procaspase-3 levels in 3xTg mice over-expressing 5LO compared to the
control group (Fig. 6a,b). The opposite was true for both 3xTg mice genetically deficient for 5LO and receiving 5LO inhibitor zileuton (Fig. 6c–f). Similar results were observed in brains
from WT mice (Fig. 6g–l). Finally, immunofluorescence studies showed that compared with 3xTg control mice the co-localization for GSAP and 5LO, caspase-3 and 5LO and caspase-3 and GSAP were
higher in 3xTg mice overexpressing 5LO, whereas this was absent in 3xTg mice genetically deficient for 5LO (Fig. 7a–c). DISCUSSION In the current paper we provide _in vitro_ and _in vivo_
experimental evidence that 5LO is involved in the proteolytic processing of the GSAP-FL, the precursor protein that by generating the biologically active fragment GSAP 16 kDa is ultimately
responsible for controlling Aβ biogenesis and that this biological effect is mediated by the activation of caspase-3. In addition, our findings demonstrate that this process is selective for
GSAP, since it does not influence other γ-secretase modulators and specific for 5LO since another LO, the 12/15LO, is without effect. The 5LO is an enzyme widely expressed in the central
nervous system and is up-regulated in AD brains19. Consistent evidence has demonstrated that this protein is directly involved in the production and subsequent deposition of Aβ peptides _in
vitro_ and _in vivo_20. GSAP is a key molecule responsible for the rate-limiting step in Aβ production by directly interacting with the γ-secretase complex and for this reason it is an
emerging potential new player in molecular and cellular mechanisms of relevance to AD brain amyloidosis21,22. Because of these two pieces of information, in the current paper we wanted to
test the hypothesis that 5LO act as a direct regulator of GSAP formation and if this was the case, investigate the underlying mechanism(s). Initially, we demonstrated that over-expression of
5LO, but not 12/15LO, did not alter steady state and mRNA levels of the GSAP precursor protein, but selectively increased the amount of its active form, the GSAP16 kDa. To further
investigate the specificity of this biological effect we looked at 2 different γ-secretase protein modulators, namely TMP21 and CD147 and we observed that 5LO was without any effect. These
data were affirmed in the inverse by using pharmacological and genetic strategies. Thus, incubation with two distinct and structurally unrelated 5LO inhibitors or the use of GSAP siRNA
reduced GSAP 16 kDa levels. Next, by using biochemistry and immunofluorescence assay approaches we showed that in neuronal cells, GSAP physically and directly interacts with 5LO but not with
another member of the LO family (i.e,12/15LO). Because our previous study identified a caspase-3 processing domain in the GSAP-FL precursor protein sequence and provided experimental
evidence that this caspase is essential for GSAP 16 kDa formation and biogenesis of Aβ peptides12, we wanted to investigate whether the 5LO effect above described was also mediated by a
caspase-3 dependent mechanism. Interestingly, this hypothesis was supported by some previous data in the literature suggesting a biological link between 5LO and caspase-3. Thus, ApoEˉ/ˉ mice
lacking 5LO have reduced caspase-3 activity23, while overexpression of 5LO significantly enhanced caspase-3 activation in PC12 cells24 and 5LO pharmacological inhibition significantly
down-regulated caspase-3 activation in the brains of rats undergoing middle cerebral artery occlusion25. To prove that 5LO regulation of GSAP 16 kDa formation is dependent upon caspase-3
activation, we adopted a pharmacological and a genetic approach. First, we showed that 5LO overexpression was not able to influence GSAP precursor protein processing to form the 16 kDa
fragment in the presence of a caspase-3 inhibitor. Under this experimental condition we observed a significant reduction in the activity of this caspase and in the amount of Aβ in the
supernatants. Similar results were obtained when levels of procaspase-3 were significantly reduced in neuronal cells treated with caspase-3 siRNA. Taken together our findings provide _in
vitro_ experimental support for the novel hypothesis that 5LO specifically regulates the processing of GSAP-FL and amyloidogenesis via the activation of caspase-3. This observation does not
contrast with our previous report in which we showed that 5LO can act as a modulator of Aβ formation by regulating s the transcription of the four components of the γ-secretase complex9. On
the other hand, we believe that this newly discovered function of 5LO add a new interesting facet to the complex neurobiology of this protein within the central nervous system. The
biological relevance of our discovery was corroborated by the implementation of ex-vivo studies in a transgenic mouse model of AD in which the 5LO pathway was genetically or
pharmacologically modulated. Thus, genetic absence or pharmacological inhibition of 5LO resulted in a significant reduction in GSAP 16 kDa, but no changes in its precursor protein, TMP21 nor
CD147 levels in the brains of 3xTg mice. By contrast, brain tissues from 3xTg mice over-expressing 5LO had a significant increase in GSAP16 kDa, but no changes in GSAP-FL, TMP21 and CD147
steady state levels. These results were reproduced in WT mice, suggesting that 5LO acts as an endogenous modulator of GSAP fragment expression independently from the presence of a transgene.
Importantly, the amount or availability of 5LO was a direct predictor of the levels of active caspase-3 in the brains of these animals, where immunofluorescence studies revealed an increase
or decrease in co-localization between GSAP and 5LO, caspase-3 and 5LO and caspase-3 and GSAP, respectively. In summary, our studies provide the first direct experimental evidence that GSAP
cleavage via caspase-3, which generates the 16 kDa fragment ultimately responsible for controlling the γ-secretase activity and amyloidogenesis _in vivo_, is specifically regulated and
dependent upon the availability of 5LO. They further establish 5LO as an attractive and viable Aβ lowering therapeutic target for AD without the toxic effect of classic γ-secretase
inhibitors. METHODS CELL CULTURE AND TREATMENTS The N2A (neuro-2 A neuroblastoma) neuronal cells stably expressing human APP carrying the K670 N, M671 L Swedish mutation (APP swe) were used
in our _in vitro_ studies and grown, as previously described12,26. For transfection, cells were grown to 70% confluence and transfected with 1 μg of empty vector (pcDNA3.1) or human 5LO
pcDNA3.1 (Dr. Colin Funk, Queen’s University, Kingston, Canada) by using Lipofectamine 2000® (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. After 24 h transfection,
supernatants were collected and cells pellets harvested in lytic buffer for biochemical analyses. For pharmacological treatment, cells were incubated with 5-HETE (10 μM), or zileuton (10
μM, 50 μM, 100 μM), or AA-861 (10 μM, 15 μM, 30 μM), or with the caspase-3 inhibitor (z-DEVD-fmk) (100 μM, 500 μM) for 24 h after transient transfection with human 5LO pcDNA3.1, or singly
incubated with the caspase-3 inhibitor (z-DEVD-fmk) (10 μM, 25 μM, 50 μM, 100 μM, 500 μM) for 24 hours, after which supernatants were collected and cells pellets harvested in lytic buffer
for biochemical analyses. For siRNA knock-down studies, GSAP siRNA (sc-140659) and caspase-3 siRNA (sc-29927) and a negative control siRNAs (Control siRNA-A, sc-37007) were all obtained from
Santa Cruz Biotech. N2Asw-APP cells were reverse transfected with siRNA (100 nM) using Lipofectamine® 2000 Transfection Reagent (Invitrogen, Carslab, CA) according to the manufacturer’s
instruction and as previously described12,26. MICE AND TREATMENTS All animal procedures were approved by the Animal Care and Usage Committee of Temple University, in accordance with the U.S.
National Institutes of Health guidelines. The 3xTg mice, 3xTg/H5LO mice over-expressing 5LO (3xTg/H5LO), 3xTg mice genetically deficient for 5LO (3xTg/5LOKO), the 3xTg mice receiving
zileuton and their wild type control mice were described previously5,17,18. At sacrifice, brains were removed and dissected in two hemihalves by mid-sagittal dissection. One half was
immediately stored at 80 oC for biochemistry assays, the other immediately fixed for immunofluorescence studies. QUANTITATIVE ANALYSIS OF AΒ PEPTIDES Levels of Aβ 1–40 and 1–42 in
supernatants were assayed by a sensitive sandwich ELISA kit (WAKO Chem., Richmond, VA), as previously described17,18. IMMUNOBLOT ANALYSIS Proteins were extracted, sonicated, centrifuged at
13,000 rpm for 45 min at 4 oC and supernatants used for immunoblot analysis, as previously described17,18. Actin was always used as an internal loading control. Primary antibodies used were
as follows: anti GSAP full length (1:200) and GSAP-16 kDa (1:150) (Thermo Scientific); anti-5LO (1:500) (BD Bioscience); anti-12-15LO (1:200) (Santa Cruz Biotech., Dallas, TX); anti-TMP21
(1:200), anti-CD147(1:200), anti-caspase-3 (1:200) (Santa Cruz Biotech., Dallas, TX); anti-β-actin (1:200) (Santa Cruz Biotech., Dallas, TX). IRDye infrared secondary antibodies were from
LI-COR Bioscience (Lincoln, NE). CO-IMMUNOPRECIPITATION STUDIES Cells were grown to 85–90% confluence and then lysed in 50 mM HEPES, 150 mM NaCl, 5 mM MgCl2, 5 mM CaCl2, 1% CHAPSO containing
a protease inhibitor mixture. Prior to immunoprecipitation, cell lysates were diluted in lysis buffer lacking CHAPSO to give 0.25% final CHAPSO concentration. Cell lysates were incubated
for 3 hr at room temperature with 5 μg of anti-GSAP antibody. DynabeadsM-280 sheep anti-rabbit IgG (50 μl; Invitrogen, Carslab, CA) were added and samples were incubated overnight at 4 °C. A
control incubation of cell lysates with Dynabeads alone was also conducted. Dynabeads were collected and washed 5 times with lysis buffer containing 0.25% CHAPSO. Bound proteins were eluted
with SDS sample buffer containing reducing agent and subject to Western blot analysis as described in the previous paragraph. IMMUNOFLUORESCENCE MICROSCOPY Immunofluorescence studies were
performed as previously described12. Briefly, cells were placed on glass coverslips and the following day fixed in 4% paraformaldehyde in PBS for 15 min at 22 °C. For the brain tissues,
sections were deparaffinized, hydrated and subsequently with 3% H2O2 in methanol and then retrieved antigen with citrate (10 mM). After five rinses with PBS, cells or sections were incubated
in a blocking solution (5% normal serum/0.4% TX-100) for 1 h at 22 °C and then with the primary antibody separately against GSAP, 5LO, caspase-3 overnight at 4 °C. After washing with PBS,
samples were incubated for 1 h with a secondary Texas Red or Alexa Fluor 488- conjugated antibody (Invitrogen, Carslab, CA). Coverslips were mounted using VECTASHIELD mounting medium (Vector
Laboratories, Burlingame, CA, USA) and analyzed with Confocal Laser Scanning Microscope Carl Zeiss LSM 710 (Carl Zeiss, Germany). Control coverslips were processed as described above except
that no primary antibody was added to the solution. CASPASE-3 ACTIVITY ASSAY Cells were rinsed with PBS once and lysed in buffer A (50 mM Tris–HCl [pH 8.0], 150 Mm sodium chloride, 1%
NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 0.02% sodium azide and freshly added protease inhibitors [100 μg/ml phenylmethysulfonyl fluoride and 1 μg/ml Aprotinin]).
Following incubation on ice for 0.5 h, the samples were centrifuged at 16,000 g at 4 °C for 15 min and the supernatant was collected. The caspase-3 activity was measured by using a
colorimetric substrate Ac-DEVDpNA and the production of pNA was monitored over 20 min by microplate reader at OD405. One unit activity was defined as the amount of the enzyme required to
cleave 1 pmol of pNA/min/mg protein. The activity of caspase-3 was also measured indirectly by cleavage of PARP, which is a recognized substrate of caspase-3. REAL-TIME QUANTITATIVE REVERSE
TRANSCRIPTION-PCR AMPLIFICATION RNA was extracted and purified using the RNeasy Mini Kit (Qiagen, Valencia, CA), as previously described4. Briefly, mouse GSAP gene was amplified by using the
corresponding primers (SA Biosciences, Valencia, CA) and β-actin was always used as an internal control gene. DATA ANALYSIS One-way ANOVA followed by the Bonferroni’s Multiple Comparison
tests and non-parametric t-tests were performed using GraphPad Prism 5.0. All data are presented as mean ± s.e.m. Significance was set at p < 0.05. ADDITIONAL INFORMATION HOW TO CITE THIS
ARTICLE: Chu, J. _et al._ Regulation of gamma-secretase activating protein by the 5Lipoxygenase: _in vitro_ and _in vivo_ evidence. _Sci. Rep._ 5, 11086; doi: 10.1038/srep11086 (2015).
REFERENCES * Gandy, S. Lifelong management of amyloid beta metabolism to prevent Alzheimer’s disease. N. Engl. J. Med. 367, 864–866 (2012). Article CAS Google Scholar * Goedert, M. &
Spillantini, M. G. A century of Alzheimer’s disease. Science 314, 777–781 (2006). Article ADS CAS Google Scholar * Firuzi, O., Zhuo, J., Chinnici, C., Wisnieski, T. & Praticò, D.
2008 5-Lipoxygenase gene disruption reduces amyloid-β pathology in a mouse model of Alzheimer’s disease. FASEB J. 22, 1169–1178 (2008). * Chu, J. & Praticò, D. 5-Lipoxygenase as an
endogenous modulator of amyloid-β formation _in vivo_. Ann. Neurol. 69, 34–46 (2011). Article CAS Google Scholar * Giannopoulos, P. F. et al. Gene Knockout of 5-Lipoxygenase Rescues
Synaptic Dysfunction and Improves Memory in the Triple-Transgenic Model of Alzheimer’s Disease. Mol Psychiatry 19, 511–518 (2014). Article CAS Google Scholar * Wolfe, M. S. Inhibition and
modulation of γ-secretase for Alzheimer’s disease. Neurotherapeutics 5, 391–398 (2008). Article CAS Google Scholar * Netzer, W. J. et al. Gleevec inhibits β-amyloid production but not
Nocth cleavage. Proc. Natl. Acad. Sci. USA 100, 12444–12449 (2003). Article ADS CAS Google Scholar * He, G. et al. Gamma-secretase activating protein, a therapeutic target for
Alzheimer’s disease. Nature 467, 95–98 (2010). Article ADS CAS Google Scholar * Deatherage, C. L., Hadziselimovic, A. & Sanders, C. R. Purification and characterization of human
γ-secretase activating protein. Biochemistry 51, 5153–5159 (2012). Article CAS Google Scholar * Satoh, J., Tabunoki, H., Ishida, T., Saito, Y. & Arima, K. Immunohistochemical
characterization of γ-secretase activating protein expression in Alzheimer’s disease brain. Neuropathol. App. Neurobiol. 38, 132–140 (2012). Article CAS Google Scholar * Chu, J.,
Lauretti, E., Craige, C. P. & Praticò, D. Pharmacological modulation of GSAP reduces amyloid-β levels and tau phosphorylation in a mouse model of Alzheimer’s disease with plaques and
tangles. J. Alzheimers Dis. 41, 729–737 (2014). Article CAS Google Scholar * Chu, J. et al. Gamma secretase-activating protein is a substrate for caspase-3: implications for Alzheimer’s
disease. Biol. Psychiatry 77, 720–728 (2015). Article CAS Google Scholar * Chen, F. et al. TMP21 is a presenilin complex component that modulates gamma-secretase but not epsilon-secretase
activity. Nature 440, 1208–1212 (2006). Article ADS CAS Google Scholar * Zhou, S. & Zhou, H., Walian PJ, Jap BK. Regulation of gamma-secretase activity in Alzheimer’s disease.
Biochemistry 46, 2553–2563 (2007). Article CAS Google Scholar * Riccioni, G., DiIlio, C., Conti, P., Theoharides, T. C. & D’Orazio, N. Advances in therapy with anti-leukotriene drugs.
Ann. Clin. Lab. Sci. 34, 379–387 (2004). CAS PubMed Google Scholar * Stepanichev, M. Y. et al. Central administration of a caspase inhibitor impairs shuttle-box performance in rats.
Neuroscience 136, 579–591 (2005). Article CAS Google Scholar * Chu, J., Giannopoulos, P. F., Ceballos-Diaz, C., Golde, T. E. & Praticò, D. 5-Lipoxygenase gene transfer worsens memory,
amyloid and tau brain pathologies in a mouse model of AD. Ann. Neurol. 72, 442–454 (2012). Article CAS Google Scholar * Chu, J., Li, J. G. & Praticò, D. Zileuton improves memory
deficits, amyloid and tau pathology in a mouse model of Alzheimer’s disease with plaques and tangles. PLoS ONE 8, 1–8 (2013). Google Scholar * Ikonomovic, M. D., Abrahamson, E. E., Uz, T.,
Manev, H. & Dekosky, S. T. Increased 5-lipoxygenase immunorteactivity in hippocampus of patients with Alzheimer’s disease. J. Histochem. Cytochem. 56, 1065–1073 (2008). Article CAS
Google Scholar * Giannopoulos, P. F., Joshi, Y. B. & Praticò, D. Novel lipid signaling pathways in Alzheimer’s disease pathogenesis. Biochem. Pharmacol. 88, 560–564 (2014). Article CAS
Google Scholar * Zhu, M. et al. A common GSAP promoter variant contributes to Alzheimer’s disease liability. Neurobiol Aging. 35, 2656.e1-7 (2014). Article Google Scholar * Weintraub,
M. K. et al. Imatinib methanesulfonate reduces hippocampal amyloid-β and restores cognitive function following repeated endotoxin exposure. Brain Behav. Immun. 33, 24–28 (2013). Article CAS
Google Scholar * Martınez-Clemente, M. et al. 5-Lipoxygenase deficiency reduces hepatic inflammation and tumor necrosis factor α–induced hepatocyte damage in hyperlipidemia-prone
ApoE-null mice. Hepathology 51, 817–827 (2010). Article Google Scholar * Wang, Z. J., Zhou, B., Mao, W. W. & Yin, M. Overexpression of 5-lipoxygenase increases the neuronal
vulnerability of PC12 cells to Aβ42 . Yakugaku Zasshi 131,1843–1853 (2011). Article CAS Google Scholar * Shi, S. S. et al. 5-Lipoxygenase inhibitor zileuton inhibits neuronal apoptosis
following focal cerebral ischemia. Inflammation 36, 1209–1217 (2013). Article CAS Google Scholar * Chu, J., Giannopoulos, P. F., Ceballos-Diaz, C., Golde, T. E. & Praticò, D.
Adeno-associated virus-mediated brain delivery of 5-Lipoxygenase modulates the AD-like phenotype of APP mice. Mol. Neurodegen. 7, 1 (2012). Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS This work was supported by grants from the Alzheimer Art Quilt Initiative (to DP) and the National Institute of Health, AG33568 (to DP), HL086699, HL119306 and GM10982 (to
MM). The authors also acknowledge the assistance of Mr. Phillip F. Giannopoulos in providing some of the brain tissues. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Pharmacology, Center for Translational Medicine Temple University School of Medicine, Philadelphia, PA, USA Jin Chu, Jian-Guo Li & Domenico Praticò * Department of Biochemistry, Center
for Translational Medicine Temple University School of Medicine, Philadelphia, PA, USA Nicholas E. Hoffman, Alexandra M. Stough & Muniswamy Madesh Authors * Jin Chu View author
publications You can also search for this author inPubMed Google Scholar * Jian-Guo Li View author publications You can also search for this author inPubMed Google Scholar * Nicholas E.
Hoffman View author publications You can also search for this author inPubMed Google Scholar * Alexandra M. Stough View author publications You can also search for this author inPubMed
Google Scholar * Muniswamy Madesh View author publications You can also search for this author inPubMed Google Scholar * Domenico Praticò View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS J.C. and D.P. designed experiments; J.C. performed major _in vivo_ and _in vitro_ biochemistry experiments, including quantitative analysis
of Aβ peptides, immunoblot analysis, caspase-3 activity assay, as well as real-time PCR; J.-G.L. performed co-immunoprecipitation studies; J.C., N.-E. H. and A.M.S. performed confocal
microscopy and analysis; M.M. provided constructive suggestions; J.C. and D.P. wrote the paper. All the authors read and approved the final version of the paper. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing financial interests. RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included
under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chu, J., Li, JG., Hoffman, N. _et al._ Regulation of gamma-secretase activating
protein by the 5Lipoxygenase: _in vitro_ and _in vivo_ evidence. _Sci Rep_ 5, 11086 (2015). https://doi.org/10.1038/srep11086 Download citation * Received: 26 February 2015 * Accepted: 14
May 2015 * Published: 16 June 2015 * DOI: https://doi.org/10.1038/srep11086 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative








