- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Toll-like receptors (TLRs), as innate immunity sensors, play critical roles in immune responses. Six SNPs of _TLR3_, _TLR7_ and _TLR8_ were genotyped to determine their associations
with systemic lupus erythematosus (SLE) and clinical manifestations of SLE. TLR7 SNP rs3853839 was independently associated with SLE susceptibility in females (G _vs._ C: _p_ = 0.0051).
TLR7 rs3853839-G (G _vs._ C: _p_ = 0.0100) and TLR8 rs3764880-G (recessive model: _p_ = 0.0173; additive model: _p_ = 0.0161) were associated with pericardial effusion in females relative to
healthy females. Anti-SSA positive cases were more likely to have the dominant TLR7 rs179010-T allele than normal controls (_p_ = 0.0435). TLR3 rs3775296-T was associated with
photosensitivity (_p_ = 0.0020) and anemia (_p_ = 0.0082). The “G-G” haplotype of TLR7 rs3853839 and TLR8 rs3764880 increased risk of SLE in females (age adjusted _p_ = 0.0032). These
findings suggest that TLR variations that modify gene expression affect risk for SLE susceptibility, clinical phenotype development and production of autoantibodies. SIMILAR CONTENT BEING
VIEWED BY OTHERS GENETIC VARIANTS IN UNC93B1 PREDISPOSE TO CHILDHOOD-ONSET SYSTEMIC LUPUS ERYTHEMATOSUS Article Open access 03 June 2024 AN ESSENTIAL ROLE FOR TASL IN MOUSE AUTOIMMUNE
PATHOGENESIS AND TOLL-LIKE RECEPTOR SIGNALING Article Open access 24 January 2025 SYSTEMIC LUPUS ERYTHEMATOSUS GENETICS: INSIGHTS INTO PATHOGENESIS AND IMPLICATIONS FOR THERAPY Article 04
September 2024 INTRODUCTION System lupus erythematosus (SLE) is a systemic autoimmune disease characterized by widespread loss of immune tolerance to self-antigens. Genetic and environmental
factors contribute to the development of SLE and patients typically experience alternating periods of flare-up and remission1. The generation of numerous autoantibodies that react with
self-nuclear and -cytoplasmic antigens is associated with the dysfunction of multiple organ systems2,3,4,5. The genetic transmission and patterns of inheritance of SLE have not yet been
elucidated. In particular, it has been difficult to identify specific genetic polymorphisms associated with SLE due to the presence of polygenic inheritance, extensive genetic heterogeneity,
the small size of most genetic studies and the low disease prevalence6,7. Genome-wide association studies (GWASs) of various populations have identified several common immune response
pathways and the presence of genetic variants in some ethnic groups that are involved in the pathogenesis of SLE8,9,10,11,12,13. However, genetic dissection of SLE and other autoimmune
diseases is difficult because these are complex diseases that involve alterations of multiple biologic pathways14. The advent of modern genomics and the availability of new technologies have
made it possible to fine-map candidate genes for SLE and other specific diseases based on knowledge of gene map position and functional relevance15,16,17,18. Such studies may help to
identify the roles of candidate genes for SLE and the roles of different genes in the expression of different clinical manifestations4,19,20. Previous studies have indicated that innate
pattern recognition receptors, such as Toll-like receptors (TLRs), play important roles in the development of autoimmunity. TLR proteins are localized on the cell surface or in endosomes and
play critical roles in innate immune responses against different pathogens21,22. Internalized nucleic acid immune complexes act as endogenous ligands that activate intracellular TLRs and
these initiate several signaling pathways that lead to increased production of type I interferons (IFNs) in plasmacytoid dendritic cells (pDCs)22,23,24,25,26. Increased production of type I
IFNs increases apoptosis, neutrophil cell death _via_ neutrophil extracellular trap (NETosis), innate immune signaling and viral infection-induced autoimmunity27,28,29. Aberrant stimulation
of the innate immune system through intracellular TLRs may lead to hyperactive immune responses and contribute to the pathogenesis of SLE30,31,32. TLR3, TLR7 and TLR8 are primarily
associated with endosomal membranes and they recognize microbial nucleic acids. TLR3 binds to viral double-stranded RNA (dsRNA) and induces antiviral immune responses by promoting the
production of type I IFN and pro-inflammatory cytokines. TLR7 binds to single-stranded RNA (ssRNA) from RNA viruses and triggers pDCs to produce type I IFN. TLR8 is phylogenetically related
to TLR7 and also mediates recognition of viral ssRNA. Thus, these 3 TLRs are responsible for pathogen clearance, antigen recognition and induction of cytokine production30,33,34. Genetic and
hormonal factors appear to partially explain the female predominance of SLE35. In particular, TLR7 and TLR8 are on the X chromosome (Xp22.2) and play critical roles in innate immunity and
inflammatory responses30. Studies of animal models indicated that TLR7 gene dosage significantly affected the hyperactivity of B cells36,37,38,39,40,41. Mice with a Y-linked autoimmune
accelerator (Yaa) that carries an extra copy of TLR7 develop autoimmunity to RNA-associated autoantigens, but mice with a low TLR7 copy number require additional susceptibility loci to
develop autoimmunity39,40. In humans, males with Klinefelter syndrome carry an extra X chromosome (47, XXY) and are more likely to develop SLE; females with Turner syndrome lack one X
chromosome (45, X) and are less likely to develop SLE42,43. In addition, a study of a Mexican population indicated that increased TLR7 copy number correlated with TLR7 mRNA levels and
susceptibility to childhood-onset SLE44 although TLR7 copy number variations (CNVs) are infrequent in human SLE45. Other studies indicated that increased expression of TLRs in peripheral
blood mononuclear cells and lymphocytes led increased IFN-α expression in SLE patients46,47,48. Previous research indicated that a functional TLR7 SNP (rs3853839-G > C) affects TLR7
expression by modulation of microRNA-3148 (miR-3148)49 and that two other intron SNPs (rs5935436 and rs179010) were associated with increased SLE susceptibility45,49. The TLR8 SNP rs3764880
is a functional polymorphism that affects TLR8 transcription and translations of TLR8 isoforms, which leads to the activation difference of NF-κB, is also associated with SLE
susceptibility45,49,50,51,52. A study of cell cultures indicated that TLR3 rs3775296 (in the promoter) and rs3775291 (in exon 4) affected TLR3 cell surface expression and localization and
subsequently influenced NF-κB cascade induction although the effect of these mutations has not been assessed in clinical studies53. The present study examined the associations of the above
mentioned 6 SNPs (3 in TLR7, 1 in TLR8, 2 in TLR3) with SLE and with specific clinical manifestations of SLE. RESULTS We investigated the role of 6 SNPs in the susceptibility to SLE
(rs3775296 and rs3775291 from TLR3; rs5935436, rs179010 and rs3853839 from TLR7; and rs3764880 from TLR8) by examination of 795 SLE patients (68 males and 727 females) and 1162 healthy
controls (513 males and 649 females). The average age of cases was 30.71 years (SD = 11.62, range: 8 to 77 years), 8.55% were males (31.46 ± 12.52 years-old) and 91.45% were females (30.64 ±
11.56 years-old). The healthy controls had a mean age of 40.24 years (SD = 10.88, range 18–64 years), with similar average ages of males and females (40.26 ± 9.26 years _vs._ 40.23 ± 12.02
years). The age difference of SLE patients and healthy controls was statistically significant for both males and females, so we adjusted for age in the subsequent association analysis. Table
1 shows the clinical characteristics of the 795 SLE patients, with independent statistical analyses for males and females. In the female cases and controls, there were no deviations from
HWE in the six candidate SNPs. Table 2 shows the single-locus associations between the six candidate SNPs and susceptibility to SLE. In males, there were no significant case-control
associations after the false discovery rate (FDR) correction, possibly due to the small sample size. In females, there was a significant allelic association between SLE and rs3853839 in TLR7
(PFDR = 0.013, OR = 1.38, 95% CI = 1.12–1.69). Moreover, the risk allele G had a recessive effect in females (GG _vs._ GC + CC: PFDR = 0.017, OR = 1.44, 95% CI = 1.14–1.83), suggesting that
rs3853839 plays a role in development of SLE. We also evaluated the independent contributions of the 6 candidate SNPs (adjusted for age) to SLE risk in females and performed a multivariate
logistic regression analysis that included the significant SNPs with the same genetic models. The rs3853839 SNP was the only significant susceptibility marker among the 6 examined SNPs (G
_vs._ C: _p_ = 0.0051, OR = 1.42, 95% CI = 1.11–1.82). TLR SNP POLYMORPHISMS AFFECTED SLE PHENOTYPE AND PRODUCTION OF AUTOANTIBODIES Patients with SLE are present with heterogeneous clinical
features and have significant variations in the severity, nature and spectrum of clinical involvement. Thus, we examined the effect of TLR SNP polymorphisms on SLE clinical parameters and
phenotypes. Based on the clinical characteristics of males and females (Table 1), we initially performed two comparisons: _(i)_ allele frequencies of SLE patients with each characteristic
(“+” in Tables S1–S3) and SLE patients without the characteristic (“−” in Tables S1–S3); and _(ii)_ allele frequencies of SLE patients with each characteristic and the normal controls
(“normal” in Tables S1–S3). In males, there were no significant associations of the 6 candidate SNPs with SLE clinical manifestations after the FDR correction (Table 3, with more details in
Tables S1 and S3). Again, this may result from the small sample size. In females, TLR7 rs3853839 G risk allele was associated with several clinical manifestations of SLE (Table 4). The
comparison of phenotype-positive cases and normal controls indicated significant associations of this allele with oral ulcer, arthritis, malar rash, photosensitivity, pericardial effusion,
depressed complement, anti-dsDNA, anti-Sm and anti-SSA (PFDR < 0.05 for all comparisons). Multivariate logistic regression analysis of the independent association of each clinical
characteristic with rs3853839 indicated that only pericardial effusion was significantly associated with the TLR7 rs3853839 G risk allele. In particular, cases with pericardial effusion were
more likely to have the G risk allele than normal controls (_p_ = 0.0100, OR = 2.82, 95% CI = 1.28–6.19). Table S2 shows that anti-SSA was associated with the rs179010 T risk allele (T
_vs._ C: PFDR = 0.0366, OR = 1.47, 95% CI = 1.11–1.94; CT + TT _vs._ CC: PFDR = 0.0037, OR = 1.93, 95% CI = 1.33–2.81). Multivariate logistic regression analysis also showed that cases who
were anti-SSA positive were more likely to carry the dominant rs179010 T risk allele than normal controls (_p_ = 0.0435, OR = 1.33, 95% CI = 1.01–1.75), but cases who were anti-SSA negative
had the opposite tendency (_p_ = 0.0299, OR = 0.69, 95% CI = 0.49–0.96). Although rs3764880 in TLR 8 was not associated with SLE susceptibility, the risk allele G was associated with oral
ulcer with significant additive effects (PFDR = 0.0232: OR = 1.66, 95% CI = 1.18–2.35, G _vs._ A: PFDR = 0.0300, OR = 1.65, 95% CI = 1.17–2.32). However, incorporation of clinical variables
with nominal _p_ values below 0.05 into the multivariate logistic regression model indicated that pericardial effusion was the only clinical characteristic associated with rs3764880. In
particular, pericardial effusion-positive cases were more likely to carry the rs3764880 G risk allele than normal controls, either with a recessive effect (_p_ = 0.0173, OR = 2.91, 95% CI =
1.21–7.02) or with an additive effect (_p_ = 0.0161, OR = 2.96, 95% CI = 1.22–7.14). Analysis of the association of TLR3 SNPs with SLE clinical characteristics indicated no significant
associations for males (Tables 3 and S3). In females, comparison of phenotype-positive cases with phenotype-negative cases and of phenotype-positive cases with normal controls indicated
positive associations of rs3775296 with anemia (Table 4, TT _vs._ GG + GT: PFDR = 0.0244, OR = 2.41, 95% CI = 1.33–4.39; TT _vs._ GG + GT: PFDR = 0.0373, OR = 2.11, 95% CI = 1.23–3.65,
respectively). Multivariate logistic regression analysis indicated that photosensitivity-negative cases and anemia-negative cases were associated with recessive rs3775296 T risk allele
relative to normal controls (photosensitivity: _p_ = 0.0020, OR = 2.40, 95% CI = 1.38–4.19; anemia: _p_ = 0.0082, OR = 0.44, 95% CI = 0.24–0.81). TLR HAPLOTYPES WERE ASSOCIATED WITH SLE
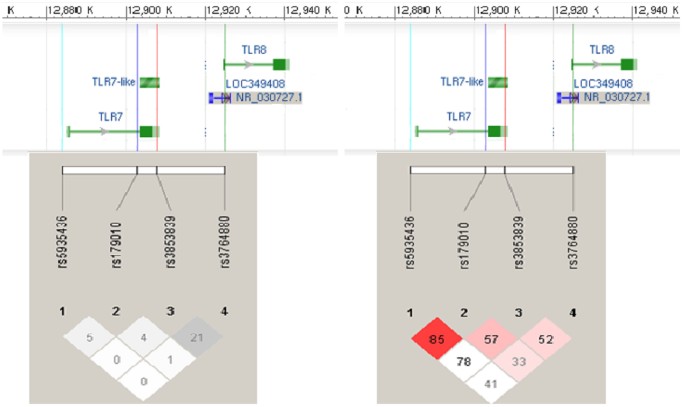
SUSCEPTIBILITY IN FEMALES In Figure 1, pair-wise LD measures r2 and D′ for the four TLR7 and TLR8 SNPs rs3853839, rs5935436, rs179010 and rs3764880 in the female healthy controls were
presented. We estimated the frequencies of haplotypes formed by these 4 SNPs in females to determine the associations of different haplotypes with susceptibility to SLE. Table 5 shows the 4
inferred haplotypes that had estimated frequencies more than 5%. Performance of haplotype-trait association tests in female SLE patients and healthy controls indicated that the “C-C-C-G”
haplotype protected against development of SLE (5.78% in cases _vs._ 8.55% in controls; permutation _p_-value = 0.0033, OR = 0.60, 95% CI = 0.44–0.82 after age adjustment). In Table 6 shows
the estimates for haplotypes from rs3853839 and rs3764880 SNPs only. These results show that the effect of “C-G” on SLE susceptibility remained significant when we controlled for age (7.08%
in cases _vs._ 8.39% in controls; permutation _p_-value = 0.0073, OR = 0.62, 95% CI = 0.46–0.83). Nevertheless, females with “G-G” haplotypes of rs3853839 and rs3764880 were significantly
more likely to develop SLE (age adjusted _p_-value = 0.0032, OR = 1.32, 95% CI = 1.10–1.60). DISCUSSION Previous research with large samples indicated that the functional TLR7 SNP
rs3853839-G > C was significantly associated with SLE in East Asians, especially in males45. The current study identified the rs3853839-G allele of TLR7 as the main susceptibility marker
in female SLE patients from Taiwan based on single-locus and multivariate logistic regression analyses. This confirms the role of this SNP in the pathogenesis of SLE. The genetic effect of
TLR7 on SLE appears to vary among different ethnic groups45,54,55,56. In particular, a previous study indicated that the TLR7 intronic SNPs rs179019 and rs179010 were associated with SLE,
independent of the 3′ UTR SNP rs3853839 in Japanese females54. Other research indicated that the TLR7 SNP rs179008 was not associated with SLE in a European population, but was significantly
associated with SLE in Brazilians55,56. Recently, Deng et al. conducted a large trans-ancestral fine-mapping of European Americans, African Americans and Amerindian/Hispanics and identified
rs3853839-G as the only genetic risk variant for SLE in the TLR7-TLR8 region, although they did not confirm the male specific association. Notably, rs3853839-G appears to increase risk for
SLE in different populations, although there are different frequencies in different ethnic groups. This highlights the critical role of elevated TLR7 expression in the pathogenesis of SLE,
which in this case is mediated by slower mRNA degradation due to miR-3148 expression49. TLR7 and TLR8 contribute to antigen recognition and antibody production in the pathogenesis of SLE.
TLR ligands, which are present in viruses and virus-like particles (VLPs), can directly stimulate B cells and B cell responses are integrated with dual antigen-specific B cell receptor (BCR)
and TLR engagement57,58. B cells with up-regulated MYD88 expression become more responsive to TLR ligands and promote class-switch recombination59. The inactivation or overexpression of
genes that encode TLRs or of molecules that alter TLR signaling provides a bridge between the innate and adaptive immune systems and this is critical to the presence of B cells defects in
the pathogenesis of SLE60,61. Moreover, the recognition of endogenous RNA-containing antigens by TLR7/3 may trigger autoreactive B cells in the germinal center and this is accompanied by the
suppression of T regulatory cells, leading to disruption of self-tolerance62,63,64. In this regard, numerous innate and adaptive immunity related genes involving IFN-alpha mediated
signature pathway as well as T and B cells activation signaling pathways participate in the SLE pathogenesis65. The present study found that TLR7 rs3853839 G risk allele was associated with
several clinical manifestations of SLE, including oral ulcer, arthritis, malar rash, photosensitivity, pericardial effusion, depressed complement and anti-dsDNA, anti-Sm and anti-SSA
autoantibodies. The TLR7 rs179010 T risk allele was also associated with anti-SSA autoantibodies. These findings suggest that TLR7 may play a key role in autoantibody production because it
increases B-cell sensitivity to RNA-containing autoantigens in the development of systemic autoimmunity. However, given the limited sample sizes of our stratified phenotype groups, this
finding requires replication by larger future studies. We also observed that the non-synonymous TLR8 SNP rs3764880-G allele was a risk factor for oral ulcer and pericardial effusion, with
significant additive effects. Interestingly, previous research indicated that the TLR8 rs3764880-G allele protected against tissue damage in active tuberculosis and predicted a slower
disease course in patients with HIV infections52,66. The _TLR8_ SNP rs3764880 alters the ATG start codon of TLR8 isoform B into a GTG. A methionine at position 4 of isoform B is used as the
start codon for the _TLR8_-rs3764880G allele, resulting in a truncated TLR8 isoform B with a shorter signal peptide (1038 residues for the _TLR8_-rs3764880G allele _vs._ 1041 residues for
the _TLR8_-rs3764880A allele). Immune cells carrying the _TLR8_-rs3764880G allele had augmented TNFα-responses, but decreased translation of truncated TLR8 isoform B and NF-κB production
relative to those carrying the _TLR8_-rs3764880A allele50,66. Therefore, TLR8 appears to have important roles in autoimmune diseases and in response to infections. Previous research
indicated that TLR7 and TLR8 have closely related functions in immune responses. We observed that the TLR7 and TLR8 SNP haplotype rs5935436-C/rs179010-C/rs3853839-C/rs3764880-G protected
against development of SLE, but the effect of rs3853839-C/rs3764880-G on SLE susceptibility remained significant when controlled for age. On the other hand, females with G-G haplotypes of
rs3853839 and rs3764880 were significantly more likely to develop SLE. These results indicate that TLR8 may play a regulatory role in TLR7 function in innate immunity, similar to that
documented in mice67. TLR3 recognizes dsRNA and its elevated level in human SLE peripheral blood cells suggests that it may play a key role in IFN signature gene activation22,46,47,48. In
addition, previous research indicated that TLR3 aggravated lupus nephritis in lupus-prone mice68. We observed that TLR3 rs3775296-T risk allele had recessive effects on photosensitivity and
anemia-negative SLE patients. Thus, our data indicate that TLR3 may play a role in the development of different SLE phenotypes and in antiviral responses that trigger expression of
pro-inflammatory genes. In conclusion, the present study confirmed that certain functional TLR7 and TLR8 variations, particularly TLR7 rs3853839-G > C, modify gene expression and increase
the risk for SLE, development of certain SLE phenotypes and production of autoantibodies. These results suggest that TLR7/8 genetic variations are potential biomarkers for prediction of SLE
phenotypes and also have implications for the development of therapeutic measures that may prevent various pathological conditions that are characteristic of SLE. METHODS CHARACTERISTICS OF
THE STUDY POPULATIONS All patients were recruited from the clinics of Chang Gung Memorial Hospital and rheumatology specialists confirmed that all patients fulfilled the 1982 and 1997
American College of Rheumatology (ACR) diagnostic criteria for SLE69. For the purpose of this study, healthy controls were selected following a questionnaire to assure that they did not have
any autoimmune disease phenotypes. This study was approved by the ethics committees of Chang Gung Memorial Hospital and all patients provided written informed consent according to the
Declaration of Helsinki. GENOMIC DNA EXTRACTION Genomic DNA was extracted from EDTA-anticoagulated peripheral blood using the Purgene DNA isolation kit as described previously70. SNPS
GENOTYPING ASSAYS Validated Applied Biosystem TaqMan SNP assays were used for genotype determination of TLRs according the vendor's instructions (Life Technologies, Grand Island, NY).
The TaqMan allele discrimination assays were performed on an ABI ViiA 7 Real-time System (Life Technology) and probes were labeled with a fluorescent dye (FAM and VIC). STATISTICAL ANALYSIS
The functional candidate gene approach was used to perform a case-control association study by examination of 795 SLE patients (68 males and 727 females) and 1162 healthy controls (513 males
and 649 females). The Hardy-Weinberg equilibrium (HWE) was examined for the 6 selected SNPs using chi-square tests in females with and without SLE. Differences of allele frequencies in each
of the 6 SNPs were separately assessed in males and females to investigate the single-locus associations. Additionally, for females, the significance of differences in genotype frequencies
were also evaluated and dominant and recessive models were tested for each SNP. The _p_-values, odds ratios (ORs) and 95% confidence intervals (CIs) were then calculated based on the risk
allele identified. TLR 8 and TLR7 are located on the X chromosome (Xp22.2), so female and male data were analyzed separately. Meta-analysis with generation of meta _p_-values using the
Cochran-Mantel-Haenzsel (CMH) method was used to assess the allelic associations of the 6 SNPs in all samples. We initially stratified clinical phenotypes according to diagnostic criteria to
investigate the association of each SNP with SLE clinical manifestations. SLE patients with the phenotype under investigation were classed as “+” cases, those without this phenotype as “−”
cases and healthy controls as “normal”. Then, allele frequencies were compared for “+” cases and “−” cases and for “+” cases and “normal” controls. In females, additional analyses also
considered additive, dominant and recessive effects for the risk allele of each SNP in order to assess genotype-phenotype associations. To investigate the independent association of SLE
clinical characteristics with the six SNPs, multivariate logistic regression analysis was subsequently carried out to identify the independent statistical association of significant
phenotypes (identified above) with the six SNPs. The additive, dominant and recessive allele effects for each SNP were modeled as the response variable and the three categories of
individuals: “+” cases, “−” cases and “Normal” pertaining to each clinical characteristic were used as the independent variables. Linkage disequilibrium (LD) patterns of neighboring SNPs on
the same chromosome were analyzed by Haploview 4.2 (Broad Institute, Cambridge, MA, USA; http://www.broad.mit.edu/mpg/haploview). Haplotype information was inferred and frequencies were
estimated using the HAPLOTYPE procedure in SAS 9.2 (SAS Institute, Cary, NC). Differences in haplotype frequencies were assessed for SLE cases and controls were separately assessed in males
and females. The permutation (N = 10,000) _p_-values of each haplotype were calculated using the expectation-maximization (EM) algorithm, conditional on the other haplotypes, to evaluate the
independent association of each category of haplotypes. Benjamini and Hochberg's linear step-up method in the SAS MULTTEST procedure was used to account for multiple testing71. The
False Discovery Rate (FDR)-adjusted _p_-values are defined in a step-up fashion, with less conservative multipliers and control. A corrected _p_-value (PFDR) less than 0.05 indicated
statistical significance. REFERENCES * Tsokos, G. C. Systemic lupus erythematosus. N Engl J Med 365, 2110–2121 (2011). Article CAS PubMed Google Scholar * Hughes, T. & Sawalha, A. H.
The role of epigenetic variation in the pathogenesis of systemic lupus erythematosus. Arthritis Res Ther 13, 245 (2011). Article CAS PubMed PubMed Central Google Scholar * Pathak, S.
& Mohan, C. Cellular and molecular pathogenesis of systemic lupus erythematosus: lessons from animal models. Arthritis Res Ther 13, 241 (2011). Article PubMed PubMed Central Google
Scholar * Liu, Z. & Davidson, A. Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat Med 18, 871–882 (2012). Article PubMed PubMed Central CAS
Google Scholar * Gualtierotti, R., Biggioggero, M., Penatti, A. E. & Meroni, P. L. Updating on the pathogenesis of systemic lupus erythematosus. Autoimmun Rev 10, 3–7 (2010). Article
CAS PubMed Google Scholar * Wakeland, E. K., Liu, K., Graham, R. R. & Behrens, T. W. Delineating the genetic basis of systemic lupus erythematosus. Immunity 15, 397–408 (2001).
Article CAS PubMed Google Scholar * Lauwerys, B. R. & Wakeland, E. K. Genetics of lupus nephritis. Lupus 14, 2–12 (2005). Article CAS PubMed Google Scholar * Remmers, E. F. et
al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N Engl J Med 357, 977–986 (2007). Article CAS PubMed PubMed Central Google Scholar * Harley, J. B. et
al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet 40, 204–210 (2008). Article
CAS PubMed PubMed Central Google Scholar * Hom, G. et al. Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. N Engl J Med 358, 900–909 (2008). Article CAS
PubMed Google Scholar * Yang, W. et al. Genome-wide association study in Asian populations identifies variants in ETS1 and WDFY4 associated with systemic lupus erythematosus. PLoS Genet 6,
e1000841 (2010). Article PubMed PubMed Central CAS Google Scholar * Han, J. W. et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci
for systemic lupus erythematosus. Nat Genet 41, 1234–1237 (2009). Article CAS PubMed Google Scholar * Deng, Y. & Tsao, B. P. Genetic susceptibility to systemic lupus erythematosus in
the genomic era. Nat Rev Rheumatol 6, 683–692 (2010). Article CAS PubMed PubMed Central Google Scholar * Cui, Y., Sheng, Y. & Zhang, X. Genetic susceptibility to SLE: Recent
progress from GWAS. J Autoimmun (2013). * Harley, I. T., Kaufman, K. M., Langefeld, C. D., Harley, J. B. & Kelly, J. A. Genetic susceptibility to SLE: new insights from fine mapping and
genome-wide association studies. Nat Rev Genet 10, 285–290 (2009). Article CAS PubMed PubMed Central Google Scholar * Lessard, C. J. et al. Identification of IRF8, TMEM39A and
IKZF3-ZPBP2 as susceptibility loci for systemic lupus erythematosus in a large-scale multiracial replication study. Am J Hum Genet 90, 648–660 (2012). Article CAS PubMed PubMed Central
Google Scholar * Gateva, V. et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet 41, 1228–1233
(2009). Article CAS PubMed PubMed Central Google Scholar * Yang, W. et al. Meta-analysis followed by replication identifies loci in or near CDKN1B, TET3, CD80, DRAM1 and ARID5B as
associated with systemic lupus erythematosus in Asians. Am J Hum Genet 92, 41–51 (2013). Article CAS PubMed PubMed Central Google Scholar * Guerra, S. G., Vyse, T. J. & Cunninghame
Graham, D. S. The genetics of lupus: a functional perspective. Arthritis Res Ther 14, 211 (2012). Article PubMed PubMed Central Google Scholar * Patel, D. R. & Richardson, B. C.
Dissecting complex epigenetic alterations in human lupus. Arthritis Res Ther 15, 201 (2013). Article PubMed PubMed Central Google Scholar * Akira, S., Uematsu, S. & Takeuchi, O.
Pathogen recognition and innate immunity. Cell 124, 783–801 (2006). Article CAS PubMed Google Scholar * Barbalat, R., Ewald, S. E., Mouchess, M. L. & Barton, G. M. Nucleic acid
recognition by the innate immune system. Annu Rev Immunol 29, 185–214 (2011). Article CAS PubMed Google Scholar * Ewald, S. E. & Barton, G. M. Nucleic acid sensing Toll-like
receptors in autoimmunity. Curr Opin Immunol 23, 3–9 (2011). Article CAS PubMed Google Scholar * Means, T. K. et al. Human lupus autoantibody-DNA complexes activate DCs through
cooperation of CD32 and TLR9. J Clin Invest 115, 407–417 (2005). Article CAS PubMed PubMed Central Google Scholar * Bave, U. et al. Fc gamma RIIa is expressed on natural
IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J Immunol 171, 3296–3302 (2003).
Article CAS PubMed Google Scholar * Baccala, R., Hoebe, K., Kono, D. H., Beutler, B. & Theofilopoulos, A. N. TLR-dependent and TLR-independent pathways of type I interferon induction
in systemic autoimmunity. Nat Med 13, 543–551 (2007). Article CAS PubMed Google Scholar * Ronnblom, L., Alm, G. V. & Eloranta, M. L. The type I interferon system in the development
of lupus. Semin Immunol 23, 113–121 (2011). Article PubMed CAS Google Scholar * Garcia-Romo, G. S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric
systemic lupus erythematosus. Sci Transl Med 3, 73ra20 (2011). Article PubMed PubMed Central Google Scholar * Elkon, K. B. & Wiedeman, A. Type I IFN system in the development and
manifestations of SLE. Curr Opin Rheumatol 24, 499–505 (2012). Article CAS PubMed Google Scholar * Bauer, S., Pigisch, S., Hangel, D., Kaufmann, A. & Hamm, S. Recognition of nucleic
acid and nucleic acid analogs by Toll-like receptors 7, 8 and 9. Immunobiology 213, 315–328 (2008). Article CAS PubMed Google Scholar * Kontaki, E. & Boumpas, D. T. Innate immunity
in systemic lupus erythematosus: sensing endogenous nucleic acids. J Autoimmun 35, 206–211 (2010). Article CAS PubMed Google Scholar * Christensen, S. R. & Shlomchik, M. J.
Regulation of lupus-related autoantibody production and clinical disease by Toll-like receptors. Semin Immunol 19, 11–23 (2007). Article CAS PubMed PubMed Central Google Scholar * Heil,
F. et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303, 1526–1529 (2004). Article CAS ADS PubMed Google Scholar * Gilliet, M., Cao,
W. & Liu, Y. J. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol 8, 594–606 (2008). Article CAS PubMed Google Scholar *
Rubtsov, A. V., Rubtsova, K., Kappler, J. W. & Marrack, P. Genetic and hormonal factors in female-biased autoimmunity. Autoimmun Rev 9, 494–498 (2010). Article CAS PubMed PubMed
Central Google Scholar * Pisitkun, P. et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 312, 1669–1672 (2006). Article CAS ADS PubMed
Google Scholar * Subramanian, S. et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A 103, 9970–9975 (2006). Article CAS ADS PubMed
PubMed Central Google Scholar * Santiago-Raber, M. L. et al. Evidence for genes in addition to Tlr7 in the Yaa translocation linked with acceleration of systemic lupus erythematosus. J
Immunol 181, 1556–1562 (2008). Article CAS PubMed Google Scholar * Hwang, S. H. et al. B cell TLR7 expression drives anti-RNA autoantibody production and exacerbates disease in systemic
lupus erythematosus-prone mice. J Immunol 189, 5786–5796 (2012). Article CAS PubMed Google Scholar * Moisini, I. et al. The Yaa locus and IFN-alpha fine-tune germinal center B cell
selection in murine systemic lupus erythematosus. J Immunol 189, 4305–4312 (2012). Article CAS PubMed Google Scholar * Walsh, E. R. et al. Dual signaling by innate and adaptive immune
receptors is required for TLR7-induced B-cell-mediated autoimmunity. Proc Natl Acad Sci U S A 109, 16276–16281 (2012). Article CAS ADS PubMed PubMed Central Google Scholar * Scofield,
R. H. et al. Klinefelter's syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum 58,
2511–2517 (2008). Article PubMed PubMed Central Google Scholar * Cooney, C. M. et al. 46,X,del(X)(q13) Turner's syndrome women with systemic lupus erythematosus in a pedigree
multiplex for SLE. Genes Immun 10, 478–481 (2009). Article CAS PubMed PubMed Central Google Scholar * Garcia-Ortiz, H. et al. Association of TLR7 copy number variation with
susceptibility to childhood-onset systemic lupus erythematosus in Mexican population. Ann Rheum Dis 69, 1861–1865 (2010). Article CAS PubMed Google Scholar * Shen, N. et al. Sex-specific
association of X-linked Toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proc Natl Acad Sci U S A 107, 15838–15843 (2010). Article CAS ADS PubMed PubMed Central
Google Scholar * Komatsuda, A. et al. Up-regulated expression of Toll-like receptors mRNAs in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clin Exp
Immunol 152, 482–487 (2008). Article CAS PubMed PubMed Central Google Scholar * Wong, C. K. et al. Activation profile of Toll-like receptors of peripheral blood lymphocytes in patients
with systemic lupus erythematosus. Clin Exp Immunol 159, 11–22 (2010). Article CAS PubMed PubMed Central Google Scholar * Midgley, A., Thorbinson, C. & Beresford, M. W. Expression
of Toll-like receptors and their detection of nuclear self-antigen leading to immune activation in JSLE. Rheumatology (Oxford) 51, 824–832 (2012). Article CAS Google Scholar * Deng, Y. et
al. MicroRNA-3148 modulates allelic expression of toll-like receptor 7 variant associated with systemic lupus erythematosus. PLoS Genet 9, e1003336 (2013). Article CAS PubMed PubMed
Central Google Scholar * Gantier, M. P. et al. Genetic modulation of TLR8 response following bacterial phagocytosis. Hum Mutat 31, 1069–1079 (2010). Article CAS PubMed Google Scholar *
Cervantes, J. L., Weinerman, B., Basole, C. & Salazar, J. C. TLR8: the forgotten relative revindicated. Cell Mol Immunol 9, 434–438 (2012). Article CAS PubMed PubMed Central Google
Scholar * Davila, S. et al. Genetic association and expression studies indicate a role of toll-like receptor 8 in pulmonary tuberculosis. PLoS Genet 4, e1000218 (2008). Article PubMed
PubMed Central CAS Google Scholar * Ranjith-Kumar, C. T. et al. Effects of single nucleotide polymorphisms on Toll-like receptor 3 activity and expression in cultured cells. The Journal
of biological chemistry 282, 17696–17705 (2007). Article CAS PubMed Google Scholar * Kawasaki, A. et al. TLR7 single-nucleotide polymorphisms in the 3′ untranslated region and intron 2
independently contribute to systemic lupus erythematosus in Japanese women: a case-control association study. Arthritis Res Ther 13, R41 (2011). Article CAS PubMed PubMed Central Google
Scholar * Sanchez, E. et al. Investigation of TLR5 and TLR7 as candidate genes for susceptibility to systemic lupus erythematosus. Clin Exp Rheumatol 27, 267–271 (2009). CAS PubMed Google
Scholar * dos Santos, B. P. et al. TLR7/8/9 polymorphisms and their associations in systemic lupus erythematosus patients from southern Brazil. Lupus 21, 302–309 (2012). Article CAS
PubMed Google Scholar * Lau, C. M. et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med 202, 1171–1177 (2005).
Article CAS PubMed PubMed Central Google Scholar * Rawlings, D. J., Schwartz, M. A., Jackson, S. W. & Meyer-Bahlburg, A. Integration of B cell responses through Toll-like receptors
and antigen receptors. Nat Rev Immunol 12, 282–294 (2012). Article CAS PubMed PubMed Central Google Scholar * Giltiay, N. V., Chappell, C. P. & Clark, E. A. B-cell selection and
the development of autoantibodies. Arthritis Res Ther 14 Suppl 4, S1 (2012). Article PubMed CAS Google Scholar * Avalos, A. M., Busconi, L. & Marshak-Rothstein, A. Regulation of
autoreactive B cell responses to endogenous TLR ligands. Autoimmunity 43, 76–83 (2010). Article CAS PubMed PubMed Central Google Scholar * Green, N. M. & Marshak-Rothstein, A.
Toll-like receptor driven B cell activation in the induction of systemic autoimmunity. Semin Immunol 23, 106–112 (2011). Article CAS PubMed PubMed Central Google Scholar * Rubtsov, A.
V. et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood 118, 1305–1315 (2011). Article CAS
PubMed PubMed Central Google Scholar * Green, N. M., Moody, K. S., Debatis, M. & Marshak-Rothstein, A. Activation of autoreactive B cells by endogenous TLR7 and TLR3 RNA ligands. J
Biol Chem 287, 39789–39799 (2012). Article CAS PubMed PubMed Central Google Scholar * Hackl, D., Loschko, J., Sparwasser, T., Reindl, W. & Krug, A. B. Activation of dendritic cells
via TLR7 reduces Foxp3 expression and suppressive function in induced Tregs. Eur J Immunol 41, 1334–1343 (2011). Article CAS PubMed Google Scholar * Bronson, P. G., Chaivorapol, C.,
Ortmann, W., Behrens, T. W. & Graham, R. R. The genetics of type I interferon in systemic lupus erythematosus. Curr Opin Immunol 24, 530–537 (2012). Article CAS PubMed Google Scholar
* Oh, D. Y. et al. A functional toll-like receptor 8 variant is associated with HIV disease restriction. J Infect Dis 198, 701–709 (2008). Article CAS PubMed Google Scholar * Demaria,
O. et al. TLR8 deficiency leads to autoimmunity in mice. J Clin Invest 120, 3651–3662 (2010). CAS PubMed PubMed Central Google Scholar * Patole, P. S. et al. Viral double-stranded RNA
aggravates lupus nephritis through Toll-like receptor 3 on glomerular mesangial cells and antigen-presenting cells. J Am Soc Nephrol 16, 1326–1338 (2005). Article CAS PubMed Google
Scholar * Hochberg, M. C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40, 1725 (1997). Article
CAS PubMed Google Scholar * Chen, J. Y. et al. Association of a transmembrane polymorphism of Fcgamma receptor IIb (FCGR2B) with systemic lupus erythematosus in Taiwanese patients.
Arthritis Rheum 54, 3908–3917 (2006). Article CAS PubMed Google Scholar * Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to
multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 289–300 (1995). Download references ACKNOWLEDGEMENTS We greatly appreciate the Shin Chu Blood Donor
Center for providing samples. This work was supported by National Science Council, Taiwan and CMRPG 391813 from Chang Gung Memorial Hospital. AUTHOR INFORMATION Author notes * Wang Chin-Man
and Chang Su-Wei contributed equally to this work. AUTHORS AND AFFILIATIONS * Department of Physical Medicine and Rehabilitation, Chang Gung Memorial Hospital, Chang Gung University, College
of Medicine, No. 5, Fu-Shin St, Kwei-Shan, Tao-Yuan, 33375, Taiwan Chin-Man Wang * Clinical Informatics and Medical Statistics Research Center, Chang Gung University, College of Medicine,
259 Wenhua 1st Road, Kwei-Shan, Tao-Yuan, 33375, Taiwan Su-Wei Chang & Tse-Chih Chou * Department of Medicine, Division of Allergy, Immunology and Rheumatology, Chang Gung Memorial
Hospital, Chang Gung University, College of Medicine, No. 5, Fu-Shin St, Kwei-Shan, Tao-Yuan, 33375, Taiwan Yeong-Jian Jan Wu, Jing-Chi Lin, Huei-Huang Ho & Ji-Yih Chen * Xin-Jhu Blood
Center, Taiwan Blood Services Foundation, No.8, Ln.215, Guang Ming 11th Rd., Jhubei City, Xin-Jhu County, 30269, Taiwan Bing Yang * Deptartment of Veterinary and Biomedical Sciences, 235B
Animal Science/Vet. Med. Bldg., University of Minnesota, 1988 Fitch Avenue, St. Paul, MN, 55108, USA Jianming Wu Authors * Chin-Man Wang View author publications You can also search for this
author inPubMed Google Scholar * Su-Wei Chang View author publications You can also search for this author inPubMed Google Scholar * Yeong-Jian Jan Wu View author publications You can also
search for this author inPubMed Google Scholar * Jing-Chi Lin View author publications You can also search for this author inPubMed Google Scholar * Huei-Huang Ho View author publications
You can also search for this author inPubMed Google Scholar * Tse-Chih Chou View author publications You can also search for this author inPubMed Google Scholar * Bing Yang View author
publications You can also search for this author inPubMed Google Scholar * Jianming Wu View author publications You can also search for this author inPubMed Google Scholar * Ji-Yih Chen View
author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS C.M.W. and J.Y.C. performed study design, manuscript preparation and coordination. S.W.C.
performed statistical analysis, data interpretation and manuscript preparation. T.C.C. participated in the statistical analysis. Y.J.J., J.C.L., H.H.H. and P.Y. participated in sample
acquisition and data interpretation. J.W. conceived of the study, participated in its design and helped to draft the manuscript. All authors reviewed the manuscript. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION Genetic variations in Toll-like receptors (TLRs 3/7/8)
are associated with systemic lupus erythematosus in a Taiwanese population RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0
Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wang, CM., Chang, SW.,
Wu, YJ. _et al._ Genetic variations in Toll-like receptors (TLRs 3/7/8) are associated with systemic lupus erythematosus in a Taiwanese population. _Sci Rep_ 4, 3792 (2014).
https://doi.org/10.1038/srep03792 Download citation * Received: 11 November 2013 * Accepted: 27 December 2013 * Published: 21 January 2014 * DOI: https://doi.org/10.1038/srep03792 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative






