- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Building of hierarchical core-shell hetero-structures is currently the subject of intensive research in the electrochemical field owing to its potential for making improved
electrodes for high-performance micro-supercapacitors. Here we report a novel architecture design of hierarchical MnO2@silicon nanowires (MnO2@SiNWs) hetero-structures directly supported
onto silicon wafer coupled with Li-ion doped 1-Methyl-1-propylpyrrolidinium bis(trifluromethylsulfonyl)imide (PMPyrrBTA) ionic liquids as electrolyte for micro-supercapacitors. A unique 3D
mesoporous MnO2@SiNWs in Li-ion doped IL electrolyte can be cycled reversibly across a voltage of 2.2 V and exhibits a high areal capacitance of 13 mFcm−2. The high conductivity of the SiNWs
arrays combined with the large surface area of ultrathin MnO2 nanoflakes are responsible for the remarkable performance of these MnO2@SiNWs hetero-structures which exhibit high energy
density and excellent cycling stability. This combination of hybrid electrode and hybrid electrolyte opens up a novel avenue to design electrode materials for high-performance
micro-supercapacitors. SIMILAR CONTENT BEING VIEWED BY OTHERS FABRICATION OF BINARY METAL–ORGANIC FRAMEWORKS OF NI–MN@ZIFS(COX·ZN1−XO) DECORATED ON CF/CUO NANOWIRE FOR HIGH-PERFORMANCE
ELECTROCHEMICAL PSEUDOCAPACITORS Article Open access 12 June 2024 HIERARCHICAL FE2O3 HEXAGONAL NANOPLATELETS ANCHORED ON SNO2 NANOFIBERS FOR HIGH-PERFORMANCE ASYMMETRIC SUPERCAPACITOR DEVICE
Article Open access 02 September 2022 INCREMENTAL SUBSTITUTION OF NI WITH MN IN NIFE2O4 TO LARGELY ENHANCE ITS SUPERCAPACITANCE PROPERTIES Article Open access 02 July 2020 INTRODUCTION
Micro-supercapacitors are miniaturized electrochemical energy storage devices, recently developed, which can offer power densities several orders of magnitude larger than those of
conventional batteries and supercapacitors due to their short ion diffusion lengths1,2,3,4. Remarkably, such microdevices can be directly integrated into other miniaturized electronic
devices such as sensors-actuators or energy-harvesting microsystems providing excellent nano-/micro-scale peak power5,6,7. Recently, great efforts have been devoted to increase the energy
and power densities of micro-supercapacitors via the fabrication of nanostructured electroactive materials such as carbide-derived carbon2, carbon onions3 and the development of thin-film
manufacture technologies for example electrochemical polymerization1, inkjet printing8 and layer-by-layer assembly9. In spite of such great advancements, the development of high performance
micro-supercapacitors is still a challenge. The last few years have witnessed a burst of reports on the use of silicon nanowires (SiNW) as electrode materials for micro-supercapacitors due
to their fascinating capacitive properties. These include plain silicon nanowires (SiNWs)10, as well as doped SiNWs11,12, silicon carbide nanowires13, porous silicon coated with gold14,15.
Moreover, in order to improve the capacitive properties of SiNWs, the development of core-shell nanostructures has been intensively investigated very recently, including NiO/SiNWs16,17,
poly(3,4-ethylenedioxythiophene) (PEDOT)/SiNW18 etc. However, due to the intrinsically poor electrical conductivity of metal oxides and the short diffusion distance of electrolytes into
pseudocapacitor electrodes, only the surface of electroactive materials can effectively contribute to the total capacitance while the large portion of material underneath the surface could
hardly participate in the electrochemical charge storage process, leading to values of areal specific capacitance (ASC) lower than expected. Therefore, it is still a great challenge to boost
the electrochemical utilization and ASC of pseudocapacitive materials by rationally designing electrodes with novel microstructures. An emerging attractive concept is to directly grow smart
integrated array architectures with the combination of two types of materials and/or nanostructures on conducting substrates as binder-free electrodes for micro-supercapacitors. In this
way, many advantages such as multiple accessible electroactive sites, short ion transport pathways, superior electron collection efficiency and even fascinating synergetic properties are
simultaneously achieved to deliver high ASC, sustained cycle life and rate performance. The overall performance of a supercapacitor depends not only on the electrode materials employed but
also on the electrolytes used. Ionic liquids are more expensive than aqueous electrolytes but their relatively superior properties such as high thermal stability, large potential window etc.
makes them more promising in supercapacitors. MnO2 films with common ionic liquid (IL) electrolyte-based supercapacitors have been investigated with an electrochemical quartz-crystal
microbalance (EQCM), X-ray photoemission spectroscopy (XPS)19 and _in situ_ X-ray absorption spectroscopy (XAS)20. It is reported that the most common cations, such as
n-butyl-n-methylpyrrolidinium+, 1-ethyl-3-methylimidazolium+ and 1-butyl-3-methyl-imidazolium+, adsorb only onto the electrode surface of the MnO2 films and do not penetrate into tunnels
within the [MnO6] octahedral framework. Thus, a low percentage of Mn in the structure undergoes redox processes with ionic liquid (IL) electrolytes, indicating that ion insertion is
correspondingly low. Thus, novel ionic liquid with appropriate cations that enable compensation of the redox reaction during charge and discharge cycles are crucial to improve the capacity
performance of MnO2 films. Based on the above considerations, we have fabricated and patented [ref] a unique design of hierarchical MnO2@SiNWs core-shell hetero-structure coupled with a
novel Li-ion doped ionic liquid as electrolyte, which is based on LiClO4 and 1-Methyl-1-propylpyrrolidinium bis(trifluromethylsulfonyl)imide (PMPyrrBTA) for high-performance
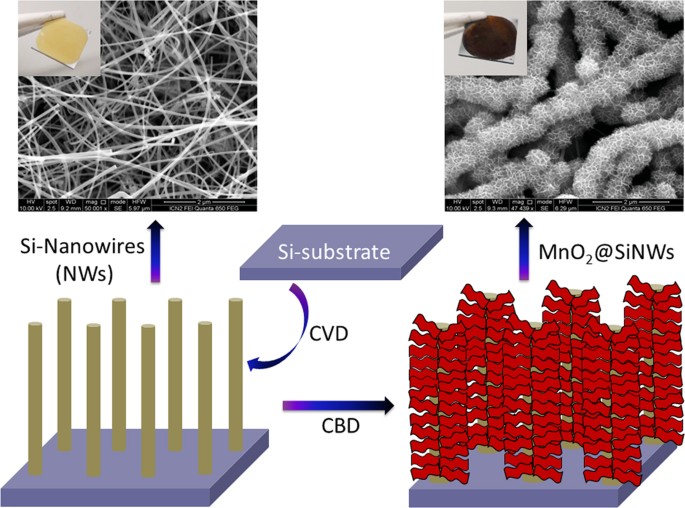
micro-supercapacitors. In this case the slim SiNWs are the “core” and ultrathin MnO2 nanoflakes the “shell” layer. Initially, SiNWs were grown on silicon wafer by chemical vapor deposition
(CVD) technique on which subsequent deposition of ultrathin MnO2 nanoflakes using chemical bath deposition (CBD) method was carried out. Figure 1 shows a schematic illustration of steps
involved in the fabrication of MnO2@SiNWs core-shell hetero-structure along with SEM and digital photographs. This MnO2@SiNWs device can be cycled reversibly at a high operating voltage of
2.2 V with good capacitance, energy density and excellent cycling stability in a LiClO4-PMPyrrBTA IL electrolyte. RESULTS Figure 2 presents SEM images of the MnO2@SiNWs hetero-structures
prepared for different deposition times at two different magnifications. From Fig. 2a,b, one can see that surfaces of SiNWs nanowires are partly covered by MnO2 nanoflakes after just 5
minutes of reaction. Yet, many of the nanowires remain uncoated, which indicates an insufficient deposition time. As the reaction time increased to 10 min, almost the whole surfaces of Si
nanowires are homogenously covered by ultrathin MnO2 nanoflakes (Fig. 2c,d). Further increase in reaction time (15 min) results in SiNWs surfaces covered by highly mesoporous MnO2 nanoflakes
(Fig. 2e,f), indicative of a sufficiently long reaction time with KMnO4. On closer inspection, the individual hierarchical MnO2@SiNWs hetero-structure is determined to have a much larger
diameter (Fig. 2f), than the pristine Si nanowires. Finally, when the reaction time is 20 min, the resulting hetero-structure is extra thick but less porous and begins to show signs of
damage. Indeed, high magnification images show some cracks on the surfaces, not observed in the structures grown during shorter times and therefore most likely due to the over-loading of
MnO2 on SiNWs (Fig. 2g,h). Figure 3a,b shows TEM images of the MnO2@SiNWs core-shell hetero-structure prepared in 15 min time. The surfaces of Si nanowires are uniformly covered by ultrathin
nanoflakes (Fig. 3b). The surface of the nanoflake is highly transparent, suggesting very small thickness (~2–5 nm). Further analysis of the SAED pattern (inset of Fig. 3b) taken from the
nanoflake edge reveals the formation of birnessite-type polycrystalline MnO221,22. From the HRTEM image (Fig. 3c), one can clearly see the lattice fringes with an interplanar spacing of 0.69
nm for the two curling nanosheets, which is identified as the characteristic interplanar spacing of the (001) plane of birnessite-type MnO2. The proposed growth mechanism for MnO2@SiNWs
core-shell hetero-structure is as follows: Initially, MnO–4 nuclei are produced and adsorbed on surfaces of SiNWs and form MnO2 nuclei upon reduction. With the increase in reaction time, the
MnO2 nuclei grow and are aggregated and transformed to nanoflakes since thermodynamically, surface energy of individual nanoflakes is high hence they start to self-aggregate (supporting
information S1). At the end, the MnO2 nanoflake is compact and totally covers the surface of Si nanowires, resulting in the formation of the hierarchical MnO2@SiNWs core-shell
hetero-structure. Such process is supported by the morphology evolution at different growth stages via tuning the reaction time. In order to determine the crystal phases present in the
MnO2@SiNWs hetero-structures, X-ray diffraction (XRD) analyses were carried out, as shown in Fig. 3d, where XRD patterns of MnO2 grown SiNWs at different deposition times are presented. The
diffraction peaks can be indexed as (001), (002), (−111) and (020) corresponding to the birnessite manganese dioxide phase (JCPDS card no. 80–1098, space group of _C_2/m) and confirming the
expected formation of MnO2. Figure 3e,f shows XPS spectra of the MnO2@SiNWs, which are calibrated with reference to C1s peak at 285 eV (supporting information S3). The Mn2p XPS spectrum
exhibits two major peaks at binding energies of 642.2 and 654 eV with a spin-energy separation of 11.8 eV (Fig. 3e), in agreement with other reports on MnO2 phases23. As reported
previously24, the average oxidation state of Mn in manganese oxides can be determined by the energy separation of Mn3s peaks. The MnO2@SiNWs hybrid structures exhibit an energy separation of
4.85 eV for the Mn3s doublet (Fig. 3f), indicating that Mn in the hetero-structure has an oxidation state of Mn(IV). To further investigate the surface properties of hierarchical MnO2@SiNWs
core-shell hetero-structures, we performed Brunnauer-Emmett-Teller (BET) analysis on adsorption isotherms shown in Fig. 4(a). The MnO2@SiNWs hybrid structure shows a typical IV- type
isotherm with hysteresis loop in a relative pressure (p/p0) range of 0.4–1.0, implying the formation of slit-like pores, a type of porosity which can be easily understood as a result of the
stacking of MnO2 flakes. The BET surface area of the MnO2@SiNWs core-shell hetero-structure is calculated to be 142 m2g−1 which is much higher than plate-like (23–43 m2g−1) or comparable to
nanorods (100–150 m2g−1), hollow spheres (52–108 m2g−1) and urchin-like (80–119 m2g−1) MnO2 structures25. Figure 4b shows the Barett-Joyner-Halenda (BJH) pore size distribution curve with a
distinct maximum centered at ~3.5 nm. This confirms the mesoporous nature of MnO2@SiNWs hybrid structure. The mesoporosity of MnO2@SiNWs samples results from a combination of internal space
of the agglomerated nanoflakes and surface rugosity of the individual nanoflakes. Such type of hierarchical surface morphologies with high surface area and mesoporous nature can enhance
electrochemical properties since large pore channels permit rapid electrolyte transport, while the small pores provide more active sites for chemical reactions26,27. To evaluate the
electrochemical performance of the MnO2@SiNWs hetero-structure, two-electrode configuration was used in the electrochemical measurements. Figure 5(a) presents the CV curves of MnO2@SiNWs
electrodes in three different electrolytes, namely i) LiClO4/propylene carbonate, ii) 1-Methyl-1-propylpyrrolidinium bis(trifluromethylsulfonyl)imide (PMPyrrBTA) and iii) LiClO4 doped ionic
liquid electrolyte (LiClO4-PMPyrrBTA). Although there are no distinct redox peaks, the shape of CV curve in LiClO4/PC electrolyte deviates from the ideal rectangle, implying that the
electrode shows faradaic pseudocapacitive nature. However, in PMPyrrBTA and even more clearly so in Li+-doped PMPyrrBTA, the shape of the CV curve is nearly rectangular, indicating that the
MnO2@SiNWs electrode has satisfactory capacitive behavior in these electrolytes. Furthermore, it is interesting to note that the MnO2@SiNWs electrode in Li+-doped PMPyrrBTA electrolyte has a
substantially larger CV area than in pure PMPyrrBTA. The rectangular CV response, which reflects the pseudocapacitive behavior, was attributed to a continuous and reversible faradaic
reaction of the Mn-oxide. Thus, the addition of Li salt in ionic liquid electrolyte significantly increases the electrochemical performance of the MnO2@SiNWs electrodes. In order to
investigate the relationship between the deposition time of MnO2 and the performances of the devices, we have varied MnO2 deposition time from 0 to 20 min. CV curves corresponding to the
different MnO2 deposition times were measured and are illustrated in Fig. 5(b). All the CV curves were measured at a scan rate of 100 mV/s but show obvious differences. From CV curves, it is
seen that, the areal capacitance (related to the area under the CV curves) increases proportionally to deposition time up to 15 min, then gets stabilized at ca. 5.2 mFcm−2 as deposition
time reaches 20 min Fig. 5(c). We should recall that the microstructural analysis by electron microscopy indicated that the mesoporous structure of MnO2 coating on SiNWs started to damage
for deposition times longer than 15 min (see SEM micrographs in Fig. 2). Hence, we can conclude that both from a microstructural and an electrochemical point of view, 15 min is an optimal
deposition time for MnO2. Next, in order to highlight the merits of this unique hybrid architecture, we tested the hierarchical MnO2@SiNWs nanowires as electrodes in symmetrical
supercapacitors (two-electrode configuration). For reference, CV curves of MnO2 and SiNWs have been tested and in Li-ion doped ionic liquid electrolyte (LiClO4-PMPyrrBTA) and shown in
supporting information S4. Figure 5(d–e) shows the cyclic voltammograms (CVs) of MnO2@SiNWs at different scan rates from 0.01 Vs−1 to 10 Vs−1 suggesting that the MnO2@SiNWs devices can be
operated over a wide range of scan rates. Moreover, the CV profiles of the MnO2@SiNWs electrodes show the rectangular shape characteristic of capacitive energy storage. This shape remains
unchanged as the scan rate increases from 0.01 Vs−1 to 10 Vs−1, demonstrating good capacitive properties and high-rate capability. Furthermore, the area integrated within the
current-potential curves greatly increases for the core-shell arrays as compared with bare SiNWs. This represents a much larger capacity for the hybrid nanowires, which must be attributed to
the additional pseudocapacitance provided by the superficial intercalation of Li+ions into the thin MnO2 flakes that form the nanowires shell. Also, the high scan rate that MnO2@SiNWs can
achieve (10 Vs−1) implies an ultrahigh power density for these unique core-shell hetero-structures. Figure 5(f) shows the variation of areal and specific capacitance with scan rate for
MnO2@SiNWs electrodes. The highest areal capacitance obtained for the MnO2@SiNWs electrode was 13.38 mFcm−2 (51.46 Fg−1, for mass loading of 0.26 mgcm−2) at 0.01 Vs−1, which is much higher
than the values obtained for the pristine SiNWs (ranging from 0.01–0.05 mFcm−2) 11,12,13,14,15 and SiNWs based nanocomposites (for details see supporting information S5). The high areal
capacitances we report here are also superior to those found for any of other recently reported hybrid nanostructures. For example MnO2/onion like carbon (MnO2/OLC) (7.04 mFcm−2 at 0.02
mAcm−2)28, MWCNT/MnO2 (2.43 mFcm−2 at 0.5 mA)29, CNT/MnO2 (3.01 mFcm−2 at 0.002 mA)30, or conducting polymer (PEDOT) coated SiNWs (9 mFcm−2 at 0.1 mAcm−2)18. Moreover, MnO2@SiNWs electrodes
exhibit a good rate capability with capacity retention of 34.90% of the initial capacitance as the scan rate increases from 0.01 to 0.1 Vs−1. Furthermore, it should be remarked that the
MnO2@SiNWs hybrid symmetric micro-supercapacitors reported here also show better performance in terms of specific capacitance (13.9 mFcm−2 at 0.01 Vs−1) than interdigitated on-chip
micro-supercapacitors based on carbide derived carbon films (e.g. SC: 1.5 mFcm−2 at 0.1 Vs−1)31, or onion-like carbon based micro-supercapacitor electrodes prepared by electrophoretic
deposition (e.g. SC: 1.1 mFcm−2 at 0.2 Vs−1)32. To further investigate electrochemical performances of the MnO2@SiNWs symmetric device, we carried out galvanostatic charge-discharge cycles
at various current densities (Fig. 6(a)). The charging and discharging parts of the curves are not perfectly linear which indicates a contribution from the pseudocapacitive mechanism
associated to surface intercalation of Li+onto MnO2. Additionally, a very small _iR_ drop (where _i_ and _R_ represent the current and resistance) for the MnO2@SiNWs electrode was observed.
This small ohmic drop can be the result of a series of low-resistance connections provided by the solid connection between the silicon substrate and Si nanowires, between the nanowires and
MnO2 thin sheets as well as the improved ionic conductivity resulting from the addition of a small amount of LiClO4 to the PMPyrrBTA-ionic liquid. The areal, volume and specific capacitances
of the MnO2@SiNWs symmetric device were derived from the discharging curves measured at different current densities and are plotted in Fig. 6(b,c). Remarkably, the MnO2@SiNWs device
exhibits very high areal capacitance with values up to 13.92 mFcm−2 (51 Fg−1, 0.26 Fcm−3) at a current density of 0.4 mAcm−2. These exceptionally good capacitance values can be attributed to
the highly porous structure and high specific surface area which facilitate ion transfer and thus enhance redox faradaic reactions and surface adsorption of electrolyte cations. Figure 6(d)
compares the areal power and energy densities of the MnO2@SiNWs device reported in this work to the values reported for other supercapacitors. The as-fabricated MnO2@SiNWs symmetric device
features a maximum areal energy density of 9.1 μWhcm−2 at a current density of 0.4 mAcm−2, which stays in values of 4.02 μWhcm−2 (0.07 mWhcm−3) at 1 mAcm−2, again confirming the excellent
rate performance of the MnO2@SiNWs hybrid device as shown in Fig. 6(d). Moreover, the obtained maximum volumetric energy density (0.17 mWhcm−3) is comparable to carbon/MnO2 (0.22 mWhcm−3 at
0.02 Acm−3)33 whereas the areal energy density is considerably higher than SiNWs and carbon based materials34. For example, CNT/OMC (1.77 μWhcm−2, 0.08 mAcm−2)35, graphene (0.17 μWhcm−2,
0.017 mAcm−2)36, plastic wire/ZnO nanowires on gold films (0.027 microWcm−2, 2 microampere)37, pen ink (2.7 μWhcm−2 37, 0.083 mAcm−2)38, CNT and Ti fibers (0.15 μWhcm−2, 0.25 μA)39,
PANI/stainless steel (0.95 μWhcm−2, 0.32 mAcm−2)40. Moreover, Fig. 6(e) shows volumetric power density versus volumetric energy density for the MnO2@SiNWs sample plotted and compared with
other energy storage devices such as electrolytic capacitors and carbon onion micro-supercapacitors41. It is seen that MnO2@SiNWs electrode demonstrates relatively higher energy density than
conventional capacitors and higher power density than carbon onion micro-supercapacitors. This observation is quite promising in the context of utilizing MnO2@SiNWs samples for fabricating
electrodes in supercapacitor devices. The electrochemical stability of MnO2@SiNWs hybrid device was examined by repeated charge-discharge processes at 1 mAcm−2. Figure 6(f), shows the
evolution of areal capacitance and capacity retention for 5000 cycles. The areal capacitance decreases from 5.9 to 5.31 mFcm−2 after 5000 cycles. The overall capacitance loss for MnO2@SiNWs
device is about 9.1% (90.9% stability) after 5000 cycles. Thus, the unique 3D hierarchical hybrid electrode shows high electrochemical stability for long cycle life applications at high
current densities. DISCUSSION As described above, hierarchical ultrathin MnO2 nanoflakes can be controllably grown on SiNWs in order to fabricate MnO2@SiNWs core-shell hybrid electrodes by a
simple solution method followed by a thermal annealing treatment. We would like to discuss here the various reasons why this smartly designed core-shell hetero-structure offers multiple
noticeable advantages over previous materials used for micro-supercapacitors applications. For example, (1) well wrapped ultrathin MnO2 nanoflakes on SiNWs enable a fast, reversible faradaic
reaction and provide a short ion diffusion path. (2) Moreover, a unique 3D mesoporous structure of MnO2 on SiNWs provides a large-area contact for the electrode and electrolyte and enables
accommodation of the large volume change and release of the associated strain generated during rapid charge and discharge cycling. (3) Electrically conducting slim SiNWs directly grown on Si
wafer serve both as the backbone and electron superhighway for charge storage and delivery. (4) MnO2@SiNWs core-shell hetero-structure are strongly supported on Si wafer, avoiding the use
of polymer binder/conductive additives and ensuring a sufficiently porous structure and consequently the “inactive” surface is significantly reduced. (5) Last but not least, The Li+doped
ionic liquid used here offers additional advantages. Thus, the ionic liquid electrolytes provide a high operating potential of the electrode of 2.2 V whereas LiClO4 as the primary ionic
working species is reversibly inserted into and out of lattice tunnels between the [MnO6] octahedral subunits and cause a large amount of a large amount of the manganese oxide to take part
in surface redox reactions. To conclude, we have developed a facile and cost-effective method to grow hierarchical ultrathin MnO2 nanoflakes on SiNWs in order to fabricate MnO2@SiNWs hybrid
nanocomposite electrodes and demonstrate improved electrochemical performance with Li-ion doped PMPyrrBTA ionic liquid for micro-supercapacitors. By taking advantage of the hybridization of
MnO2 ultrathin nanoflakes and silicon nanowires (SiNWs), we demonstrate that the device fabricated by the MnO2@SiNWs electrodes can be cycled reversibly at a high operating voltage of 2.2 V
and exhibits highest areal capacitance of 13 mFcm−2. The maximum energy density of 9.1 μWhcm−2 (0.17 mWhcm−3) and maximum power density of 388 μWcm−2 (16 mWcm−3) obtained from symmetrical
MnO2@SiNWs devices with a LiClO4-PMPyrrBTA IL electrolyte constitute record-breaking values compared with areal energy and power densities reported in the literature for other
micro-supercapacitors. Moreover, it exhibits excellent cycling performance with 91% retention after 5000 cycles. This exciting capacitive behavior is attributed to the unique hierarchical
MnO2@SiNWs core-shell hybrid structure coupled with Li ion doped IL liquid. This novel double-hybrid approach (with hybridization at the electrode and the electrolyte) has led to the recent
filing of a patent43 and suddenly adds a novel practical route for the elegant design of high-performance micro-supercapacitors. METHOD FABRICATION OF SINWS ON SILICON WAFER Silicon
nanowires were fabricated by following the procedure reported elsewhere42. SiNWs electrodes with a length of approximately 50 μm and a diameter of 50 nm were grown in a CVD reactor
(EasyTube3000 First Nano, a Division of CVD Equipment Corporation) by using the vapor-liquid-solid (VLS) method via gold catalysis on highly doped n-Si (111) substrate. Gold colloids with
size of 50 nm were used as catalysts, H2 as carrier gas, silane (SiH4) as silicon precursor, phosphine (PH3) as n-doping gas and HCl as additive gas. The use of HCl has been proven to reduce
the gold surface migration and improve the morphology of SiNWs. Prior to the growth, wafer surface was cleaned by successive dipping in acetone, isopropanol and Caro (H2SO4-H2O2, 3:1 v/v)
solutions in order to remove organic impurities, after that, the substrates were dipped in HF 10% and NH4F solution to remove the native oxide layer. Finally, the gold catalyst was deposited
on the surface. The deposition was carried out using HF 10% from an aqueous gold colloid solution. The growth was performed at 600 °C, under 6 Torr total pressure, with 40 sccm (standard
cubic centimeters) of SiH4, 100 sccm of PH3 gas (0.2% PH3 in H2), 100 sccm of HCl gas and 700 sccm of H2 as supporting gas. The doping level (dl) of the SiNWs was managed by the pressure
ratio: dopant gas/SiH4, which was evaluated in previous works (dl: 4 × 1019 cm−3). GROWTH OF ULTRATHIN MNO2 NANOFLAKES ON SINWS Growth of ultrathin MnO2 nanoflakes on SiNWs was carried out
by a simple chemical bath deposition (CBD) method. Briefly, 2 millimoles KMnO4 was dissolved in 50 ml of deionized water and then 2 ml of hydrochloric acid (98 wt%) was slowly dropped into
the above solution. The solution was transparent and free from any precipitate. Then, silicon wafer with pre-deposited SiNWs was immersed in the bath at a temperature of 323 K. After a few
minutes, the solution became blurred and a brown precipitate was formed in the bath. During the precipitation an heterogeneous reaction occurred and the deposition of MnO2 took place on
SiNWs. In order to get uniform coating of MnO2, different time intervals such as 5, 10, 15 and 20 min were tested. Finally, MnO2@SiNWs substrates were removed, rinsed and dried in vacuum at
373 K for 2 h. CHARACTERIZATION TECHNIQUES The surface morphology was studied by scanning electron microscopy (FEI Quanta 650 F Environmental SEM). TEM images were obtained with a field
emission gun transmission electron microscope (Tecnai G2 F20 S-TWIN HR(S) TEM, FEI). Crystallographic study was carried out using Panalytical X’pert Pro-MRD instrument (Cu Kα radiation and
PIXel detector). The X-ray photoelectron spectra (XPS) data were obtained by X-ray photoelectron spectroscopy (XPS, SPECS Germany, PHOIBOS 150). N2 adsorption/desorption was determined by
Brunauer-Emmett-Teller (BET) measurements using Micromeritics instrument (Data Master V4.00Q, Serial#:2000/2400). Electrochemical characterization of MnO2@SiNW hybrid electrodes were carried
out in 2-electrode configuration with Biologic VMP3 potentiostat. All samples were measured in the typical two-electrode coin cells with MnO2@SiNW hybrid (1 cm × 1 cm) used as both the
cathode and anode electrodes. The two electrodes were sandwiched by a PVDF separator and assembled into a coin cell. The material’s mass loading on the sponge is obtained by measuring the
weight difference before and after MnO2 deposition by using a microbalance. The electrolytes used in this study include 0.1 M of LiClO4/propylene carbonate, 1-Methyl-1-propylpyrrolidinium
bis(trifluromethylsulfonyl)imide (PMPyrrBTA) (purchased from IOLITEC (Ionic Liquids Technologies GmbH, Germany) and 0.01 M LiClO4 doped ionic liquid electrolyte (LiClO4-PMPyrrBTA). All cells
were assembled and sealed in an Argon-filled glove box. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Dubal, D. P. _et al._ 3D hierarchical assembly of ultrathin MnO2 nanoflakes on
silicon nanowires for high performance micro-supercapacitors in Li- doped ionic liquid. _Sci. Rep._ 5, 9771; doi: 10.1038/srep09771 (2015). REFERENCES * Sung, J. H., Kim, S. J., Jeong, S.
H., Kim, E. H. & Lee, K. H. Flexible microsupercapacitors. J. Power Sources 162, 1467–1470 (2006). ADS CAS Google Scholar * Chmiola, J., Largeot, C., Taberna, P. L., Simon, P. &
Gogotsi, Y. Monolithic carbide-derived carbon films for micro-supercapacitors. Science 328, 480–483 (2010). ADS CAS PubMed Google Scholar * Pech, D. et al. Ultrahigh-power
micrometer-sized supercapacitors based on onion-like carbon. Nat. Nanotech. 5, 651–654 (2010). ADS CAS Google Scholar * Gao, W. et al. Direct laser writing of micro-supercapacitors on
hydrated graphite oxide films. Nat. Nanotech. 6, 496–500 (2011). ADS CAS Google Scholar * Adriaanse, C. Microsupercapacitors plugging electronics into T-shirts. Chem. Ind. 11–11 (2011). *
Rolison, D. R. et al. Multifunctional 3D nanoarchitectures for energy storage and conversion. Chem. Soc. Rev. 38, 226–252 (2009). CAS PubMed Google Scholar * Beidaghi, M. & Wang, C.
L. Recent advances in design and fabrication of on-chip micro-supercapacitors. Proc. SPIE 8377, 837708 (2012). Google Scholar * Pech, D. et al. Elaboration of a microstructured
inkjet-printed carbon electrochemical capacitor. J. Power Sources 195, 1266–1269 (2010). ADS CAS Google Scholar * Beidaghi, M. & Wang, C. L. Micro-supercapacitors based on
interdigital electrodes of reduced graphene oxide and carbon nanotube composites with ultra-high power handling performance. Adv. Funct. Mater. 22, 4501–4510 (2012). CAS Google Scholar *
Aradilla, D. et al. High performance of symmetric micro-supercapacitors based on silicon nanowires using N-methyl-N-propylpyrrolidinium bis (trifluoromethylsulfonyl)imide as electrolyte.
Nano Energy, 9, 273–281 (2014). CAS Google Scholar * Thissandier, F., Pauc, N., Brousse, T ., Gentile, P. & Sadki, S. Micro-ultracapacitors with highly doped silicon nanowires
electrodes. Nanoscale Res. Lett. 8, 38 (2013). ADS PubMed PubMed Central Google Scholar * Berton, N. et al. Wide-voltage-window silicon nanowire electrodes for micro-supercapacitors via
electrochemical surface oxidation in ionic liquid electrolyte. Electrochem. Commun., 41, 31–34 (2014). CAS Google Scholar * Alper, J. P., Vincent, M., Carraro, C. & Maboudian, R.
Silicon carbide coated silicon nanowires as robust electrode material for aqueous micro-supercapacitor. Appl. Phys. Lett., 100, 163901 (2012). ADS Google Scholar * Rowlands, S. E. &
Latham, R. J. Supercapacitor devices using porous silicon electrodes. Ionics, 5, 144–149 (1999). CAS Google Scholar * Desplobain, S., Gautier, G., Semai, J., Ventura, L. & Roy, M.
Investigations on porous silicon as electrode material in electrochemical capacitor. Phys. Stat. Sol. (C), 4, 2180–2184 (2007). CAS Google Scholar * Tao, B., Zhang, J., Miao, F., Hui, S.
& Wan, L. Preparation and electrochemistry of NiO/SiNW nanocomposite electrodes for electrochemical capacitors. Electrochim. Acta, 55, 5258–5262 (2010). CAS Google Scholar * Lu, F. et
al. Electrochemical properties of high power supercapacitors using ordered NiO coated Si nanowire arrays electrodes, Appl. Phys. A, 104, 545–550 (2011). ADS CAS Google Scholar * Aradilla,
D. et al. Novel hybrid micro-supercapacitor based on conducting polymer coated silicon nanowires for electrochemical energy storage. RSC Adv., 4, 26462–26467 (2014). CAS Google Scholar *
Chang, J. K., Lee, M. T., Tsai, W. T., Deng, M. J. & Sun, I. W. X-ray photoelectron spectroscopy and _in situ_ X-ray absorption spectroscopy studies on reversible insertion/desertion of
dicyanamide anions into/from manganese oxide in ionic liquid. Chem. Mater., 21, 2688–2695 (2009). CAS Google Scholar * Lee, M. T. et al. Pseudocapacitance of MnO2 originates from
reversible insertion/desertion of thiocyanate anions studied using _in situ_ X-ray absorption spectroscopy in ionic liquid electrolyte. J. Power Sources, 195, 919–922 (2010). ADS CAS
Google Scholar * Liu, J. et al. Co3O4 nanowire@MnO2 ultrathin nanosheet core/shell arrays: a new class of high-performance pseudocapacitive materials. Adv. Mater., 23, 2076–2081 (2011). CAS
PubMed Google Scholar * Zhou, J. et al. Novel synthesis of birnessite-type MnO2 nanostructure for water treatment and electrochemical capacitor. Ind. Eng. Chem. Res. 52, 9586–9593
(2013). CAS Google Scholar * Xia, H. et al. Hierarchically structured Co3O4@Pt@MnO2 nanowire arrays for high-performance supercapacitors. Sci. Rep. 3, 2978 (2013). PubMed PubMed Central
Google Scholar * Huang, M. et al. Merging of Kirkendall growth and Ostwald ripening: CuO@MnO2 core-shell architectures for asymmetric supercapacitors. Sci. Rep. 4, 4518 (2014). PubMed
PubMed Central Google Scholar * Wei, W., Cui, X., Chen, W. & Ivey, D. G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev., 40, 1697–1721
(2011). CAS PubMed Google Scholar * Dubal, D. P., Kim, J. G., Kim, Y., Holze, R. & Kim, W. B. Demonstrating the highest supercapacitive performance of branched MnO2 nanorods grown
directly on flexible substrates using controlled chemistry at ambient temperature. Energy Technol. 1, 125–130 (2013). CAS Google Scholar * Dubal, D. P., Gund, G. S., Holze, R. &
Lokhande, C. D. Mild chemical strategy to grow micro-roses and micro-woolen like arranged CuO nanosheets for high performance supercapacitors. J. Power Sources, 242, 687–698 (2013). ADS CAS
Google Scholar * Wang, Y. et al. Printed all-solid flexible microsupercapacitors: towards the general route for high energy storage devices. Nanotechnol. 25, 094010 (2014). ADS Google
Scholar * Li, L., Chen, C., Xie, J., Shao, Z. & Yang, F. The preparation of carbon nanotube/MnO2 composite fiber and its application to flexible micro-supercapacitor. J. Nanomaters .
2013, 1–5 (2013). Google Scholar * Ren, J. et al. Twisting carbon nanotube fibers for both wire-shaped micro-supercapacitor and micro-battery. Adv. Mater., 25, 1155–1159 (2013). CAS PubMed
Google Scholar * Huang, P. et al. Micro-supercapacitors from carbide derived carbon (CDC) films on silicon chips. J. Power Sources, 225, 240–244 (2013) ADS CAS Google Scholar * Huang,
P. et al. On-chip micro-supercapacitors for operation in a wide temperature range. Electrochem. Commun., 36, 53–56 (2013). CAS Google Scholar * Xiao, X. et al. Fiber-based all-solid-state
flexible supercapacitors for self-powered systems. ACS Nano, 6, 9200–9206 (2012). CAS PubMed Google Scholar * Le, V. T. et al. Coaxial fiber supercapacitor using all-carbon material
electrodes. ACS Nano, 7, 5940–5947 (2013). CAS PubMed Google Scholar * Ren, J., Bai, W., Guan, G., Zhang, Y. & Peng, H. Flexible and weaveable capacitor wire based on a carbon
nanocomposite fiber. Adv. Mater., 25, 5965–5970 (2013). CAS PubMed Google Scholar * Meng, Y. et al. All-graphene core-sheath microfibers for all-solid-state stretchable fibriform
supercapacitors and wearable electronic textiles. Adv. Mater., 25, 2326–2331 (2013). CAS PubMed Google Scholar * Bae, J. et al. Fiber supercapacitors made of nanowire-fiber hybrid
structures for wearable/flexible energy storage, Angew. Chem. Int. Ed., 50, 1683–1687 (2011). CAS Google Scholar * Fu, Y. et al. Fiber supercapacitors utilizing pen ink for
flexible/wearable energy storage. Adv. Mater., 24, 5713–5718 (2012). CAS PubMed Google Scholar * Chen, T. et al. An integrated ‘energy wire’ for both photoelectric conversion and energy
storage. Angew. Chem. Int. Ed., 41, 11977–11980 (2012). ADS Google Scholar * Fu, Y. et al. Integrated power fiber for energy conversion and storage. Energy Environ. Sci., 6, 805–812
(2013). CAS Google Scholar * Xiong, G., Meng, C., Reifenberger, R. G., Irazoqui, P. P. & Fisher, T. S. A review of graphene-based electrochemical microsupercapacitors. Electroanalysis,
26, 30–51 (2014). CAS Google Scholar * Gentile, P. et al. Effect of HCl on the doping and shape control of silicon nanowires. Nanotechnology, 23, 215702 (2012). ADS CAS PubMed Google
Scholar * Dubal, D. P. et al. European Patent EU15158311 March 9, 2015. Electrode Material Comprising Silicon Nanowires Covered By Mesoporous Oxide Nanostructured Coating And Ionic Liguid
Electrolytes For Energy Storage Applications. Download references ACKNOWLEDGEMENTS The authors acknowledge the financial support from the European Commission’s Seventh Framework Program for
Research, Technological Development and Demonstration under Grant agreement no.309143 (NEST, 2012-2015). ICN2 acknowledges support of the Spanish MINECO through the Severo Ochoa Centers of
Excellence Program under Grant SEV-2013-0295. Authors appreciate the award to DPD of a Marie-Curie Fellowship through Beatriu de Pinos Program (BP-DGR-2013) for Catalan system of science and
technology, Spain. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Catalan Institute of Nanoscience and Nanotechnology, CIN2, ICN2 (CSIC-ICN), Campus UAB, Bellaterra, E-08193, Barcelona
Deepak P. Dubal & Pedro Gomez-Romero * Univ. Grenoble Alpes, INAC-SPRAM, F-38000, Grenoble, France David Aradilla & Saïd Sadki * CNRS, SPRAM, F-38000, Grenoble, France David Aradilla
& Saïd Sadki * CEA, INAC-SPRAM, F-38000 Grenoble, France David Aradilla & Saïd Sadki * Univ. Grenoble Alpes, INAC-DIR, F-38000 Grenoble, France, CEA, INAC-DIR F-38000, Grenoble,
France Gérard Bidan * Univ. Grenoble Alpes, INAC-SP2M-SiNaPs, F-38000 Grenoble, France Pascal Gentile * CEA, INAC-SP2M-SiNaPS, F-38000 Grenoble, France Pascal Gentile * IOLITEC Ionic Liquids
Technologies GmbH, Salzstrasse 184, 74076, Heilbronn, Germany Thomas J.S. Schubert & Jan Wimberg * Consejo Superior de Investigaciones Científicas (CSIC), Spain Pedro Gomez-Romero
Authors * Deepak P. Dubal View author publications You can also search for this author inPubMed Google Scholar * David Aradilla View author publications You can also search for this author
inPubMed Google Scholar * Gérard Bidan View author publications You can also search for this author inPubMed Google Scholar * Pascal Gentile View author publications You can also search for
this author inPubMed Google Scholar * Thomas J.S. Schubert View author publications You can also search for this author inPubMed Google Scholar * Jan Wimberg View author publications You can
also search for this author inPubMed Google Scholar * Saïd Sadki View author publications You can also search for this author inPubMed Google Scholar * Pedro Gomez-Romero View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS D.P.D. and P.G.R. designed the experiments, analyzed the data and wrote the manuscript. D.A., G.B.,
P.G. and S.S. carried out synthesis and characterization of silicon nanowires. T.J.S.S. and J.W. provide the ionic liquids. D.P.D. and P.G.R. designed and carried out synthesis and
electrochemical measurements of hybrid thin films. To the preparation and reviewing manuscript, all authors contributed equally. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare
no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0
International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the
material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Dubal, D., Aradilla, D., Bidan, G. _et al._ 3D hierarchical assembly of ultrathin
MnO2 nanoflakes on silicon nanowires for high performance micro-supercapacitors in Li- doped ionic liquid. _Sci Rep_ 5, 9771 (2015). https://doi.org/10.1038/srep09771 Download citation *
Received: 03 December 2014 * Accepted: 16 March 2015 * Published: 18 May 2015 * DOI: https://doi.org/10.1038/srep09771 SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative






