- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Confirmatory tests for the diagnosis of brain death in addition to clinical findings may shorten observation time required in some countries and may add certainty to the diagnosis
under specific circumstances. The practicability of Gadolinium-enhanced magnetic resonance angiography to confirm cerebral circulatory arrest was assessed after the diagnosis of brain death
in 15 patients using a 1.5 Tesla MRI scanner. In all 15 patients extracranial blood flow distal to the external carotid arteries was undisturbed. In 14 patients no contrast medium was noted
within intracerebral vessels above the proximal level of the intracerebral arteries. In one patient more distal segments of the anterior and middle cerebral arteries (A3 and M3) were filled
with contrast medium. Gadolinium-enhanced MRA may be considered conclusive evidence of cerebral circulatory arrest, when major intracranial vessels fail to fill with contrast medium while
extracranial vessels show normal blood flow. SIMILAR CONTENT BEING VIEWED BY OTHERS COMPUTED TOMOGRAPHY ANGIOGRAPHY SCORING SYSTEMS AND THE ROLE OF SKULL DEFECTS IN THE CONFIRMATION OF BRAIN
DEATH Article Open access 23 July 2021 SILENT MAGNETIC RESONANCE ANGIOGRAPHY DIAGNOSTIC VALUE OF INTRACRANIAL UNRUPTURED ANEURYSMS Article Open access 07 February 2025 THE ESAH SCORE: A
SIMPLE PRACTICAL PREDICTIVE MODEL FOR SAH MORTALITY AND OUTCOMES Article Open access 28 December 2024 INTRODUCTION Death is the irreversible loss of all functions. The diagnosis of brain
death in most countries is based on the evidence of the loss of brain function and confirmation of the irreversibility of that loss of function1,2,3,4. The clinical criteria giving evidence
of the loss of brain function (coma, cranial nerve areflexia and apnea) are universally accepted. “_Most physicians recognize that the diagnosis of death based upon clinical judgement alone
is subject to human error_”5. The evidence for the irreversibility of loss of function, a waiting period and/or a confirmatory test, is viewed variably and reflects different concepts of
safety6,7. Two main groups of confirmatory tests focus either on the demonstration of the cessation of cerebral blood flow or the absence of cerebral bioelectrical brain activity.
Conventional angiography and electroencephalography (EEG) have frequently been recommended4. As the complete arrest of cerebral blood flow will result in a global brain infarction after a
short interval8 Bernat1 considered the registration of the absence of intracranial blood flow the most reliable finding to demonstrate an irreversible loss of all brain functions.
Identification of cerebral circulatory arrest has been reported using a number of techniques: computed tomography (CT) angiography, four-vessel angiography, cerebral digital subtraction
angiography, intravenous radionuclide angiography, single photon emission computed tomography (SPECT) and transcranial Doppler ultrasonography (TCD)9,10,11,12,13,14. All these techniques
have their limitations and their availability is variable. While TCD depends on the expertise of the investigator and requires a high degree of experience, CT angiography (CTA) involves
contrast media with a possibly harmful side effect on the donor or the transplantable organs. We consider contrast-enhanced MR-angiography (MRA) particularly suitable to identify cerebral
circulatory arrest. Subsequently we report our MRA findings, which we obtained in 15 patients after brain death had been diagnosed. RESULTS The clinical data and the MR findings of all brain
dead patients are presented in Table 1. Common MRI findings were diffuse gyral swelling, parenchymal hemorrhage; diffuse cerebral white matter injury and tonsilar herniation. In 14 of 15
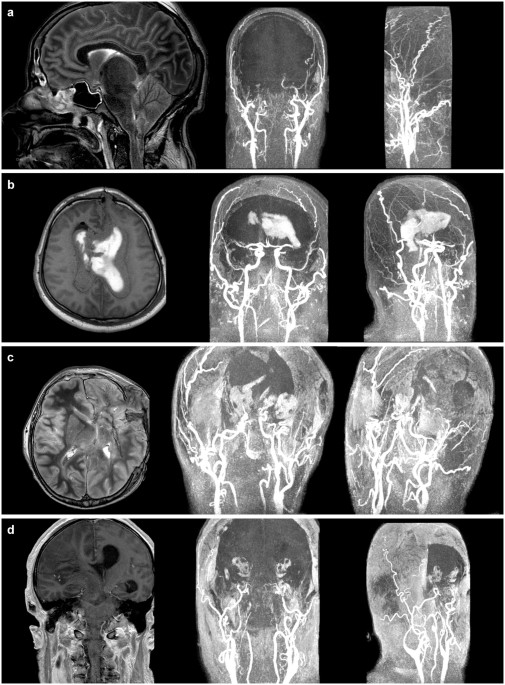
patients the Gadolinium-enhanced MRA showed no evidence of arterial blood flow in the intracranial circulation above the level of the proximal A2-segment of the anterior cerebral artery
(ACA) and the M1-segment of the middle cerebral artery (MCA) as depicted in Figure 1. One patient exhibited some extremely slow cerebral blood flow of contrast medium into the A3 and M3
segments after the diagnosis of brain death (Figure 2). DISCUSSION Confirmatory tests for the diagnosis of BD in addition to the clinical findings of coma and apnoeic cranial nerve areflexia
are not required in many countries4. It may be mandatory in some countries under specific clinical conditions6. Confirmatory tests are used in cases of unreliable clinical examinations, to
shorten the time of observation and in some countries in primary brain lesions of the posterior fossa. The treatment with CNS depressant medication (e.g. barbiturates) is the most frequent
reason for the delay of the diagnosis of brain death based on clinical criteria. As long as CNS depressant drugs have not been eliminated to a neglectable level or until other confounding
factors such as metabolic or endocrine disorders have not been excluded, brain death cannot be diagnosed on clinical criteria alone. A delay of the diagnosis of brain death has been linked
to an increased risk of organ failure in transplant recipients15 as timing has proved to be an important factor for successful transplantation. In order to preserve the integrity of the
donor organs and to decrease the rate of transplant failures some authors therefore call for a regular use of reliable confirmatory tests to shorten the time needed to diagnose brain
death16. Confirmatory tests may be divided into two groups: in those that demonstrate the cessation of cerebral blood flow (CBF) and those, which prove the absence of bioelectrical activity.
In the countries in which confirmatory tests are recommended or eventually even required, the most often used ancillary test is electroencephalography (EEG), which has been reported to have
a 90% sensitivity2. Other accepted electrophysiological tests rely on the evidence of the loss of evoked potentials17. A reliable confirmatory test would be most helpful particularly in
cases when cranial nerve areflexia cannot be verified because of lesions to the midface and the eyes or in concomitant drug intoxication. In recent years great efforts have been made to
develop new confirmatory tests for the reliable diagnosis of brain death. Magnetic resonance imaging (MRI) is believed to offer several sophisticated techniques that may improve the
diagnostics of BD. Aichner et al.18 observed uniform patterns of diffuse brain swelling and tonsilar herniation in patients with clinical signs of brain death. Kumada et al.19 found
decreased ADC values up to 40% as compared to a healthy control group emphasizing that DWI and ADC mapping show areas that correspond to edema of cytotoxic nature and ischemic tissue.
Several other authors confirmed these results20,21,22,23. In a recent study we evaluated the reliability of diffusion weighted imaging in the diagnosis of brain death. We concluded that the
unpredictable effects of pseudonormalization and possible susceptibility artifacts due to micro hemorrhages preclude a leading role of DWI in the diagnosis of brain death22. It is widely
accepted that the cessation of cerebral blood flow for about 30 minutes results in a global brain infarction and subsequently in brain death8. Hence, Bernat1 argued that the proof of a
complete cerebral circulatory arrest is the most reliable confirmation of an irreversible loss of all brain functions. The precise pathophysiology of the cessation of cerebral blood flow may
still be discussed, but several mechanisms have been proposed to explain the arrest of brain circulation in BD. A massively increased intracerebral pressure (ICP) leads to an increased
vascular resistance of the brain. When the ICP exceeds the systolic blood pressure, the brain cannot be perfused. As the tolerance of brain tissue to withdrawal of oxygen is very short, thus
irreversible destruction of brain tissue will ensue within minutes, leading to further swelling of the brain. Undoubtedly, a total cerebral circulatory arrest documents the “death of all
intracranial neurons”1,24. Various techniques have been used to demonstrate this arrest: four-vessel angiography12, cerebral intravenous digital subtraction angiography9, radionuclide
angiography10, single photon emission tomography25, MRI angiography26,27,28,29, CT angiography13 and a few more2. Transcranial Doppler sonography (TCD) is a non-invasive technique and is
independent from any contrast media. It is cost-effective and additionally fast and easy to use on the intensive care unit (ICU)30. Some authors have investigated the reliability of MR
angiography26,27,29. Ishii et al.26 suggested first that absence of the cerebral blood flow above the supraclinoid portions of ICA and their intracranial branches on MR angiograms is
specific to brain death. Karantanas et al.27 and Sohn et al.29 confirmed these findings using sophisticated time-of-flight (TOF) imaging techniques. TOF imaging has been increasingly
accepted as a technique for the examination of the intracranial circulation. The advantages of time-of-flight imaging include high spatial resolution, relative short imaging time, high
signal-to-noise ratio and the independence from any contrast agents31. It is based on the flow-related signal enhancement of the blood entering into the volume-of-interest32. TOF imaging,
however, underlies some technical limitations and potential pitfalls. Large intracranial hematomas excite high signal intensities leading to an extremely difficult and unreliable
interpretation of the intracranial circulation33. The dependence on adequate flow enhancement imposes certain constraints on time-of-flight imaging. Thus, slowed circulation causes dephasing
and saturation leading to signal losses. Furthermore, when vessels run parallel to the imaging slice orientation, the signal may become saturated with a subsequently undetectable blood
flow, even when the blood flow is not severely impaired31. As we are not aware of any preceding study examining the diagnostic value of gadolinium-enhanced MR angiography (MRA) for the
detection of brain death we examined its practicability to confirm cerebral circulatory arrest. In 14 patients the MRA showed no intracranial contrast signal media above the level of the
proximal A2-segment of the ACA and the M1-segment of the MCA indicating an irreversible loss of all brain functions (Figure 1). These results are consistent with the findings of several
other authors who investigated the intracranial circulation using TOF-based MR angiography26,27,29. No blood flow above the level of the supraclinoid portions of the ICA was found. One brain
dead patient presented with contrast medium in the A3 and M3 segments (Figure 2). Such findings are not unusual, as “_total cessation of circulation is less common than reduced cerebral
blood flow in the early phases of brain death_”5. Flower and Patel34 observed some markedly reduced residual intracranial arterial flow in 6 patients who were brain dead using radionuclide
angiography. Shah et al.35 evaluated the reliability of cerebral perfusion scintigraphy (CPS) in the diagnosis of brain death. They reported the case of a patient who suffered brain death as
a result of a ruptured giant aneurysm and following subarachnoid hemorrhage. A partially persistent intracranial perfusion was detected by using CPS. Several authors presented persistent
but reduced CBF in patients after diagnosis of brain death using various imaging techniques36,37 in up to 2–3%38. Decreased intracranial pressure e.g. after decompressive craniectomy,
extensive decompressing fractures or ventricular shunts may lead to preserved but markedly decreased cerebral blood flow or some reperfusion after the onset of complete and irreversible loss
of brain function38. In the present study patient #10 exhibited a markedly reduced intracranial circulation after unilateral decompressive craniectomy and subsequent diagnosis of brain
death (Figure 2). These observations from angiography have led to the concept that the documentation of cerebral circulatory arrest is conclusive evidence of brain death. Some residual blood
flow is compatible with brain death but less conclusive than large vessels failing to fill with contrast medium while extracranial vessels exhibit normal circulation39. In the case of
residual cerebral blood the diagnosis of brain death cannot be ruled out and diagnosis should be based either on the duration of observation time or other confirmatory tests. An ideal
confirmatory test for the diagnosis of brain death is safe, extremely sensitive, reliable and widely available. None of the existing ancillary tests fulfill all these criteria. Validities
and sensitivities of 90% and more were reported for the widely accepted ancillary tests that confirm brain death by either demonstrating the absence of cerebral bioelectrical activity or
cerebral circulation2,14. Bedside examinations are more practicable on the intensive care unit (ICU) and MRI of ventilated patients is quite a burden in terms of time and personnel required.
All confirmatory tests, however, have their limitations. TCD is investigator-dependent and requires a high degree of experience. Four-vessel and CT angiography are based on contrast media
with a potentially harmful impact on the possibly alive patients and on possible donor organs. Direct arterial angiography is hardly performed in Germany because of legal concerns, since
adverse effects of iodine contrast media and arterial infarction are a rare but realistic risk40. EEG is useless in hypothermia, CNS depressants and hypotonic conditions2. Angiographic
methods that demonstrate the absence of cerebral blood flow are independent from confounders like drug-intoxication or metabolic disturbances that preclude the BD diagnosis on clinical
criteria solely. The presented preliminary results of gadolinium-enhanced MRA likewise show highest sensitivity and validity. To date, now study compared the validity and reliability between
contrast-enhanced (CE-MRA) and non-contrast-enhanced magnetic resonance angiography (NCA-MRA) and its practicability in the diagnosis of BD. Both methods showed sensitivities and
specificities up to 100%. The substantial differences, however, are based on the methodical issues26,27,29. Thus, CE-MRA relies on the paramagnetic properties of the applied contrast agents
like Gadolinium (Gd) that shorten the T1 relaxation time. In contrast, NCE-MRA techniques, such as TOF, are based on the suppression of the background signal by slice-selective
radiofrequency pulses that saturate the signal from the stationary tissue. NCE-MRA techniques fully refrain from the use of contrast agents. Advantages of CE-MRA relative to NCE-MRA
techniques include considerably shorter acquisition times, improved anatomical coverage and decreased susceptibility artifacts caused by hemorrhages or other pathological conditions like
increased intracranial pressure and reduced intracranial blood flow41. The major drawback of CE-MRA is the dependence on contrast agents such as Gd that is known to induce nephrogenic
systemic fibrosis (NSF); a very rare syndrome however, which appears to occur exclusively in patients with renal impairment. Severe, life-threatening anaphylactoid or nonallergic
anaphylactic reactions are exceedingly rare with a likelihood of about 1:1,000,000 patients42. No case of NSF has been reported in patients with unimpaired renal function as of yet43. The
effect of contrast media on organ transplants is unknown as of yet. The unwanted side effects of invasive arterial angiography with iodine contrast media have raised generally accepted legal
concerns. The likelihood of severe unwanted side effects of iodine contrast media is 1:170,00044. Thus, MR angiography with Gd appears to pose a tenfold lower risk to the patient than CT
angiography with iodine contrast media. We concluded that contrast-enhanced MRA techniques may be useful to confirm brain death and that its inherent risk is acceptably low. A comparison of
CE- and NCE-MRA in future studies may be interesting. METHODS The present study was approved by the Local Ethics Committee of the University of Magdeburg in compliance with national
legislation and the Code of Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association (Declaration of Helsinki). PATIENTS Gadolinium-enhanced MRA of
the brain was prospectively obtained in fifteen patients (13 males and 2 females; mean age 58.20±19.6 years; age ranged between 20 and 80 years). The primary underlying brain lesion was
caused by: traumatic brain injury (n = 6), intracerebral hemorrhage (n = 7) and subarachnoid hemorrhage (n = 2). Brain death was diagnosed according to the guidelines of the
Bundesärztekammer [Richtlinien zur Feststellung des Hirntodes, Deutsches Ärzteblatt 95, Heft 30, 24.07.1998]. Clinical tests included the confirmation of coma, cranial nerve areflexia, apnea
and the loss of cerebral bioelectrical activity using EEG for at least 30 minutes as a sign of irreversible bioelectrical silence. The presence of a brain lesion and exclusion of other
factors that can cause coma was a prerequisite (e.g. CNS-depressant drugs, hypothermia, imbalance of electrolytes, metabolic or endocrine disturbances). IMAGING All patients were examined
within the first 24 hours after the clinical diagnosis of brain death. MR imaging was conducted on a 1.5 Tesla Intera scanner (Philips Medical Systems, Best, Netherlands) using a 16-channel
head coil. Details of the MRA protocol are listed in Table 2. The MR angiography was visualized by a maximum intensity projection (MIP). DATA ANALYSIS Image data of all observed brain dead
patients were pseudonymized and transferred to a separate workstation running Osirix [Version 5.5.2, http://www.osirix-viewer.com/]. The MRA findings were evaluated by neuroradiologists.
REFERENCES * Bernat, J. L. On irreversibility as a prerequisite for brain death determination. Adv Exp Med Biol 550, 161–167 (2004). Article Google Scholar * Machado, C. Diagnosis of brain
death. Neurol Int 2, e2, 10.4081/ni.2010.e2 (2010). Article CAS PubMed PubMed Central Google Scholar * Stevens, R. D. & Bhardwaj, A. Approach to the comatose patient. Crit Care Med
34, 31–41 (2006). Article Google Scholar * Wijdicks, E. F., Varelas, P. N., Gronseth, G. S. & Greer, D. M. Evidence-based guideline update: determining brain death in adults: report
of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 74, 1911–1918, 10.1212/WNL.0b013e3181e242a8 (2010). Article PubMed Google Scholar * Walker, A. E.
Cerebral death. 3rd edn, 95 (Urban & Schwarzenberg, 1985). * Haupt, W. F. & Rudolf, J. European brain death codes: a comparison of national guidelines. J Neurol 246, 432–437 (1999).
Article CAS Google Scholar * Wijdicks, E. F. Brain death worldwide: accepted fact but no global consensus in diagnostic criteria. Neurology 58, 20–25 (2002). Article Google Scholar *
Ingvar, D. H. Brain death–total brain infarction. Acta Anaesthesiol Scand Suppl 45, 129–140 (1971). Article CAS Google Scholar * Albertini, A., Schonfeld, S., Hiatt, M. & Hegyi, T.
Digital subtraction angiography–a new approach to brain death determination in the newborn. Pediatr Radiol 23, 195–197 (1993). Article CAS Google Scholar * Braunstein, P., Korein, J.,
Kricheff, II. & Lieberman, A. Evaluation of the critical deficit of cerebral circulation using radioactive tracers (bolus technique). Ann N Y Acad Sci 315, 143–167 (1978). Article CAS
ADS Google Scholar * Combes, J. C., Chomel, A., Ricolfi, F., d'Athis, P. & Freysz, M. Reliability of computed tomographic angiography in the diagnosis of brain death. Transplant
Proc 39, 16–20, 10.1016/j.transproceed.2006.10.204 (2007). Article PubMed Google Scholar * Munari, M. et al. Confirmatory tests in the diagnosis of brain death: comparison between SPECT
and contrast angiography. Crit Care Med 33, 2068–2073 (2005). Article Google Scholar * Qureshi, A. I., Kirmani, J. F., Xavier, A. R. & Siddiqui, A. M. Computed tomographic angiography
for diagnosis of brain death. Neurology 62, 652–653 (2004). Article Google Scholar * Welschehold, S. et al. Technical aids in the diagnosis of brain death: a comparison of SEP, AEP, EEG,
TCD and CT angiography. Dtsch Arztebl Int 109, 624–630, 10.3238/arztebl.2012.0624 (2012). Article PubMed PubMed Central Google Scholar * Ramjug, S., Hussain, N. & Yonan, N. Prolonged
time between donor brain death and organ retrieval results in an increased risk of mortality in cardiac transplant recipients. Interact Cardiovasc Thorac Surg 12, 938–942,
10.1510/icvts.2010.252809 (2011). Article PubMed Google Scholar * Lopez-Navidad, A. et al. Early diagnosis of brain death in patients treated with central nervous system depressant drugs.
Transplantation 70, 131–135 (2000). CAS PubMed Google Scholar * Firsching, R. & Frowein, R. A. in _Head Injuries_ Vol. 17 _Advances in Neurosurgery_ (eds ReinholdA Frowein, Mario
Brock, & Margareta Klinger) Ch. 48, 275–280 (Springer Berlin Heidelberg, 1989). * Aichner, F. et al. Magnetic resonance: a noninvasive approach to metabolism, circulation and morphology
in human brain death. Ann Neurol 32, 507–511, 10.1002/ana.410320405 (1992). Article CAS PubMed Google Scholar * Kumada, K. et al. Diffusion-weighted imaging of brain death: study of
apparent diffusion coefficient. No To Shinkei 53, 1027–1031 (2001). CAS PubMed Google Scholar * Lovblad, K. O. & Bassetti, C. Diffusion-weighted magnetic resonance imaging in brain
death. Stroke 31, 539–542 (2000). Article CAS Google Scholar * Nakahara, M., Ericson, K. & Bellander, B. M. Diffusion-weighted MR and apparent diffusion coefficient in the evaluation
of severe brain injury. Acta Radiol 42, 365–369 (2001). Article CAS Google Scholar * Luchtmann, M. et al. Controversies of diffusion weighted imaging in the diagnosis of brain death. J
Neuroimaging 23, 463–468 (2013). Article Google Scholar * Selcuk, H. et al. Diffusion-weighted imaging findings in brain death. Neuroradiology 54, 547–554, 10.1007/s00234-011-0912-9
(2012). Article PubMed Google Scholar * Pallis, C. Whole-brain death reconsidered–physiological facts and philosophy. J Med Ethics 9, 32–37 (1983). Article CAS Google Scholar * Facco,
E. et al. 99mTc-HMPAO SPECT in the diagnosis of brain death. Intensive Care Med 24, 911–917 (1998). Article CAS Google Scholar * Ishii, K. et al. Brain death: MR and MR angiography. AJNR
Am J Neuroradiol 17, 731–735 (1996). CAS PubMed Google Scholar * Karantanas, A. H., Hadjigeorgiou, G. M., Paterakis, K., Sfiras, D. & Komnos, A. Contribution of MRI and MR angiography
in early diagnosis of brain death. Eur Radiol 12, 2710–2716, 10.1007/s00330-002-1336-z (2002). Article CAS PubMed Google Scholar * Matsumura, A. et al. Magnetic resonance imaging of
brain death. Neurol Med Chir (Tokyo) 36, 166–171 (1996). Article CAS Google Scholar * Sohn, C. H. et al. Imaging findings of brain death on 3-tesla MRI. Korean J Radiol 13, 541–549,
10.3348/kjr.2012.13.5.541 (2012). Article PubMed PubMed Central Google Scholar * Conti, A. et al. Transcranial Doppler ultrasonography in the assessment of cerebral circulation arrest:
improving sensitivity by transcervical and transorbital carotid insonation and serial examinations. Neurocrit Care 10, 326–335, 10.1007/s12028-009-9199-7 (2009). Article PubMed Google
Scholar * Lohan, D. G. S., R. Nael, K., Krishnam, M. & Finn, J. P. Contrast-enhanced MRA versus nonenhanced MRA: Pros and cons. Applied Radiology 36, 3–15 (2007). Google Scholar *
Nishimura, D. G. Time-of-flight MR angiography. Magn Reson Med 14, 194–201 (1990). Article CAS Google Scholar * Wilcock, D. J., Jaspan, T. & Worthington, B. S. Problems and pitfalls
of 3-D TOF magnetic resonance angiography of the intracranial circulation. Clin Radiol 50, 526–532 (1995). Article CAS Google Scholar * Flowers, W. M. & Patel, B. R. Radionuclide
angiography as a confirmatory test for brain death: A review of 229 studies in 219 patients. South Med J 90, 1091–1096 (1997). Article Google Scholar * Shah, R., Miron, S. & Sodee, D.
B. Visualization of cerebral aneurysm on Tc-99m HMPAO brain perfusion scintigram for brain death. Clin Nucl Med 19, 457–458 (1994). Article CAS Google Scholar * Nau, R. et al. Results of
four technical investigations in fifty clinically brain dead patients. Intensive Care Med 18, 82–88 (1992). Article CAS Google Scholar * Rosenklint, A. & Jorgensen, P. B. Evaluation
of angiographic methods in the diagnosis of brain death. Correlation with local and systemic arterial pressure and intracranial pressure. Neuroradiology 7, 215–219 (1974). Article CAS
Google Scholar * Flowers, W. M., Jr & Patel, B. R. Persistence of cerebral blood flow after brain death. South Med J 93, 364–370 (2000). Article Google Scholar * Vlahovitch, B. F., P.
Kuhner, A. Stopak, B. Allais, B. & Gros, C. Arrêt circulatoire intracranien dans la mort du cerveau: Angiographie avec injection sous pression. Acta Radiologica 13, 334–349 (1972).
Article Google Scholar * König, B. Todesbegriff, Todesdiagnostik und Strafrecht. Kieler Schriften zum Strafrecht. 1st edn, Vol. 9 (Alfred Metzner Verlag, 1989). * Hartung, M. P., Grist, T.
M. & Francois, C. J. Magnetic resonance angiography: current status and future directions. J Cardiovasc Magn Reson 13, 19, 10.1186/1532-429X-13-19 (2011). Article PubMed PubMed
Central Google Scholar * Prince, M. R., Zhang, H., Zou, Z., Staron, R. B. & Brill, P. W. Incidence of immediate gadolinium contrast media reactions. Am J Roentgenol 196, W138–143,
10.2214/AJR.10.4885 (2011). Article Google Scholar * Wang, Y. et al. Incidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium-based contrast agent guidelines.
Radiology 260, 105–111, 10.1148/radiol.11102340 (2011). Article PubMed Google Scholar * Morcos, S. K. & Thomsen, H. S. Adverse reactions to iodinated contrast media. Eur Radiol 11,
1267–1275 (2001). Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Neurosurgery, Otto-von-Guericke-University Magdeburg, M.
Luchtmann, J. Kohl & R. Firsching * Institute of Neuroradiology, Otto-von-Guericke-University Magdeburg, O. Beuing, M. Skalej & S. Serowy * Institute of Biometry and Medical
Informatics, Otto-von-Guericke-University Magdeburg, J. Bernarding Authors * M. Luchtmann View author publications You can also search for this author inPubMed Google Scholar * O. Beuing
View author publications You can also search for this author inPubMed Google Scholar * M. Skalej View author publications You can also search for this author inPubMed Google Scholar * J.
Kohl View author publications You can also search for this author inPubMed Google Scholar * S. Serowy View author publications You can also search for this author inPubMed Google Scholar *
J. Bernarding View author publications You can also search for this author inPubMed Google Scholar * R. Firsching View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS M.L. prepared the manuscript including all figures/tables and was responsible for the clinical part of the data collection and analysis. O.B. and M.S. were responsible
for the technical part of the data collection. J.K. was responsible for the clinical part of the data collection. S.S. analysed the collected data especially the maximum intensity
projections (MIP) of the MRA. J.B. prepared parts of the manuscript and analyzed the collected data. R.F. designed the study and was responsible for manuscript preparation and data analysis.
All authors reviewed the manuscript. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. RIGHTS AND PERMISSIONS This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Luchtmann, M., Beuing, O., Skalej, M. _et al._ Gadolinium-enhanced magnetic resonance angiography in brain death. _Sci Rep_ 4, 3659 (2014).
https://doi.org/10.1038/srep03659 Download citation * Received: 08 October 2013 * Accepted: 16 December 2013 * Published: 13 January 2014 * DOI: https://doi.org/10.1038/srep03659 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative



:max_bytes(150000):strip_icc():focal(506x311:508x313)/Lexi-Reed-Finally-Able-to-Walk-After-Months-in-the-Hospital-for-Calciphylaxis-092722-1-62b2912a57a54519a7ccc481183817cb.jpg)



