- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Supercapacitors, as one of alternative energy devices, have been characterized by the rapid rate of charging and discharging and high power density. But they are now challenged to
achieve their potential energy density that is related to specific capacitance. Thus it is extremely important to make such materials with high specific capacitances. In this report, we have
gained homogenous Ni(OH)2 on graphene by efficiently using of a facile and effective electrostatic induced stretch growth method. The electrostatic interaction triggers advantageous change
in morphology and the ordered stacking of Ni(OH)2 nanosheets on graphene also enhances the crystallization of Ni(OH)2. When the as-prepared Ni(OH)2/graphene composite is applied to
supercapacitors, they show superior electrochemical properties including high specific capacitance (1503 F g−1 at 2 mV s−1) and excellent cycling stability up to 6000 cycles even at a high
scan rate of 50 mV s−1. SIMILAR CONTENT BEING VIEWED BY OTHERS FACILE SYNTHESIS OF PEDOT-RGO/HKUST-1 FOR HIGH PERFORMANCE SYMMETRICAL SUPERCAPACITOR DEVICE Article Open access 03 June 2021
SUPERCAPACITORS BASED ON TI3C2TX MXENE EXTRACTED FROM SUPERNATANT AND CURRENT COLLECTORS PASSIVATED BY CVD-GRAPHENE Article Open access 12 January 2021 TUNING THE HIERARCHICAL PORE STRUCTURE
OF GRAPHENE OXIDE THROUGH DUAL THERMAL ACTIVATION FOR HIGH-PERFORMANCE SUPERCAPACITOR Article Open access 22 January 2021 INTRODUCTION Supercapacitors (SCs), also known as ultracapacitors
or electrochemical capacitors, have attracted numerous attentions for energy storage due to their high power performance, long cycle life and low maintenance cost1,2,3. In general, SCs can
be classified into electrical double layer capacitors (EDLCs) and pseudocapacitors based on their energy storage mechanisms4,5,6. EDLCs store the charge using reversible adsorption and
desorption of the electrolyte ions onto the surface of active materials. EDLCs, mainly based on carbon materials, that are electrochemically stable and physical processes, hence they can
perform ultrahigh power density and excellent cycle life, yet their further applications are confined by the limited energy density7,8,9,10. On the contrary, pseudocapacitors based on fast
reversible surface redox reactions hold the potential of much higher energy density11,12,13,14. Although extensive studies have been made, there is still an urgent demand for
pseudocapacitors that allow for fast charging and discharging with high stability for the storage of electrical energy in practical application15,16,17,18,19. Recently, two-dimensional
nanomaterials, which possess nanoscale dimension in thickness and infinite length in the plane, have attracted tremendous attention owing to their unique properties and potential
applications in the areas of energy storage and conversion such as supercapacitors20,21,22. Ni(OH)2, with unique layer structure has been developed for pseudocapacitive
applications23,24,25,26. However, its practical application in SCs is still hindered by the poor conductivity of Ni(OH)2. To address this problem, downsizing it to nanoscale and constructing
hybrid materials with highly electrical conductive, flexible and chemically stable conductive substrates have been investigated to be promising approaches27,28,29,30. Graphene, emerging as
a 2D single layer of carbon atoms with a hexagonal packed structure, has attracted considerable attention in scientific researches as conductive substrate due to its ultrahigh surface area
(~2630 m2 g−1 for single-layer graphene), low cost and high electrical conductivity (103–104 S m−1)31,32,33,34,35,36. Graphene can be obtained through reduction of graphene oxide (GO)
obtained by modified Hummer's methods. It is an inexpensive processes, which is currently used for large-scale production of graphene37,38. Typically, GO contains carboxylic acid, epoxy
and hydroxyl groups and these unique features make it an excellent 2D support to load Ni(OH)2 and then improving the electrochemical properties of Ni(OH)239,40,41,42,43. However, there is
still a great challenge to reduce aggregation-derived degradation in capacitance especially at a high scan rate associated with untight contact between graphene nanosheets and metal
hydroxide. Herein, we report a facile electrostatic induced stretch growth method to achieve homogenous coating of Ni(OH)2 nanosheets on graphene under mild reflux condition. The
electrostatic interaction triggers advantageous change in morphology and the ordered stacking of Ni(OH)2 nanosheets on graphene and also enhances the crystallization of Ni(OH)2. Functional
groups of GO (hydroxyl, epoxy, carboxyl and carbonyl groups) act as anchor sites for in-situ growth. The combined advantages of unique structure, high conductivity and structural stability
endow the as-prepared composite (Ni(OH)2/GS) with superior performances including high specific capacitance (1503 F g−1 at 2 mV s−1) and excellent cycling stability up to 6000 cycles even at
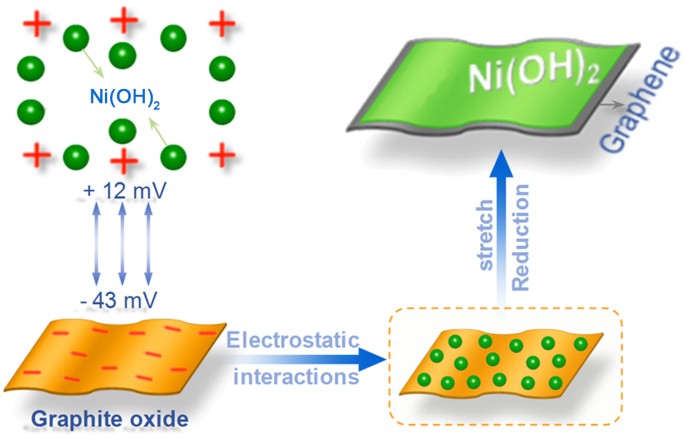
a high scan rate of 50 mV s−1. RESULTS MORPHOLOGY AND STRUCTURE ANALYSIS Figure 1 shows the formation procedure of homogenous coating of precursor Ni(OH)2 on graphene under mild reflux
condition. First, precursor Ni(OH)2 is obtained by facile precipitation reaction. Then, precursors are deposited onto the surface of GO. The Zeta potential of precursor Ni(OH)2 and GO are
measured under reaction condition by dynamic light scattering. The precursor Ni(OH)2 is positively charged (+12 mV) and GO are negatively charged (−43 mV). With the help of electrostatic
interacting between reverse charged reactants, Ni(OH)2 is anchored onto the surface of GO nanosheets. Thereafter, Ni(OH)2/GS composites are obtained after reflux in DMF at 95°C for 16 h.
Different Ni(OH)2/GS composites are prepared by control the mass ratio of GO to Ni(OH)2 as 1:20, 1:5 and 1:2 and the corresponding composites are denoted as Ni(OH)2/GS-20, Ni(OH)2/GS-5 and
Ni(OH)2/GS-2, respectively. For comparison, pure Ni(OH)2 is also synthesized under the similar conditions except for the absence of graphene. The crystal structures of the obtained samples
are characterized by X-ray diffraction (XRD) pattern. As shown in Figure 2a, all the diffraction peaks of the precursor Ni(OH)2, pure Ni(OH)2 and Ni(OH)2/GS composites can be indexed to
beta-Ni(OH)2 (JCPDS: 14-0117). The 2θ degrees for diffractions of (001), (100), (101), (102), (110) and (111) planes are at 19.258, 33.064, 38.541, 52.100, 59.052 and 62.726°, respectively.
For precursor Ni(OH)2 and pure Ni(OH)2, the XRD diffraction peaks are low and broad, revealing the poor crystallinity and small size of crystallite. Interestingly, with the presence of
graphene, the diffraction peaks become obviously stronger, indicating the enhanced crystallization of the Ni(OH)2/GS. To further confirm the contribution of graphene, we also investigated
other composites with different mass ratio of GO to Ni(OH)2. With the addition of GO, it can be found that the crystallization can be optimized with increasing amount of GO, as is displayed
by the XRD patterns of Ni(OH)2/GS-20, Ni(OH)2/GS-5 and Ni(OH)2/GS-2 in Figure 2b. These results show the existence of graphene can enhance the crystallization of Ni(OH)2. The morphology and
structure of precursor Ni(OH)2, pure Ni(OH)2 and Ni(OH)2/GS composites are studied by field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). As shown
in Figure S1, precursor Ni(OH)2 is stacked particle and the obtained pure Ni(OH)2 is observed as aggregated nanoplate. Figure 3a and b display the morphology and structure of Ni(OH)2/GS-20
composite, we can also observe stacked particles of Ni(OH)2 with low mass ratio of graphene substrates. The morphology and structure of Ni(OH)2/GS-5 and Ni(OH)2/GS-2 composites are shown in
Figure 3c–f, respectively. Interestingly, it is found that Ni(OH)2 and graphene are so much alike that it is impossible to discriminate one from the other, which indicates that Ni(OH)2 and
graphene are homogenously contacted and intercalated with each other (vide infra). Moreover, the graphene sheets overlap with each other and thus result in a three-dimensional and porous
network. Combined with the results from XRD patterns, it can be speculated that the functional groups between graphene sheets chelate Ni(OH)2 disordered stacks with the help of electrostatic
interaction. And then these groups can act as anchoring sites, enabling Ni(OH)2 stretch on graphene sheets and forming ordered stack on interfaces of graphene sheets, which is depicted in
Figure S2. The high-resolution TEM image of Ni(OH)2/GS-5 in Figure 4a shows lattice fringes of 0.27 and 0.23 nm, which are consistent to the _d_ spacing of the (100) and (101) planes of
Ni(OH)2, confirming its crystalline nature in accordance with the XRD results. Furthermore, the distribution of graphene and Ni(OH)2 in the Ni(OH)2/GS-5 composite is then analyzed using
energy dispersive X-ray (EDX) spectroscopy mapping technique (Figure 4b–e). The region of nickel (Ni), oxygen (O) and carbon (C) is similar to the selected area in Figure 4b. These results
clearly show that the elements of Ni, O and C are distributed homogeneously, further indicating that the coating of Ni(OH)2 on graphene is uniform and complete. X-ray photoelectron
spectroscopy (XPS) technique is employed to analyze the chemical state of all elements in Ni(OH)2/GS-5 composite (Figure 5a–d). As shown in Figure 5a, the survey spectrum of Ni(OH)2/GS-5
also certify the presence of Ni, O and C in the composite, which is consistent with the above FESEM and EDX results. Figure 5b shows the deconvolution of C 1 s spectrum with different oxygen
containing functional groups, including the non-oxygenated C in C-C at 284.5 eV, the carbon in C-O at 286.4 eV and the carbon in C = O at 288.8 eV. These hydrophilic functional groups in
Ni(OH)2/GS-5 are believed to play dual roles here. On the one hand, these hydrophilic groups can enhance the wettability of the electrode and further improve the compatibility between the
electrode and the electrolyte. On the other hand, these groups can act as anchoring sites to interact with precursors, enabling Ni(OH)2 to directly grow on graphene sheets. The peaks of the
Ni 2p spectrum in Figure 5c can be assigned to the Ni 2p3/2 (850–865 eV) and Ni 2p1/2 (870–885 eV) spin orbit levels, which show Ni ion is +2 valence state. Figure 5d shows the deconvolution
of O 1 s spectrum with three peaks. The peak at 530.2 eV is ascribed to C-O-Ni bond. The peak at 531.2 eV is assigned to C = O groups or shoulder peak of O 1 s in Ni(OH)2 and the peak at
533.1 eV is ascribed to C-OH and/or C-O-C groups (hydroxyl and/or epoxy). Raman spectra of the Ni(OH)2/GS-5 composites before and after reduction are shown in Figure 5e and f. There are two
prominent peaks at 1357 and 1595 cm−1, which correspond to the D and G bands of carbon in the Ni(OH)2/GS-5 composite before reflux treatment. For Ni(OH)2/GS-5 composite, the peaks for D and
G bands are 1356 and 1600 cm−1,respectively. The G band shifts in carbon-based composites relate to the charge transfer between the carbon and other compounds. Therefore, the observed shift
by 6 cm−1 indicates the presence of a charge transfer from graphene to Ni(OH)2. These intimate bindings afford facile electron transports through Ni(OH)2 to graphene and further facilitates
electrons to the current collectors, leading to low electrical resistances. In addition, the N2-adsorption-desorption isotherm and the pore-size distribution of Ni(OH)2/GS-5 composite and
pure Ni(OH)2 are collected in Figure S3a and b. The actual mass ratio of graphene in Ni(OH)2/GS-5 composite after reflux treatment is determined by thermogravimetric analysis in Figure S3c
to be 10 wt%. ELECTROCHEMICAL MEASUREMENTS Cyclic voltammetry (CV) is then employed to investigate the capacitance mechanism and the specific capacitances at various scan rates. Figure 6a
and b show CV curves of Ni(OH)2/GS-5 and pure Ni(OH)2 electrodes from 2 to 200 mV s−1, respectively. The two strong peaks indicate that the capacitive behavior mainly results from
pseudocapacitive capacitance based on redox mechanism. The positive peak observed at 0.42 V (vs Hg/HgO) could be reasonably attributed to the following reaction: Ni(II) → Ni(III), indicating
an oxidation process. And the corresponding negative peak occurs at 0.30 V (vs Hg/HgO), indicating a reduction process: Ni(III) → Ni(II). The specific capacitances can be calculated by
integrating the area under the CV curves (see supplementary information for details). The average specific capacitances of Ni(OH)2/GS-5 and pure Ni(OH)2 electrodes are calculated to be 1503
and 1064 F g−1 at the scan rate of 2 mV s−1, which are shown in Figure 6c. For Ni(OH)2/GS-5 electrodes, interestingly, CV curves are found to retain a similar shape to that of a scan rate of
2 mV s−1 even at 200 mV s−1. Although the specific capacitances decrease with the increasing scan rates, a high value of 299 F g−1 can still be obtained at 200 mV s−1. However, a lower
value of 142 F g−1 is obtained at 200 mV s−1 for pure Ni(OH)2 electrodes. In addition to CV curves, galvanostatic discharge curves (GC) (Figure S4) are also employed to estimate the specific
capacitance, which are shown in supplementary information. The enhanced performance of Ni(OH)2/GS-5 can be attributed to the improved electrical conductivity and facilitated ion transport
and diffusion rate. It can be explained according to Figure 6d, where illustrates the relationship between anodic peak current and square root of scan rates for Ni(OH)2/GS-5 and pure Ni(OH)2
electrode. Note that the anodic peak current (_i_) increase linearly with square root of scan rates (_v_1/2), which satisfies Cottrell equation and indicates a diffusion-controlled process.
According to Cottrell equation, _i_ = _nFACD_1/2/(π_t_)1/2, where _n_ is the number of electrons transferred during the redox reaction, _F_ is a faradic constant, _A_ is the effective
surface area of the working electrode, _C_ is the concentration of the electrolyte, _D_ is the diffusion coefficient and Cottrell equation can be simplified as _i_ = a ν1/243 We can conclude
that the anodic peak current (_i_) increases with square root of the diffusion coefficient (_D_) in the certain situation. From Figure 6d, we can find that the Ni(OH)2/GS-5 composite
represents fast diffusion velocity. For comparison, the electrochemical properties of other composites with different mass ratios of GO to Ni(OH)2 are also measured by galvanostatic
discharge curves (GC) and CV curves, as shown in Figure S5. Table S1 and S2 summarize the specific capacitances of Ni(OH)2/GS-20, Ni(OH)2/GS-5, Ni(OH)2/GS-2 composites and pure Ni(OH)2
calculated from CV curves and galvanostatic discharge curves. Graphene prevents the aggregation of Ni(OH)2 nanosheets, which enables an effective redox reaction at the interface of Ni(OH)2
and electrolyte. Besides, the oxygen-containing functional groups enhance the wettability of the electrode and thereby the electroactive species, Ni(OH)2, can be easily infiltrated by
electrolyte ions for charge-transfer reactions. It can be noted that insufficient and excess graphene are harmful for the improved performance. For Ni(OH)2/GS-20, excess Ni2+ ions around GO
prevent themself from dispersing on GO nanosheets well thus resulting in low capacitance with a capacitance of 1052 F g−1 at 2 mV s−1. On the other hand, too many graphene in Ni(OH)2/GS-2
induce the aggregation of graphene sheets and the lower capacitance of graphene ascribe to EDLC behavior compromise the capacitance based on the total mass of the composite. The highest
capacitance of Ni(OH)2/GS-2 is 1334 F g−1 at 2 mV s−1. The optimal ratio of GO to Ni(OH)2 is 1:5 by mass. DISCUSSION Figure 7a shows CV curves of Ni(OH)2/GS-5, pure Ni(OH)2 and Ni foam at a
scan rate of 50 mV s−1. Note that the background signal deriving from Ni foam is negligible. The average specific capacitance of the Ni(OH)2/GS-5 at a scan rate of 50 mV s−1 is 515 F g−1, a
factor of ~1.7 higher than that of pure Ni(OH)2 at the same scan rate. For practical application, cyclic stability is crucial for an electrode material in electrochemical capacitors. As
shown in Figure 7b, the loss in capacity for Ni(OH)2/GS-5 is only 3.5% within 6000 cycles (from 515 to 497 F g−1). In general, the excellent cycling stability is attributed the intimate
bindings of Ni(OH)2 nanosheets and graphene nanosheets affording facile electron transports through Ni(OH)2 to graphene and further facilitating electrons to the current collectors, which
can improve their electronic and ionic conductivities. Besides, it should be noted that a decrease can be found before 2000 cycles and there is a slight increase with the cycles ranging from
2000 to 4000 cycles and then the tendency holds nearly stable after 4000 cycles. Taking the properties of the composite into account, the slight increase may be attributed to the
improvement of ion accessibility owing to the functional groups on graphene which have been confirmed by previous XPS results (Figure 5). To further analyze the reason that Ni(OH)2/GS-5
composite performs more excellent capacitive behavior than pure Ni(OH)2, the transport characteristics of the charge carriers within electrode are investigated using electrochemical
impedance spectroscopy (EIS) as shown in Figure 7c. Nyquist plots are composed by two distinct parts, a semicircle at high frequency and a linear line at low frequency. The high frequency
semicircle intercepts the real axis at _R_s and (_R_s + _R_ct), while _R_s means a bulk solution resistance and _R_ct means a charge-transfer resistance, respectively. _R_s is related with
several parts including the electrolyte resistance, the collector/electrode contact resistance and the electrode/electrolyte interface resistance. But the morphological difference has little
effect on the ohmic resistance of the supercapacitor device, as shown in the zoom-in of the intersection part. We can note that there is no obvious distinction between Ni(OH)2/GS-5 and pure
Ni(OH)2 electrode with regard to _R_s. In terms of _R_ct, the radius of the semicircle is about 0.5 ohm, which is smaller than that of pure Ni(OH)2 (about 1 ohm), suggesting the lower
charge-transfer resistance. We can assume that synergistic effects between Ni(OH)2 and graphene are favorable to the penetration of electrolytes into the whole electrode matrix and then
reduce electrical resistance at the interface between the electrode material and the current collector. The inclined portion of the curve (about 45°) in the low frequency is ascribed to the
Warburg impedance, which is related to ion diffusion/transport in the electrolyte. The more vertical shape at low frequency for Ni(OH)2/GS-5 indicates a more capacitive behavior of the
electrode. Figure 7d shows the EIS data of Ni(OH)2/GS-5 composite before and after 6000 cycles. In terms of _R_ct, the value after 6000 cycles is about 0.2 ohm, a lower resistance than the
previous result. It may be attributed to the improvement of ion accessibility resulting from the increased wettability during the cycling process, which provides an evidence for the trend of
cycling stability in Figure 6b. However, we can find that there is a little deviation from a vertical line in terms of the inclined portion of the curve in the low frequency after 6000
cycles, indicating the lack of good ion diffusion/transport in the electrolyte to the electrode surface. In summary, we have developed a facile and effective stretch growth technique for
homogenous coating Ni(OH)2 on graphene to tackle the challenges of pseudocapacitor materials in SCs. The electrostatic interaction triggers advantageous change in morphology and the ordered
stacking of Ni(OH)2 nanosheets on graphene and also enhances the crystallization of Ni(OH)2. Many functional groups of GO (hydroxyl, epoxy, carboxyl and carbonyl groups) act as anchor sites
for growth. This novel Ni(OH)2/graphene composite inherits the advantages of graphene nanosheets and two-dimensional Ni(OH)2 materials thus exhibit higher specific capacitance, better rate
capability and cycling stability compared to pure Ni(OH)2. The obtained novel Ni(OH)2/GS hybrid with optimization ratio holds high specific capacitance and excellent cycling stability up to
6000 cycles even at a high scan rate of 50 mV s−1. The significant improvement is rarely reported in previous literatures and is attributed to its tailored properties, which are vital to the
operation of SCs, including the intimate bindings, high conductivity, structural stability and good wettability. It is concluded that the synergetic effect between graphene and
two-dimensional Ni(OH)2 structure benefits the improvement of the electrochemical properties of the hybrid composites. This facile method may offer an attractive alternative approach for
preparation of the graphene based two-dimensional composites as high performance electrodes for supercapacitors. METHODS SYNTHESIS OF GRAPHENE OXIDE Graphene oxide was prepared by the
oxidation of natural graphite powder via an improved Hummers method42. Briefly, a 9:1 mixture of concentrated H2SO4/H3PO4 (45:5 mL) was added to a mixture of graphite flakes (0.375 g) and
KMnO4 (2.25 g). The reaction was then heated to 50°C and stirred for 24 h. The reaction was cooled to room temperature and poured onto ice (200 mL) with 30% H2O2 (3 mL). Then, the mixture
was centrifuged (10000 rpm for 5 min). The remaining solid material was then washed in succession with 200 mL of 30% HCl for twice and 400 mL of water for three times. For each wash, the
mixture was centrifuged (12000 rpm for 10 min) and graphite oxide was obtained. The as-prepared graphite oxide was dispersed into deionized water to form a homogenous solution (about 1 mg
mL−1). SYNTHESIS OF PRECURSOR NI(OH)2 NiSO4·7H2O (2.5759 g) and NaOH (0.132 g) was dissolved in 100 mL deionized water and stirred for 30 min. Then we obtained precursor Ni(OH)2
precipitation by centrifuging and washing the mixture with copious deionized water for several times. SYNTHESIS OF PURE NI(OH)2 The as-obtained Ni(OH)2 precursor particles were dispersed
into 150 mL DMF and sonicated for 60 min to separate stacked precursors and thereafter are used for refluxing at 95°C for 16 h. SYNTHESIS OF NI(OH)2/GS COMPOSITES The as-obtained Ni(OH)2
precursors were dispersed into deionized water and then different amount of GO was added. The ratio of GO to Ni(OH)2 is 1:20, 1:5 and 1:2 by mass. After stirring and sonicating for 60 min to
separate stacked precursors, the precursor was first deposited on GO uniformly in aqueous solution. And then the intermediate products were centrifuged with deionized water and dispersed
into 150 mL DMF for refluxing at 95°C for 16 h. Ni(OH)2/GS composites were obtained by centrifuging and washing the mixture with copious de-ionized water and ethanol for several times and
then collected by lyophilization. MATERIALS CHARACTERIZATION X-ray diffraction (XRD) patterns were collected on Bruker D8 Focus Powder X-ray diffractometer using Cu Kα radiation (40 kV, 40
mA). The scanning electron microscopy was performed by using a field emission scanning electron microscopy (FESEM, HITACHI, S-4800). Transmission electron microscopy (TEM), high-resolution
transmission electron microscopy (HTEM), scanning transmission electron microscopy (STEM) and energy dispersive X-ray spectroscopy mapping technique were taken on a FEI Tacnai G2 electron
microscope operated at 200 kV. X-Ray photoelectron spectrometry data was acquired using an ESCALAB X-ray photoelectron spectrometer with monochromatic Al Kα X-rays. Zeta potential (ζ,
effective surface charge) was measured by dynamic light scattering (Malvern Nano-ZS, UK). Raman spectra were collected with a Renishaw 1000 model confocal microscopy Raman spectrometer with
a CCD detector and a holographic notch filter at ambient conditions. The specific surface area and porosity were determined by nitrogen sorption using a Micrometritics ASAP 2020 analyzer.
Specific surface areas were calculated by the Brunaure-Emmert-Teller (BET) method. Pore volumes and sizes were estimated from pore size distribution curves from the adsorption isotherms
using the Barrett-Joyner-Halenda (BJH) method. Thermogravimetric analysis (TGA) was performed at a heating rate of 5°C/min in flowing air (NETZSCH STA 449 F3, Germany). PREPARATION OF THE
ELECTRODES To evaluate the electrochemical properties of pure Ni(OH)2 and Ni(OH)2/GS composites, working electrodes were prepared by mixing the as-obtained powder (80 wt%) as active material
with acetylene black (10 wt%) and PTFE (10 wt%). The mixtures were grounded in alcohol and the obtained slurries were pasted and pressed onto a nickel foam substrate at 10 MPa and then
dried at 80°C overnight. The mass of the active materials was about 1.5 mg. Before testing, the electrode materials should be activated for abundant cycles until they can stabilizes at a
certain condition. ELECTROCHEMICAL EVALUATION The electrochemical measurements were carried out using a three-electrode mode in 6 M KOH aqueous solution. Hg/HgO electrode filled with 1 M KOH
was used as reference electrode and a platinum plate was used as counter electrode. Electrochemical studies including cyclic voltammetry (CV), galvanostatic charge-discharge (GC) and
electrochemical impedance spectroscopy (EIS) were carried out using VMP3 electrochemical workstation (Bio-logic Inc.). All tests were performed at room temperature. Typical CV curves were
measured at different scan rates from 2 to 200 mV s−1 between 0.1 and 0.6 V. GC measurements were conducted under various current densities from 1 to 100 A g−1 between 0.0 and 0.5 V. EIS
tests were performed for the working electrode in a frequency range of 100 kHz–0.01 Hz with ac perturbation of 5 mV. The EIS data were analyzed using Nyquist plots, which represent the
imaginary part (Z″) and real part (Z′) of impedance. REFERENCES * Chmiola, J. et al. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 313, 1760–1763
(2006). Article CAS ADS Google Scholar * Miller, J. R. & Simon, P. Electrochemical capacitors for energy management. Science 321, 651–652 (2008). Article CAS Google Scholar *
Chmiola, J., Largeot, C., Taberna, P. L., Simon, P. & Gogotsi, Y. Monolithic carbide-derived carbon films for micro-supercapacitors. Science 328, 480–483 (2010). Article CAS ADS
Google Scholar * Simon, P. & Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 7, 845–854 (2008). CAS ADS Google Scholar * Stoller, M. D. & Ruoff, R. S. Best
practice methods for determining an electrode material's performance for ultracapacitors. Energy Environ. Sci. 3, 1294–1310 (2010). Article CAS Google Scholar * Zhu, H., Wang, X.,
Liu, X. & Yang, X. Integrated synthesis of poly(o-phenylenediamine)-derived carbon materials for high performance supercapacitors. Adv. Mater. 24, 6524–6529 (2012). Article CAS Google
Scholar * Liu, C., Li, F., Ma, L. P. & Cheng, H. M. Advanced materials for energy storage. Adv. Mater. 22, E28–E62 (2010). Article CAS Google Scholar * Guo, Y. G., Hu, J. S. &
Wan, L. J. Nanostructured materials for electrochemical energy conversion and storage devices. Adv. Mater. 20, 2878–2887 (2008). Article CAS Google Scholar * Jiang, H., Lee, P. S. &
Li, C. 3D carbon based nanostructures for advanced supercapacitors. Energy Environ. Sci. 6, 41–53 (2013). Article CAS Google Scholar * Choi, N. S. et al. Challenges facing lithium
batteries and electrical double-layer capacitors. Angew. Chem. Int. Ed. 51, 9994–10024 (2012). Article CAS Google Scholar * Zhao, X., Sanchez, B. M., Dobson, P. J. & Grant, P. S. The
role of nanomaterials in redox-based supercapacitors for next generation energy storage devices. Nanoscale 3, 839–855 (2011). Article CAS ADS Google Scholar * Wang, G., Zhang, L. &
Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 41, 797–828 (2012). Article CAS Google Scholar * Yuan, C. et al. Ultrathin mesoporous
NiCo2O4 nanosheets supported on Ni foam as advanced electrodes for supercapacitors. Adv. Funct. Mater. 22, 4592–4597 (2012). Article CAS Google Scholar * Jiang, J. et al. Large-scale
uniform alpha-Co(OH)2 long nanowire arrays grown on graphite as pseudocapacitor electrodes. ACS Appl. Mater. Interfaces 3, 99–103 (2011). Article CAS Google Scholar * Zhang, G. & Lou,
X. W. Controlled growth of NiCo2O4 nanorods and ultrathin nanosheets on carbon nanofibers for high-performance supercapacitors. Sci. Rep. 3, 1470–1475 (2013). Article Google Scholar *
Guan, C. et al. Nanoporous walls on macroporous foam: rational design of electrodes to push areal pseudocapacitance. Adv. Mater. 24, 4186–4190 (2012). Article CAS Google Scholar * Jung,
S. M., Jung, H. Y., Dresselhaus, M. S., Jung, Y. J. & Kong, J. A facile route for 3D aerogels from nanostructured 1D and 2D materials. Sci. Rep. 2, 849–853 (2012). Article ADS Google
Scholar * Chien, H. C., Cheng, W. Y., Wang, Y. H. & Lu, S. Y. Ultrahigh specific capacitances for supercapacitors achieved by nickel cobaltite/carbon aerogel composites. Adv. Funct.
Mater. 22, 5038–5043 (2012). Article CAS Google Scholar * Shao, M. et al. Core–shell layered double hydroxide microspheres with tunable interior architecture for supercapacitors. Chem.
Mater. 24, 1192–1197 (2012). Article CAS Google Scholar * Tang, P. Y., Zhao, Y. Q., Wang, Y. M. & Xu, C. L. Metal decorated nickel foam inducing regulatable manganese dioxide
nanosheet arrays architecture for high-performance supercapacitor application. Nanoscale. 10.1039/C3NR02119J (2013). * Sumboja, A., Foo, C. Y., Wang, X. & Lee, P. S. Large Areal Mass,
Flexible and Free-Standing Reduced Graphene Oxide/Manganese Dioxide Paper for Asymmetric Supercapacitor Device. Adv. Mater. 25, 2809–2815 (2013). Article CAS Google Scholar * Chen, H.,
Hu, L., Yan, Y., Che, R., Chen, M. & Wu, L. One-Step Fabrication of Ultrathin Porous Nickel Hydroxide-Manganese Dioxide Hybrid Nanosheets for Supercapacitor Electrodes with Excellent
Capacitive Performance. Adv. Energy Mater. 10.1002/aenm.201300580 (2013). * Yang, G. W., Xu, C. L. & Li, H. L. Electrodeposited nickel hydroxide on nickel foam with ultrahigh
capacitance. Chem. Commun. 0, 6537–6539 (2008). Article CAS Google Scholar * Wu, M. S. & Huang, K. C. Fabrication of nickel hydroxide electrodes with open-ended hexagonal nanotube
arrays for high capacitance supercapacitors. Chem. Commun. 47, 12122–121224 (2011). CAS Google Scholar * Lu, Z., Chang, Z., Zhu, W. & Sun, X. Beta-phased Ni(OH)2 nanowall film with
reversible capacitance higher than theoretical Faradic capacitance. Chem. Commun. 47, 9651–9653 (2011). Article CAS Google Scholar * Jin, M. et al. Sponge-like Ni(OH)2-NiF2 composite film
with excellent electrochemical performance. Phys. Chem. Chem. Phys. 5, 1601–1605 (2013). Article Google Scholar * Zhong, J. H. et al. Co3O4/Ni(OH)2 composite mesoporous nanosheet networks
as a promising electrode for supercapacitor applications. J. Mater. Chem. 22, 5656–5665 (2012). Article CAS Google Scholar * Mohana Reddy, A. L., Gowda, S. R., Shaijumon, M. M. &
Ajayan, P. M. Hybrid nanostructures for energy storage applications. Adv. Mater. 24, 5045–5064 (2012). Article Google Scholar * Wang, Y. et al. High performance hybrid supercapacitor based
on graphene-supported Ni(OH)2-nanowites and ordered mesoporous carbon CMK-5. J. Electrochem. Soc. 160, A98–A104 (2013). Article CAS Google Scholar * Huang, X., Qi, X., Boey, F. &
Zhang, H. Graphene-based composites. Chem. Soc. Rev. 41, 666–686 (2012). Article CAS Google Scholar * Stoller, M. D., Park, S., Zhu, Y., An, J. & Ruoff, R. S. Graphene-based
ultracapacitors. Nano Lett. 8, 3498–3502 (2008). Article CAS ADS Google Scholar * Zhang, L. et al. Porous 3D graphene-based bulk materials with exceptional high surface area and
excellent conductivity for supercapacitors. Sci. Rep. 3, 1408–1416 (2013). Article Google Scholar * Sheng, K., Sun, Y., Li, C., Yuan, W. & Shi, G. Ultrahigh-rate supercapacitors based
on eletrochemically reduced graphene oxide for ac line-filtering. Sci. Rep. 2, 247–251 (2012). Article ADS Google Scholar * Lei, Z., Christov, N. & Zhao, X. S. Intercalation of
mesoporous carbon spheres between reduced graphene oxide sheets for preparing high-rate supercapacitor electrodes. Energy Environ. Sci. 4, 1866–1873 (2011). Article CAS Google Scholar *
Qu, Q., Yang, S. & Feng, X. 2D sandwich-like sheets of iron oxide grown on graphene as high energy anode material for supercapacitors. Adv. Mater. 23, 5574–5580 (2011). Article CAS
Google Scholar * Xu, B. et al. What is the choice for supercapacitors: graphene or graphene oxide. Energy Environ. Sci. 4, 2826–2830 (2011). Article CAS Google Scholar * Tour, J. M. et
al. Improved synthesis of graphene oxide. Acs Nano 4, 4806–4814 (2010). Article Google Scholar * Hummers, W. S. & Offeman, R. E. Preparation of graphitic oxide. J. Am. Chem. Soc. 80,
1339–1339 (1958). Article CAS Google Scholar * Lee, J. W., Ahn, T., Soundararajan, D., Ko, J. M. & Kim, J. D. Non-aqueous approach to the preparation of reduced graphene
oxide/alpha-Ni(OH)2 hybrid composites and their high capacitance behavior. Chem. Commun. 47, 6305–6307 (2011). Article CAS Google Scholar * Yan, J. et al. Advanced asymmetric
supercapacitors based on Ni(OH)2/graphene and porous graphene electrodes with high energy density. Adv. Funct. Mater. 22, 2632–2641 (2012). Article CAS Google Scholar * Wang, H.,
Casalongue, H. S., Liang, Y. & Dai, H. Ni(OH)2 nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. J. Am. Chem. Soc. 132, 7472–7477 (2010). Article CAS
Google Scholar * Dong, X. C. et al. 3D graphene–cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 6, 3206–3213 (2012). Article CAS
Google Scholar * Sathiya, M., Prakash, A. S., Ramesha, K., Tarascon, J. M. & Shukla, A. K. V2O5-anchored carbon nanotubes for enhanced electrochemical energy storage. J. Am. Chem. Soc.
133, 16291–16299 (2011). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work is financially supported by 100 Talents Program of The Chinese Academy of Sciences,
National Program on Key Basic Research Project of China (973 Program, Grant No. 2012CB215500), National Natural Science Foundation of China (Grant No. 21101147 and 21203176), China
Postdoctoral Science Foundation (2011M500624) and Special Foundation of China Postdoctoral Science (2012T50293). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * State Key Laboratory of Rare
Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022, China Zhong Wu, Xiao-Lei Huang, Zhong-Li Wang, Ji-Jing Xu, Heng-Guo Wang
& Xin-Bo Zhang * University of Chinese Academy of Sciences, Beijing, 100124, China Zhong Wu Authors * Zhong Wu View author publications You can also search for this author inPubMed
Google Scholar * Xiao-Lei Huang View author publications You can also search for this author inPubMed Google Scholar * Zhong-Li Wang View author publications You can also search for this
author inPubMed Google Scholar * Ji-Jing Xu View author publications You can also search for this author inPubMed Google Scholar * Heng-Guo Wang View author publications You can also search
for this author inPubMed Google Scholar * Xin-Bo Zhang View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Z.W. carried out the experiments and
wrote the paper. Z.W., X.L.H., Z.L.W., J.J.X. and H.G.W. discussed the results. X.B.Z. supervised the research and revised the manuscript. All authors reviewed the manuscript. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION Supporting Information RIGHTS AND
PERMISSIONS This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit
http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wu, Z., Huang, XL., Wang, ZL. _et al._ Electrostatic Induced Stretch Growth of
Homogeneous β-Ni(OH)2 on Graphene with Enhanced High-Rate Cycling for Supercapacitors. _Sci Rep_ 4, 3669 (2014). https://doi.org/10.1038/srep03669 Download citation * Received: 15 August
2013 * Accepted: 05 December 2013 * Published: 13 January 2014 * DOI: https://doi.org/10.1038/srep03669 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative


:max_bytes(150000):strip_icc():focal(319x0:321x2)/people_social_image-60e0c8af9eb14624a5b55f2c29dbe25b.png)






![[photograph]: honeycomb sandwich | science news](https://i0.wp.com/www.sciencenews.org/wp-content/uploads/2025/01/webfallback_dark.jpg)