- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT A modified smoothed particle hydrodynamic (MSPH) computational technique was utilized to simulate molten particle motion and infiltration speed on multi-scale analysis levels. The
radial velocity and velocity gradient of molten alumina, iron infiltration in the TiC product and solidification rate, were predicted during centrifugal self-propagating high-temperature
synthesis (SHS) simulation, which assisted the coating process by MSPH. The effects of particle size and temperature on infiltration and solidification of iron and alumina were mainly
investigated. The obtained results were validated with experimental microstructure evidence. The simulation model successfully describes the magnitude of iron and alumina diffusion in a
centrifugal thermite SHS and Ti + C hybrid reaction under centrifugal acceleration. SIMILAR CONTENT BEING VIEWED BY OTHERS CHOICE OF REACTION PROGRESS VARIABLE UNDER PREFERENTIAL DIFFUSION
EFFECTS IN TURBULENT SYNGAS COMBUSTION BASED ON DETAILED CHEMISTRY DIRECT NUMERICAL SIMULATIONS Article Open access 27 June 2024 A NOVEL SPIRAL INFINITY REACTOR FOR CONTINUOUS HYDROTHERMAL
SYNTHESIS OF NANOPARTICLES Article Open access 21 May 2022 PARTICLE FLOW NUMERICAL SIMULATION MODEL FOR COAL AND GAS OUTBURST AND ITS MICROSCOPIC OUTBURST MECHANISM DESCRIPTION Article Open
access 03 February 2024 INTRODUCTION Self-propagating high-temperature synthesis (SHS), or combustion synthesis (CS), is an advanced method of producing high-temperature materials, such as
refractory or intermetallic materials and cermets. The CS process is characterized by extreme heating rates, short reaction times and expelling impurities during reaction1,2. Ceramic-lined
steel pipes are a sort of high-performance, heat-resistant, anti-corrosion and wear-resistant pipe that can be produced by a centrifugally-assisted thermite (CT) process3. The ceramic-lined
steel pipe produced by SHS technique has occupied the market rapidly. Not only are the quality superior and the capabilities outstanding, but the price ratio is higher than of other
wearable, anti-abrasion and anti-heat pipes. Moreover, they weigh less compared to other wearable, cast alloy steel pipes4,5. In a research by Gao, et al.6, in China, W-C-Fe cermet-lined
steel pipes were produced through a SHS-centrifugal process. Finite element analysis of residual thermal stress was performed on ceramic-lined composite pipes prepared by centrifugal SHS.
The finite element method of simulating the temperature and residual thermal stress distribution of the ceramic-lined composite pipe made by centrifugal SHS was discussed.Wang and Yang7 also
employed various SHS layer thicknesses to fabricate centrifugal-SHS composite pipes with the aim of reducing residual stress. Therefore, the reliability of the ceramic-lined composite pipes
should improve. Local reinforcement of functionally graded coating produced using a centrifugal-assisted thermite process has recently come to the attention of researchers8,9,10. The
thermite reaction (Al2O3 and Fe) infiltrated the TiC pellets and created a strong, titanium aluminide intermetallic layer. There is a compelling need to simulate the mechanism of locally
reinforcing lined ceramic tubes. Several simulation techniques are applied in SHS, among which finite difference numerical method11, numerical modeling of field-activated combustion
synthesis process12, modeling the solidification of functionally graded materials by centrifugal casting10,13 and analytical modeling14,15. Initially developed in197716,17, Smoothed Particle
Hydrodynamics (SPH) is a meshless Lagrangian particle method for obtaining approximate numerical solutions of fluid dynamics equations. The SPH method was originally meant for astrophysical
applications. It gradually extended to problems concerning incompressible fluids using either weakly compressible fluid models, or algorithms designed to solve full incompressible
equations18. In this method, the fluid is replaced by a set of particles19,20. At present, SPH has a wide range of applications in numerous fields, such as heat conduction, gas explosions,
micromachining, micro-forming and granular flows that have demonstrated the ability to simulate highly non-linear free-surface flows including wave overturning, jets and the formation of
spray and droplets21,22. SPH has been vastly developed for different applications like underwater explosion20, wave propagation19, magnetohydrodynamics23, bulk deformation24 and particle
motion18. SPH has also been implemented to solve complex splashing free-surface flows and the differential motion of multiple solid-casting processes owing to its mesh-free nature. It can
additionally handle boundaries and apply fragmenting free-surface flow of solid particles25. In the SPH technique, the spatial gradients of speed and deposition rate at the boundaries are
difficult to calculate due to insufficient data. This method also employs ghost particles at the boundary, which are not real or adequate for solving approximation problems at the boundary.
Therefore, to mitigate such drawbacks, modified smoothed particle hydrodynamics (MSPH) is adopted based on the Taylor expansion as opposed to ghost particles26. Mathematically, the range of
problems that can be solved by MSPH is much broader than by mesh-based methods, since approximation is not based on elements whose distortion may degrade calculation accuracy, something
helpful in both the fluid and solid domains27. However, to the best of the authors' knowledge, there are no reports on meshless particle methods such as MSPH to simulate SHS particle
behavior, e.g., velocity, viscosity and displacement. Therefore, the purpose of this work is to implement MSPH to predict the radial velocity and velocity gradient of molten alumina, iron
infiltration in the TiC product and solidification rate during the centrifugal SHS-assisted functional coating process. The effects of particle size and temperature on the infiltration and
solidification of iron and alumina are mainly investigated. The obtained results are validated with experimental evidence of microstructure. RESULTS Temperature data obtained from the
solution of the heat balance equation was employed to calculate the fluids' (iron and alumina) viscosity, particle speed in the radial direction, gradient of speed and particles'
position. A microstructural analysis is also presented in this section. In order to verify the MSPH code, the exact solution of velocity equation (7) and values obtained from the MSPH
solution are plotted accordingly. In addition, the measured temperatures are compared with the MSPH solution results from equation (6). MICROSTRUCTURAL ANALYSIS A typical area of
functionally graded TiC-Fe-Alumina functionally graded coating, processed using centrifugally assisted combustion synthesis method, was taken for microstructural observations. A field
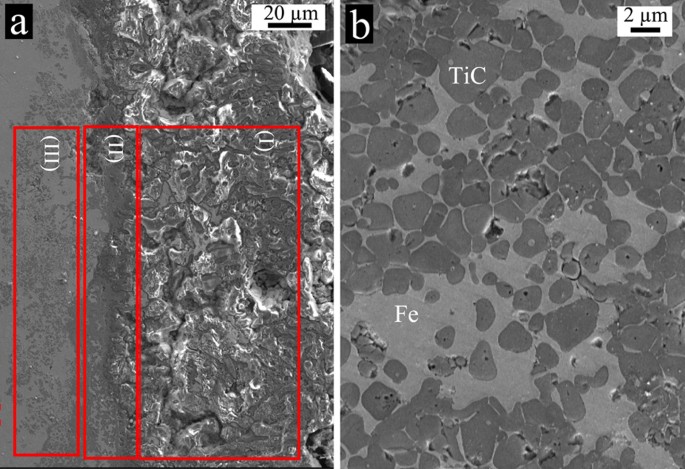
emission scanning electron microscopy (FESEM) micrograph of the typical area is shown in Figure 1. Figure 1 (a) illustrates a region next to the pellet's inner surface relative to the
rotation axis, while Figure 1 (b) depicts a higher magnification of zone (III) from Figure 1(a). Corresponding energy dispersive spectroscopy (EDS) elemental analysis of Figure 1 (a) for
zones (I), (II) and (III) is listed in Table 1. Zones (I), (II) and (III) in Figure 1 (a) are marked relative to the tube's axis of rotation. In accordance with the EDS and FESEM
results, the thickness of the alumina-rich layer comprising zones (I) and (II) is roughly 110 μm. Moreover, zone (I) is a composite-coated layer with an alumina-rich constituent. However,
based on micrograph measurements, alumina diffusion occurs around zone (II), whose thickness is around 30 μm. On the other hand, iron particles diffuse deeper into the Ti + C pellet compared
with alumina. PARTICLE VISCOSITY VERSUS TIME The time-viscosity plot of a centrifugal thermite reaction is illustrated in Figure 2 along with the corresponding temperature cooling reaction.
The time-viscosity plot is the MSPH solution of the exact calculations using Arrhenius' equation adopted in eq. (8). PARTICLE VELOCITY AND VELOCITY GRADIENT The exact particle solution
is derived based on fundamentals of fluid mechanics, which provides a closed-form solution for the velocity and position of alumina and iron as given by equations (7) and (11). The exact
solution of particle velocity and the corresponding MSPH simulation in three different particle size cases (dp, dp2 and dp3) are plotted in Figure 3. The simulation parameters are listed in
Table 2. Figure 4 shows the radial velocity of molten alumina particles for three different particle sizes as predicted by MSPH and the exact solution. Figure 5 illustrates the particle
velocity gradient in the radial direction as estimated by equation (11) based on the MSPH model. CHANGE IN PARTICLE POSITION The instantaneous change in radial particle position versus time
during the deposition process is calculated for iron and alumina particles and the results are given in Figure 6. Evidently, there is an indirect correlation with viscosity change over time.
DISCUSSION Once the thermite reaction occurred under the influence of centrifugal acceleration, the molten Al2O3-Fe thermite products infiltrated the Ti + C pellet. The infrared pyrometer
recorded the reaction temperature released up to 2830°C (Figure 2). The thermite molten products were pushed to the inner surface of the Ti + C pellet under the applied centrifugal force.
The molten semi-products were forcibly trying to penetrate the porous Ti + C media to form an in-situ TiC-Fe-Al2O3 composite. According to the temperature observation, the reaction lasted
around 2.5 seconds from ignition until Fe solidification, at which point the reaction and infiltration presumably stopped. The thermite reaction products (Fe and Al2O3) deposited according
to their densities28 at the graphite mold's innermost layer, after which they diffused into the TiC zone. The reactions created a porous TiC product. A more in-depth discussion on the
current method is given elsewhere8. In Figure 1 (a), zone (I) is alumina-rich and zones (II) and (III) are Fe-rich. Zone (I) is located at the innermost layer relative to the pipe's
axis. Alumina mainly formed a coating layer on the TiC pellet and slightly diffused into it, whereas the iron phase mostly diffused inside the TiC pellet. The composition of the phases
gradually changed with the specimen's volume. Figure 1 (b) represents the magnified micrograph corresponding to zone (III). It is clear how the molten iron diffused inside the porous
area of the compacted Ti + C pellet and formed an interesting composite. Iron particles exist in all layers, with a declining rate from the inner to the outermost layer relative to the axis
of rotation. Centrifugal acceleration significantly affected both metallurgical alloying and mechanical interlocking between different specimen layers during product formation. It is
apparent that the alumina phase did not significantly diffuse in zone (III), whereas the iron phase diffused into the TiC pellet. As revealed in Figure 2, the viscosity of molten alumina
particles exhibits higher values and abrupt changes at 0.8 and 2.4 s after the reaction started, compared to iron particles. This is because the melting point of alumina is above 2200°C and
it starts to solidify at 0.7 s, while Fe is in a molten state seeing that its melting point is 1535°C. This explains why Fe was able to penetrate deeper into TiC, as well as according to the
FESEM microstructure observation of Figure 1, zone (iii). The viscosity analysis serves to calculate the particle velocity and location at any given time. With respect to Figure 3, in the
early process stages and shortly after the reaction began, the iron particles' radial velocity slowed down rapidly as viscosity increased. It completely stopped after three seconds of
reaction process. Physically, the particle migration rate (particle velocity) becomes zero when the viscosity sharply increases during solidification or when the particles reach a boundary
that halts further movement. Under the centrifugal field where the particles were larger, the initial speed would be higher, compared with the smaller particles. The particle velocity of
MSPH prediction is in close correlation with the exact solution results from Figure 3. This validates the simulation model and permits for the application of MSPH in the SHS process. As seen
in Figure 4, the alumina particle velocity seems to be approximately half the velocity of iron particles for corresponding particle sizes. This may be attributable to the density of iron,
which is about twice that of alumina. Equation (11) is simpler than deriving and using the exact expression for velocity gradient calculations. Obviously, the velocity gradient can describe
continuous particle deceleration for particles of different sizes. The exponential deceleration of iron particles was predicted because iron was in a molten state for longer than alumina, as
observed from the temperature-time curve of the thermite reaction. With increasing viscosity (Figure 2), particle motion would naturally be affected. For instance, in Figure 5, as the
particle size increases, the velocity gradient slope is greater and the particles decelerate faster. However, this does not drastically happen for alumina, whereby the particle speed
gradient curve tends to become zero almost at the same time regardless of particle size. Nevertheless, the gradient of velocity in the radial direction for iron significantly correlates to
particle size. When the velocity gradient becomes zero at this stage, there might not be molten phase diffusion; but the solid-state diffusion phenomenon is probably still mainly
happening29,30 for iron particles. Solid-state diffusion is widely accepted with respect to combustion synthesis processing. When the material has less motion in the liquid phase and owing
to the in-situ high temperature gradient, the particles diffuse into each other and form intermetallics, alloys or cermets. If this phenomenon combines with centrifugal force, there would be
extra external force acting beyond the viscosity increment, which poses resistance to the particles' motion. Finally, there would be a greater amount of the obtained product than
expected. Velocity and velocity gradient results are in agreement with the experimental evidence of the centrifugal thermite and molten iron infiltration in the TiC pellet discussed
earlier8. Moreover, the simulation results help infer the reason why alumina particles are observed at the TiC pellet boundary and why iron particles infiltrated the TiC porous medium
(Figure 1). The indirect correlation with viscosity change over time is manifest in Figure 6. The particle position becomes zero exactly when the velocity gradient becomes zero. The particle
motion completely stops after the solidification process takes place for 3 seconds. Sudden changes in molten particle position are obvious when the viscosity decreases and the particles
have no relative motion and displacement after 0.7 s from the SHS reaction. However, the particles may experience solid diffusion. Consequently, the particle diffusion speed tends toward
zero as time increases. Again, it is understood that alumina particles are not moving toward the TiC pellet as the iron particles are (Figure 6). The alumina particles are mostly deposited
at the internal boundary of the TiC pellet (Figure 1), so-called the top surface, due to the rapid increase in relative viscosity (Figure 2). Meanwhile, the viscosity of alumina increases in
a fraction of a second, whereas the iron viscosity does not increase to the same extent. Therefore, iron will continue to penetrate or diffuse into the TiC porous media and alumina flow
stops. The results in Figure 6 indicate that the alumina particles are expected to diffuse into TiC at a depth between 15 to 45 μm, depending on particle size. The FESEM micrograph in Figure
1 (a) and its corresponding EDS elemental analysis (Table 1) strongly support the simulation MSPH results. It is thus clear why alumina accumulated at the innermost layer relative to the
rotation axis, but the solid alumina layer isolated the iron particles. However, the iron particles remained in liquid phase and were able to stay in the molten medium for a longer period
than alumina (Figure 8). This phenomenon allows the iron particles to wet and infiltrate the TiC porous media and form a stronger composite structure. Figure 8 (b) displays how the iron
particles surround TiC in zone (III). Therefore, the above-mentioned simulation has excellent potential in controlling particle diffusion. As explained earlier, the total mass and volume of
the solution domain were approximated based in the particle method. As the number of particles approaches infinity, the approximation error moves toward zero. Essentially, both real and
approximated solution domains are identical, thus, the exact analytical and MSPH solutions must be the same. However, because the simulation process included a limited number of particles,
simulation error was present. Therefore, for a small number of particles the approximation error is big given that the particles are large. In the case of size dp2, shown in Figure 3 and
Figure 5, the particle size was 120 μm, which is the largest among the simulated cases. Thus, it is anticipated that the simulation error would be the largest. This helps clarify the
significant difference in the two models' results. Moreover, the viscosity equation was also approximated based on particle size. Similarly, for particle size dp2, the viscosity
approximation error was the largest. Especially at 0.9 and 2.1 seconds from the beginning of the reaction, the approximation error was quite significant (Figure 3). Given that at these two
points abrupt changes in viscosity value occurred, particle approximation is presumed to be even poorer. The MSPH simulation results are in agreement with the experimental observations.
Alumina viscosity increases faster than iron and alumina protects iron from external heat exchange, the iron remains in the molten medium longer than alumina. This phenomenon allows the iron
particles to wet and infiltrate the TiC porous media and form a strong composite. Mathematical modeling of particle deposition velocity and viscosity can describe the process of Fe
infiltration versus time. Melt viscosity increment due to the decrease in temperature leads to a significant speed reduction of particle deposition onto TiC. The MSPH method provides a very
good estimation of velocity and velocity gradient of iron and alumina molten particles. The MSPH estimates the magnitude of molten iron and alumina infiltration, the depth, velocity and
velocity gradient. The velocity gradient of particles reveals that the product velocity decelerates as the viscosity increases versus time. The prediction is in agreement with the
experimental results, microstructure and EDS observations. METHODS EXPERIMENTAL METHODS A centrifugal thermite (CT) machine facilitated rapid centrifugal acceleration as well as the
temperature increase during the experiment. A bilayer graphite-steel compacted mold was fixed inside the CT reaction chamber. A high performance infrared thermometer, Raytek MM1MHSF3L,
recorded the experimental real-time temperature data. The detailed procedure of using the centrifugal machine for thermite processing in metallic pipes is explained in recently published
literature31. The starting materials, namely Al (<75 μm, 99% purity, Sigma Aldrich) and Fe2O3 (<5 μm, 97% purity, Sigma Aldrich) powders were dried for 8 hours and mixed in a Retsch PM
200 Planetary Ball Mill for 4 hours at 30-minute intervals. The green powder stoichiometric mixture was prepared according to the following reaction equation (1)32. Titanium (Sigma Aldrich,
−100 mesh, 99.7% purity) and carbon (Sigma Aldrich, −1000 mesh, 99.9% purity) elemental powders were dried for 7 hours at 125°C, following which they were mixed using a Planetary Ball Mill
(Retsch PM 200) according to the reaction equation (2): A 75 mm long carbon steel pipe with inner and outer diameters of 69 mm and 75 mm, respectively, served as a bulk holder for graphite.
The dried Ti + C green powder was compressed into a pellet. The pellet was then inserted into the graphite mold's pre-defined location as shown in Figure 7. The pellet was positioned
near the head of the tube because of the higher thermal gradient33. The thermite mixture was fed into the tube. The rotation speed was subsequently increased to 280 g acceleration, followed
by igniting an electric arc with a graphite electrode at the entrance of the tube. The onset of the thermite reaction was trailed by the titanium and carbon reaction, which occurred in
response to the heat generated by the thermite (first) reaction. The released temperature was recorded by an infrared pyrometer. The detailed process and characterizations are explained
extensively in a recently published paper by the authors8. MODELING AND SIMULATION OF SHS USING MODIFIED SMOOTHED PARTICLE HYDRODYNAMICS (MSPH) IMPLEMENTATION OF MSPH IN THE CENTRIFUGAL
THERMITE METHOD Figure 8 shows a schematic that represents the centrifugal SHS components in particle approximation. The TiC porous pellet, Al2O3 and Fe Molten particles are shown just after
the SHS reaction started (Figure 8 (a)). The representation of the infiltration of molten alumina and iron particles into the TiC pellet are seen in Figure 8 (b) and (c), which show the TiC
pellet just after particle infiltration stopped and the particles approached the inner surface of the steel pipe. In this model, all particles are distinct in volume. Comprehensive MSPH
formulations are provided in literature26,34. The MSPH method was adapted to the TiC-Fe-Al2O3 centrifugal SHS process. Cylindrical coordinates (r,θ,z) were used to analyze the axisymmetric
motion of particles, since the reaction occurred inside a cylindrical steel pipe. The function, _F_, describing a displacement variable, is independent of the angular position, θ, of a
particle point. Particle approximation using MSPH is mainly dependent on the Taylor series expansion of function _f_(r,z) about point (ri,zi), which is given as: where the derivatives are
assessed at point x = (ri,zi) = (xi, yi). Neglecting the third and higher order derivative terms on the right side of equation (3) and multiplying both sides of the equation with a
positive-value kernel function _W_(_x_ − ζ, _h_) of compact support, or a smoothing length of 2 h, determines the number of particles involved in the approximation. The center of the
particle position, x, its first and second derivatives, then integrate the resulting equations over the solution domain. The integration domain can be replaced by the compact support of the
kernel function since the kernel function is zero when the support is above 2 _h_. In this way, it is possible to estimate and write particle position, particle velocity and particle
velocity gradient in matrix form: Where and To obtain the solution for _F_, matrix _B_ should be non-singular. This condition is already satisfied since the functions φ_i_ are linearly
independent and the number of particles in the compact support of the kernel function _W_ for particles is at least 6. All derivatives of kernel function _W_ appearing in matrix _B_, must
not be constants. Therefore, to easily approximate and calculate the radial positions and infiltration rates of iron and alumina into TiC in the centrifugal-assisted SHS method, the revised
Gauss function W (x-ξ, h) in equations (4) is used as the kernel function. In the present SHS model, smoothing length, or interaction radius (_h_), is selected such that on average 15–20
particles are interacting26. SOLVING THE HEAT BALANCE EQUATION Based on the geometric configuration of the SHS process, the temperature distribution is symmetric about the z-axis. Therefore,
the thermal condition problem is simply a two-dimensional axisymmetric problem. For a unity volume, the heat balance equation or the transient heat flow governing equation is: where _T_ is
the transient temperature, _t_ the time during the process, _k_ is thermal conductivity, ρ is density, _C__p_ is the heat capacity and _q_ is the rate of heat generated during the reaction
process. The last term in equation (6) functions as a heat sink from the reaction zone in the SHS process. The heat is consumed in preheating the reacted mixture. With the help of the
initial conditions, the MSPH method can be utilized to solve equation (6). The starting mixture temperature and activation energy are given. Equation (6) is written for particle _i_ and the
values of Trri, _T__ri__and T__zzi_, which are similar to _T__xxi__, T__xi__and T__yyi_ and are determined by solving the linear simulation equations (4). The solution is marching forward in
time by the conditionally stable forward-difference scheme. Upon obtaining the temperature distribution, it is possible to calculate the viscosity, particle velocity and velocity gradient.
SOLVING THE PARTICLE VELOCITY AND VELOCITY GRADIENT EQUATIONS Form Stoke's formula for particle motion in fluid, as well as normal gravity and equilibrium of forces on a particle in the
SHS process under centrifugal acceleration8, the radial particle velocity _V (T, t)_ as a function of temperature and time is given as: where _R_ is the current radial position of the
particles, η _(T__c_) is the current viscosity of the molten metal, _Q_ is total activation energy and the gas constant is _R__g_. The rotation speed per second is _n_, _g_ is the
acceleration of gravity and _T__c_ is the temperature. Particle size is _d_ whereas ρ_o_ and ρ_m_ are the particle density and melt density, respectively. Since the determinant of matrix _B_
is nonzero, its inverse, _B_−1, exists. Thus, equation (4) can be rewritten as: Equation (8) gives the values of function _f_ (velocity, _V_) and its first-order and second-order
derivatives at point X = (xi, yi) in terms of velocity values at points neighboring X. Based on equation (9), MSPH approximation of radial velocity and radial velocity gradient can be
explicitly written as: It is evident that for the MSPH method, kernel approximation of the velocity gradient is given in terms of the integration of the first-order derivative of W(x-ξ, h)
over its compact support. CODING AND SIMULATION PROCEDURE MATLAB, Mathworks ® version 2012a, was employed to write the MSPH for solving equations (6) and (7). A flowchart of the solution
procedure is shown in Figure 9. First, using the initial condition, the heat condition equation is solved and once the temperature distribution is known, viscosity, particle radial velocity
and radial velocity gradient are calculated. The solution marches forward in time by the conditionally stable forward-difference scheme. The time increment employed is 1 μs. Interaction
radius or smoothing length (h) is chosen such that 15–20 particles are interacting on average26, where the total number of particles is 29. The MSPH simulation parameters are listed in Table
1. MSPH simulation of iron and alumina particles was conducted with parameters extracted from existing literature. Molten iron ηo = 0.0065 kg/m.s35, total activation energy, Q = 706 kJ/mol
of Al-Fe2O3 and Ti-C36,37, gas constant Rg = 8.31441 J/K.mol38 and ηo = 0.052 N.s/m2 is the liquid viscosity of alumina at 2408 K39. The other thermophysical properties are listed in Table
3. REFERENCES * Song, M. S., Ran, M. W. & Kong, Y. Y. In situ fabrication of ZrC ceramic obtained by self-propagating high-temperature synthesis from Al-Zr-C elemental powders. Int. J.
Refract. Met. Hard Mater 29, 10.1016/j.ijrmhm.2011.01.013 (2011). * Munir, Z. A. in Encyclopedia of Materials: Science and Technology [(eds K. H. Jürgen, Buschow et al. )] 8323–8327
(Elsevier, 2001). * Odawara, O. Mass-Forced SHS Technology of Ceramic Materials. Adv. Sci. Technol. 63, 302–311, 10.4028/www.scientific.net/AST.63.302 (2010). Article CAS Google Scholar *
Zhu, Y., Sun, S., Ni, H. & Huang, M. Study on microstructure and properties of ceramic-lined composite steel pipes produced by centrifugal-SHS process. Key Eng. Mater. 464, 434–437,
10.4028/www.scientific.net/KEM.464.434 (2011). Article CAS Google Scholar * Merzhanov, A. G. Thermally coupled SHS reactions. Int. J. Self Propag. High Temp. Synth. 20, 61–63,
10.3103/s1061386211010109 (2011). Article Google Scholar * Gao, F., Guo, Z. & Lin, T. Preparation of W-C-Fe cermet lined steel pipes by SHS-centrifugal process. J. Univ. Sci. Technol.
Beijing 30, 648–651 (2008). CAS Google Scholar * Wang, Y.-F. & Yang, Z.-G. Finite element analysis of residual thermal stress in ceramic-lined composite pipe prepared by
centrifugal-SHS. Mater. Sci. Eng., A 460–461, 130–134, 10.1016/j.msea.2007.01.017 (2007). Article CAS Google Scholar * Mahmoodian, R., Hassan, M. A., Rahbari, R. G., Yahya, R. &
Hamdi, M. A novel fabrication method for TiC-Al2O3-Fe functional material under centrifugal acceleration. Composites, Part B 50, 187–192, 10.1016/j.compositesb.2013.02.016 (2013). Article
CAS Google Scholar * Yang, Y.-F., Wang, H.-Y., Liang, Y.-H., Zhao, R.-Y. & Jiang, Q.-C. Effect of C particle size on the porous formation of TiC particulate locally reinforced steel
matrix composites via the SHS reaction of Ni–Ti–C system during casting. Effect of C particle size on the porous formation of TiC particulate locally reinforced steel matrix composites via
the SHS reaction of Ni–Ti–C system during casting 474, 355–362, 10.1016/j.msea.2007.04.061 (2008). Article CAS Google Scholar * Birman, V. & Byrd, L. W. Modeling and Analysis of
Functionally Graded Materials and Structures. Appl. Mech. Rev. 60, 195–216, 10.1115/1.2777164 (2007). Article ADS Google Scholar * Gennari, S., Maglia, F., Anselmi-Tamburini, U. &
Spinolo, G. SHS (Self-sustained high-temperature synthesis) of intermetallic compounds: effect of process parameters by computer simulation. Intermetallics 11, 1355–1359,
10.1016/S0966-9795(03)00179-1 (2003). Article CAS Google Scholar * Zhang, G., Xiao, G. & Fan, Q. Numerical modeling of field-activated combustion synthesis process of the B4C system.
Mater. Res. Bull. 46, 345–349, 10.1016/j.materresbull.2010.12.019 (2011). Article CAS Google Scholar * Gao, J. W. & Wang, C. Y. Modeling the solidification of functionally graded
materials by centrifugal casting. Mater. Sci. Eng., A 292, 207–215, 10.1016/s0921-5093(00)01014-5 (2000). Article Google Scholar * Advani, A. H. et al. Dynamic modelling of material and
process parameter effects on self-propagating high-temperature synthesis of titanium carbide ceramics. J. Mater. Sci. 27, 3309–3317, 10.1007/BF01116030 (1992). Article CAS ADS Google
Scholar * Song, I., Wang, L., Wixom, M. & Thompson, L. T. Self-propagating high temperature synthesis and dynamic compaction of titanium diboride/titanium carbide composites. J. Mater.
Sci. 35, 2611–2617, 10.1023/A,1004731532616 (2000). Article CAS ADS Google Scholar * Gingold, R. A. & Monaghan, J. J. Smoothed particle hydrodynamics-theory and application to
non-spherical stars. Mon. Not. R. Astron. Soc. 181, 375–389 (1977). Article ADS Google Scholar * Lucy, L. B. A numerical approach to the testing of the fission hypothesis. Astron. J. 82,
1013–1024 (1977). Article ADS Google Scholar * Monaghan, J. J. A turbulence model for Smoothed Particle Hydrodynamics. Eur. J. Mech. B-Fluid 30, 360–370, 10.1016/j.euromechflu.2011.04.002
(2011). Article ADS MathSciNet MATH Google Scholar * Monaghan, J. J. Smoothed particle hydrodynamics. Rep. Prog. Phys. 68, 1703, 10.1088/0034-4885/68/8/R01 (2005). Article ADS
MathSciNet MATH Google Scholar * Liu, M. & Liu, G. Smoothed particle hydrodynamics (SPH): an overview and recent developments. Arch. Comput. Method E. 17, 25–76,
10.1007/s11831-010-9040-7 (2010). Article CAS MathSciNet MATH Google Scholar * Cleary, P. W. & Monaghan, J. J. Conduction Modelling Using Smoothed Particle Hydrodynamics. J. Comput.
Phys. 148, 227–264, 10.1006/jcph.1998.6118 (1999). Article ADS MathSciNet MATH Google Scholar * Muhammad, N., Rogers, B. D. & Li, L. Understanding the behaviour of pulsed laser dry
and wet micromachining processes by multi-phase smoothed particle hydrodynamics (SPH) modelling. J. Phys. D: Appl. Phys. 46, 095101, 10.1088/0022-3727/46/9/095101 (2013). Article CAS ADS
Google Scholar * Price, D. J. Smoothed particle hydrodynamics and magnetohydrodynamics. J. Comput. Phys. 231, 759–794, 10.1016/j.jcp.2010.12.011 (2012). Article ADS MathSciNet MATH
Google Scholar * Takaffoli, M. & Papini, M. Material deformation and removal due to single particle impacts on ductile materials using smoothed particle hydrodynamics. Wear 274–275,
50–59, 10.1016/j.wear.2011.08.012 (2012). Article CAS Google Scholar * Cleary, P. et al. Modeling of cast systems using smoothed-particle hydrodynamics. JOM 56, 67–70,
10.1007/s11837-004-0038-1 (2004). Article CAS Google Scholar * Zhang & Batra, R. C. Modified smoothed particle hydrodynamics method and its application to transient problems. Comput.
Mech. 34, 137–146, 10.1007/s00466-004-0561-5 (2004). Article MATH Google Scholar * Belytschko, T., Rabczuk, T., Huerta, A. & Fernández-Méndez, S. in Encyclopedia of Computational
Mechanics (John Wiley & Sons, Ltd, 2004). * Meng, Q. S. et al. Microstructure and mechanical properties of multilayer-lined composite pipes prepared by SHS centrifugal-thermite process.
Mater. Sci. Eng., A 456, 332–336, 10.1016/j.msea.2006.12.016 (2007). Article CAS Google Scholar * Feng, K. Q., Hong, M., Xiong, J., Fan, H. Y. & Guo, Z. X. Mechanism of combustion
synthesis of TiC-Fe composites under the action of an electric field. Powder Metall. 55, 235–241, 10.1179/1743290111Y.0000000005 (2012). Article CAS Google Scholar * Fan, Q., Chai, H.
& Jin, Z. Mechanism of combustion synthesis of TiC–Fe cermet. J. Mater. Sci. 34, 115–122, 10.1023/A,1004430028260 (1999). Article CAS ADS Google Scholar * Mahmoodian, R., Rahbari, R.
G., Hamdi, M. & Sparham, M. A new attempt to adopt a machine for SHS lining ceramics inside pipes. High Temp. Mater. Processes 16, 15–23, 10.1615/HighTempMatProc.2012004708 (2012).
Article CAS Google Scholar * Odawara, O. in International Symposium on Combustion and Plasma Synthesis of High Temperature Materials. [(ed Holt, J. B. & Munir, Z. A.)] [179–185] (VCH
Publishers, Inc., 1990). * Mahmoodian, R., Rahbari, R. G., Hamdi, M., Hassan, M. A. & Sparham, M. The Effects of an Unexpected Ceramic Coating Phase at the Head of a Pipe on Joining and
Postprocessing of a Ceramic-Lined Composite Pipe. JOM 65, 80–85, 10.1007/s11837-012-0498-7 (2013). Article CAS ADS Google Scholar * Batra, R. C. & Zhang, G. M. Modified Smoothed
Particle Hydrodynamics (MSPH) basis functions for meshless methods and their application to axisymmetric Taylor impact test. J. Comput. Phys. 227, 1962–1981, 10.1016/j.jcp.2007.10.001
(2008). Article ADS MathSciNet MATH Google Scholar * Ho, Y.-H. & Hwang, W.-S. The Analysis of Molten Steel Flow in Billet Continuous Casting Mold. ISIJ Int. 36, 1030–1035,
10.2355/isijinternational.36.1030 (1996). Article CAS Google Scholar * Fan, R. H., Lü, H. L., Sun, K. N., Wang, W. X. & Yi, X. B. Kinetics of thermite reaction in Al-Fe2O3 system.
Thermochim. Acta 440, 129–131, 10.1016/j.tca.2005.10.020 (2006). Article CAS Google Scholar * Munoz, J. D., Arizmendi, A., Mendoza-Allende, A. & Montemayor-Aldrete, J. A. High
temperature activation energy for plastic deformation of titanium carbide single crystals as a function of the C: Ti atom ratio. J. Mater. Sci. 32, 3189–3193, 10.1023/a,1018654818631 (1997).
Article CAS ADS Google Scholar * Balout, B. & Litwin, J. Mathematical Modeling of Particle Segregation During Centrifugal Casting of Metal Matrix Composites. J. Mater. Eng. Perform.
21, 450–462, 10.1007/s11665-011-9873-8 (2012). Article CAS Google Scholar * Quang, V. V. Dynamical, Rheological and Thermal Studies of High Temperature Oxide Liquids PhD thesis,
Aberystwyth University, (2008). * Vardelle, A., Themelis, N. J., Dussoubs, B., Vardelle, M. & Fauchais, P. Transport and chemical rate phenomena in plasma sprays. High Temp. Mater.
Processes 1, 295–313 (1997). Article CAS Google Scholar * Rahimian, M., Parvin, N. & Ehsani, N. Investigation of particle size and amount of alumina on microstructure and mechanical
properties of Al matrix composite made by powder metallurgy. Mater. Sci. Eng., A 527, 1031–1038, 10.1016/j.msea.2009.09.034 (2010). Article CAS Google Scholar * Chan, Y. & Choi, S.
Numerical simulations of inductive-heated float-zone growth. J. Appl. Phys. 72, 3741–3749, 10.1063/1.352294 (1992). Article CAS ADS Google Scholar * Mihok, Ľ., Demeter, P., Baricova, D.
& Seilerova, K. Utilization of ironmaking and steelmaking slags. Metalurgija 45, 163–168 (2006). CAS Google Scholar * Glorieux, B., Millot, F., Rifflet, J. C. & Coutures, J. P.
Density of Superheated and Undercooled Liquid Alumina by a Contactless Method. Int. J. Thermophys. 20, 1085–1094, 10.1023/A,1022650703233 (1999). Article CAS Google Scholar * Mahmoodian,
R., Hassan, M. A., Hamdi, M., Yahya, R. & Rahbari, R. G. In-situ TiC-Fe-Al2O3-TiAl/Ti3Al composite coating processing using centrifugal assisted combustion synthesis. Composites _Part B:
Engineering_IN PRESS, 10.1016/j.compositesb.2013.12.016 (2014). Download references ACKNOWLEDGEMENTS The authors would like to acknowledge the University of Malaya for providing the
necessary facilities and resources for this research. This research was fully funded by the Ministry of Higher Education, Malaysia with the high impact research (HIR) grant number of
UM.C/625/1/HIR/MOHE/ENG/27. Special thanks are due to Mr. Ali Mahmoodian, the CEO of Azarin Kar Ind. Co., for consulting and facilitating the design and fabrication of the reaction chamber.
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Mechanical Engineering, Centre of Advanced Manufacturing and Materials Processing (AMMP), University of Malaya, 50603, Kuala
Lumpur, Malaysia M. A. Hassan, Reza Mahmoodian & M. Hamdi * Department of Mechanical Engineering, Assiut University, Assiut, 71516, Egypt M. A. Hassan * Department of Research and
Development, AzarinKar IND. Co., Industrial Zone 1, 7635168361, Kerman, Iran Reza Mahmoodian Authors * M. A. Hassan View author publications You can also search for this author inPubMed
Google Scholar * Reza Mahmoodian View author publications You can also search for this author inPubMed Google Scholar * M. Hamdi View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS R.M. conducted the research experiment and contributed to the discussions, theoretical analysis and writing of the manuscript along with M.A.N.A. and
M.H. All authors reviewed the manuscript. Mr. Ali Mahmoodian has facilitated the design and fabrication of the centrifugal SHS reaction chamber at Azarin Kar IND. Co. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing financial interests. RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0
Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hassan, M.,
Mahmoodian, R. & Hamdi, M. Modified smoothed particle hydrodynamics (MSPH) for the analysis of centrifugally assisted TiC-Fe-Al2O3 combustion synthesis. _Sci Rep_ 4, 3724 (2014).
https://doi.org/10.1038/srep03724 Download citation * Received: 26 September 2013 * Accepted: 16 December 2013 * Published: 16 January 2014 * DOI: https://doi.org/10.1038/srep03724 SHARE
THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative