- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Blood ammonia levels in healthy individuals are low, but they increase in liver disease due to loss of functional liver mass and portosystemic shunting. Hyperammonemia is one of the
key factors involved in the prognosis of cirrhosis and its complications. Here we review to establish a connection between alterations in gut microbial communities and intestinal ammonia
metabolism, highlighting a key impact of gut dysbiosis on blood ammonia levels during liver disease. SIMILAR CONTENT BEING VIEWED BY OTHERS GUT MICROBIOTA ANALYSIS IN CIRRHOSIS AND
NON-CIRRHOTIC PORTAL HYPERTENSION SUGGESTS THAT PORTAL HYPERTENSION CAN BE MAIN FACTOR OF CIRRHOSIS-SPECIFIC DYSBIOSIS Article Open access 11 March 2025 IMPAIRED FLUX OF BILE ACIDS FROM THE
LIVER TO THE GUT REVEALS MICROBIOME-IMMUNE INTERACTIONS ASSOCIATED WITH LIVER DAMAGE Article Open access 07 June 2023 UTILIZING THE GUT MICROBIOME IN DECOMPENSATED CIRRHOSIS AND
ACUTE-ON-CHRONIC LIVER FAILURE Article 30 November 2020 INTRODUCTION The gut microbiome dynamically interacts with the human physiology to maintain the integrity of the intestinal
microecosystem. The metabolism of ammonia is influenced by both the host’s intestinal metabolic machinery and the gut microbial community1. The liver primarily catabolizes ammonia and in
conjunction with other organs plays a crucial role in controlling its level in the bloodstream. When ammonia metabolism (both production and clearance) in the intestine, liver, or other
organs is disturbed, for example in liver diseases, its levels in the blood are elevated (known as hyperammonemia) and this can have deleterious effects, particularly leading to
complications such as increased hospitalization and hepatic encephalopathy (HE)2. While it has been widely accepted that an imbalance in gut bacteria plays a significant role in the
development and advancement of liver disease, the connections between the gut microbiome and the metabolism of ammonia remain understudied. Here, we highlight the significance of gut
microbiota in the production and consumption of ammonia and make an effort to link changes in gut microbial populations to ammonia metabolism and levels in liver diseases. In this review, we
would be discussing ammonia metabolism in chronic liver disease and cirrhosis and not ACLF or ALF. AMMONIA METABOLISM IN HEALTH AND DISEASE SYNTHESIS OF AMMONIA IN HEALTH Ammonia is an
important metabolite that maintains cellular pH. In nature, ammonia exists in gaseous and ionic form as ammonium ion (NH4+), which are in equilibrium with each other. Recently, it has been
shown that an NH4+ uses transporters to transverse the cell membrane, where it first dissociates into ammonia and hydrogen ion (a proton), which then pass through the cell membrane and then
finally both again combine into NH4+ inside the cell3. The blood levels of ammonia must remain very low because even slightly elevated concentrations are toxic to the central nervous
system4. In healthy humans, blood ammonia levels are maintained in the range of 10–40 μM5. Most of the ammonia in circulation originates from the gastrointestinal tract from the catabolism
of dietary proteins and amino acids. Among all amino acids, glutamate and glutamine are most prominent players of nitrogen and ammonia metabolism6. In the intestine, ammonia is primarily
produced through two processes. Firstly, it is formed when glutamine is deaminated by phosphate activated glutaminase (PAG) in the enterocytes lining the mucosal layer of small intestine and
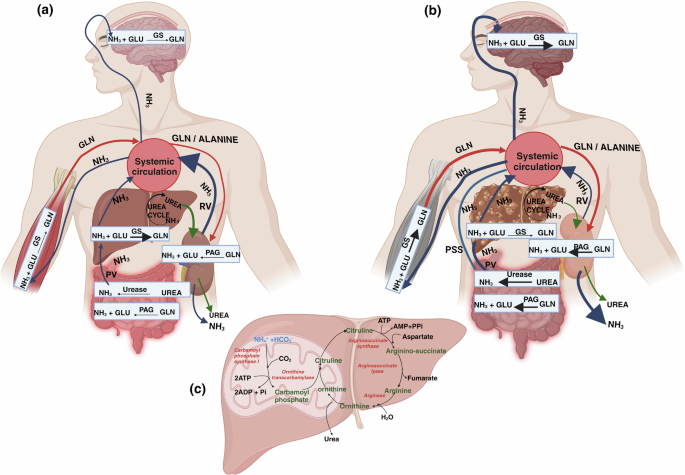
colon. Secondly, it is generated from the conversion of dietary urea (protein rich foods) or hepatic urea (15–30%) by the gut microbial urease enzyme, which is abundant in the colon7 (Fig.
1a). In healthy individuals, ~80% of intestinal PAG is located in the small intestine, whereas the remaining 20% is found in the large intestine8. During the post-absorptive state, as seen
in dogs, approximately 50% of intestinal ammonia originates from metabolism of glutamine in small intestine, 21% comes from breakdown of urea in the large intestine while 5% comes from
glutamine metabolism in large intestine9. The kidneys also play a role in ammonia production by means of PAG majorly in renal epithelial cells in the proximal tubule. During the
post-absorptive stage, glutamine coming from the liver serves as the primary substrate for renal production of ammonia through the action of PAG10. Along with ammonia, kidneys also produce
equimolar bicarbonate generation (two NH4+ and two bicarbonate molecules). Thus, ammonia metabolism in the kidneys is critical to maintain acid base homeostasis11. SYNTHESIS OF AMMONIA IN
LIVER DISEASE Arterial ammonia levels in patients with liver disease range from 40–60 μM in cirrhosis. Changes in arterial ammonia levels are better indicators of liver disease as they are
representative of changes in urea cycle enzymes in liver as compared to venous ammonia levels, which are less accurate because of peripheral uptake of ammonia into muscle and brain tissue12.
Studies have shown that elevated intestinal PAG activity plays a significant role in enhanced systemic blood ammonia or hyperammonemia during liver disease (Fig. 1b). Preclinical
investigations have reported that duodenal PAG activity in mucosal biopsies of the small intestine increase by almost four times in cirrhotic patients compared to healthy individuals8.
Studies have shown that hyperammonemia is primarily caused by preexisting intra- and/or extrahepatic portacaval shunts (that directly connect portal to systemic blood) in rats with
cirrhosis. Several studies have proven that portal-systemic shunting is the major cause of elevated ammonia levels in the systemic circulation. This has also been shown in stable individuals
with cirrhosis and TIPS (transjugular intrahepatic portosystemic shunt). In comparison to controls, both portal and arterial ammonia increases substantially after portacaval shunting13,14.
The hepatic extraction efficiency of ammonia from the portal vein is about 93%in health15. Thus, little, if any, ammonia enters the systemic circulation from the portal vein under normal
conditions, and the liver maintains the level of circulating ammonia at relatively low levels. Ammonia extraction is reduced in the presence of portosystemic shunting16. This is attributed
to a predominance of pathobionts within the gut microbiome that produces more ammonia irrespective of the degree of portosystemic shunting17. The microbiome also contributes towards
increased synthesis of intestinal ammonia through the action of the bacterial enzymes18. Studies have demonstrated that in patients with advanced cirrhosis and upper gastrointestinal
bleeding, kidneys have a six-fold increase in ammonia production due to increased alanine uptake19. However, it has also been shown in experimental and clinical studies that kidneys remove
more ammonia from the body than its production following hyperammonemia induced by portacaval shunting14. AMMONIA REMOVAL IN HEALTH LIVER Ammonia is efficiently detoxified in the liver
through either the conversion into urea via the urea cycle enzymes or the conversion into glutamine through the activity of glutamine synthetase (GS) (Fig. 1a, c). The enzymes responsible
for the urea cycle are mainly found in periportal hepatocytes, whereas GS is significantly abundant in perivenous hepatocytes20. The initial stage of the urea cycle is controlled by the
enzyme carbamoyl phosphate synthetase I (CPS I), which acts as a rate-limiting enzyme. In the first stage, carbamoyl phosphate and ornithine react together, resulting in the production of
citrulline which in further stages with the help of other enzymes, produces urea21 (Fig. 1c). Urea is passively transported across the biological membranes by diffusion and with the help of
urea transporters, it is ultimately removed by the kidneys in the urine22. The perivenous hepatocytes convert ammonia to glutamine by GS, thus providing an alternate method for scavenging
ammonia20. Hepatic glutamine metabolism, together with urea synthesis, has a vital role in maintaining the detoxification of ammonia in the body23. MUSCLES In addition to the liver, GS
activity is also found in the muscles, but it is lower compared to the liver during health. By employing genetic methods to completely eliminate GS from muscle, it has been demonstrated that
GS has a minimal role in muscle during normal fed conditions, and only becomes significant during times of stress, such as starvation or hyperammonemia24. BRAIN The brain exhibits GS
activity majorly in astrocytes and some in the neurons, rendering it an essential organ for detoxification and utilization of ammonia in the maintenance of a healthy state25. Astrocyte GS
utilizes ammonia in order to produce glutamine, which is subsequently delivered to neurons for deamination to produce crucial neurotransmitters glutamate and GABA26. KIDNEYS The kidneys
closely regulate the elimination of urinary ammonia by many mechanisms, including tubular urine flow, apical/basolateral ion exchangers (such as Na + –K + –NH4 + –ATPase), acid-base balance,
and ammonia counter-current system27. The regulation of ammonia transport by renal epithelial cells determines the amount of ammonia that is excreted in urine or returned to the systemic
circulation. Under basal conditions, 30% of the ammonia that is produced in kidneys is excreted in urine and 70% is added to the systemic circulation via renal veins10. AMMONIA REMOVAL IN
DISEASE LIVER Chronic liver disease causes a decreased clearance of ammonia in liver. A decline in the expression and activity of urea cycle enzymes leads to hyperammonemia28 (Fig. 1c). In
cirrhosis, the loss of hepatocytes results in a significant reduction in the liver’s capacity to detoxify ammonia through the urea cycle or GS activity29 (Fig. 1b). However, generally there
is enough capacity to remove ammonia from the body until the emergence of advanced chronic liver disease. Many patients with stable cirrhosis show normal systemic ammonia levels, indicating
that the liver is still capable of efficiently removing majority of ammonia from the portal vein and hepatic artery. This is also evident by the fact that a large increase in protein intake
in patients with cirrhosis enhances functional hepatic nitrogen clearance and urea synthesis30. During advanced liver disease, reduced hepatic clearance, and presence of portal-systemic
shunting, are important causes of hyperammonemia9,29. Using a 90 min constant infusion of ammonia to achieve plasma steady-state, Eriksen et al. clearly demonstrated that in patients with
cirrhosis, ammonia clearance was ∼20% lower and ammonia production nearly threefold higher than in healthy persons, indicating relevance of both ammonia clearance and production in governing
plasma ammonia levels29. KIDNEYS During liver cirrhosis and portocaval shunting, kidneys respond to early hyperammonemia by enhancing ammonia excretion in the urine (70%) and decreasing its
secretion into the renal vein (30%)14. The improvement of renal ammonia excretion following TIPS and the decrease in renal ammonia release into circulation in patients with cirrhosis
provide supporting evidence for this19. Changes in urinary ammonia excretion can result from changes in renal epithelial cell ammonia transport that determines the proportion that is
excreted in the urine versus that delivered to systemic circulation10. MUSCLES Due to the presence of GS activity, muscle is considered as a good buffer system to dispose of excess
circulating ammonia during liver disease. GS activity in muscles is known to increase during hepatic insufficiency and portosystemic shunting31. Cirrhotic patients with a normal muscle mass
may experience a less severe form of hyperammonemia while those having significant muscular atrophy are more susceptible to developing hyperammonemia13. The relationship between
hyperammonemia and muscle mass is complex. Hyperammonemia is known to have a direct negative effect on muscle turnover by causing an increased activation of myostatin (an inhibitor of muscle
growth), mitochondrial dysfunction with decreased ATP content, modifications of contractile proteins, and impaired ribosomal function32. Sarcopenia (reduced muscle mass and strength), that
is present between 30% and 70% of cirrhotic patients and low myostatin levels have been independently associated with the development of HE33. Also, due to excessive protein catabolism in a
sarcopenic muscle, glutamine synthesis by GS may be decreased and hence cirrhotic patients with sarcopenia will have a reduced GS activity and a higher risk of developing HE34,35. In
addition, it is important to note that the process of skeletal muscle ammonia uptake and glutamine release does not always result in overall detoxification of ammonia in the body. This is
because the glutamine produced by the muscles can be absorbed by the splanchnic area or kidneys and converted back into ammonia, which is then released into the bloodstream36. Maintaining an
optimum muscle mass by light exercise and increased caloric intake have been shown to be beneficial in patients with compensated cirrhosis in a few studies but long-term clinical effects in
patients with hyperammonemia need to be clarified further36. BRAIN Enhanced ammonia detoxification via the conversion of glutamate to glutamine by brain GS results in elevated levels of
glutamine, causing osmotic stress and subsequent cell swelling. Brain glutamine levels correlate with the grade of HE in patients with cirrhosis37. The underlying pathophysiology of HE in
cirrhosis is multifactorial, involving accumulation of ammonia and manganese in brain, systemic and central inflammation, activation of the GABAergic neurotransmitter system, etc. Astroglial
and microglial cells are the major cells affected during HE in cirrhosis patients38. GUT MICROBIOME IN LIVER DISEASE Microbial species have been identified in several parts of the gut, such
as the mucosa-associated microbiota (intestinal biopsies) and the luminal or stool microbiota, using 16S rRNA gene sequencing39,40. The mucosal microbiota, located within the mucus layer
that adheres to the mucosa, differs from and remains more consistent throughout time compared to the luminal counterpart. In contrast to luminal or stool microbiota, the mucosal microbiota
engages more closely with the host. Therefore, changes in the stool microbiota may not provide an adequate representation of the complex interactions that take place immediately at the
surface of the gut mucosa. Nevertheless, because of the challenge of acquiring mucosal microbiota, most of the studies have opted for stool sampling as a means of screening microbiota40. Gut
and stool microbiota have been linked to the progression of almost all liver diseases, regardless of their etiology41. GUT MICROBIOTA AND AMMONIA METABOLISM The gut microbiota is
intricately linked to the processes of nitrogen and ammonia metabolism, and any modifications in the microbiome can cause major disturbances that ultimately impact the entire system. The
small intestinal lumen harbors a substantial number of live bacteria, but this number is even higher in the large intestine. Small intestinal microbiota plays an important role in metabolism
of nitrogen and ammonia. Dietary and endogenous proteins are a potential source of amino acids for the microbiota. These amino acids are utilized by the bacteria for protein synthesis,
production of metabolic energy, and recycling of reduced co-factors42. Our understanding of ammonia metabolism by the microbiota in the small intestine remains limited in comparison to the
large intestine or colon. Hence, in this review, we have restricted our discussion to the large intestine only. In healthy individuals, the concentration of ammonia in the lumen of large
intestine is typically low due to the presence of low pH and high amounts of carbohydrates which prevent the synthesis of ammonia40. AMMONIA PRODUCTION BY MICROBIAL UREASE ACTIVITY Several
gut microbial species in the colon are involved in the production of ammonia, either through the conversion of urea by bacterial urease or through proteolysis activities. Urease is an
enzymatic protein synthesized by bacteria that facilitates the conversion of urea into carbamate and ammonia. Mammals do not possess any identified urease gene, therefore, the process of
urea breakdown in the colon, facilitated by urease, is dependent only on the gut microbiota43,44. Urea transporters are found in the colonic mucosa and are involved in delivering dietary or
systemic urea from the bloodstream to the intestinal lumen45. The majority of the bacteria that produce urease are mainly Gram-negative facultatively anaerobic bacteria belonging to the
Enterobacteriaceae and Veillonellaceae families. _Klebsiella pneumoniae_, a member of the Enterobacteriaceae family, has demonstrated significant urease activity in the in vitro
investigations. In addition, it is worth noting that anaerobic gram-positive bacteria, such as Streptococcaceae, possess significant urease activity46. More than 90% of clinical strains of
methicillin-resistant gram-positive bacteria, _Staphylococcus aureus_ also have the ability to break down urea by hydrolysis. The significance of urease in facilitating bacterial survival in
hostile microenvironments within the host’s body is particularly evident in the instance of _Helicobacter pylori_ (_H pylori_), a pathogen accountable for gastritis and peptic ulcers, which
maintains its activity at the pH of stomach. _H. pyrloi_ possesses the ability to produce ammonia as a result of its urease activity47. They demonstrate a markedly higher level of urease
activity as compared to numerous other members of the Enterobacteriaceae family48. Studies have shown that _H. pylori_ increases the generation of ammonia in laboratory conditions and in rat
models of cirrhosis. While there has been extensive research on urease activity in _H. pylori_, there is limited evidence on urease activities in other bacterial species found in the large
intestine49. The existence of commensal bacteria possessing urease activity, which generates endogenous ammonia, implies a crucial role of ammonia generation in physiological processes
conducted an excellent study that reported an advantageous function of naturally occurring ammonia synthesis by gut urease bacteria (Figure 2). A strain of urease-expressing bacteria, called
_Streptococcus thermophilus_, was found to reverse depression-like behaviors in mice. When the production of ammonia in the gut was blocked by inhibiting the activity of urease-producing
bacteria, it increased vulnerability to stress49, indicating the significance of urease bacteria in maintaining ammonia levels for physiological functions. AMMONIA PRODUCTION BY MICROBIAL
PROTEOLYTIC ACTIVITY In addition to urease, a variety of bacterial species in the colon produce ammonia by proteolytic activities. The primary organisms responsible for the breakdown of
proteins in the large intestine are the _Bacteroides, Clostridium, Propionibacterium, Fusobacterium, Streptococcus_, and _Lactobacillus_ genera. _Bacteroides_ species, including _Bacteroides
vulgatus_ and _Bacteroides fragilis_, release proteases in the intestines50 (Fig. 2). Vince et al. isolated specific proteolytic bacteria from healthy subjects and tested their ability to
produce ammonia. The study found that the bacteria responsible for ammonia production were primarily gram-negative anaerobic and aerobic rods, such as _Clostridia_, Bacillus spp, some
_Helicobacter sp., Mycobacterium TB, Mycobacterium bovis_, and _Vibrio parahaemolytic_. Research has shown that microbial proteases play a role in disorders such as inflammatory bowel
disease (IBD) and irritable bowel syndrome (IBS)51, however, their role in cirrhosis remains completely unexplored. In cirrhosis, a study by Kang et al. observed that in the large intestine,
PAG activity was decreased in germ-free cirrhotic mice as compared to conventional cirrhotic mice indicating that PAG activity is contributed by both host and gut microbiota. In the large
intestine and cecal lining of conventional cirrhotic mice, they also reported an increased abundance of _Staphylococcaceae, Enterobacteriaceae_, and _Lactobacillaceae_ and lower abundance of
indigenous families like _Lachnospiraceae, Ruminococcaceae, Clostridiales XIV_ and _Bifidobacteriaceae_. Although the proteolytic or urease activities of these bacteria were not studied,
the relative abundance of these bacterial families was positively correlated with increased systemic ammonia and neuroinflammation52. GUT MICROBIOTA AND AMMONIA PRODUCTION IN CIRRHOSIS AND
HE Pyrosequencing of the 16S rRNA V3 region has shown that the composition of the stool microbiota in the cirrhosis patients varies in terms of both phyla and families. The families
Enterobacteriaceae, Veillonellaceae (Gram-negative facultative anaerobic bacteria), and Streptococcaceae (anaerobic gram-positive bacteria) are commonly found in patients with cirrhosis at
the family level. A positive correlation has been identified between the Child-Turcotte-Pugh (CTP) score and Streptococcaceae in patients with cirrhosis53. Rai et al. revealed a positive
correlation between the severity of cirrhosis as measured by the Model for End Stage Liver Disease (MELD) score, and the presence of the harmful gut bacterial phylum, Enterobacteriaceae.
Conversely, a negative correlation between the severity of cirrhosis and the presence of Ruminococcacea in the gut was seen54. In cases of more severe liver diseases such as acute-on-chronic
liver failure (ACLF), it has been observed that the presence of harmful bacterial families, specifically Proteobacteria, Enterococcaceae, and Streptococcaceae, is associated with greater
mortality and poor outcomes55. Although no quantitative correlations have been done, yet the presence of increased abundance of gut bacterial families such as Veillonellaceae and
Streptococcaceae has been suggested to correlate with increased production of gut ammonia in cirrhosis53. An increase in ammonia production in the large intestine during cirrhosis can be
attributed to a decrease in the presence of beneficial commensal bacteria and the generation of short-chain fatty acids and organic acids. These substances increase the pH of the large
intestine, which in turn enhances bacterial metabolism and leads to an increase in ammonia production. Alterations in the microbiota during cirrhosis can also lead to modifications in the
process of bacterial nitrogen assimilation. A majority of gut bacteria have the ability to manufacture glutamate dehydrogenase and utilize ammonium in their metabolic pathways, allowing many
gut symbionts to ingest ammonium56. This is important for detoxification since high levels of ammonium can be detrimental to both microbiota and host. Some of the bacterial taxas which are
known to be utilizing ammonia for their metabolic activities are Bacillus, Clostridium, Pseudomonas, Streptomyces, Bacteroides, Methanobacteria57,58. However, as of date, there is limited
knowledge of the specific relationship between the type and quantity of gut bacteria and the generation or utilization of intestinal ammonia in cirrhosis (Fig. 2). Given the prominent role
of ammonia in the development of HE, several studies have investigated gut microbial changes in patients with HE. HE has been classified into two categories based on the severity of
symptoms: overt HE (OHE), which is fully symptomatic, and a less symptomatic state known as minimal HE (MHE)54. The main pathophysiological mechanism of OHE and MHE is hyperammonemia leading
to astrocyte dysfunction and deficits in some cognitive areas that can only be measured by neuropsychometric testing. Psychometric and behavioral changes are present with lesser or no
symptoms in MHE but hyperammonemia exists in both conditions59. The gut microbiome of cirrhotic individuals with MHE has a significant increase in _Escherichia coli_ and _Staphylococcus
spp_. compared to patients without MHE60. Zhang et al. identified distinct changes in patients with cirrhosis and MHE, including a higher abundance of _Streptococcus salivarius_ compared to
those without MHE. Remarkably, a direct association between the prevalence of _Streptococcus salivarius_ and the accumulation of ammonia in cirrhotic individuals with MHE was also seen61.
Another study demonstrated the presence of urea catabolite genes in _Streptococcus salivarius_, which were associated with increased activity of the urease enzyme. Therefore, the connection
between _Streptococcus salivarius_ and hyperammonemia in individuals with minimal hepatic encephalopathy (MHE) could be attributed to the significant urease activity exhibited by these
bacteria59. In another study, it was discovered that the presence of Alcaligeneceae (Gram-negative, aerobes) and Porphyromonadaceae (gram-negative anaerobes) in stool was positively
correlated with cognitive impairment in patients with cirrhosis. Importantly, the link between HE and cognitive impairment was attributed to the capacity of Alcaligenaceae to produce ammonia
through the breakdown of urea47. GUT MICROBIOTA-TARGETED AMMONIA-LOWERING THERAPIES Although not many studies have evaluated the contribution of gut microbiota to ammonia production in HE
and cirrhosis pathogenesis, interestingly, most of the successful ammonia-lowering therapies in HE are either based on changing the pH of the gut or modulating the gut microbiota. Since
hyperammonemia is associated with HE in patients with cirrhosis, here we discuss these therapies in context of HE. LACTULOSE Lactulose is a synthetic sugar that is not broken down by the
body until it reaches the colon. In the colon, it gets converted by bacteria into acetic acid and lactic acid. These carboxylic acids lower the pH inside the colon, inhibit the growth and
metabolism of urease bacteria, thus reducing ammonia production62. Lactulose also reduces ammonia and other nitrogenous substances from the colon by its cathartic action (which is probably
caused by the osmotic effect of the organic acid metabolites of lactulose solution) that expels the trapped ammonium ions63. Lactulose also results in dose-dependent acceleration of the
colonic transit time64. Lactulose behaves as a prebiotic and modifies the composition of the intestinal microbiota through an increase in beneficial bacteria (e.g., _Bifidobacteria_ and
_Lactobacillus_) and a decrease in potentially harmful bacteria (e.g., Enterobacteria) thereby contributing to healthier intestinal microbiota in patients with cirrhosis65. It would be
worthwhile to study the type of gut bacteria being modulated by lactulose treatment and their relation with intestinal ammonia metabolism. PROBIOTICS Probiotic therapy entails oral
administration of live microorganisms, either monocultures or mixed cultures, with the aim of enhancing beneficial properties of the intestinal microflora62. Several studies have provided
evidence that native and engineered probiotics profoundly affect many aspects of intestinal nitrogen metabolism (production or utilization) that reduce the production of ammonia in the gut
and hence hyperammonemia. In order to reduce the amount of intestinal ammonia production, Kurtz et al. modified the probiotic _Escherichia coli Nissle_ 1917 to generate a new strain called
SYNB1020 (S-ARG) by upregulating arginine biosynthesis by removing a negative regulator of l-arginine biosynthesis and adding a feedback-resistant enzyme involved in l-arginine biosynthesis.
When ingested, this novel variant had the capability to convert ammonia (NH3) into l-arginine. The SYNB1020 in vitro system demonstrated enhanced l-arginine synthesis from ammonia. SYNB1020
illustrated efficacy in reducing systemic hyperammonemia, enhancing survival in ornithine transcarbamylase-deficient mice, and reducing hyperammonemia in thioacetamide-induced liver injury
mouse models66. SYNB1020 was safe but could not lower blood ammonia in patients with cirrhosis in a phase 1b/2a trial. In another study by Sanchez et al., the efficacy of genetically
engineered _E.coli Nissle_ strains (S-ARG and S-ARG + BUT) was studied on HE. S-ARG + BUT strain along with converting ammonia to arginine, also synthesized butyrate, effectively attenuated
hyperammonemia and prevented memory impairment in bile duct ligated rats67. Another study showed that genetically engineered _Lactobacillus plantarum_ strain exhibited exceptional efficacy
in converting ammonia to alanine (NH3 hyperconsuming strain) and had significant effects on reducing levels of ammonia in the blood and feces68. ANTIBIOTICS Oral antibiotics have been
commonly used to specifically target pathogenic bacteria in the colon. Neomycin and vancomycin are antibiotics that have been proven to reduce blood ammonia levels in patients suffering from
end-stage liver disease7. Neomycin is known to reduce the endogenous production of ammonia and is used for treatment of acute or acute on chronic HE69. Vancomycin has been shown to reduce
blood ammonia and also the severity of HE in patients with cirrhosis70. Another antibiotic, rifaximin that is poorly absorbed in the blood and primarily targets gut bacteria, reduces the
formation of ammonia in HE71. A randomized, double-blind research showed that the antibiotic rifaximin effectively attenuates HE by reducing arterial blood ammonia, improving in asterixis in
patients with chronic liver disease72. Prophylactic administration of rifaximin has shown to improve hyperammonemia and prevent neurophysiological functions in patients with cirrhosis by
modulation of metabolic activity of gut bacteria73. FECAL MICROBIOTA TRANSPLANTATION (FMT) FMT involves the transfer of stool from a donor who has a healthy gut microbiota to a patient who
has an imbalanced gut microbiota, with the goal to restore healthy microbiome. A healthy donor is considered the one with no active infections and antibiotics usage within 3 months of
enrollment, obesity, diabetes, chronic alcohol intake, IBD, CKD, or any other malignancy74. The administration of FMT led to a decrease in hospitalizations and improved cognitive skills in
patients with cirrhosis who experienced repeated episodes of HE75. In patients with advanced cirrhosis, FMT decreased microbial-associated ammonia production and augmented ammonia
utilization via anaerobic metabolism of L-aspartate to Hippurate (glycine conjugate of benzoic acid) providing concrete evidence of gut microbiome’s contribution towards altered ammonia
metabolism in cirrhosis. Hippurate is a marker of gut diversity, associated with microbial degradation of certain dietary components, and is known to facilitate the disposal of ammonia by
urinary excretion76. ENGINEERED MICROBIOTA AND UREASE INHIBITORS Administration of modified Schaedler flora (ASF), that consisted of a specific assemblage of eight bacteria with decreased
abundance of bacteria containing urease gene resulted in a gradual decrease in ammonia production and fecal urease activity. ASF transplantation was also linked to lower morbidity and death
in a mouse model of acute and chronic liver injury77. Urease inhibitors have emerged as an important strategy for reducing ammonia by targeting the synthesis of ammonia by urease bacteria.
Acetohydroxamic acid (AHA) is a well-researched compound that inhibits the enzyme urease. It has been suggested as a therapy for chronic urinary infections caused by the breakdown of urea78.
Apart from AHA, other potent urease inhibitors, such as octanohydroxamic acid (OHA) and nicotinohydroxamic acid, have been studied as potential therapies for HE. In a recent study,
2-octynohydroxamic acid, a urease inhibitor exhibited minimal cytotoxic and carcinogenic effects at micromolar concentrations and also decreased ammonia levels in mouse models of both acute
and chronic liver disease79. However, more evidences that urease inhibitors are effective in liver disease are still lacking. CONCLUSION AND FUTURE PERSPECTIVES Ammonia is the most common
source of nitrogen available in the gut and given the fact that many microbial species produce and use ammonia in their metabolic processes, they play a crucial role in maintaining blood
ammonia levels. Although meager, available experimental evidence strongly suggest that the intestinal microbiota is actively involved in the synthesis and breakdown of ammonia along with the
host cells in liver physiology and pathophysiology. It is thus important to consider the contribution of gut microbiome towards ammonia production and systemic levels during liver disease.
Different animal and patient studies have yielded varying results as the net result of gut microbial ammonia metabolism to plasma ammonia levels from a quantitative perspective varies
according to many parameters like the nutritional status, liver disease severity, the animal model studied, the availability of the nitrogen sources, the composition and concentration of the
microbiota, the overall metabolic capacity of the microbiota, the bowel transit time, the luminal pH, systemic glutamine levels, etc. Nonetheless, the effectiveness of many gut
microbiota-targeted therapies in reducing hyperammonemia and cognitive impairments in cirrhosis affirms a key role of gut microbiome in ammonia production and utilization. We now need
systematic investigations to delineate the precise contribution and mechanisms of increased ammonia production by gut microbial species to hyperammonemia in cirrhosis and HE.
Microbiome-targeted therapy for lowering of ammonia levels in patients with HE has made substantial progress in last few years. However, the routine use of these generalized therapies such
as FMT and antibiotics can also disrupt the beneficial effects of intestinal flora. In this regard, we need to focus on therapies that specifically reduce ammonia formation in patients with
cirrhosis, for example targeting bacterial urease activity would be an ideal strategy to reduce ammonia production in a dysbiotic gut in cirrhosis. DATA AVAILABILITY No datasets were
generated or analysed during the current study. REFERENCES * Rowland, I. et al. Gut microbiota functions: metabolism of nutrients and other food components. _Eur. J. Nutr._ 57, 1–24 (2018).
Article CAS PubMed Google Scholar * Tranah, T. H. et al. Plasma ammonia levels predict hospitalisation with liver-related complications and mortality in clinically stable outpatients
with cirrhosis. _J. Hepatol._ 77, 1554–1563 (2022). Article CAS PubMed Google Scholar * Allen, W. J. & Collinson, I. A molecular dual carriageway. _eLife_ 9, e61148 (2020). * Cagnon,
L. & Braissant, O. Hyperammonemia-induced toxicity for the developing central nervous system. _Brain Res. Rev._ 56, 183–197 (2007). Article CAS PubMed Google Scholar * Levitt, D. G.
& Levitt, M. D. A model of blood-ammonia homeostasis based on a quantitative analysis of nitrogen metabolism in the multiple organs involved in the production, catabolism, and excretion
of ammonia in humans. _Clin. Exp. Gastroenterol._ 11, 193–215 (2018). Article CAS PubMed PubMed Central Google Scholar * Ling, Z.-N. et al. Amino acid metabolism in health and disease.
_Signal Transduct. Target. Ther._ 8, 1–32 (2023). Google Scholar * Rose, C. F. Ammonia-lowering strategies for the treatment of hepatic encephalopathy. _Clin. Pharmacol. Ther._ 92, 321–331
(2012). Article CAS PubMed Google Scholar * Romero-Gómez, M. et al. Intestinal glutaminase activity is increased in liver cirrhosis and correlates with minimal hepatic encephalopathy.
_J. Hepatol._ 41, 49–54 (2004). Article PubMed Google Scholar * Wright, G., Noiret, L., Olde Damink, S. W. M. & Jalan, R. Interorgan ammonia metabolism in liver failure: the basis of
current and future therapies. _Liver Int. Off. J. Int. Assoc. Study Liver_ 31, 163–175 (2011). Article CAS Google Scholar * Weiner, I. D. & Verlander, J. W. Renal Ammonia Metabolism
and Transport. _Compr. Physiol._ 3, 201–220 (2013). Article PubMed PubMed Central Google Scholar * Baertl, J. M., Sancetta, S. M. & Gabuzda, G. J. Relation of acute potassium
depletion to renal ammonium metabolism in patients with cirrhosis. _J. Clin. Investig._ 42, 696–706 (1963). Article CAS PubMed PubMed Central Google Scholar * Katayama, K. & Kakita,
N. Possible pathogenetic role of ammonia in liver cirrhosis without hyperammonemia of venous blood: The so-called latency period of abnormal ammonia metabolism. _Hepatol. Res. Off. J. Jpn.
Soc. Hepatol._ 54, 235–243 (2024). Article CAS Google Scholar * Eriksen, P. L., Djernes, L., Vilstrup, H. & Ott, P. Clearance and production of ammonia quantified in humans by
constant ammonia infusion - the effects of cirrhosis and ammonia-targeting treatments. _J. Hepatol._ 79, 340–348 (2023). Article CAS PubMed Google Scholar * Dejong, C. H., Deutz, N. E.
& Soeters, P. B. Renal ammonia and glutamine metabolism during liver insufficiency-induced hyperammonemia in the rat. _J. Clin. Investig._ 92, 2834–2840 (1993). Article CAS PubMed
PubMed Central Google Scholar * Cooper, A. J., Nieves, E., Coleman, A. E., Filc-DeRicco, S. & Gelbard, A. S. Short-term metabolic fate of [13N]ammonia in rat liver in vivo. _J. Biol.
Chem._ 262, 1073–1080 (1987). Article CAS PubMed Google Scholar * Nomura, F. et al. Effect of intrahepatic portal-systemic shunting on hepatic ammonia extraction in patients with
cirrhosis. _Hepatol. Baltim. Md_ 20, 1478–1481 (1994). Article CAS Google Scholar * Bajaj, J. S. et al. Altered profile of human gut microbiome is associated with cirrhosis and its
complications. _J. Hepatol._ 60, 940–947 (2014). Article CAS PubMed Google Scholar * Chen, Z. et al. The role of intestinal bacteria and gut–brain axis in hepatic encephalopathy. _Front.
Cell. Infect. Microbiol_. 10, 595759 (2021). * Olde Damink, S. W. M. et al. The kidney plays a major role in the hyperammonemia seen after simulated or actual GI bleeding in patients with
cirrhosis. _Hepatology_ 37, 1277–1285 (2003). Article PubMed Google Scholar * Moorman, A. F., Vermeulen, J. L., Charles, R. & Lamers, W. H. Localization of ammonia-metabolizing
enzymes in human liver: ontogenesis of heterogeneity. _Hepatology_ 9, 367–372 (1989). Article CAS PubMed Google Scholar * Matsumoto, S. et al. Urea cycle disorders—update. _J. Hum.
Genet._ 64, 833–847 (2019). Article PubMed Google Scholar * Leung, D. W., Loo, D. D. F., Hirayama, B. A., Zeuthen, T. & Wright, E. M. Urea transport by cotransporters. _J. Physiol._
528, 251–257 (2000). Article CAS PubMed PubMed Central Google Scholar * Häussinger, D. Hepatocyte heterogeneity in glutamine and ammonia metabolism and the role of an intercellular
glutamine cycle during ureogenesis in perfused rat liver. _Eur. J. Biochem._ 133, 269–275 (1983). Article PubMed Google Scholar * He, Y. et al. Glutamine synthetase in muscle is required
for glutamine production during fasting and extrahepatic ammonia detoxification. _J. Biol. Chem._ 285, 9516 (2010). Article CAS PubMed PubMed Central Google Scholar * Suárez, I.,
Bodega, G. & Fernández, B. Glutamine synthetase in brain: effect of ammonia. _Neurochem. Int._ 41, 123–142 (2002). Article PubMed Google Scholar * Weiler, C. T., Nyström, B. &
Hamberger, A. Glutaminase and glutamine synthetase activity in synaptosomes, bulk-isolated glia and neurons. _Brain Res._ 160, 539–543 (1979). Article CAS PubMed Google Scholar * Owen,
E. E. & Robinson, R. R. Amino acid extraction and ammonia metabolism by the human kidney during the prolonged administration of ammonium chloride*. _J. Clin. Investig._ 42, 263–276
(1963). Article CAS PubMed PubMed Central Google Scholar * Lykke Eriksen, P. et al. Non-alcoholic fatty liver disease causes dissociated changes in metabolic liver functions. _Clin.
Res. Hepatol. Gastroenterol._ 43, 551–560 (2019). Article PubMed Google Scholar * Rudman, D. et al. Maximal rates of excretion and synthesis of urea in normal and cirrhotic subjects. _J.
Clin. Invest._ 52, 2241–2249 (1973). Article CAS PubMed PubMed Central Google Scholar * Hamberg, O., Nielsen, K. & Vilstrup, H. Effects of an increase in protein intake on hepatic
efficacy for urea synthesis in healthy subjects and in patients with cirrhosis. _J. Hepatol._ 14, 237–243 (1992). Article CAS PubMed Google Scholar * Desjardins, P., Rao, K. V.,
Michalak, A., Rose, C. & Butterworth, R. F. Effect of portacaval anastomosis on glutamine synthetase protein and gene expression in brain, liver and skeletal muscle. _Metab. Brain Dis._
14, 273–280 (1999). Article CAS PubMed Google Scholar * Di Cola, S. et al. Ammonia and the muscle: an emerging point of view on hepatic encephalopathy. _J. Clin. Med._ 11, 611 (2022).
Article PubMed PubMed Central Google Scholar * Kim, G., Kang, S. H., Kim, M. Y. & Baik, S. K. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and
meta-analysis. _PloS One_ 12, e0186990 (2017). Article PubMed PubMed Central Google Scholar * Biolo, G., Zorat, F., Antonione, R. & Ciocchi, B. Muscle glutamine depletion in the
intensive care unit. _Int. J. Biochem. Cell Biol._ 37, 2169–2179 (2005). Article CAS PubMed Google Scholar * Tateyama, M. et al. Loss of skeletal muscle mass affects the incidence of
minimal hepatic encephalopathy: a case control study. _BMC Gastroenterol_ 20, 371 (2020). Article CAS PubMed PubMed Central Google Scholar * Ganda, O. P. & Ruderman, N. B. Muscle
nitrogen metabolism in chronic hepatic insufficiency. _Metabolism_ 25, 427–435 (1976). Article CAS PubMed Google Scholar * Laubenberger, J. et al. Proton magnetic resonance spectroscopy
of the brain in symptomatic and asymptomatic patients with liver cirrhosis. _Gastroenterology_ 112, 1610–1616 (1997). Article CAS PubMed Google Scholar * Butterworth, R. F. Hepatic
encephalopathy in cirrhosis: pathology and pathophysiology. _Drugs_ 79, 17–21 (2019). Article CAS PubMed PubMed Central Google Scholar * Nowicki, C. et al. Comparison of gut microbiome
composition in colonic biopsies, endoscopically-collected and at-home-collected stool samples. _Front. Microbiol._ 14, 1148097 (2023). Article PubMed PubMed Central Google Scholar * Wu,
M. et al. The differences between luminal microbiota and mucosal microbiota in mice. _J. Microbiol. Biotechnol._ 30, 287–295 (2020). * Stojic, J., Kukla, M. & Grgurevic, I. The
intestinal microbiota in the development of chronic liver disease: current status. _Diagnostics_ 13, 2960 (2023). Article CAS PubMed PubMed Central Google Scholar * Guinane, C. M. &
Cotter, P. D. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. _Ther. Adv. Gastroenterol._ 6, 295–308 (2013). Article
Google Scholar * Collins, C. M. & D’Orazio, S. E. Bacterial ureases: structure, regulation of expression and role in pathogenesis. _Mol. Microbiol._ 9, 907–913 (1993). Article CAS
PubMed Google Scholar * Dintzis, R. Z. & Hastings, A. B. The effect of antibiotics on urea breakdown in mice. _Proc. Natl. Acad. Sci. USA._ 39, 571–578 (1953). Article CAS PubMed
PubMed Central Google Scholar * Yu, L. et al. Physiological functions of urea transporter B. _Pflugers Arch_ 471, 1359–1368 (2019). Article CAS PubMed PubMed Central Google Scholar *
Chang, Y., Park, T.-E., Lee, S.-W. & Lee, E.-H. Colorimetric detection of urease-producing microbes using an ammonia-responsive flexible film sensor. _Biosensors_ 12, 886 (2022). Article
CAS PubMed PubMed Central Google Scholar * Mora, D. & Arioli, S. Microbial urease in health and disease. _PLoS Pathog_ 10, e1004472 (2014). Article PubMed PubMed Central Google
Scholar * Bajaj, J. S. et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. _Am. J. Physiol.
Gastrointest. Liver Physiol._ 303, G675–G685 (2012). Article CAS PubMed PubMed Central Google Scholar * Wang, P. et al. Gut microbiome-derived ammonia modulates stress vulnerability in
the host. _Nat. Metab._ 5, 1986–2001 (2023). Article CAS PubMed Google Scholar * Kowalchuk, G. A. & Stephen, J. R. Ammonia-oxidizing bacteria: a model for molecular microbial
ecology. _Annu. Rev. Microbiol._ 55, 485–529 (2001). Article CAS PubMed Google Scholar * Caminero, A., Guzman, M., Libertucci, J. & Lomax, A. E. The emerging roles of bacterial
proteases in intestinal diseases. _Gut Microbes_ 15, 2181922 (2023). * Kang, D. J. et al. Gut microbiota drive the development of neuro-inflammatory response in cirrhosis. _Hepatol. Baltim.
Md_ 64, 1232–1248 (2016). Article CAS Google Scholar * Chen, Y. et al. Characterization of fecal microbial communities in patients with liver cirrhosis. _Hepatology_ 54, 562 (2011).
Article PubMed Google Scholar * Patidar, K. R. & Bajaj, J. S. Covert and overt hepatic encephalopathy: diagnosis and management. _Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J.
Am. Gastroenterol. Assoc._ 13, 2048–2061 (2015). Google Scholar * Kim, S.-E. et al. The role of gut dysbiosis in acute-on-chronic liver failure. _Int. J. Mol. Sci._ 22, 11680 (2021).
Article CAS PubMed PubMed Central Google Scholar * Kim, J. N. et al. Metabolic networks for nitrogen utilization in Prevotella ruminicola 23. _Sci. Rep._ 7, 7851 (2017). Article PubMed
PubMed Central Google Scholar * Li, L., Chen, L., Shang, R., Wang, G. & Zhang, J. Improvement in bioconversion efficiency and reduction of ammonia emission by introduction of fruit
fermentation broth in a black soldier fly larvae and kitchen waste conversion system. _Insect Sci_ 30, 975–990 (2023). Article CAS PubMed Google Scholar * Kanamori, K., Weiss, R. L.
& Roberts, J. D. Ammonia assimilation pathways in nitrogen-fixing Clostridium kluyverii and Clostridium butyricum. _J. Bacteriol._ 171, 2148–2154 (1989). Article CAS PubMed PubMed
Central Google Scholar * Zhan, T. & Stremmel, W. The diagnosis and treatment of minimal hepatic encephalopathy. _Dtsch. Ärztebl. Int._ 109, 180–187 (2012). PubMed PubMed Central
Google Scholar * Liu, Q. et al. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. _Hepatology_ 39, 1441–1449 (2004). Article PubMed
Google Scholar * Zhang, Z. et al. Large-scale survey of gut microbiota associated with MHE Via 16S rRNA-based pyrosequencing. _Am. J. Gastroenterol._ 108, 1601–1611 (2013). Article CAS
PubMed Google Scholar * Fijan, S. Microorganisms with claimed probiotic properties: an overview of recent literature. _Int. J. Environ. Res. Public. Health_ 11, 4745–4767 (2014). Article
PubMed PubMed Central Google Scholar * Elkington, S. G., Floch, M. H. & Conn, H. O. Lactulose in the treatment of chronic portal-systemic encephalopathy. A double-blind clinical
trial. _N. Engl. J. Med._ 281, 408–412 (1969). Article CAS PubMed Google Scholar * Fritz, E. et al. Effects of lactulose and polyethylene glycol on colonic transit. _Aliment. Pharmacol.
Ther._ 21, 259–268 (2005). Article CAS PubMed Google Scholar * Riggio, O. et al. Effect of lactitol and lactulose administration on the fecal flora in cirrhotic patients. _J. Clin.
Gastroenterol._ 12, 433 (1990). Article CAS PubMed Google Scholar * Kurtz, C. B. et al. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent
exposure in healthy humans. _Sci. Transl. Med._ 11, eaau7975 (2019). Article CAS PubMed Google Scholar * Ochoa-Sanchez, R. et al. Genetically engineered E. coli Nissle attenuates
hyperammonemia and prevents memory impairment in bile-duct ligated rats. _Liver Int. Off. J. Int. Assoc. Study Liver_ 41, 1020–1032 (2021). CAS Google Scholar * Nicaise, C. et al. Control
of acute, chronic, and constitutive hyperammonemia by wild-type and genetically engineered Lactobacillus plantarum in rodents. _Hepatology_ 48, 1184–1192 (2008). Article CAS PubMed Google
Scholar * Greenberg, L. H. & Momary, H. Audiotoxicity and nephrotoxicity due to orally administered neomycin. _JAMA_ 194, 827–828 (1965). Article CAS PubMed Google Scholar * Tarao,
K. et al. Successful use of vancomycin hydrochloride in the treatment of lactulose resistant chronic hepatic encephalopathy. _Gut_ 31, 702–706 (1990). Article CAS PubMed PubMed Central
Google Scholar * Nakai, M. et al. Efficacy of rifaximin against covert hepatic encephalopathy and hyperammonemia in Japanese patients. _PLoS One_ 17, e0270786 (2022). Article CAS PubMed
PubMed Central Google Scholar * Loguercio, C., Federico, A., De Girolamo, V., Ferrieri, A. & Del Vecchio Blanco, C. Cyclic treatment of chronic hepatic encephalopathy with rifaximin.
Results of a double-blind clinical study. _Minerva Gastroenterol. Dietol._ 49, 53–62 (2003). CAS PubMed Google Scholar * Yu, X. et al. Rifaximin modulates the gut microbiota to prevent
hepatic encephalopathy in liver cirrhosis without impacting the resistome. _Front. Cell. Infect. Microbiol._ 11, 761192 (2022). Article PubMed PubMed Central Google Scholar * Kassam, Z.
et al. Donor screening for fecal microbiota transplantation. _N. Engl. J. Med._ 381, 2070–2072 (2019). Article PubMed Google Scholar * Bajaj, J. S. et al. Fecal microbiota transplant from
a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. _Hepatology_ 66, 1727–1738 (2017). Article CAS PubMed Google Scholar * Shawcross, D. et al. Faecal
microbiota transplant restores intestinal barrier function and augments ammonia metabolism in patients with cirrhosis: a randomised single-blind placebo-controlled trial. Preprint at
https://doi.org/10.21203/rs.3.rs-3088449/v1 (2023). * Shen, T.-C. D. et al. Engineering the gut microbiota to treat hyperammonemia. _J. Clin. Invest._ 125, 2841–2850 (2015). Article PubMed
PubMed Central Google Scholar * Griffith, D. P. & Musher, D. M. Acetohydroxamic acid. potential use in urinary infection caused by urea-splitting bacteria. _Urology_ 05, 299–302
(1975). Article CAS PubMed Google Scholar * Evstafeva, D. et al. Inhibition of urease-mediated ammonia production by 2-octynohydroxamic acid in hepatic encephalopathy. _Nat. Commun._ 15,
2226 (2024). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS The figures have been created using BioRender.com. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Molecular and Cellular Medicine, Institute of Liver and Biliary Sciences, New Delhi, India Deepika Jakhar & Savneet Kaur * Department of Hepatolopgy,
Institute of Liver and Biliary Sciences, New Delhi, India Shiv K. Sarin Authors * Deepika Jakhar View author publications You can also search for this author inPubMed Google Scholar * Shiv
K. Sarin View author publications You can also search for this author inPubMed Google Scholar * Savneet Kaur View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS D.J. and S.K. conceived the study and drafted the manuscript. D.J. designed the figures. S.K.S. and S.K. revised the manuscript. All authors read and approved the final
manuscript. CORRESPONDING AUTHOR Correspondence to Savneet Kaur. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not
have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s
Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jakhar, D., Sarin, S.K. & Kaur, S. Gut microbiota and dynamics of ammonia
metabolism in liver disease. _npj Gut Liver_ 1, 11 (2024). https://doi.org/10.1038/s44355-024-00011-x Download citation * Received: 15 July 2024 * Accepted: 11 October 2024 * Published: 04
December 2024 * DOI: https://doi.org/10.1038/s44355-024-00011-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative

:max_bytes(150000):strip_icc():focal(717x391:719x393)/Barbara-Eden-03-061323-6d67ed81017f48e1914e46ebe0797dce.jpg)







