- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Near Eastern wild boars possess a characteristic DNA signature. Unexpectedly, wild boars from Israel have the DNA sequences of European wild boars and domestic pigs. To understand
how this anomaly evolved, we sequenced DNA from ancient and modern pigs from Israel. Pigs from Late Bronze Age (until ca. 1150 BCE) in Israel shared haplotypes of modern and ancient Near
Eastern pigs. European haplotypes became dominant only during the Iron Age (ca. 900 BCE). This raises the possibility that European pigs were brought to the region by the Sea Peoples who
migrated to the Levant at that time. Then, a complete genetic turnover took place, most likely because of repeated admixture between local and introduced European domestic pigs that went
feral. Severe population bottlenecks likely accelerated this process. Introductions by humans have strongly affected the phylogeography of wild animals, and interpretations of phylogeography
based on modern DNA alone should be taken with caution. SIMILAR CONTENT BEING VIEWED BY OTHERS COMPLETE MITOCHONDRIAL GENOME SEQUENCE ANALYSIS REVEALED DOUBLE MATRILINEAL COMPONENTS IN
INDIAN GHOONGROO PIGS Article Open access 17 January 2025 ORIGIN AND SPATIAL POPULATION STRUCTURE OF MALAGASY NATIVE CHICKENS BASED ON MITOCHONDRIAL DNA Article Open access 04 January 2024
SPATIAL GENETIC STRUCTURE OF EUROPEAN WILD BOAR, WITH INFERENCES ON LATE-PLEISTOCENE AND HOLOCENE DEMOGRAPHIC HISTORY Article Open access 13 January 2023 INTRODUCTION Phylogeographic studies
use phylogenetic methods and geographical contexts to reveal evolutionary histories of species to follow migrations and contraction/expansion processes1,2. Recent studies of mitochondrial
DNA (mtDNA) of wild boar (_Sus scrofa_) found strong phylogeographic structure across its entire distribution range with European, Near Eastern and East Asian pigs falling into separate
clades3,4,5,6,7. This structure has enabled researchers to infer past events in the history of wild boars, such as postglacial recolonisation of northern Europe from southern refugia8.
Violation of this phylogeographic structure was used to infer human movements and translocations across Europe and the Pacific4,5,9. During the Epipaleolithic and Neolithic periods, humans
introduced wild boars into various regions across Eurasia (e.g.10,11,12). Since domestication, however, the assumption is that humans have mainly translocated domestic pigs rather than wild
boars. It was remarkable, then, that Giuffra _et al._3 reported that three modern wild boars from Israel possessed a European mtDNA haplotype. In contrast, studies have shown that modern
wild boars from other Near Eastern countries (i.e., Egypt, Syria, Turkey, Armenia, Iraq and Iran) possess Near Eastern haplotypes5,7,9. Patterns observed using only modern mtDNA might not
accurately represent earlier phylogeographic events such as migrations, translocations and extinctions (e.g.13,14,15). Therefore, ancient DNA is a powerful tool for revealing population
histories, and can shed light on past events (e.g.16,17,18). Through the use of ancient DNA, we aim to elucidate the apparent paradox of the modern Israeli wild boar, and to determine just
how far back this inconsistency extends. Today, wild boars are found in the Mediterranean region of Israel and south of the Dead Sea19. They have been part of the fauna of the southern
Levant since at least the middle Pleistocene, ca.0.78 Mya20. Pigs were domesticated in the Neolithic21,22 and their remains are found at most archaeological sites in Israel. Their frequency,
however, varies greatly between sites as well as between periods (e.g.23,24). Variation in the number of pig bones, and therefore in the past consumption of pork, depends on several
factors, including climate, modes of life, as well as cultural and religious preferences25,26,27. Pig remains, however, are especially abundant in the Israeli archaeological record in four
periods: Middle Bronze II (ca. 2000 BCE–1550 BCE); Iron I (ca. 1150-800 BCE) faunal assemblages in Philistia (the southern coastal plain) (e.g.23,28,29,30); assemblages from non-Jewish sites
from the Roman (634-324 BCE) and Byzantine periods (324-63 BCE)23,27. There is extensive archaeological evidence showing that during all these periods there were contacts between the Levant
and Europe (e.g.31,32,33; needless to say, connections became stronger with time). In order to resolve the phylogeographic inconsistency that the modern Israeli wild boars have a different
mtDNA signature from their surroundings, we examined a combination of modern and ancient DNA sequences of suids from Israel. We analysed DNA from a series of modern wild boars from museum
collections representing their full biogeographical distribution across the country, in order to assess the observation made by Giuffra _et al._3 and examine whether their results reflected
a genetic anomaly or a widespread pattern. Ancient DNA data enabled us to test whether this was a case of shifting spatial and temporal patterns of haplotypes, and if so to pinpoint when it
had started. For the modern wild boar samples, we amplified a combination of mtDNA control region (CR), and nuclear Melanocortin receptor 1 (MC1R) gene. This is the first time that Near
Eastern wild boars have been sequenced for the MC1R gene that is associated with coat colour variation (e.g.34,35). We chose this nuclear marker because a large geographical dataset is
available for this gene. Moreover, recent studies showed that pig coat colour is the result of strong selective pressures (e.g.36,37). Thus European and Asian pigs can be distinguished
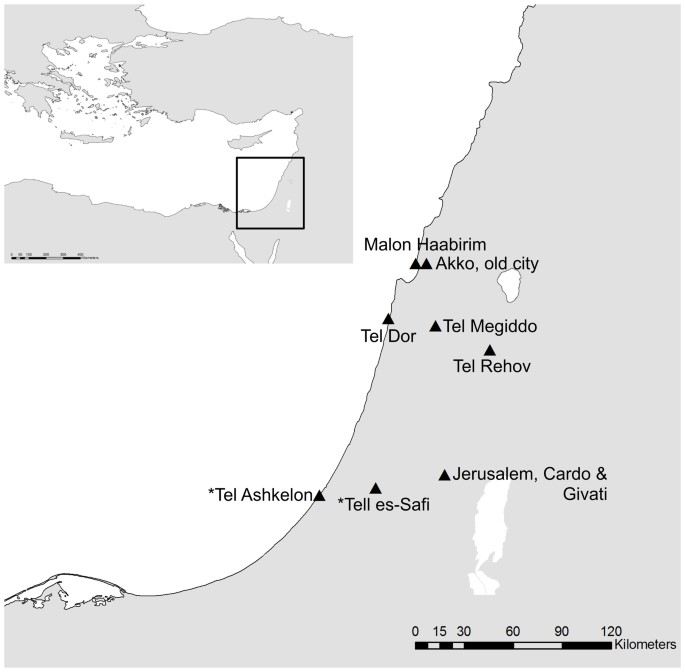
according to their MC1R gene. Using ancient DNA techniques, we studied domestic pig bones from a diachronic series of Israeli archaeological assemblages dating from the Neolithic period to
medieval times (Fig. 1). For the ancient samples we analysed a short fragment of the mtDNA CR. We then compared our results to phylogenetic data on Asian and European pigs to elucidate the
biogeographic affinities of our samples. RESULTS MODERN DNA We analysed the DNA from 25 museum specimens of modern wild boars from Israel using mtDNA CR (see Supplementary Table S1). The
phylogenetic tree of mtDNA CR was based on 621 bp, and is in agreement with previous studies of _Sus scrofa_5,6,7,9. Three supported clades are known – one European (with Italian samples
forming a divergent clade at the base of the group), and two Near Eastern – while the rest of the tree consists of East Asian pigs (Fig. 2). All 25 modern wild boars from Israel as well as
one Israeli boar sequence from GenBank (accession number AF136558) (Fig. 2) fell within the European clade. Very little genetic diversity was found in the mtDNA CR between these wild boar
sequences, with 21 samples sharing the same haplotype. We also analysed the nuclear MC1R region in 17 modern wild boar specimens from Israel. A median-joining network, based on 963 bp of
MC1R region demonstrates the differences between European and Asian suids, and confirms that extant wild boars from Israel possess a European wild boar haplotype (identical to GenBank
Accession numbers: EU443645-56 & GQ240942) (see Supplementary Fig. S1). ANCIENT DNA We successfully extracted and sequenced DNA from 34 out of 177 ancient domestic pig bones sampled from
archaeological sites in Israel (Fig. 1). The overall success rate was 19%, which is the expected rate given the climate in the region38,39 (see Supplementary Fig. S2). The samples were
collected from secure stratigraphic contexts associated with well-defined ceramic assemblages, which provide secure relative chronology (i.e., period and/or sub-period). Many of the Bronze
and Iron Age strata from which the bones were obtained have been radiocarbon dated (e.g.40,41,42,43,44). We further obtained three dates out of the four samples we submitted for radiocarbon
dating (there was insufficient collagen for dating in the fourth) (see Supplementary Table S1). Two fragments of the mtDNA CR were amplified: one (ANC1) following Larson _et al._5, the
second was chosen to increase resolution for the Near Eastern clade, and was targeted to the 3′ end of the modern CR fragment. The ancient mtDNA data reveal the existence of two Near Eastern
haplotypes in Israel in the past (Arm 1T and Y1) (Fig. 3). Haplotype Arm 1T is present in six of the 34 sequenced samples: one sample dating to the Middle Bronze I (MMP168 dated to
1895-1610 cal. BCE), three samples dating to the Iron I (11th century BCE), one to the Iron IIA (9th century BCE) and one to the Byzantine period (MMP253 dated to 400-605 cal. CE) (see
Supplementary Table S1). Haplotype Y1 is present in the Late Bronze III to the Iron IIA (ca. 1200-800 BCE). The major turnover in the pig population occurred at the beginning of the Iron II,
around 900 BCE, when the European haplotype first became predominant relative to the Near Eastern haplotypes (one sample is dated, MMP175 1005-805BC cal. BCE). One sample with a European
haplotype comes from a Middle Bronze context (2000-1550 BCE) in the port city of Ashkelon. Unfortunately, there was insufficient soluble collagen to radiocarbon date this bone and confirm
its age. The sample was re-extracted three times and multiple amplifications resulted in the same sequence. In order to follow changes in the frequency of haplotypes through time and space,
and understand the relationship between the samples, we created a three-dimensional statistical parsimony network of mtDNA CR (Fig. 4). The network is in agreement with the modern
phylogenetic tree of _Sus scrofa_ in which Europe, the Near East and East Asia fall into separate groups. The two Near Eastern haplotypes (Arm 1T and Y1) from this region are missing from
modern Israeli pigs but are still found elsewhere in the Near East. In the ancient Israeli specimens, we found additional mutations to the Near Eastern haplotypes Arm 1T and Y1: a mutation
in the combined fragment in one Iron Age sample (MMP63), and an insertion in the Y1 haplotype in three samples from the Late Bronze and Iron I (MMP121, 129, 242). We also observed mutations
in the European haplogroup in two specimens from the Roman period (MMP150, 211). These samples were re-extracted and multiple amplifications resulted in the same sequence. However, they are
still confined to the general haplotypes that are of interest to us. DISCUSSION Our results show that all modern Israeli wild boar populations share a European mitochondrial genetic
signature (Fig. 2). This is despite the fact that the samples derive from different habitats–coastal, mountainous and desert, and represent a period of 60 years. Our results confirm those of
Giuffra _et al._3, who found a distinction between wild boars from Israel and those from the rest of the Near East, where local haplotypes are dominant in the modern populations5,6,7,9. The
ancient DNA data offer an explanation for this paradox. The data demonstrate that domestic pigs from the region possessed Near Eastern haplotypes (Arm 1T and Y1) (Fig. 3). These haplotypes
were also found in Anatolian wild boars before domestication, starting in the Neolithic period, meaning that these haplotypes were the local genetic signature in the region (Fig. 5a). During
the early phases of the Iron Age, however, domestic European pigs were introduced into the region. A millennium or so later, this genetic signature reached fixation in terms of mtDNA (Fig.
3). Subsequent introductions of European pigs into the southern Levant might have occurred after the Iron Age—for instance in Roman-Byzantine times and during the Crusades—and this may have
contributed to the dominance of the European haplotype. The introduced domestic European pigs had a crucial effect on the mtDNA of the indigenous wild boar populations of Israel. Feral
European pigs could have driven the local wild boars to extinction, or, more likely, they could have hybridized the local wild boar population. Although the MC1R data show that modern wild
boars from Israel possess a European wild boar coat colour (see Supplementary Fig. S1), we cannot distinguish whether this signal is a true local signature or whether it comes from the
introduced European domestic pigs that went feral and retained the wild boar coat colour. Data regarding this gene from other parts of the Near East are missing and hence we cannot reach
firmer conclusions. The outcome of these introductions, in terms of mtDNA, was a complete turnover (Fig. 3). In Anatolia, a similar pattern is observed in the Late Bronze Age (samples dated
to ca. 1,600-1,440 BCE), when European haplotypes first appeared. In Anatolia too the major transition to a European haplotype took place during the Iron Age9. But, in contrast to the
situation in Israel, where the European haplotype became the dominant genetic signature, in Anatolia the modern wild boar populations still possess Near Eastern haplotypes9 (Fig. 5). In
Israel this could have happened through repeated admixture with introduced European domestic pigs that went feral as well as severe population bottlenecks. A recent documented bottleneck
that occurred during the 1930s and 1940s, when wild boar populations were decimated by overhunting19, may explain the lack of genetic diversity across the modern populations and the fixation
of the European signature. Although we cannot refute the possibility that European pigs were brought to the southern Levant in an earlier period (see the sample from Middle Bronze Ashkelon,
but note that it could not be radiocarbon dated and may be intrusive), their first main appearance occurred during the Iron Age. The major shift between Near Eastern to European haplotypes
in Israel took place around 900 BCE. This could have been connected to the migration and settlement of the Sea Peoples – groups that originated in the Aegean Basin, Cyprus and/or southern
Anatolia. According to textual material and archaeological finds, in the 12th to early 10th centuries BCE, they settled along the coastal plain of the Levant. The Philistines are the
best-known and most studied group among these peoples. They established themselves mainly in urban centres in the southern coastal plain, such as Gath (Tell es-Safi), Tel Miqne-Ekron and
Ashkelon (e.g.30,31,33,45). The arrival of the Philistines was accompanied by a sharp rise in the ratio of pig bone in their main urban sites30,45. The exceptionally high frequencies (up to
20–25% of the faunal assemblage) stand in stark contrast to the low numbers of pigs in most contemporaneous assemblages from local Canaanite and early Israelite settlements (usually <2%
of the faunal assemblage)23,29,30,46,47. Morphological evidence from Tel Miqne-Ekron also points to the possible entry of a new pig population into the site with the arrival of the
Philistines48. Since the distribution of Philistine pottery indicates at least cultural diffusion to broader areas of the region, beyond the coast of the Levant, e.g., to the north and
inland of the Levant, it is possible that the introduction of European pigs might have been a broader phenomenon. Note that later migrations from the west, for instance in the
Hellenistic-to-Byzantine periods and the time of the Crusades, could have re-introduced European pigs into coastal areas as well as inland parts of the Levant. The abundance of pig remains,
together with the genetic results presented here, hint at the possibility that pigs were translocated by the Sea Peoples in general and the Philistines in particular during their migration
to the southern Levant. A ca. 250 year gap exists between their first arrival and our first European mtDNA signal. This can be explained by several factors: 1) the Sea Peoples migration was
a several-decades-long process (e.g.49) that seems to have peaked in the 11th century BCE; 2) most of our Iron Age samples come from non-Sea Peoples' sites; 3) the sample-size is too
small to track minute chronological processes within the early phases of the Iron Age; 4) it took some time for the haplotype to become significant. The results of this study show that
modern DNA by itself cannot reflect the entire history of a species, and emphasize the effect of turnovers in misinterpreting phylogeographic patterns. In the past, people transported pigs
over significant distances and this played an important role in shaping the phylogenetic patterns we observe today. Ancient DNA is a powerful tool that aids in deciphering species histories,
since phylogeographic patterns are far more complex than surmised from present-day biogeography. METHODS SAMPLE COLLECTION We examined a total of 38 bone, hair, skin and soft tissue samples
of modern wild boars from Israeli museum collections (see Supplementary Table S1). The samples cover the entire biogeographic distribution of wild boars in the country, and all four
populations as suggested in Davidowitz and Horwitz50 based on morphological characteristics. We also obtained 177 samples of ancient pig bones from archaeological sites across Israel,
spanning the Neolithic to Crusader periods (ca. 7000 BCE to 1300 CE) (Fig. 1, Supplementary Table S1). Most of the samples are of domestic pigs as evident from demographic and osteometric
data. They derive from well-excavated sites with secure stratigraphic contexts, in many cases accompanied by multiple radiocarbon-dates (e.g.40,41,42,43,44). Four samples were directly
radiocarbon dated at the Weizmann Institute, Rehovot, Israel. The dates throughout the text are stated as calibrated dates. DNA EXTRACTION AND AMPLIFICATION MODERN SAMPLES DNA from bones was
extracted according to a method modified from Yang _et al_.51. Specifically, about 50 mg of bone powder was incubated with 0.44 M EDTA (pH = 8) (AMRESCO, USA), 0.1 M urea, and 20 mg/ml
proteinase K (AMRESCO, USA) overnight at 56°C. After decalcification and digestion, the supernatant was concentrated to about 100 μl using Vivaspin filter 3000 MWCO (Sartorius Stedim
Biotech), and then directly purified using silica based spin columns (Minelute PCR Purification kit, QIAGEN, Inc). DNA from tissue, skin, hair, and other non-bone samples were extracted
using QIAamp DNA Micro Kit (QIAGEN, Inc) following a protocol for forensic casework samples. Following Larson _et al._5,7 a total of 620 bp of the Control Region (CR) of the mitochondrial
DNA (mtDNA) was amplified using two overlapping fragments. The primers; PIG1F (5′-CATTCCATTCGTATGCAAACC-3′) and PIG3R (5′-ACCAGATGCCTGTTAAAGTT-3′) were used to amplify a 372 bp fragments5,
and L119 5′-(CAGTCAACATGCATATCACC-3′) and H16108 (5′-GCACCTTGTTTGGATTRTCG-3′)7 amplified a 352 bp fragment. In addition, following Li _et al._37, a total of 1552 bp of the MC1R region was
amplified. This region includes 425 bp of the 5′ untranslated region, 963 bp of the coding region and 164 bp of the 3′ untranslated region. One pair of primers was designed to amplify the
whole region; MF1 (5′-GTGCGGCGGCTCTGCGCTCCAA-3′) and MR1 (5′-CCCCCACTCCCCATGCCTCCTG-3′)37. Another set of inner primers was designed for samples that were badly preserved and for which the
amplification of the long fragment failed; MF2 (5′-GCTGCTGCTGGAGGCGGGC-3′), MR2 (5′-ACACCATGGAGCCGCAGATGAGC-3′). Each PCR reaction contained a total volume of 25 μl, consisting of 1× PCR
Buffer, 1-1.5 U of Platinum Taq DNA polymerase, 1.5–2 mM of MgCl2, 0.2 mM of each deoxynucleotide triphosphate (dNTP) (all from Invitrogen, UK), 0.1 mg/ml of Bovine Serum Albumin (New
England, BioLabs, UK), 0.2–0.4 μM of each primer (Sigma-Aldrich Inc.), and, 2–3 μl of DNA extract. Reaction profiles included a 2-min initial denaturation step at 94°C, followed by 30–35
cycles of the following: 30 sec denaturation at 94°C, 30 sec annealing at 52–68°C, 0.5–1.5 min of extension at 72°C, and a final extension of 10 min at 72°C. The amplicons were cleaned from
unincorporated primers using Exonuclease I, and Shrimp Alkaline Phosphatase (Thermo Fisher Scientific, UK). The samples were sequenced and analysed in ABI 3100 Genetic Analyzer or
Beckman-Coulter CEQT 8000 Genetic Analysis System. Sequencing was conducted on both strands. Sequencing chromatograms were assembled and analysed using analysis software (Geneious version
5.6 created by Biomatters. Available from http://www.geneious.com/). ANCIENT SAMPLES DNA extractions and preparations for PCR reactions of the ancient samples were set up at the Institute of
Archaeology of Tel Aviv University (Israel) in a dedicated lab for ancient DNA, in a building where no molecular work has previously taken place or is currently being conducted. The bone
extraction procedure was similar to that used for modern samples. Following Larson _et al._5, a short fragment of mtDNA CR was amplified ANC1F (5′- CTTTAAAACAAAAAAACCCATAAAAA-3′), and ANC1R
(5′- TTAATGCACGACGTACATAGG-3′). This 74 bp fragment is highly variable and can distinguish between European, Near Eastern and East Asian haplotypes. In addition, in order to increase
resolution between the European and Near Eastern haplotypes, another pair of primers was used at the 3′ end of modern fragment to amplify 79 bp of the mtDNA CR. The forward was designed for
this study, namely ANC4 F (5′- GCACCTTGTTTGGATTRTCG), and the reverse primer was the same as in the modern samples H16108 (5′-GCACCTTGTTTGGATTRTCG-3′) 7. PCRs and post-PCR work were
performed at the Zoology Department of Tel Aviv University (Israel). PCR amplifications were performed in 25 μl reactions with: 1× PCR buffer, 1.5 U of Platinum Taq DNA polymerase High
Fidelity, 2 mM of MgSO4, 0.2 mM of each dNTP (all Invitrogen, UK) 0.1 mg/ml of Bovine Serum Albumin (New England, BioLabs, UK), 0.4 μM of each primer (Sigma-Aldrich Inc.) and 2–4 μl of DNA
extract. The PCR amplification consisted of an initial denaturation at 94°C for four minutes, followed by 55 cycles of denaturation at 94°C for 30 sec, annealing at 52°C for 30 sec, and
extension at 68°C for 40 sec with a final extension period of 5 minutes at 68°C. The samples were cleaned, sequenced and analysed the same way as the modern samples. AUTHENTICITY CRITERIA
FOR THE ANCIENT DNA DATA For both modern and ancient samples multiple negative controls were used during DNA extractions (every fourth or fifth sample) and in all PCR reactions. All reagents
used were molecular biology grade, and when possible, were decontaminated using UV irradiation. All working areas and equipment were decontaminated using bleach, and/or UV irradiation. All
extracts were independently amplified and sequenced at least twice for each fragment to ensure that there were no misincorporated bases. In addition, two thirds of all ancient samples were
re-extracted and the two fragments were amplified. For the ANC1 fragment, another forward primer was designed, to insure repeatability. The primer ANC2F (5′- AAATTGCGCACAAACATACAAAT-3′)
amplifies a shorter fragment (54 bp), and was used to re-amplify 80% of the samples. For more details about the archaeological sites information, DNA success ratio and radiocarbon sample
pre-treatment and measurement, please see Supporting Information. PHYLOGENETIC ANALYSES MODERN SAMPLES The modern DNA sequence data for mtDNA CR and MC1R were aligned with modern pig
sequences available on GenBank (Supplementary Tables S1 and S2). Phylogenetic relationships for mtDNA CR region were estimated using Maximum Likelihood (ML)52, and Bayesian algorithms53,54.
jMODELTEST version 2.155 was used to determine which molecular substitution model best fitted the data based on the Bayesian Information Criterion. The chosen model was implemented in ML and
Bayesian inference. ML phylogeny was estimated using PAUP* 4.0b1056. A full heuristic search was performed starting with a Neighbour Joining tree and tree-bisection-reconnection (TBR)
branch swapping. The parameter values identified by jMODELTEST were used to construct the first tree. The parameters were then re-estimated from the resulting tree and used in a new ML
analysis. The procedure was repeated until the estimated parameter values remained unchanged, and these values were used for 100 bootstrap replicates. MrBayes v3.2.153,54 was used to conduct
the Bayesian Markov Chain Monte Carlo (MCMC) phylogenetic inference using best-fit model indicated by jMODELTEST version 2.155 based on the Akaike and Bayesian Information Criterion. The
molecular substitution model was Hasegawa-Kishino-Yano (HKY)57 with gamma-distribution and a portion of Invariable sites to account for among site rate variation. MCMC sampling was performed
using two separate runs of three million generations with sampling every 100 generations, discarding the first 10% as burn-in. Four chains (three heated, one cold) were used in the analysis
in each of the two independent runs. Mixing and convergence to stationary distributions were checked with Tracer v1.5 (http://tree.bio.ed.ac.uk/software/tracer/). The desert Warthog
(_Phacochoerus aethiopicus_) served as an outgroup. Network software version 4.6.1.10 (www.fluxus-engineering.com) was used to construct median-joining network for MC1R region (Supplementary
Table S1, Table S2)58. To be able to use the large dataset from Fang _et al._36, the analysis for the creation of the network was carried out for 963 bp, which was mainly based on the
coding region of MC1R. ANCIENT SAMPLES The two mtDNA CR fragments amplified for ancient DNA were concatenated and aligned with modern sequences from across Eurasia, given the large dataset
that has been generated by previous studies5,6,9. Haplotype name for each specimen was based on the first fragment- ANC1 terminology. Thirty-four ancient samples and 179 modern _Sus scrofa_
sequences (including the 25 Israeli wild boars) were used to construct a 3D statistical parsimony network using the script TempNet59 in R60 (Supplementary Table S1, Table S2). Gaps were
assigned as a fifth character. Using the Bayesian phylogenetic analysis, samples were assigned to one of four time categories: modern, Hellenistic to Crusader (330 BCE – 1200/1300 CE), Iron
Age (1150-586 BCE) and Bronze Age (3500-1150 BCE). DNA sequences have been deposited in GenBank (http://www.ncbi.nlm.nih.gov/), with accession numbers KF525706-KF525782. REFERENCES * Avise,
J. C. et al. Intraspecific phylogeography: the mitochondrial DNA bridge between population genetics and systematics. _Annual Reviews of Ecology and Systematic_ 18, 489–522 (1987). Article
Google Scholar * Avise, J. C. _Phylogeography: The history and formation of species_ (Harvard University Press, Cambridge, MA, 2000). Google Scholar * Giuffra, E. et al. The origin of the
domestic pig: independent domestication and subsequent introgression. _Genetics_ 154, 1785–91 (2000). CAS PubMed PubMed Central Google Scholar * Haile, J., Larson, G., Owens, K., Dobney,
K. & Shapiro, B. Ancient DNA typing of archaeological pig remains corroborates historical records. _J. Archaeol. Sci._ 37, 174–177 (2010). Article Google Scholar * Larson, G. et al.
Ancient DNA, pig domestication, and the spread of the Neolithic into Europe. _Proc. Natl. Acad. Sci. USA_ 104, 15276–81 (2007). Article ADS Google Scholar * Larson, G. et al. Phylogeny
and ancient DNA of _Sus_ provides insights into neolithic expansion in Island Southeast Asia and Oceania. _Proc. Natl. Acad. Sci. USA_ 104, 4834–9 (2007). Article ADS CAS Google Scholar
* Larson, G. et al. Worldwide phylogeography of wild boar reveals multiple centers of pig domestication. _Science_ 307, 1618–21 (2005). Article ADS CAS Google Scholar * Alexandri, P. et
al. The Balkans and the colonization of Europe: the post-glacial range expansion of the wild boar,. _Sus scrofa. J. Biogeogr._ 39, 713–723 (2012). Article Google Scholar * Ottoni, C. et
al. Pig domestication and human-mediated dispersal in western Eurasia revealed through ancient DNA and geometric morphometrics. _Mol. Biol. Evol._ 10.1093/molbev/mss261 (2013). * Hongo, H.,
Anezaki, T., Yamazaki, K., Takahashi, O. & Sugawara, H. Hunting or management? The status of _Sus_ in the Jomon period in Japan. in _Pigs And Humans 10,000 Years of Interaction_ (eds.
Albarella, U., Dobney, K., Ervynck, A. & Rowley-Conwy, P.) 109–130 (Oxford University Press, Oxford, 2007). Google Scholar * Rowley-Conwy, P. & Dobney, K. Wild boar and domestic
pigs in Mesolithic and Neolithic southern Scandinavia. in _Pigs And Humans 10,000 Years Of Interaction_ (eds. Albarella, U., Dobney, K., Ervynck, A. & Rowley-Conwy, P.) 131–155 (Oxford
University Pres, Oxford, 2007). Google Scholar * Vigne, J. D. et al. Pre-Neolithic wild boar management and introduction to Cyprus more than 11,400 years ago. _Proc. Natl Acad. Sci. USA_
106, 16135–38 (2009). Article ADS CAS Google Scholar * Hewitt, G. M. Post-glacial re-colonization of European biota. _Biol. J. Linn. Soc._ 68, 87–112 (1999). Article Google Scholar *
Hewitt, G. M. Some genetic consequences of ice ages, and their role in divergence and speciation. _Biol. J. Linn. Soc._ 58, 247–276 (1996). Article Google Scholar * Taberlet, P.,
Fumagalli, L., Wust-Saucy, A. G. & Cosson, J. F. Comparative phylogeography and postglacial colonization routes in Europe. _Mol. Ecol._ 7, 453–464 (1998). Article CAS Google Scholar *
Hofreiter, M. et al. Lack of phylogeography in European mammals before the last glaciation. _Proc. Natl. Acad. Sci. USA_ 101, 12963–12968 (2004). Article ADS CAS Google Scholar *
Valdiosera, C. E. et al. Staying out in the cold: glacial refugia and mitochondrial DNA phylogeography in ancient European brown bears. _Mol. Ecol._ 16, 5140–5148 (2007). Article CAS
Google Scholar * Bray, S. C. E. et al. Ancient DNA identifies post-glacial recolonisation, not recent bottlenecks, as the primary driver of contemporary mtDNA phylogeography and diversity
in Scandinavian brown bears. _Diversity and Distributions_ 19, 245–256 (2012). Article Google Scholar * Mendelssohn, H. & Yom-Tov, Y. A report of birds and mammals which have increased
their distribution and abundance in Israel due to human activity. _Israel J. Zool._ 45, 35–47 (1999). Google Scholar * Geraads, D. & Tchernov, E. Femurs humains du Pleistocene moyen de
Gesher Benot Ya'acov (Israel). _L'Anthropologie_ 87, 138–141 (1983). Google Scholar * Haber, A. & Dayan, T. Analyzing the process of domestication: Hagoshrim as a case study.
_J. Archaeol. Sci._ 31, 1587–1601 (2004). Article Google Scholar * Marom, N. & Bar-Oz, G. The prey pathway: A regional history of cattle (_Bos taurus_) and pig (_Sus scrofa_)
domestication in the northern Jordan Valley, Israel. _PLoS One_ 8, e55958 (2013). Article ADS CAS Google Scholar * Hesse, B. Pig Lovers and Pig Haters: Patterns of Palestinian Pork
Production. _Journal of Ethnobiology_ 10, 195–225 (1990). Google Scholar * Grigson, C. Plough and Pasture in the early economy of the southern Levant. in _The Archaeology of Society in the
Holy Land_ (ed. Levy, T. E.) 245–68 (Leicester University Press, London, 1998). Google Scholar * Hesse, B. & Wapnish, P. Can pig remains be used for ethnic diagnosis in the Ancient Near
East? in _The Archaeology of Israel,_ Vol. 237, (eds. Silberman, A. S. & Small, D.) 238–270 (1996). Google Scholar * Hesse, B. & Wapnish, P. Pig use and abuse in the ancient
Levant: Ethnoreligious boundary-building with swine. in _Ancestors for the Pigs: Pigs in Prehistory._ Vol. 15, (ed. Nelson, S. M.) 123–135 (1998). Google Scholar * Horwitz, L. K. &
Studer, J. Pig production and exploitation during the Classical periods in the Southern Levant. in _Archaeozoology of the Near East_, Vol. VI, (eds. Buitenhuis, H., Choyke, A. M., Martin,
L., Bartosiewicz, L. & Mashkour, M.) 222–239 (ARC-Publicaties 123, Groningen, 2005). Google Scholar * Lev-Tov, J. S. E. Pigs, Philistines, and the ancient animal economy of Ekron from
Late Bronze to Iron Age II University of Tennessee. (2000). * Sapir-Hen, L., Bar-Oz, G., Gadot, Y. & Finkelstein, I. Pig husbandry in Iron Age Israel and Judah: New insights regarding
the origin of the “Taboo”. _Zeitschriftfirt des Deutschen Palästina-Vereins_ 129.1, 1–20 (2013). Google Scholar * Maeir, A. M., Hitchcock, L. A. & Horwitz, L. K. On the constitution and
transformation of Philistine identity. _Oxford J. Archaeology_ 32, 1–38 (2013). Article Google Scholar * Yasur-Landau, A. _The Philistines and Aegean migration at the end of the Late
Bronze Age_ (Cambridge University Press, Cambridge, 2010). Book Google Scholar * Kislev, M. E., Artzy, M. & Marcus, E. Import of an Aegean food plant to a Middle Bronze IIA coastal
site in Israel. _Levant_ 25, 145–154 (1993). Article Google Scholar * Oren, E. D. _The Sea People and their world: A reassessment_, (The University Museum, University of Pennsylvania Press
Philadelphia, 2000). Google Scholar * Kijas, J. M. H. et al. Melanocortin receptor 1 (MC1R) mutations and coat color in pigs. _Genetics_ 150, 1177–1185 (1998). CAS PubMed PubMed Central
Google Scholar * Vage, D. I., Klungland, H., Lu, D. & Cone, R. D. Molecular and pharmacological characterization of dominant black coat color in sheep. _Mamm. Genome_ 10, 39–43
(1999). Article CAS Google Scholar * Fang, M. Y., Larson, G., Ribeiro, H. S., Li, N. & Andersson, L. Contrasting mode of evolution at a coat color locus in wild and domestic pigs.
_PLoS Genet._ 5, e1000341 10.1371/journal.pgen.1000341 (2009). Article CAS PubMed PubMed Central Google Scholar * Li, J. et al. Artificial selection of the melanocortin receptor 1 gene
in Chinese domestic pigs during domestication. _Heredity_ 105, 274–281 (2010). Article CAS Google Scholar * Allentoft, M. E. et al. The half-life of DNA in bone: measuring decay kinetics
in 158 dated fossils. _P. Roy. Soc. Lond. B Bio._ 279, 4724–4733 (2012). Article CAS Google Scholar * Bollongino, R., Tresseta, A. & Vignea, J. D. Environment and excavation: Pre-lab
impacts on ancient DNA analyses. _Comptes Rendus Palevol_ 7, 91–98 (2008). Article Google Scholar * Gilboa, A. & Sharon, I. An archaeological contribution to the Early Iron Age
chronological debate: Alternative chronologies for Phoenicia and their effects on the Levant, Cyprus, and Greece. _B. Am. Sch. Oriental Re._ 332, 7–80 (2003). Article Google Scholar *
Mazar, A., Bruins, H. J., Panitz-Cohen, N. & Van der Plicht, J. Ladder of time at Tel Rehov: Stratigraphy, archaeological context, pottery and radiocarbon dates. in _The Bible and
Radiocarbon Dating: Archaeology, Text and Science_ (eds. Levy, T. E. & Higham, T.) 193–255 (Equinox, London, 2005). Google Scholar * Boaretto, E. Radiocarbon Dates. in _Megiddo IV: The
1998–2002 Seasons_ (eds. Finkelstein, I., Ussishkin, D. & Halpern, B.) 550–557 (Tel Aviv, 2006). Google Scholar * Sharon, I., Gilboa, A., Jull, T. A. J. & Boaretto, E. Report on the
first stage of the Iron Age dating project in Israel: Supporting a low chronology. _Radiocarbon_ 49, 1–46 (2007). Article CAS Google Scholar * Finkelstein, I. & Piasetzky, E.
Radiocarbon dating the Iron Age in the Levant: A bayesian model for six ceramic phases and six transitions. _Antiquity_ 84, 374–385 (2010). Article Google Scholar * Hitchcock, L. A. &
Maeir, A. M. Beyond Creolization and Hybridity: Entangled and Transcultural Identities in Philistia. _Archaeological Review from Cambridge_ 28, 51–72 (2013). Google Scholar * Faust, A.
& Lev-Tov, J. The constitution of Philistine identity: Ethnic dynamics in twelfth to tenth century Philistia. _Oxford Journal of Archaeology_ 30, 13–31 (2011). Article Google Scholar *
Bunimovitz, S. & Lederman, Z. Canaanite resistance: The Philistines and Beth-Shemesh - A case study from the Iron Age I. _B. Am. Sch. Oriental Res._ 364, 37–51 (2011). Article Google
Scholar * Owen, J. The rise and fall of the Philistine pig: An investigation of a pig assemblage from Tel Miqne-Ekron. BSc Dissertation, University of Durham. (2005). * Cifola, B. The role
of the Sea Peoples at the end of the Late Bronze Age: A reassessment of textual and archaeological evidence. _Orientis Antiqvi Miscellanea_ I, 1–23 (1994). Google Scholar * Davidowitz, G.
& Horwitz, L. K. Morphometric variation between populations of recent wild boar in Israel. in _Pigs and Humans 10,000 Years of Interaction_ (eds. Albarella, U., Dobney, K., Ervynck, A.
& Rowley-Conwy, P.) (Oxford University Press, Oxford, 2007). Google Scholar * Yang, D. Y., Eng, B., Waye, J. S., Dudar, J. C. & Saunders, S. R. Technical note: Improved DNA
extraction from ancient bones using silica-based spin columns. _Am. J. of Phys. Anthropol._ 105, 539–543 (1998). Article CAS Google Scholar * Felsenstein, J. Evolutionary trees from DNA
sequences: a maximum likelihood approach. _J. Mol. Evol._ 17, 368–76 (1981). Article ADS CAS Google Scholar * Ronquist, F. & Huelsenbeck, J. P. MrBayes 3: Bayesian phylogenetic
inference under mixed models. _Bioinformatics_ 19, 1572–1574 (2003). Article CAS Google Scholar * Huelsenbeck, J. P. & Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees.
_Bioinformatics_ 17, 754–755 (2001). Article CAS Google Scholar * Posada, D. jModelTest: phylogenetic model averaging. _Mol. Biol. Evol._ 25, 1253–6 (2008). Article CAS Google Scholar
* Swofford, D. L. _PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4_ (Sinauer Associates, Sunderland, Massachusetts, 2003). Google Scholar * Hasegawa, M.,
Kishino, H. & Yano, T. A. Dating of the human ape splitting by a molecular clock of mitochondrial-DNA. _J. Mol. Evol._ 22, 160–174 (1985). Article ADS CAS Google Scholar * Bandelt,
H. J., Forster, P. & Rohl, A. Median-joining networks for inferring intraspecific phylogenies. _Mol. Biol. Evol._ 16, 37–48 (1999). Article CAS Google Scholar * Prost, S. &
Anderson, C. N. K. TempNet: a method to display statistical parsimony networks for heterochronous DNA sequence data. _Methods in Ecology and Evolution_ 2, 663–667 (2011). Article Google
Scholar * Team, R. D. C. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (2010). Download references ACKNOWLEDGEMENTS We
thank the following archaeologists, zooarchaeologists and museum curators for kindly providing us samples: Noha Agha, Amani Abu Hamid, Doron Ben-Ami, Daniel Berkowic, Fanny Bocquentin, Eric
H. Cline, Ehud Galili, Ayelet Gilboa, Zvi Greenhut, Gila Kahila Bar Gal, Seymour Gitin, Amir Golani, Hamudi Khalaily, Thomas E. Levy, Nimrod Marom, Daniel Master, Assaf Nativ, Amihai Mazar,
Ilan Sharon, Danny Syon, Assaf Yasur-Landau, Edna Stern, Karin Tamar, David Ussishkin, Shlomit Weksler-Bdolah. We also want to thank to the following museums for providing us samples: the
Steinhardt National Collection of Natural History, Zoological Museum at Tel Aviv University (Israel), and the Wildlife Tissue Collection at the Laboratory of Molecular Evolution, National
Natural History Collections of The Hebrew University of Jerusalem (Israel). This study was funded by the European Research Council under the European Community's Seventh Framework
Program (FP7/2007–2013) ERC grant agreement no. 229418. Partial funding (relating to the excavation of the samples from Tell es-Safi/Gath and the research conducted by L.K.H) was provided by
a grant (#32/11) from the F.I.R.S.T. (Bikura) track of the Israel Science Foundation to A.M.M. Ehud Weiss, and L.K.H. A small funding was also provided by the Jacob M. Alkow Chair in the
Archaeology of Israel in the Bronze and Iron Ages. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute of Archaeology, Tel Aviv University, Tel Aviv 69978, Israel, Meirav Meiri, Lidar
Sapir-Hen & Israel Finkelstein * Department of Zoology, Tel Aviv University, Tel Aviv 69978, Israel, Meirav Meiri & Dorothée Huchon * Department of Archaeology, University of Haifa,
Haifa 31905, Israel, Guy Bar-Oz * Weizmann Institute-Max Planck Center for Integrative Archaeology, D-REAMS Radiocarbon Dating Laboratory, Weizmann Institute of Science, Rehovot 76100,
Israel, Elisabetta Boaretto * National Natural History Collections, Faculty of Life Science, The Hebrew University of Jerusalem, Jerusalem 91905, Israel, Liora Kolska Horwitz * The Martin
(Szusz) Department of Land of Israel Studies and Archaeology, The Institute of Archaeology, Bar-Ilan University, Ramat Gan 52900, Israel, Aren M. Maeir * Department of Archaeology, Durham
Evolution and Ancient DNA, University of Durham, Durham DH1 3LE, United Kingdom, Greger Larson * Department of Structural Biology, Kimmel Center for Archaeological Science, Weizmann
Institute of Science, Rehovot 76100, Israel, Steve Weiner Authors * Meirav Meiri View author publications You can also search for this author inPubMed Google Scholar * Dorothée Huchon View
author publications You can also search for this author inPubMed Google Scholar * Guy Bar-Oz View author publications You can also search for this author inPubMed Google Scholar * Elisabetta
Boaretto View author publications You can also search for this author inPubMed Google Scholar * Liora Kolska Horwitz View author publications You can also search for this author inPubMed
Google Scholar * Aren M. Maeir View author publications You can also search for this author inPubMed Google Scholar * Lidar Sapir-Hen View author publications You can also search for this
author inPubMed Google Scholar * Greger Larson View author publications You can also search for this author inPubMed Google Scholar * Steve Weiner View author publications You can also
search for this author inPubMed Google Scholar * Israel Finkelstein View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.M., G.B.O., S.W. and
I.F. designed research; M.M. performed research; M.M., D.H., G.L., S.W. and I.F. analysed data; G.B.O., L.K.H., A.M.M., L.S.H. and I.F. provided key samples and contextual information; E.B.
performed the radiocarbon dating of the samples; M.M., D.H., G.B.O., G.L., S.W. and I.F. wrote the paper. CORRESPONDING AUTHOR Correspondence to Meirav Meiri. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Information (DOC 1589 kb) RIGHTS AND PERMISSIONS This work
is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Meiri, M., Huchon, D., Bar-Oz, G. _et al._ Ancient DNA and Population Turnover in Southern Levantine Pigs- Signature of the Sea Peoples
Migration?. _Sci Rep_ 3, 3035 (2013). https://doi.org/10.1038/srep03035 Download citation * Received: 30 May 2013 * Accepted: 23 July 2013 * Published: 04 November 2013 * DOI:
https://doi.org/10.1038/srep03035 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative







