- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Today, many essential industrial processes depend on syngas. Due to a high energy demand and overall cost as well as a dependence on natural gas as its precursor, alternative routes
to produce this valuable mixture of hydrogen and carbon monoxide are urgently needed. Electrochemical syngas production via two competing processes, namely carbon dioxide (CO2) reduction
and hydrogen (H2) evolution, is a promising method. Often, noble metal catalysts such as gold or silver are used, but those metals are costly and have limited availability. Here, we show
that metal-organic chalcogenolate assemblies (MOCHAs) combine several properties of successful electrocatalysts. We report a scalable microwave-assisted synthesis method for highly
crystalline MOCHAs ([AgXPh] ∞: X = Se, S) with high yields. The morphology, crystallinity, chemical and structural stability are thoroughly studied. We investigate tuneable syngas production
via electrocatalytic CO2 reduction and find the MOCHAs show a maximum Faraday efficiency (FE) of 55 and 45% for the production of carbon monoxide and hydrogen, respectively. SIMILAR CONTENT
BEING VIEWED BY OTHERS A DURABLE AND PH-UNIVERSAL SELF-STANDING MOC–MO2C HETEROJUNCTION ELECTRODE FOR EFFICIENT HYDROGEN EVOLUTION REACTION Article Open access 22 November 2021 SCALABLE
SYNTHESIS OF CU-CLUSTER CATALYSTS VIA SPARK ABLATION FOR THE ELECTROCHEMICAL CONVERSION OF CO2 TO ACETALDEHYDE Article 03 January 2025 ELECTRIFIED SYNTHESIS OF _N_-PROPANOL USING A DILUTE
ALLOY CATALYST Article 28 February 2025 INTRODUCTION Syngas is a widely used gaseous mixture of carbon monoxide and hydrogen. Today´s population is highly dependent on syngas as it is used
as a precursor to many essential chemicals, including bulk chemical intermediates such as methanol, thus being relevant for the production of polymers and pharmaceuticals1,2,3,4. Thanks to
the development of Fischer–Tropsch process, syngas can be considered a potential source of synfuels and synthetic petroleum5,6,7. Furthermore, syngas is a valuable good due to its high
hydrogen content. Hydrogen is known for its good power generation capabilities2,8. It is advantageous, as it provides the highest energy per unit mass of all known fuels and does not lead to
CO2 emissions upon combustion3,9. Another use of hydrogen is its capability in saturating double bonds and acting as a reducing agent2. Hydrogen is also essential in the Haber Bosch process
for ammonia synthesis2,10. Syngas is, therefore indispensable for the world’s food supply, as approximately 88% of ammonia is used in fertilizers10. Nowadays, Syngas is mainly produced via
three processes: steam reforming2,11,12,13, partial oxidation2, or thermal decomposition3. All three processes however require high temperatures and pressures, fossil fuel precursors, and
rare and expensive metal catalysts2,3,12,13,14. Thus, alternative synthesis routes for syngas production are of great need. Besides using biomass14,15,16, thermocycles1,17,18,19,20,21 or
photocatalysis1,22,23, electrochemical CO2 reduction to CO and H2 is a promising syngas production method1,24,25,26,27,28,29. So far various metals, alloys, metal oxides, and
metal-chalcogenide catalysts have been tested30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47. Silver shows high selectivity towards CO due to its lower binding energy towards CO
intermediate, making a further reduction to hydrocarbons hardly possible31. Silver-chalcogenides, including Ag2Se and Ag2S were recently reported as highly efficient catalysts for carbon
dioxide reduction reaction (CO2RR)48,49. Optimization of selectivity, efficiency and cost of suitable catalysts for electrochemical CO2RR and syngas production is still a huge challenge in
the field30,36. In 2002 Cuthbert et al. reported the space group and structural alignment of an organic-inorganic 2D hybrid material consisting of silver metal centres with coordination to a
chalcogenide covalently binding to phenyl rings50. This coordination polymer was named _Mithrene_ ([AgSePh]∞) by the group of Nathan Hohman in 2018. Since then, the Hohman group has done
extensive research on the synthesis, characterization and the properties of these metal organic chalcogenolate assemblies (MOCHAs)51,52,53,54,55,56,57,58,59. In 2020 they introduced the
so-called tarnishing approach and a biphasic synthesis of MOCHAs52,53,60. Tarnishing is based on the reaction of a diphenyl dichalcogenide precursor with a metal substrate at slightly
elevated temperatures. This method requires highly pure metal substrates or metal films as precursors. A MOCHA film bound to the substrate obtained via this method limits the use of various
substrates for growing MOCHA films. In addition, such metal thin films require a physical vapour deposition setup which is not a common laboratory infrastructure. Furthermore, it was shown
that the reaction of diphenyl dichalcogenides with silver oxide did not lead to MOCHA formation. Silver oxide formed MOCHAs only with benzeneselenol or -thiol precursors. These reactants
however, are highly toxic and thus their handling and utilization should be kept to a minimum. Crystallographic and optical studies on MOCHAs in particular on the silver benzeneselenolate
MOCHA “_Mithrene_” have been carried out since then51,53,54,55,57,61,62,63,64. However, either the quantities are very low (biphasic synthesis) or MOCHA film thickness (tarnishing method)
does not exceed 20 nm. Such limitations seem to hinder further applications of MOCHAs. We developed a microwave-assisted process for metal organic chalcogenolate assemblies that is capable
to shorten the synthesis time from 3 days to only 1–5 h and increases the absolute yield of the material by a factor of ~100 compared to the biphasic synthesis method suggested by Schriber
et al. Furthermore, we evaluated the performance of [AgSePh]∞ and [AgSPh]∞ for electrocatalytic syngas production. In addition to catalytic studies, we perform long-term stability studies of
MOCHAs in various environments. RESULTS AND DISCUSSION SYNTHESIS AND STRUCTURAL CHARACTERIZATION OF MOCHAS So far, different synthesis methods for MOCHAs did not result in high yields,
which hinder further use of MOCHAs. Here we report a new and more advantageous approach for MOCHA synthesis with a microwave reactor using a suspension. Consequently, product isolation is
easily achieved by filtration and consecutive washing and drying of the powder. Furthermore, we were able to achieve yields up to 83% (taking a molecular weight of 264 g mol−1 for [AgSePh]∞,
as suggested by Schriber et al.51), where the absolute amount obtained (110 mg) is only limited by the reactor size. Another advantage of this routes is that the microwave synthesis does
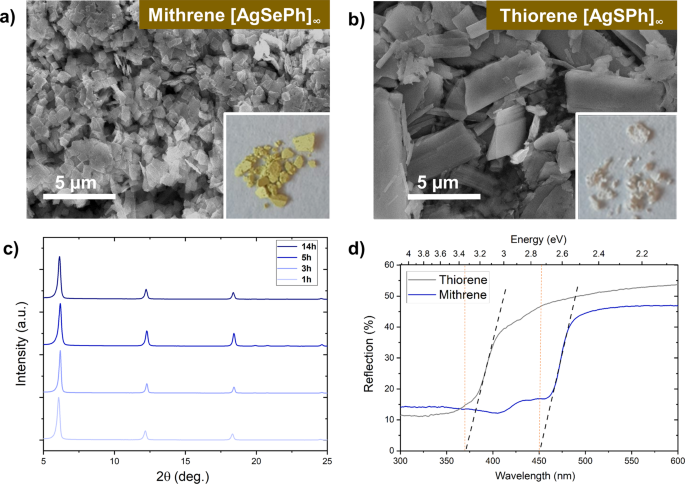
not require the use of toxic and smelly precursors (e.g., thiophenol, benzeneselenol)51 or the use of high purity metals52. X-ray diffraction (XRD) and scanning electron microscopy (SEM)
confirms the successful synthesis of _Mithrene_ ([AgSePh]∞) and _Thiorene_ ([AgSPh]∞) (Fig. 1a, b). Note that the XRD pattern exhibits three characteristic peaks corresponding to the {002},
{004}, and {006} planes, respectively51,59. We were interested in reducing the synthesis time, which is why we performed MOCHA synthesis via a microwave assisted method for 1, 3, 5, and 14
h. We obtain the same characteristic pattern using microwave synthesis at 110 °C with all tested durations (Fig. 1c). We also employed hydrothermal synthesis at 150 °C as an alternative
synthetic route. However, hydrothermal synthesis yielded products only after 72 h, manifesting again the advantage of microwave synthesis, where only 1 h was required. This suggests that the
reaction between AgNO3 and diphenyldichalcogene (the precursors) has thermal/kinetic limitations. Microwave synthesis is, in general, known to increase the reaction rate, which helps to
break the limitations by introducing a more uniform heating throughout the reaction vessel, decreasing the reaction time tremendously65. ATR-FTIR measurements can help to reveal eventual
impurities. An incomplete reaction, for instance, would result in a prominent band at around 2305 cm−1, corresponding to the benzeneselenol or benzenethiol species. As we do not observe any
bands at 2305 cm−1 (Fig. S1) we conclude that the powders contain only the products. Both MOCHA samples display similar vibrations as they can be attributed mainly to the aromatic network in
[AgSePh]∞ and [AgSPh]∞. At 1434, 1471, and 1572 cm−1, aromatic C–C stretches are observed. Vibrations at 995, 1018, and 1067 cm−1 correspond to C–H in plane stretching while C–H aromatic
out of plane vibrations can be seen at lower wavenumbers around 615, 688, 725, and 738 cm−1. Our measurements are in good agreement with literature spectra66. The band gap energies of
[AgSePh]∞ and [AgSPh]∞ were measured via UV-Vis-DRS (Fig. 1d) and calculated as 2.7 and 3.4 eV, respectively. The difference in bandgap can be traced back to the structural difference
between [AgSePh]∞ and [AgSPh]∞. In [AgSePh]∞ Ag–Ag network is trigonal, whereas in [AgSPh]∞ the Ag-Ag network is part of a linear chain51. Thus, excitons are eventually delocalized in two
dimensions above and below the silver network in [AgSePh]∞, causing absorption and emissive behaviour. [AgSPh]∞ on the other hand, does not exhibit a delocalization of excitons in two
dimensions due to Ag-Ag linear chains, causing a lack of emission in the visible range for [AgSPh]∞. Further characterization results, including energy dispersive X-ray (EDX) (Fig. S2) and
X-ray photoelectron spectroscopy (XPS) (Fig. S3) data can be found in the supplementary information in Supplementary Table 1. These measurements focussed on the determination of elemental
ratio in MOCHAs. EDX measurements lead to overestimation of carbon content by 10% (atomic) and underestimated the metal to chalcogenide ratio. XPS measurements of both MOCHAs showed results
that are in good agreement to theoretical calculations. ELECTRODE PREPARATION The MOCHAs were deposited on carbon paper comparing various techniques, including drop-casting, spin-coating,
dip-coating, and simple painting. The effect of the deposition technique on the substrate coverage was investigated using SEM. As seen in Fig. 2, drop-casting led to a full coverage of CP,
spin-coating gave a rather poor coverage. Painting and dip-coating resulted in comparable amounts of MOCHA on CP. However, dip-coating yielded more reproducible results compared to painting.
Hence, dip-coating was chosen as our standard approach for electrode preparation. CHEMICAL AND STRUCTURAL STABILITY MOCHAs are chemically and structurally stable, when stored in dark at
room temperature52,53. We further investigated their stability in harsher environments/conditions, using [AgSePh]∞ films deposited onto a Si substrate (for ease of characterization) and
polycrystalline [AgSePh]∞ powder. For the sake of brevity, we only display the results of the stability tests in organic solvents (Fig. 3). Results of further stability tests can be found in
Supplementary Information (Figs. S4–S6). Variations of pH exposure showed no change in structural or morphological appearance of [AgSePh]∞ from pH 0 to 9. The size and shape of [AgSePh]∞
crystals remained unchanged, and the powder XRD pattern displayed the three characteristic peaks. We conclude that [AgSePh]∞ is stable in acidic and basic conditions. Exposure of [AgSePh]∞
to various temperatures in the range of −25 to 120 °C was investigated as well. The results are shown in Fig. S5. We do not observe any change in shape, morphology, or size of the crystals
by means of SEM. Also, the XRD pattern confirmed no structural changes to the unexposed [AgSePh]∞ pattern. Stability tests upon light illumination with different wavelengths (365 nm, 456 nm,
Solar Simulator) showed that [AgSePh]∞ powder turned from a bright yellow colour before illumination into a darker brownish-yellow powder upon illumination times longer than 24 h.
(Illumination was performed from 10 min until 72 h). This colour change was retained even after months of storing. However, no changes were observed in XRD. Thus, we can conclude that the
synthesized MOCHAs are highly stable upon light illumination. Initial inspection of the Si substrate immersed in DMF showed no traces of [AgSePh]∞ (see Fig. 3a). However, it was noted that
the DMF solution turned yellowish. After evaporation of the DMF, a yellow residue remained. XRD pattern of this residue showed the three characteristic peaks of [AgSePh]∞ corresponding to
the {002}, {004}, and {006} planes. This implies, DMF does not alter the chemical and structural composition of [AgSePh]∞. DMF leads only to lifting-off of [AgSePh]∞ from the surface. In the
other tested solvents (ethyl acetate, isopropanol and methanol) No lift-off of [AgSePh]∞ could be seen even after being immersed for one week (Fig. 3b–d) and their stability is confirmed by
the measured XRD pattern (Fig. 3e). ELECTROCATALYTIC PERFORMANCE Electrocatalytic CO2 reduction was performed at three different potentials (−0.6, −0.8, and −1.0 V _vs_. RHE) for 30 min.
Each experiment was done a minimum of two times, with their average values shown in Fig. 4. The products were analyzed by means of gas chromatography. The faradaic efficiency (FE) and rate
of the products CO and H2 is given for these electrodes: bare carbon paper, [AgSePh]∞ on carbon paper, [AgSPh]∞ on carbon paper, (CP, [AgSePh]∞/CP and [AgSPh]∞/CP) in Fig. 4. The bare CP
electrode at all potentials produced only H2. For MOCHA/CP electrodes, the combined product formation (CO and H2) also decreased with decreasing potential, due to the lower energy input and
thus less help in overcoming the activation barrier. However, a distinct behaviour can be noted when comparing the ratio of CO to H2. While both FECO and CO rate decreased with decreasing
potentials, FEH2 increased and H2 rate decreased. Thus, the CO:H2 ratio was in favour of CO @−1.0 V _vs_. RHE, and in favour of hydrogen at potentials lower or equal to −0.8 V _vs_. RHE. At
−1.0 V vs. RHE both MOCHA electrodes achieved 55% FECO. Their rates, however, showed distinct behaviour. A rate of 61 µmol h−1 CO was obtained with [AgSePh]∞/CP, while [AgSPh]∞/CP achieved
85 µmol h−1 CO. Faraday efficiency and rate for carbon monoxide were clearly higher in the case of [AgSPh]∞ electrode compared to [AgSePh]∞ at −0.8 V _vs_. RHE. With [AgSPh]∞/CP 37% FECO and
21 µmol h−1 CO were obtained whilst [AgSePh]∞/CP only reached 6% FECO and 2 µmol h−1. From these observations we conclude that [AgSPh]∞ can be considered as a more selective catalyst for
CO2RR to CO with a higher performance compared to [AgSePh]∞. On average [AgSPh]∞ produced more CO per hour (an additional 17 µmol) compared to [AgSePh]∞. At −0.6 V vs. RHE with both
electrodes almost no CO could be detected, and also hydrogen rate was rather low reaching only values up to 40 µmol h−1. Notably, overall Faraday efficiencies lay below 100% at lower
overpotentials (−0.6 V). An explanation for that could be an unsaturated electrolyte. Although the solubility of the products hydrogen and carbon monoxide in the electrolyte 0.5 M KHCO3 is
low, a saturation of the liquid has to be obtained before the gas phase is filled. When taking a sample from the head space (gas phase) at low potentials, oversaturation in the liquid phase
might not have been achieved, leading to a reduced Faraday Efficiency. Comparing MOCHAs to similar literature examples proved to be a challenging task as such material class has not been
shown before for catalysis. We see silver chalcogenides as fitting counterparts for the performance comparison. [AgSPh]∞ was compared to Ag2S on carbonaceous electrode substrates as a
reference, which reached FECO values up to 67%49. Ma et al. investigated the difference in CO2 reduction performance between _monoclinic (m)_ and _orthorhombic (o)_ Ag2Se catalysts48. They
found, that _m_-Ag2Se was clearly favouring CO formation compared to _o_-Ag2Se. [AgSePh]∞ electrode showed a 15% higher FECO compared to their _o_-Ag2Se electrode. As [AgSePh]∞ MOCHA,
however; crystallizes in a monoclinic space group, comparison to _m_- Ag2Se (FECO 98%@−0.9 V vs. RHE48) is a fair comparison. Silver chalcogenides lead to a high CO:H2 ratio, whereas MOCHAs
are able to produce a well-suited ratio of 1:1 for syngas production. Electrocatalytic CO2RR in aqueous environment is accompanied by the hydrogen evolution reaction (HER) due to the
simultaneous reduction of water (or protons H+) and CO2. The following two reactions ocurr:27 $$\begin{array}{cc}{{{{{\rm{CO}}}}}}_{2}+2{{{{{\rm{H}}}}}}^{+}+2{e}^{-}\rightleftarrows
{{{{{{\rm{CO}}}}}}}+{{{{{\rm{H}}}}}}_{2}{{{{{\rm{O}}}}}} & {{E}}_{0}=-0.53\,{{{{{\rm{V}}}}}}\; {{{{{\rm{vs}}}}}}.{{{{{\rm{SHE}}}}}}\end{array}$$ (1)
$$\begin{array}{cc}2{{{{{\rm{H}}}}}}^{+}+2{e}^{-}\rightleftarrows {{{{{\rm{H}}}}}}_{2} & {{E}}_{0}=0.42\,{{{{{\rm{V}}}}}}\; {{{{{\rm{vs}}}}}}.{{{{{\rm{SHE}}}}}}\end{array}$$ (2) By
producing Syngas electrocatalytically, the drawback of a competing reaction can be bypassed and the HER fully integrated in the use. Another advantage of electrocatalytic Syngas production
compared to other Syngas production techniques, including solid oxide electrolysis cells (SOECs), steam reforming and thermocycles is its room temperature application1,2,13,25.
Electrocatalytic syngas production is thus a cost effective, environmentally friendly alternative for producing Syngas. However, the finding and development of cheap and suitable catalysts
is still a huge challenge in the field. A summary of potential catalysts and their Faraday Efficiencies, current densities, and experimental parameters is summarized in Table 1. and compared
to the results obtained with MOCHA/CP electrodes. RECYCLABILITY AND POST CATALYTIC STABILITY To further study the stability/evolution of MOCHAs in detail, the electrodes were investigated
before and after electrolysis. Dip-coated MOCHA/CP electrodes were sonicated in isopropanol after catalytic testing. The obtained MOCHA residue was examined by SEM and XRD (Supplementary
Fig. 7). The measurements reveal that [AgSePh]∞ and [AgSPh]∞ were both present on the carbon paper electrode after electrolysis without any structural changes. Next the Recyclability of
[AgSePh]∞/CP electrode was studied. We performed four consequent cycles of electrolysis at −1.0 V vs. RHE. The rates of CO and H2 obtained in each run are summarized in Fig. 5. The CO
formation rate decreased from 85 µmol h−1 (1st run) to 67 µmol h−1 upon consecutive electrolyses which corresponds to a ~21% decrease in activity. Exact mechanism of deactivation is subject
to further studies. CONCLUSION We developed a new, high-yield microwave assisted synthesis method for Metal Organic Chalcogenolate Assemblies. We report a MOCHA synthesis with a yield of 83
w% where the MOCHA is obtained as a powder, enabling a wide variety of characterization, application and handling possibilities with this 2D material. Compared to previously reported
methods, the microwave synthesis can compete in terms of reaction time63 or even shorten them (comparison to tarnishing52 and biphasic method53,59). Furthermore, we could circumvent the use
of toxic (thiophenol, selenophenol) precursors. The synthesis we report here requires no tedious sample separation. Stability measurements indicate that MOCHAs are stable in various
solvents, temperature range and pH media as well as upon light irradiation. Electrocatalytic Syngas production was successfully performed with [AgSePh]∞ and [AgSPh]∞ electrodes. Faraday
efficiencies and rates up to 55% and 81 µmol h−1 CO respectively, show that MOCHAs have a huge potential for electrochemical Syngas production. Further studies will focus on the underlying
mechanism and the working principle of MOCHAs in electrocatalytic CO2RR. EXPERIMENTAL Unless stated otherwise all chemicals were used as received. MOCHA SYNTHESIS MOCHAs were fabricated in
an Anton Parr Monowave 300 Microwave Synthesis Reactor. 0.5 mmol AgNO3 (abcr, 99.9%) were dissolved in 2 mL H2O. 0.75 mmol of the diphenyl dichalcogenide (DPDS, DPDSe) (Acros Organics, 99%)
were dissolved in 5 mL methanol by sonicating the suspension for 45 min in an ultrasonic bath. These two solutions were combined in the microwave vial which was closed and heat by microwave
irradiation at 110 °C for 5 h under continuous stirring at 600 rpm. The obtained MOCHA was rinsed and washed with methanol by vacuum filtration and stored in a drying oven at 40 °C for 24 h.
INK PREPARATION 100 mg of the MOCHA ([AgSePh]∞ or [AgSPh] ∞) were suspended in a mixture of 9.5 mL isopropanol (technical) and 500 µL NafionTM (Sigma Aldrich, 5 w% in aliphatic alcohols and
water) by 30 min sonication. ELECTRODE PREPARATION Toray Carbon Paper (Thermo Fisher Scientific TGP-H-60, 45356.KS) was cut into 2 × 1 cm pieces and used without further treatment. The CP
was dip coated with the “ink” for 24 h. After removing from the ink the electrodes were rinsed with isopropanol (technical) and dried. ELECTROCHEMISTRY Electrochemical measurements were
performed in a custom-designed H-cell (Perfectlight). 0.5 M KHCO3 was used as an electrolyte for all electrochemical measurements. The electrolyte was sparged with N2 or CO2 until saturation
before usage. The cathodic side was equipped with a stirring bar and the working electrode (CP, [AgSePh]∞/CP, [AgSPh]∞/CP) and the headspace was purged for an additional 30 min with CO2
before electrolysis. Ag/AgCl in 3 M KCl was used as the reference electrode whereas a platinum electrode served as the counter electrode. For electrolysis a constant potential of −1.0, −0.8,
−0.6 V vs. RHE was applied for 30 min. STABILITY TESTING The chosen irradiation wavelengths and lamps were a Thorlabs UV-lamp (365 nm), a Kessil (PR160) 456 nm lamp and a solar simulator.
Stability tests at different temperatures and illuminations were carried out on powder samples of [AgSePh]∞ placed in a glass vial. Testing in different organic solvents and pH media was
done on [AgSePh]∞ films. The films were obtained via ink preparation (see 4.2 “Ink preparation”) followed by drop-casting the ink onto a Si (711) substrate. ATR-FTIR MEASUREMENTS ATR-FTIR
spectra were taken on a Bruker Tensor 27 instrument in the range of 4000–600 cm−1. Prior a background spectrum of air was taken and directly subtracted from the measurement spectrum. DRS
MEASUREMENTS DRS measurements were recorded on a JASCO V-670 Spectrophotometer in a wavelength range from 200 to 900 nm. MgSO4 (Sigma Aldrich, >99.5%) was used as baseline. SEM/EDX
MEASUREMENTS SEM and EDX images and spectra were taken on a FEI Quanta FEG 250. SEM measurements were performed with an acceleration voltage of 10 kV and a working distance of 10 mm. For EDX
measurements an _Octane Elite Super_ detector was used. Spectra were recorded using an acceleration voltage of 20 kV, a resolution of 125.2 eV and a take-off angle of 31.7°. XPS
MEASUREMENTS All measurements were carried out on a custom-built SPECS XPS-spectrometer equipped with a monochromatised Al-Kα X-ray source (µFocus 350) and a hemispherical WAL-150 analyzer
(acceptance angle: 60°). All samples were mounted onto the sample holder using double-sided carbon tape. Pass energies of 100 eV and energy resolutions of 1 eV were used for the survey
(excitation energy: 1486.6 eV, beam energy and spot size: 70 W onto 400 μm, angle: 51° to sample surface normal, base pressure: 5 × 10−10 mbar, pressure during measurements: 2 × 10−9 mbar).
Data analysis was performed using CASA XPS software, employing transmission corrections (as per the instrument vendor’s specifications), Shirley/Tougaard backgrounds67,68 and Scofield
sensitivity factors69. Charge correction was applied so the adventitious carbon peak (C–C peak) was shifted to 284.8 eV binding energy (BE). All content values shown are in units of relative
atomic percent (at%), where the detection limit in survey measurements usually lies around 0.1–1 at%, depending on the element. The accuracy of XPS measurements is around 10–20% of the
values shown. Assignment of different components was primarily done using Reference databases70,71. XRD MEASUREMENTS Powder XRD pattern were recorded on a PANalytical X´Pert Pro
multi-purpose diffractometer (MPD) in Bragg Brentano geometry. The 2θ ranges were set between 5 and 90 degrees and a scan rate of 4° min−1 was applied. The anode material was copper, leading
to the emission of CuKα and CuKβ radiation (ratio 2:1) with a wavelength of 1.5406 Å. DATA AVAILABILITY The datasets generated during and/or analyzed during the current study are available
from the corresponding author on reasonable request. REFERENCES * Nguyen, V. N. & Blum, L. Syngas and synfuels from H2O and CO2: current status. _Chem. Ing. Tech._ 87, 354–375 (2015).
Article CAS Google Scholar * Martín, M. M. _Industrial Chemical Process Analysis and Design_ 199–297 (Elsevier, 2016). * Dalena, F. et al. _Methanol_ 3–28 (Elsevier, 2018). * Bozzano, G.
& Manenti, F. Efficient methanol synthesis: perspectives, technologies, and optimization strategies. _Prog. Energy Combust. Sci._ 56, 71–105 (2016). Article Google Scholar * Ott, J. et
al. _Ullmann’s Encyclopedia of Industrial Chemistry_ (Wiley-VCH Verlag GmbH & Co. KGaA, 2012). * Hagos, F. Y., Aziz, A. R. A. & Sulaiman, S. A. Trends of syngas as a fuel in
internal combustion engines. _Adv. Mech. Eng._ 6, 401587 (2014). Article Google Scholar * Boehman, A. L. & Corre, O. L. Combustion of Syngas in internal combustion engines. _Combust.
Sci. Technol._ 180, 1193–1206 (2008). Article CAS Google Scholar * Fan, L., Tu, Z. & Chan, S. H. Recent development of hydrogen and fuel cell technologies: a review. _Energy Rep._ 7,
8421–8446 (2021). Article Google Scholar * Paykani, A. et al. Synthesis gas as a fuel for internal combustion engines in transportation. _Prog. Energy Combust. Sci._ 90, 100995 (2022).
Article Google Scholar * Pattabathula, V. & Richardson, J. Introduction to ammonia production. _Back to Basics_ 7, 69–75 (2016). * Wilson, S. M. W., Kennedy, D. A. & Tezel, F. H.
Adsorbent screening for CO2/CO separation for applications in syngas production. _Sep. Purif. Technol._ 236, 116268 (2020). Article CAS Google Scholar * Liu, K., Song, C. & Subramani,
V. _Hydrogen and Syngas Production and Purification Technologies_ (John Wiley & Sons, 2010). * Roseno, K. T. et al. _Biofuels - State of Development_ (ed Biernat, K.) (InTech, 2018). *
Ren, J., Liu, Y.-L., Zhao, X.-Y. & Cao, J.-P. Methanation of syngas from biomass gasification: an overview. _Int. J. Hydrog. Energy_ 45, 4223–4243 (2020). Article CAS Google Scholar *
Niu, M. et al. A novel two-stage enriched air biomass gasification for producing low-tar high heating value fuel gas: pilot verification and performance analysis. _Energy_ 173, 511–522
(2019). Article CAS Google Scholar * Aziz, M. A. A., Jalil, A. A., Triwahyono, S. & Ahmad, A. CO 2 methanation over heterogeneous catalysts: recent progress and future prospects.
_Green. Chem._ 17, 2647–2663 (2015). Article CAS Google Scholar * Steinfeld, A. Solar hydrogen production via a two-step water-splitting thermochemical cycle based on Zn/ZnO redox
reactions. _Int. J. Hydrog. Energy_ 27, 611–619 (2002). Article CAS Google Scholar * Roeb, M. et al. Materials-related aspects of thermochemical water and carbon dioxide splitting: a
review. _Materials_ 5, 2015–2054 (2012). Article CAS PubMed Central Google Scholar * Serpone, N., Lawless, D. & Terzian, R. Solar fuels: status and perspectives. _Sol. Energy_ 49,
221–234 (1992). Article CAS Google Scholar * Funk, E. & Thermochemical, J. hydrogen production: past and present. _Int. J. Hydrog. Energy_ 26, 185–190 (2001). Article CAS Google
Scholar * Smestad, G. P. & Steinfeld, A. Review: photochemical and thermochemical production of solar fuels from H2O and CO2 using metal oxide catalysts. _Ind. Eng. Chem. Res._ 51,
11828–11840 (2012). Article CAS Google Scholar * Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. _Nature_ 238, 37–38 (1972). Article CAS
PubMed Google Scholar * Lin, F. & Boettcher, S. W. Adaptive semiconductor/electrocatalyst junctions in water-splitting photoanodes. _Nat. Mater._ 13, 81–86 (2014). Article CAS PubMed
Google Scholar * Bodner, M., Hofer, A. & Hacker, V. H2 generation from alkaline electrolyzer. _Wiley Interdiscip. Rev. Energy Environ._ 4, 365–381 (2015). CAS Google Scholar *
Ursúa, A., Gandía, L. M. & Sanchis, P. Hydrogen production from water electrolysis: current status and future trends. _Proc. IEEE_ https://doi.org/10.1109/JPROC.2011.2156750 (2012). *
Graves, C., Ebbesen, S. D., Mogensen, M. & Lackner, K. S. Sustainable hydrocarbon fuels by recycling CO2 and H2O with renewable or nuclear energy. _Renew. Sustain. Energy Rev._ 15, 1–23
(2011). Article CAS Google Scholar * Sun, Z., Ma, T., Tao, H., Fan, Q. & Han, B. Fundamentals and challenges of electrochemical CO2 reduction using two-dimensional materials. _Chem_
3, 560–587 (2017). Article CAS Google Scholar * Ivy, J. Summary of Electrolytic Hydrogen Production: Milestone Completion Report. _NREL/MP-560-36734_ 2–7 (2004). * Mergel, J., Glüsen, A.
& Wannek, C. _Hydrogen and Fuel Cells: Fundamentals, Technologies and Applications_ 41–60 (Wiley-VCH Verlag GmbH & Co. KGaA) (2010). * Lu, S. et al. Electrosynthesis of syngas via
the co-reduction of CO2 and H2O. _Cell Rep. Phys. Sci._ 1, 100237 (2020). Article Google Scholar * Hori, Y., Kikuchi, K. & Suzuki, S. Production of CO and CH4 in electrochemical
reduction of CO2 at metal electrodes in aqueous hydrogencarbonate solution. _Chem. Lett._ 14, 1695–1698 (1985). Article Google Scholar * Zhu, W. et al. Monodisperse Au nanoparticles for
selective electrocatalytic reduction of CO2 to CO. _J. Am. Chem. Soc._ 135, 16833–16836 (2013). Article CAS PubMed Google Scholar * Zhu, W. et al. Active and selective conversion of CO2
to CO on ultrathin Au nanowires. _J. Am. Chem. Soc._ 136, 16132–16135 (2014). Article CAS PubMed Google Scholar * Feng, X., Jiang, K., Fan, S. & Kanan, M. W. Grain-boundary-dependent
CO2 electroreduction activity. _J. Am. Chem. Soc._ 137, 4606–4609 (2015). Article CAS PubMed Google Scholar * Liu, S. et al. Shape-dependent electrocatalytic reduction of CO2 to CO on
triangular silver nanoplates. _J. Am. Chem. Soc._ 139, 2160–2163 (2017). Article CAS PubMed Google Scholar * Zhu, Y., Yu, J., Fan, Z. & Zhang, Z. _Nanomaterials for CO2 Capture,
Storage, Conversion and Utilization_ 183–196 (Elsevier, 2021). * Yang, S.-C. et al. Oxygen vacancy engineering of cerium oxides for carbon dioxide capture and reduction. _ChemSusChem_ 6,
1326–1329 (2013). Article CAS PubMed Google Scholar * Geng, Z. et al. Oxygen vacancies in ZnO nanosheets enhance CO2 electrochemical reduction to CO. _Angew. Chem. Int. Ed._ 57,
6054–6059 (2018). Article CAS Google Scholar * Xie, H. et al. Boosting tunable syngas formation via electrochemical CO2 reduction on Cu/In2O3 core/shell nanoparticles. _ACS Appl. Mater.
Interfaces_ 10, 36996–37004 (2018). Article CAS PubMed Google Scholar * Li, Q. et al. Tuning Sn-catalysis for electrochemical reduction of CO2 to CO via the core/shell Cu/SnO 2
structure. _J. Am. Chem. Soc._ 139, 4290–4293 (2017). Article CAS PubMed Google Scholar * Bi, W., Wu, C. & Xie, Y. Atomically thin two-dimensional solids: an emerging platform for
CO2 electroreduction. _ACS Energy Lett._ 3, 624–633 (2018). Article CAS Google Scholar * Gao, S. et al. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to
liquid fuel. _Nature_ 529, 68–71 (2016). Article CAS PubMed Google Scholar * Zhang, S., Kang, P. & Meyer, T. J. Nanostructured tin catalysts for selective electrochemical reduction
of carbon dioxide to formate. _J. Am. Chem. Soc._ 136, 1734–1737 (2014). Article CAS PubMed Google Scholar * Peng, X. et al. Efficient electroreduction CO2 to CO over MnO2 nanosheets.
_Inorg. Chem._ 58, 8910–8914 (2019). Article CAS PubMed Google Scholar * Lü, F. et al. Selective electrolysis of CO2 to CO on ultrathin In2Se3 nanosheets. _Electrochem. Commun._ 103,
127–132 (2019). Article Google Scholar * Asadi, M. et al. Robust carbon dioxide reduction on molybdenum disulphide edges. _Nat. Commun._ 5, 4470 (2014). Article CAS PubMed Google
Scholar * Xu, J. et al. Carbon dioxide electroreduction into syngas boosted by a partially delocalized charge in molybdenum sulfide selenide alloy monolayers. _Angew. Chem. Int. Ed._ 56,
9121–9125 (2017). Article CAS Google Scholar * Ma, D. et al. Greatly enhanced CO2 electrocatalytic reduction performance of Ag2Se nanocatalyst via phase-engineering. _Appl. Catal. B
Environ._ 316, 121658 (2022). Article CAS Google Scholar * Chen, Q. S. et al. Efficient carbon dioxide electroreduction over rationally designed heterogeneous Ag2S-Au nanocomposites. _J.
Colloid Interface Sci._ 623, 1172–1180 (2022). Article CAS Google Scholar * Cuthbert, H. L., Wallbank, A. I., Taylor, N. J. & Corrigan, J. F. Synthese und strukturelle
Charakterisierung von [Cu20 Se 4 (µ 3 -SePh) 12 (PPh 3) 6] und. _Z. Anorg. Allg. Chem._ 628, 2483–2488 (2002). Article CAS Google Scholar * Schriber, E. A. et al. Chemical crystallography
by serial femtosecond X-ray diffraction. _Nature_ 601, 360–365 (2022). Article CAS PubMed PubMed Central Google Scholar * Trang, B. et al. Tarnishing silver metal into mithrene. _J.
Am. Chem. Soc._ 140, 13892–13903 (2018). Article CAS PubMed Google Scholar * Schriber, E. A. et al. Mithrene is a self-assembling robustly blue luminescent metal-organic chalcogenolate
assembly for 2d optoelectronic applications. _ACS Appl. Nano Mater._ 1, 3498–3508 (2018). Article CAS Google Scholar * Yao, K. et al. Strongly quantum-confined blue-emitting excitons in
chemically configurable multiquantum wells. _ACS Nano_ 15, 4085–4092 (2021). Article CAS PubMed Google Scholar * Yan, H. et al. Hybrid metal-organic chalcogenide nanowires with
electrically conductive inorganic core through diamondoid-directed assembly. _Nat. Mater._ 16, 349–355 (2017). Article CAS PubMed Google Scholar * Yeung, M. et al. Corrosion of late-and
post-transition metals into metal-organic chalcogenolates and implications for nanodevice architectures. _ACS Appl. Nano Mater._ 3, 3568–3577 (2020). Article CAS Google Scholar * Kastl,
C., Schwartzberg, A. M. & Maserati, L. Picoseconds-limited exciton recombination in metal–organic chalcogenides hybrid quantum wells. _ACS Nano_ 16, 3715–3722 (2022). Article CAS
PubMed Google Scholar * Mills, H. A. et al. Sterically invariant carborane-based ligands for the morphological and electronic control of metal–organic chalcogenolate assemblies. _Chem.
Mater._ 34, 6933–6943 (2022). Article CAS Google Scholar * Popple, D. C., Schriber, E. A., Yeung, M. & Hohman, J. N. Competing roles of crystallization and degradation of a
metal-organic chalcogenolate assembly under biphasic solvothermal conditions. _Langmuir_ 34, 14265–14273 (2018). Article CAS PubMed Google Scholar * Hohman, J. N., Collins M. S., Smidt
T. E. “Mithrene and methods of fabrication of mithrene.” U. S. Patent US20190194128A1. Applied August 2017 and issued April 2020. * Maserati, L. et al. Anisotropic 2D excitons unveiled in
organic–inorganic quantum wells. _Mater. Horiz._ 8, 197–208 (2021). Article CAS PubMed Google Scholar * Paritmongkol, W. et al. Morphological control of 2D hybrid organic–inorganic
semiconductor AgSePh. _ACS Nano_ 16, 2054–2065 (2022). Article CAS PubMed Google Scholar * Paritmongkol, W. et al. Size and quality enhancement of 2D semiconducting metal–organic
chalcogenolates by amine addition. _J. Am. Chem. Soc._ 143, 20256–20263 (2021). Article CAS PubMed Google Scholar * Maserati, L. et al. Photo-electrical properties of 2D quantum confined
metal–organic chalcogenide nanocrystal films. _Nanoscale_ 13, 233–241 (2021). Article CAS PubMed Google Scholar * Kappe, C. O. Controlled microwave heating in modern organic synthesis.
_Angew. Chem. Int. Ed._ 43, 6250–6284 (2004). Article CAS Google Scholar * Maserati, L., Pecorario, S., Prato, M. & Caironi, M. Understanding the synthetic pathway to large-area,
high-quality [AgSePh]∞ nanocrystal films. _J. Phys. Chem. C_ 124, 22845–22852 (2020). Article CAS Google Scholar * Shirley, D. A. High-resolution X-ray photoemission spectrum of the
valence bands of gold. _Phys. Rev. B_ 5, 4709–4714 (1972). Article Google Scholar * Tougaard, S. Universality classes of inelastic electron scattering cross-sections. _Surf. Interface
Anal._ 25, 137–154 (1997). Article CAS Google Scholar * Scofield, J. H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. _J. Electron Spectrosc. Relat. Phenom._
8, 129–137 (1976). Article CAS Google Scholar * High Resolution XPS of Organic Polymers. The Scienta ESCA300 Database (Beamson, G.; Briggs, D.). _J. Chem. Educ._ 70, A25 (1993). Article
Google Scholar * Powell, C. X-ray Photoelectron Spectroscopy Database XPS, Version 4.1, NIST Standard Reference Database 20. https://doi.org/10.18434/T4T88K (1989). * Sheng, W. et al.
Electrochemical reduction of CO2 to synthesis gas with controlled CO/H2 ratios. _Energy Environ. Sci._ 10, 1180–1185 (2017). Article CAS Google Scholar * Qin, B. et al. Electrochemical
reduction of CO2 into tunable syngas production by regulating the crystal facets of Earth-abundant Zn catalyst. _ACS Appl. Mater. Interfaces_ 10, 20530–20539 (2018). Article CAS PubMed
Google Scholar * Mistry, H. et al. Exceptional size-dependent activity enhancement in the electroreduction of CO2 over Au nanoparticles. _J. Am. Chem. Soc._ 136, 16473–16476 (2014). Article
CAS PubMed Google Scholar * Hua, Y., Zhang, B., Hao, W. & Gao, Z. Boosting CO desorption on dual active site electrocatalysts for CO2 reduction to produce tunable syngas. _Cell Rep.
Phys. Sci._ 3, 100703 (2022). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to the AIC, USTEM, and X-Ray Centre of TU Wien for providing their
facilities. The authors acknowledge TU Wien Bibliothek for financial support through its Open Access Funding Program. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute of Materials
Chemistry, TU Wien, Getreidemarkt 9, 1060, Vienna, Austria Hannah Rabl, Stephen Nagaraju Myakala, Jakob Rath, Bernhard Fickl, Jasmin S. Schubert, Dogukan H. Apaydin & Dominik Eder
Authors * Hannah Rabl View author publications You can also search for this author inPubMed Google Scholar * Stephen Nagaraju Myakala View author publications You can also search for this
author inPubMed Google Scholar * Jakob Rath View author publications You can also search for this author inPubMed Google Scholar * Bernhard Fickl View author publications You can also search
for this author inPubMed Google Scholar * Jasmin S. Schubert View author publications You can also search for this author inPubMed Google Scholar * Dogukan H. Apaydin View author
publications You can also search for this author inPubMed Google Scholar * Dominik Eder View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
H.R. synthesized the compounds, performed the electrolyses, performed structural characterization, analyzed the catalytic performance and wrote the manuscript. S.N.M. performed SEM and EDX
measurements. J.R., B.F., and J.S.S. performed XPS measurements. D.H.A. conceptualized and supervised the research and wrote the manuscript together with H.R. using the comments from all
co-authors. D.E. coordinated the project, provided resources and contributed to the writing of the manuscript. CORRESPONDING AUTHOR Correspondence to Dogukan H. Apaydin. ETHICS DECLARATIONS
COMPETING INTERESTS All authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Communications Chemistry_ thanks Luis De Jesus and the other, anonymous, reviewer for
their contribution to the peer review of this work. Peer reviewer reports are available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION PEER REVIEW FILE SUPPORTING INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under
a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Rabl, H., Myakala, S.N., Rath, J. _et al._ Microwave-assisted
synthesis of metal-organic chalcogenolate assemblies as electrocatalysts for syngas production. _Commun Chem_ 6, 43 (2023). https://doi.org/10.1038/s42004-023-00843-3 Download citation *
Received: 31 October 2022 * Accepted: 17 February 2023 * Published: 01 March 2023 * DOI: https://doi.org/10.1038/s42004-023-00843-3 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative