- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Ozonolysis of isoprene, the most abundant alkene, produces three distinct Criegee intermediates (CIs): CH2OO, methyl vinyl ketone oxide (MVKO) and methacrolein oxide (MACRO). The
oxidation of SO2 by CIs is a potential source of H2SO4, an important precursor of aerosols. Here we investigated the UV-visible spectroscopy and reaction kinetics of thermalized MACRO. An
extremely fast reaction of _anti_-MACRO with SO2 has been found, _k_SO2 = (1.5 ± 0.4) × 10−10 cm3 s−1 (±1_σ_, _σ_ is the standard deviation of the data) at 298 K (150 − 500 Torr), which is
ca. 4 times the value for _syn_-MVKO. However, the reaction of _anti_-MACRO with water vapor has been observed to be quite slow with an effective rate coefficient of (9 ± 5) × 10−17 cm3 s−1
(±1_σ_) at 298 K (300 to 500 Torr), which is smaller than current literature values by 1 or 2 orders of magnitude. Our results indicate that _anti_-MACRO has an atmospheric lifetime (best
estimate ca. 18 ms at 298 K and RH = 70%) much longer than previously thought (ca. 0.3 or 3 ms), resulting in a much higher steady-state concentration. Owing to larger reaction rate
coefficient, the impact of _anti_-MACRO on the oxidation of atmospheric SO2 would be substantial, even more than that of _syn_-MVKO. SIMILAR CONTENT BEING VIEWED BY OTHERS DIRECT GAS-PHASE
FORMATION OF FORMIC ACID THROUGH REACTION OF CRIEGEE INTERMEDIATES WITH FORMALDEHYDE Article Open access 22 June 2023 HIGHLY OXIDIZED PRODUCTS FROM THE ATMOSPHERIC REACTION OF HYDROXYL
RADICALS WITH ISOPRENE Article Open access 28 February 2025 MOLECULAR MECHANISM FOR RAPID AUTOXIDATION IN _Α_-PINENE OZONOLYSIS Article Open access 09 February 2021 INTRODUCTION Isoprene is
the most abundant unsaturated hydrocarbon in the atmosphere1. Ozonolysis of isoprene produces three kinds of carbonyl oxides (also called Criegee Intermediates, CIs): CH2OO, methyl vinyl
ketone oxide (MVKO: CH3C(C2H3)OO), and methacrolein oxide (MACRO: CH2=C(CH3)CHOO)2,3,4. CIs are very reactive species. In 2012, their reactions with SO2 were found to be faster than
previously thought by orders of magnitude5. The oxidation of SO2 (SO2 → SO3) has gained wide attention because it is an important process in the formation of secondary aerosols (SO3 →
H2SO4)6,7,8,9,10,11,12,13,14,15. Field and chamber studies, pioneered by Mauldin et al.9, indicate that there is a non-OH oxidant contributing to the oxidation of atmospheric SO2 and this
new oxidant may be Criegee intermediates9,10,11,12,13,14,15. However, it is impractical to measure the very reactive CIs in the atmosphere. Their atmospheric concentrations can only be
estimated through kinetics analysis. For example, Novelli et al. have given an average estimate of the CI concentration of ca. 5 × 104 molecules cm−3 (with an order of magnitude uncertainty)
for the two environments they studied16. (For simplicity, we will use ‘cm−3’ for the unit of molecular number density, instead of the more formal ‘molecules cm−3’.) Note that older
estimations may have larger uncertainties in the CI concentrations since the related reaction kinetics were not well determined at that time. On the other hand, laboratory studies on
individual CI reactions have revealed that the reactivity (thus the atmospheric fate) of a CI would strongly depend on its structure17,18. For CH2OO and _anti_-CH3CHOO, which have a H-atom
at the _syn_ position, the main decay pathway is their reactions with water vapor (H2O monomer and dimer)18,19,20,21,22,23. These reactions are extremely fast, resulting in very low
steady-state concentrations of such CIs, which are too low to oxidize atmospheric SO2 at any substantial level17. For _syn_-CH3CHOO and (CH3)2COO, which have an alkyl group at the _syn_
position, their unimolecular reactions via intramolecular 1,4-H-atom transfer are the major decay process, which also generates OH radicals24,25,26,27,28,29,30,31. These unimolecular
processes are not slow and essentially limit the steady-state concentrations of such CIs17,32. Different from alkyl-substituted CIs, MVKO and MACRO have a C=C double bond, which forms
extended conjugation with the carbonyl oxide functional group (resonance stabilized). The pioneering works of Lester and coworkers have demonstrated a photolytic synthesis method that allows
direct detection of MVKO and MACRO33,34. Via this new synthesis scheme, recent studies have shown that the resonance-stabilization would affect the reactivity and thus the atmospheric fate
of MVKO35,36. For MVKO, there are four possible isomers (or conformers)2,33,35,37. Similar to simpler CIs, the barrier of rotating the carbonyl C=O bond is high, resulting in
non-interconverting _syn_ and _anti_ isomers (following the nomenclature of Barbar et al.)33,38. However, the barrier of rotating the C─C single bond between the C=C and C=O bonds is low,
giving essentially an equilibrium mixture of _cis_ and _trans_ conformers32,39,40. It has been predicted that _anti_-_trans_-MVKO would quickly interconvert to _anti_-_cis_-MVKO (>106
s−1)32, which decays quickly via fast 1,5-ring closure to form dioxole with a rate coefficient of ca. 2100 s−1 at 298 K32,33,41,42. As a result, _anti_-MVKO was not observed experimentally,
presumably due to short lifetime and/or low yield35. Caravan et al. have found that _syn_-MVKO reacts with SO2 and formic acid as fast as other alkyl CIs do. Furthermore, based on their
global chemistry and transport model, they have shown that _syn_-MVKO could significantly increase the atmospheric oxidation of SO2 and the removal of formic acid, where the isoprene
emission is high. The high impact of _syn_-MVKO is mostly due to the abundance of isoprene (its source) and its slow decay (slow unimolecular decay and slow reaction with water vapor)35. The
slow decay of _syn_-MVKO is related to its resonance-stabilized electronic structure32,36, which would be disrupted at the transition state of the unimolecular reaction33,43. Another
interesting aspect of this resonance stabilization is that the iodine-atom adduct of MVKO is relatively less stable compared to the cases of alkyl CIs36. MACRO also has a
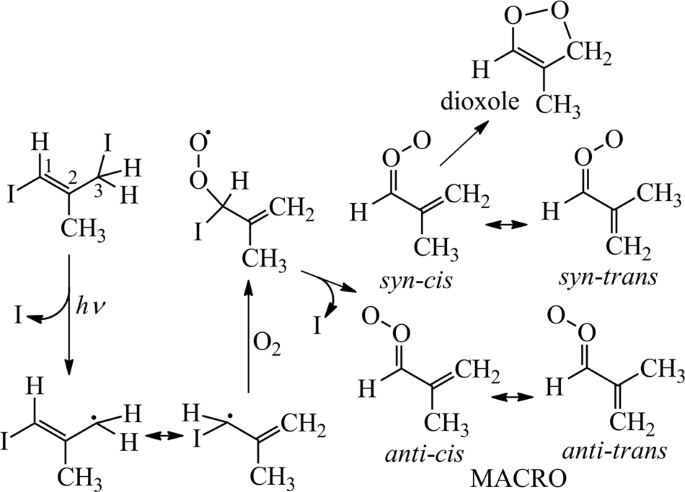
resonance-stabilized electronic structure and two non-interconverting families of conformers (Fig. 1). It has also been predicted that _syn_-_trans_-MACRO (following the nomenclature of
Vansco et al.)34 would interconvert quickly (>106 s−1) to _syn_-_cis_-MACRO, which would undergo fast unimolecular decay (_k_uni = 2500 s−1) to form dioxole, while _anti_ conformers are
expected to have slow unimolecular decay (ca. 10 s−1)32. These theoretically predicted values are from Vereecken et al.32 who utilized the structure–activity relationships which considered
the best available theoretical and experimental results at that time. However, similar to other _anti_ types of CIs (CH2OO and _anti_-CH3CHOO)17,18, the reaction of _anti_-MACRO with water
vapor was predicted to be fast (_k_water_-_eff = 7.2 × 10−15 cm3 s−1 by Anglada et al.44 or 6.3 × 10−16 cm3 s−1 by Vereecken et al.32 at relative humidity (RH) = 70% and 298 K, considering
both water monomer and dimer reactions). If so, the fast reaction with water vapor (ca. 103 s−1) would result in a very low steady-state concentration of _anti_-MACRO, diminishing its
atmospheric impact. Nonetheless, as will be shown later, this picture is incorrect. Very recently, Vansco et al. have reported the electronic spectroscopy and photochemistry of MACRO; as the
authors have mentioned, “This UV–visible detection scheme will enable study of its unimolecular and bimolecular reactions under thermal conditions of relevance to the atmosphere.”34
Following their method, here we prepared MACRO starting from the photolysis of _E_-1,3-diiodo-2-methylprop-1-ene precursor (Fig. 1). The time-resolved UV–visible spectrum of MACRO was
recorded by using a continuous broadband light source and a grating spectrometer equipped with an ultrafast CMOS camera. Analyzing the time series of the spectra allowed us to retrieve the
spectrum of MACRO and its time-dependent concentration. To our surprise, the reaction of MACRO with water vapor was found to be much slower than previous predictions32,44 by one or two
orders of magnitude, implying much longer atmospheric lifetime (ca. 18 ms vs. 3 or 0.3 ms32,44) and higher steady-state concentrations for atmospheric MACRO. On the other hand, the
resonance-stabilized MACRO still reacts extremely fast with SO2. Based on the results of a recent global chemistry and transportation model of MVKO35, our data suggest that the impact of
MACRO on the oxidation of atmospheric SO2 would also be substantial. RESULTS AND DISCUSSION ANALYSIS OF THE OBSERVED UV SPECTRUM Figure 2a shows the time-resolved difference absorption
spectra recorded in the photolysis reactor. Here ‘difference’ means the change after the photolysis laser pulse. We can see three spectral features in the spectrum: (i) a very broad and
structureless absorption band peaking at ca. 397 nm; (ii) absorption of IO which has distinctive sharp peaks between 400 and 460 nm45; (iii) broad absorption band of I2 extending to 520
nm46. The presence of IO and I2 is similar to previous investigations of CH2OO47,48,49, CH3CHOO23,50, (CH3)2COO51, and MVKO35,36. UV ABSORPTION SPECTRUM OF MACRO Under the same experimental
conditions, SO2 was added to scavenge CIs (see Supplementary Fig. 1). We found that the spectral feature (i) disappears, indicating its spectral carrier is a Criegee intermediate. We further
subtracted the time-resolved spectra recorded at [SO2] = 1 × 1014 cm−3 from those without adding SO2 at each photolysis-probe delay time. This operation removed most of the absorption
signals of IO and I2 (and also other minor byproducts), and the resulted spectra would be mainly from the Criegee intermediate. Considering that we were using the same precursor and
preparation method of Vansco et al.34, we assigned this CI to MACRO. The spectrum of MACRO can be well fitted with a Gaussian function (Fig. 3). This spectrum is similar to that of Vansco et
al. who reported a broad spectrum of MACRO peaked at 380 nm with weak oscillatory structure at long wavelengths ascribed to vibrational resonances34. However, we do not observe such
oscillatory structure; the differences are presumably due to different temperatures of the MACRO samples (thus, the conformer populations may be different), as Vansco et al. recorded their
spectrum under a jet cool condition34. We may decompose the observed spectra into the contributions of MACRO, IO, and I2 (Fig. 2a and Supplementary Fig. 4) with a least-squares fit. The
resulted signal intensities (converted to concentrations) of MACRO, IO, and I2 are plotted in Fig. 2b as a function of the delay time. The intensities of IO and I2 grow with time, indicating
they are secondary products. While the kinetics of IO and I2 formation may be interesting, we like to focus on MACRO in this work. We can see that the lifetime of MACRO in this particular
experiment is ca. 3 ms, much longer than the predicted value for _syn_-MACRO (<0.4 ms, based on its _k_uni = 2500 s−132; and other chemical processes would further shorten the lifetime).
Therefore, we conclude that the observed spectral carrier should be _anti_-MACRO, similar to the case of MVKO35. Note that the long lifetime conformer of MVKO is _syn_-MVKO (following the
nomenclature of Barbar et al.)33 which has a structure similar to _anti_-MACRO. For simplicity, we will use MACRO to represent _anti_-MACRO in the following analysis. KINETICS OF MACRO
REACTION WITH SO2 The above analysis has been repeated for experiments adding various [SO2]. The resulted MACRO signal intensities at each photolysis-probe delay time are plotted in Fig. 4a.
The decay of MACRO signal can be fitted with a single exponential function to yield a pseudo-first-order rate coefficient, _k_obs, at each [SO2]. $${\Delta}Abs\left( {{\mathrm{MACRO}}}
\right) = \sigma L\left[ {{\mathrm{MACRO}}} \right]\left( t \right) = \sigma L\left[ {{\mathrm{MACRO}}} \right]_0{\mathrm{exp}}\left( { - k_{{\mathrm{obs}}}t} \right)$$ where _σ_ is the
absorption cross section of MACRO34 and _L_ is the optical path length (when reporting Δ_Abs_(MACRO), we use its peak value at 397 nm). Figure 4b shows that _k_obs increases linearly with
[SO2]. $$k_{{\mathrm{obs}}} = k_0 + k_{{\mathrm{SO}}2}\left[ {{\mathrm{SO}}_2} \right]$$ The slope would correspond to the rate coefficient _k_SO2 of the bimolecular reaction of MACRO with
SO2, while the intercept _k_0 would account for other decay processes of MACRO that are independent on [SO2], like reactions with radical byproducts (including MACRO self-reaction),
unimolecular decay, etc. Because that MACRO can be fully scavenged within 0.3 ms under a high [SO2] (≥9.3 × 1013 cm−3), we may further improve the analysis by subtracting the high [SO2]
spectrum (the average spectrum at the two highest [SO2]) from other low [SO2] spectra to remove most of the byproduct contributions while some minor amounts of IO and I2 may still remain
(SO2 scavenge method). The resulted spectra were then decomposed into the contributions of MACRO and IO and I2. The time profiles of MACRO are plotted in Supplementary Fig. 2. When using
this SO2 scavenge method, we did not include the data point at the first delay time (0.18 ms) due to incomplete scavenging. The kinetic results of MACRO + SO2 reaction are summarized in
Supplementary Table 1. The data at 150 and 500 Torr do not show significant difference after considering the experimental uncertainties. For these four sets of experimental data, we report
the rate coefficient to be (1.5 ± 0.4) × 10−10 cm3 s−1 at 298 K and 150−500 Torr (±1_σ_, _σ_ is the standard deviation of the data). The rate coefficients of SO2 reactions with other CIs
(CH2OO5,52, _anti_- and _syn_-CH3CHOO21,23, (CH3)2COO30,51, MVKO35,36) are in the range of (0.4–2.2) × 10−10 cm3 s−117,18. It appears that the resonance-stabilization of _anti_-MACRO does
not reduce its reactivity towards SO2. KINETICS OF MACRO REACTION WITH H2O The same method has been applied to investigate the kinetics of MACRO reaction with water vapor. To our surprise,
the effect of water in the decay of MACRO is rather weak as shown in Fig. 5, indicating slow reaction. From the plots of _k_obs as a function of [H2O] (Fig. 5b), we can see that the slopes
are quite insignificant; some of them are even negative, indicating that these rate measurements are close to our measurement limit (see Supplementary Table 2). Note that the highest [H2O]
used is ca. 6 × 1017 cm−3 (ca. 18 Torr), which has replaced a larger portion (18/150 = 12%) of the bath gas if the total pressure is only 150 Torr (N2 balance). Thus, we think it may require
some cautions to view the data of 150 Torr, because the reaction environment (type of bath gas) changes at various [H2O]. Nonetheless, no trend can be found for pressures from 150 to 500
Torr. Finally, we chose the weighted average from six experimental sets (300 and 500 Torr and 298 K, Supplementary Table 2), to report the effective rate coefficient for the reaction of
MACRO with water vapor, _k_water_-_eff = (9 ± 5) × 10−17 cm3 s−1 (±1_σ_). As mentioned above, we are not confident enough to determine the lower limit of _k_water_-_eff. MACRO ISOMERS As
pointed out by Vereecken et al., the _cis_–_trans_ interconversion (Supplementary Fig. 6) (>106 s–1) is orders-of-magnitude faster than other chemical processes, such that the _cis_ and
_trans_ conformers will be in near-equilibrium and should be considered as a single pool of species32. Following this idea, we summarize the rate coefficients of the unimolecular processes
and reactions with water vapor (monomer and dimer53) in Table 1 for relevant CIs32,35,43,44. As shown in Table 1, the predicted unimolecular decay rates are very different for _syn_- and
_anti_-MACRO. _Syn_-MACRO would have a rather short lifetime of ca. 1/2500 = 4 × 10−4 s32, which means its steady-state concentration would be very low. The experimental lifetime of MACRO is
found to depend on the signal intensity—the higher the signal is, the shorter the lifetime. This is due to the fact that the inevitable reactions of MACRO with radical byproducts, including
I atoms, IO radicals, MACRO itself and the products from the fast decomposition of _syn_-MACRO (similar to the case of _anti_-MVKO)42 would shorten its lifetime. Supplementary Fig. 3 shows
the plot of _k_obs against [MACRO]0. The linear relationship supports the above mechanism. Extrapolating _k_obs to zero [MACRO]0 would effectively remove the bimolecular contributions and
give an estimate for the unimolecular lifetime of MACRO. The preliminary data of Supplementary Fig. 3 are consistent with _k_uni ≅ _k_obs([MACRO]0 = 0) < 50 s−1, which gives a lifetime
> 20 ms. This long-lived MACRO cannot be _syn_-MACRO. Thus, the observed signal should belong to _anti_-MACRO. COMPARE OF _K_ WATER-EFF WITH PREVIOUS THEORY The value of _k_water_-_eff of
_anti_-MACRO (Table 1) is smaller than those of CH2OO and _anti_-CH3CHOO17,18 by orders of magnitude, suggesting that the extended conjugation of _anti_-MACRO correlates with the lower
reactivity towards water vapor (monomer and dimer), since the alkyl-substituted CIs lack the resonance-stabilized electronic structure of the extended conjugation. Anglada et al. have
predicted the rate coefficients for _anti_-MACRO reactions with water monomer and dimer, giving _k_water-eff = 7.2 × 10−15 cm3 s−1 at RH = 70% and 298 K (Table 1)44. To our surprise, this
value is ca. 80 times larger than our experimental value. However, Vereecken et al. have pointed out that the level of theory used by Anglada et al.44 tends to underestimate the barriers for
the CI reactions with water monomer and dimer32. Using ‘structure–activity relationship’, Vereecken et al. scaled the barrier heights of a number of CI reactions by considering the best
known theoretical and experimental data (mainly for CH2OO and _anti_-/_syn_-CH3CHOO) at that time32. The resulted rate coefficients of MACRO are also shown in Table 1. We can see this
ʻscalingʼ does reduce the gap (from 80 times to 7 times) between the theoretical predictions and our experimental data. Since the reference data in the work of Vereecken et al.32 do not
contain trustable data for reactions of CIs having a conjugated C=C group (i.e., there is no good anchor point for the scaling), this difference may be reasonable. Also note that Vereecken
et al.32 have estimated an uncertainty of one order of magnitude for their rate coefficients at 298 K. With details given in Supplementary Note 3, we found that it is important to calculate
the reaction barrier heights at a high level of quantum chemistry theory like QCISD(T)/CBS//B3LYP/6-311+G(2_d_,2_p_) (CBS = complete basis set extrapolation)54,55,56,57,58,59,60. For
example, the QCISD(T)/CBS barriers are ca. 1.4 or 2.0 kcal mol−1 higher than those calculated at QCISD(T)/aug-cc-pVTZ (AVTZ) for various reactions between (CH2=CH)CHOO conformers with H2O
monomer or dimer (Supplementary Fig. 7 and Supplementary Table 4), indicating that only using the AVTZ barrier heights would overestimate the reaction rates significantly. After properly
scaling the effect of the basis sets (CBS vs. AVTZ) by using the results of (CH2=CH)CHOO, which has a structure similar to MACRO, as an anchor point, (Supplementary Fig. 7)54, our
calculation (Table 1) also predicts slower rates compared to previous ones. COMPARE OF _K_ WATER-EFF WITH OZONOLYSIS EXPERIMENT Newland et al. have analyzed the effect of water vapor in the
system of isoprene ozonolysis; in their two-CI model, the isoprene-derived non-CH2OO CI (sum of MVKO and MACRO) has an effective reaction rate coefficient with water vapor of (1.1 ± 0.27) ×
10−15 cm3 s−161. While all conformers of MVKO are expected to react with water vapor much slower (_k_water-eff ≤ 10−17 cm3 s−1)44, the value of Newland et al. is much larger than our
_k_water-eff for MACRO. At the time (2015) when the work of Newland et al. was published, the knowledge of the reaction kinetics of MVKO and MACRO was not available at all. As multiple CIs
are produced in the isoprene ozonolysis system, the kinetics is rather complicated, especially when these CIs have very different reactivities towards water vapor. For example, CH2OO, which
has the predominant yield in the isoprene ozonolysis system2,3, would be quickly consumed by its reaction with water vapor, but MVKO and MACRO would not. See Supplementary Note 2 for an
alternative analysis to fit the data of Newland et al.61. In fact, Newland et al. have mentioned that the competing effects of the different kinetics of two distinct forms (_syn_ and _anti_
conformers) in the system may effectively lead to one masking the other under the experimental conditions applied61. BEST ESTIMATION OF _K_ UNI It is very difficult to experimentally measure
the very slow rate of the _anti_-MACRO unimolecular reaction. While our preliminary experimental data (Supplementary Fig. 3) suggest that the unimolecular reaction is slow, we cannot nail
down the value of _k_uni by the experimental results. On the theoretical side, the unimolecular reaction of _anti_-MACRO proceeds through the OO bending channel forming dioxirane32,34,39,
similar to that of CH2OO41,62. By comparing with the results of high-accuracy extrapolation protocols like HEAT-345(Q)62 or high-level multireference methods like MRCI+Q (Davidson
correction)/CBS41, Yin and Takahashi have found that the QCISD(T)/CBS method slightly underestimates the barrier height of this channel (by ca. 0.4 or 1.2 kcal mol–1, respectively) for
CH2OO41. Our analysis in Supplementary Note 3 shows that for the MACRO unimolecular reaction, the electronic energy obtained by QCISD(T)/CBS would consistently underestimate the barrier
height and other factors in the rate calculation, like hindered-rotor partition function calculation and tunneling correction, have very minor effects compared to that of the electronic
energy. Therefore, our theoretical value (25 s−1) of _k_uni of _anti_-MACRO would only represent an upper limit. Assuming such underestimation in the barrier heights (0.4 or 1.2 kcal mol–1)
is similar for the unimolecular reactions of MACRO and CH2OO, we may have an overestimation of a factor of 2 or 7 for the reaction rate coefficient at 298 K. Thus, we think the best
estimated _k_uni at 298 K would be ca. 25/(2 × 7)0.5 = 7 s−1 (the uncertainty may be up to a factor of 3), which is (almost) coincident with the theoretical value of 10 s−1 by Vereecken et
al. (claimed uncertainty is ca. a factor of 5 for non-H-migration reactions)32. Although the uncertainty is still not very small, “for many assessments, however, it is sufficient to
determine whether the reaction is significantly faster or slower than competing reactions”, mentioned by Vereecken et al.32. ATMOSPHERIC LIFETIME Because the unimolecular decay and reaction
with water vapor are the predominant processes that determine the atmospheric lifetime of a CI17,18,32, we may estimate the effective decay rate coefficient _k_atm for atmospheric
_anti_-MACRO. $$k_{{\mathrm{atm}}} = k_{{\mathrm{uni}}} + k_{{\mathrm{water}} - {\mathrm{eff}}}\left[ {{\mathrm{H}}_2{\mathrm{O}}} \right]$$ Taking the best estimated _k_uni (7 s−1) and our
experimental data of _k_water-eff (Table 1), we have _k_atm = 56 s−1 (or <74 s−1, if taking our theoretical upper limit of 25 s−1 for _k_uni) for _anti_-MACRO at RH = 70% and 298 K. Note
that the water reaction may still predominate in the decay processes of atmospheric _anti_-MACRO under humid conditions that are typical for tropical forests where the isoprene emission is
large. And this atmospheric lifetime (ca. 18 ms, best estimate) is much longer than previously thought (0.3 or 3 ms, see Table 1), indicating that the atmospheric concentration of
_anti_-MACRO would be much higher than previously expected. If using the upper limits of _k_uni (25 s−1) and _k_water-eff[H2O] (49 + 54 = 103 s−1, 2_σ_ upper bound, at RH = 70% and 298 K),
we then have _k_atm < 128 s−1, which would correspond to a lifetime longer than 7.8 ms. IMPACT OF _ANTI_-MACRO ON THE OXIDATION OF ATMOSPHERIC SO2 This would depend on three factors: (i)
the yield of _anti_-MACRO in the ozonolysis of atmospheric alkenes (mainly isoprene), (ii) the atmospheric lifetime of _anti_-MACRO, and (iii) the rate coefficient of _anti_-MACRO reaction
with SO2. Each factor is discussed below. First, based on the recent analysis of Nguyen et al., _anti_-MACRO has a yield of 15% among all stabilized CIs in isoprene ozonolysis, which is very
similar to that of _syn_-MVKO (14%)2,17. In addition, an earlier study of Zhang and Zhang has shown that the activation energies of O3 cycloaddition to the two double bonds of isoprene are
comparable and the barrier heights from the primary ozonides to _syn_-MVKO and _anti_-MACRO are also similar, implying that the _syn_-MVKO and _anti_-MACRO pathways are both accessible4.
Second, given that _syn_-MACRO and _anti_-MVKO have much shorter lifetimes (_τ_ <1 ms), the oxidation of atmospheric SO2 by the C4 CIs from isoprene ozonolysis would be mainly by
_anti_-MACRO and _syn_-MVKO (_τ_ > 10 ms)32,35. The order of magnitude of _k_atm of _anti_-MACRO (56 s−1) is comparable to that of _syn_-MVKO (_k_atm ≅ _k_uni = 33 s−1 or 70 s−133,43;
_k_water-eff ~ 10−18 cm3 s−1)32,35. Combined with their similar yields in the isoprene ozonolysis, this suggests that _anti_-MACRO and _syn_-MVKO would have similar steady-state
concentrations ([_anti_-MACRO]ss ≈ [_syn_-MVKO]ss) in the troposphere. Finally, the rate coefficient of SO2 reaction with _anti_-MACRO, (1.5 ± 0.4) × 10−10 cm3 s−1, is larger than that with
_syn_-MVKO, (4.0−4.2) × 10−11 cm3 s−135,36, by a factor of ca. 4. Overall, the oxidation rate of SO2 by _anti_-MACRO would be larger than that by _syn_-MVKO by a factor of
([_anti_-MACRO]ss/[_syn_-MVKO]ss)(_k_SO2__anti_-MACRO/_k_SO2__syn_-MVKO). This factor would be larger than unity, if we assume that these two CIs are mainly from isoprene ozonolysis with
similar yields. Although CH2OO has the highest yield (ca. 58%) among the stabilized CIs produced in the ozonolysis of isoprene2,17, its fast reaction with water vapor results in a rather
short atmospheric lifetime (<1 ms)17,18,19,20, too short for CH2OO to reach any substantial concentration to oxidize atmospheric SO2. Recently Caravan et al., who utilized the up-to-date
data of MVKO kinetics, show that _syn_-MVKO has the largest modeled steady-state concentration among all stabilized CIs globally (33% by molecules, 49% by weight)35. The above analysis shows
that _anti_-MACRO would have similar concentrations as those of _syn_-MVKO and an even larger impact on the SO2 oxidation. CONCLUSION Following the method of Vansco et al.34, MACRO has been
synthesized and its UV–visible spectroscopy and reaction kinetics have been investigated. Similar to MVKO, MACRO has two non-interconverting isomers, _syn_ and _anti_ forms. _Syn_-MACRO
would undergo fast 1,5-ring closure with a predicted thermal lifetime of <0.4 ms. In our experiments, a much longer lifetime (_τ_ > 4 ms) has been observed, indicating that the
spectral carrier is _anti_-MACRO. The rate coefficient of _anti_-MACRO reaction with SO2 has been determined to be (1.5 ± 0.4) × 10−10 cm3 s−1 at 298 K, which is substantially larger than
that of the _syn_-MVKO + SO2 reaction. However, the reaction of _anti_-MACRO with H2O was found to be quite slow with an effective rate coefficient of (9 ± 5) × 10−17 cm3 s−1 at 298 K, which
is smaller than previous theoretical values by 1 or 2 orders of magnitude. Theoretical calculations that properly treat the effect of the conjugated C=C substitution may reproduce the
experimental trend. A recent global chemistry and transport modeling based on the most up-to-date knowledge of MVKO chemistry has shown that _syn_-MVKO is important in the tropospheric
processes of SO2 oxidation and formic acid removal35. Our results indicate that _anti_-MACRO has an atmospheric lifetime similar to that of _syn_-MVKO, resulting in a similarly substantial
steady-state concentration. Combined with the larger rate coefficient of its reaction with SO2, the impact of _anti_-MACRO on the oxidation of atmospheric SO2 would be larger than (at least
comparable to) that of _syn_-MVKO. As mentioned above, to serve as an efficient oxidant of SO2, it is required to have a long-enough lifetime under atmospherically relevant conditions. As
shown above and in the literature35, a resonance-stabilized electronic structure plays an interesting role for CIs. It reduces the reactivity for unimolecular decay and reactions with water
vapor, but not for the reactions with SO2. Thus, having a resonance-stabilized electronic structure may be a new direction for searching for a long-lived CI that is able to oxidize
atmospheric SO2. METHODS MACRO PREPARATION The experimental setup has been published19,36. We prepared MACRO following Vansco et al.: ICHC(CH3)CH2I (1,3-diiodo-2-methylprop-1-ene, Accela,
97.8% by gas chromatography) + _hν_ (248 nm) → CH2=C(CH3)CHI + I, CH2=C(CH3)CHI + O2 → CH2=C(CH3)CHOO + I (Fig. 1)34. The precursor concentrations were determined by its UV absorption
spectra; the absolute cross sections (Supplementary Fig. 4) have been determined by measuring the weight loss of the precursor sample and the volume flow rate of the dilution gas63,64 (see
Supplementary Note 1). CMOS CAMERA SPECTROMETER A grating spectrometer (Andor SR303i) and fast CMOS camera (Andor, Marana-4BU11) were used to obtain the time-resolved absorption spectra of
the reaction system. A series of spectra (exposure time 0.21 ms (or 0.43 ms) each) were recorded for every photolysis event. The spectrum taken before the photolysis laser pulse was used as
the reference spectrum; therefore, the change of absorbance caused by the photolysis laser pulse was recorded transiently. Accumulation of 256, 512 (0.43 ms exposure time), or 1280, 2560
(0.21 ms exposure time) laser pulses was performed to improve the signal-to-noise ratio. A background spectrum (without adding the MACRO precursor 1,3-diiodo-2-methylprop-1-ene) was recorded
under the same experimental condition. This background was due to the interaction between the photolysis laser beam and the used optics (mainly the long-pass filters that reflected the
photolysis laser beam and transmitted the probe beam). All the reported spectra are background corrected. THEORETICAL CALCULATIONS We optimized the reactant and transition state geometries
on the singlet ground electronic state using B3LYP/6-311+G(2_d_,2_p_)54,58,59. See Supplementary Data 1 for the optimized XYZ geometries. The electronic energies were corrected at
QCISD(T)/CBS level54,55,56,57,58,59,60, except for the transition states of MACRO + 2H2O, of which the energies were estimated with a correction method detailed in Supplementary Note 3
(Supplementary Figs. 7–9, Supplementary Tables 4–6). The rate coefficients were calculated using the conventional transition state theory method using rigid rotor harmonic oscillator
approximations including tunneling correction. DATA AVAILABILITY The data supporting the findings of this study are available within the article, its Supplementary Information, and
Supplementary Data 1 (XYZ geometries), and from the corresponding author upon reasonable request. REFERENCES * Sindelarova, K. et al. Global data set of biogenic VOC emissions calculated by
the MEGAN model over the last 30 years. _Atmos. Chem. Phys._ 14, 9317–9341 (2014). Article CAS Google Scholar * Nguyen, T. B. et al. Atmospheric fates of Criegee intermediates in the
ozonolysis of isoprene. _Phys. Chem. Chem. Phys._ 18, 10241–10254 (2016). Article CAS PubMed Google Scholar * Aschmann, S. M. & Atkinson, R. Formation yields of methyl vinyl ketone
and methacrolein from the gas-phase reaction of O3 with isoprene. _Environ. Sci. Technol._ 28, 1539–1542 (1994). Article CAS PubMed Google Scholar * Zhang, D. & Zhang, R. Mechanism
of OH formation from ozonolysis of isoprene: a quantum-chemical study. _J. Am. Chem. Soc._ 124, 2692–2703 (2002). Article CAS PubMed Google Scholar * Welz, O. et al. A direct kinetic
measurements of Criegee intermediate (CH2OO) formed by reaction of CH2I with O2. _Science_ 335, 204–207 (2012). Article CAS PubMed Google Scholar * Srivastava, R. K., Miller, C. A.,
Erickson, C. & Jambhekar, R. Emissions of sulfur trioxide from coal-fired power plants. _J. Air Waste Manag. Assoc._ 54, 750–762 (2004). Article CAS PubMed Google Scholar * Liu, T.
et al. Formation of secondary aerosols from gasoline vehicle exhaust when mixing with SO2. _Atmos. Chem. Phys._ 16, 675–689 (2016). Article CAS Google Scholar * Sipilä, M. et al. The role
of sulfuric acid in atmospheric nucleation. _Science_ 327, 1243 (2010). Article PubMed CAS Google Scholar * Mauldin Iii, R. L. et al. A new atmospherically relevant oxidant of sulphur
dioxide. _Nature_ 488, 193–196 (2012). Article CAS Google Scholar * Boy, M. et al. Oxidation of SO2 by stabilized Criegee intermediate (sCI) radicals as a crucial source for atmospheric
sulfuric acid concentrations. _Atmos. Chem. Phys._ 13, 3865–3879 (2013). Article CAS Google Scholar * Stangl, C. M. et al. Sulfur dioxide modifies aerosol particle formation and growth by
ozonolysis of monoterpenes and isoprene. _J. Geophys. Res. Atmos._ 124, 4800–4811 (2019). Article CAS Google Scholar * Díaz-de-Mera, Y. et al. Formation of secondary aerosols from the
ozonolysis of styrene: effect of SO2 and H2O. _Atmos. Environ._ 171, 25–31 (2017). Article CAS Google Scholar * Ye, J., Abbatt, J. P. D. & Chan, A. W. H. Novel pathway of SO2
oxidation in the atmosphere: reactions with monoterpene ozonolysis intermediates and secondary organic aerosol. _Atmos. Chem. Phys._ 18, 5549–5565 (2018). Article CAS Google Scholar *
Liu, L. et al. Effects of stabilized Criegee intermediates (sCIs) on sulfate formation: a sensitivity analysis during summertime in Beijing–Tianjin–Hebei (BTH), China. _Atmos. Chem. Phys._
19, 13341–13354 (2019). Article CAS Google Scholar * Green, J. R. et al. Rates of wintertime atmospheric SO2 oxidation based on aircraft observations during clear-sky conditions over the
eastern United States. _J. Geophys. Res. Atmos._ 124, 6630–6649 (2019). Article CAS Google Scholar * Novelli, A. et al. Estimating the atmospheric concentration of Criegee intermediates
and their possible interference in a FAGE-LIF instrument. _Atmos. Chem. Phys._ 17, 7807–7826 (2017). Article CAS Google Scholar * Cox, R. A. et al. Evaluated kinetic and photochemical
data for atmospheric chemistry: Volume VII—Criegee intermediates. _Atmos. Chem. Phys._ 20, 13497–13519 (2020). Article CAS Google Scholar * Lin, J. J.-M. & Chao, W.
Structure-dependent reactivity of Criegee intermediates studied with spectroscopic methods. _Chem. Soc. Rev._ 46, 7483–7497 (2017). Article CAS Google Scholar * Chao, W., Hsieh, J.-T.,
Chang, C.-H. & Lin, J. J.-M. Direct kinetic measurement of the reaction of the simplest Criegee intermediate with water vapor. _Science_ 347, 751 (2015). Article CAS PubMed Google
Scholar * Smith, M. C. et al. Strong negative temperature dependence of the simplest Criegee intermediate CH2OO reaction with water dimer. _J. Phys. Chem. Lett._ 6, 2708–2713 (2015).
Article CAS PubMed Google Scholar * Taatjes, C. A. et al. Direct measurements of conformer-dependent reactivity of the Criegee intermediate CH3CHOO. _Science_ 340, 177–180 (2013).
Article CAS PubMed Google Scholar * Lin, L.-C., Chao, W., Chang, C.-H., Takahashi, K. & Lin, J. J.-M. Temperature dependence of the reaction of _anti_-CH3CHOO with water vapor.
_Phys. Chem. Chem. Phys._ 18, 28189–28197 (2016). Article CAS PubMed Google Scholar * Sheps, L., Scully, A. M. & Au, K. UV absorption probing of the conformer-dependent reactivity of
a Criegee intermediate CH3CHOO. _Phys. Chem. Chem. Phys._ 16, 26701–26706 (2014). Article CAS PubMed Google Scholar * Li, Y.-L., Kuo, M.-T. & Lin, J. J.-M. Unimolecular
decomposition rates of a methyl-substituted Criegee intermediate _syn_-CH3CHOO. _Rsc Adv._ 10, 8518–8524 (2020). Article CAS PubMed PubMed Central Google Scholar * Smith, M. C., Chao,
W., Takahashi, K., Boering, K. A. & Lin, J. J.-M. Unimolecular decomposition rate of the Criegee intermediate (CH3)2COO measured directly with UV absorption spectroscopy. _J. Phys. Chem.
A_ 120, 4789–4798 (2016). Article CAS PubMed Google Scholar * Stephenson, T. A. & Lester, M. I. Unimolecular decay dynamics of Criegee intermediates: energy-resolved rates, thermal
rates, and their atmospheric impact. _Int. Rev. Phys. Chem._ 39, 1–33 (2020). Article CAS Google Scholar * Fang, Y., Barber, V. P., Klippenstein, S. J., McCoy, A. B. & Lester, M. I.
Tunneling effects in the unimolecular decay of (CH3)2COO Criegee intermediates to OH radical products. _J. Chem. Phys._ 146, 134307 (2017). Article PubMed CAS Google Scholar * Fang, Y.
et al. Deep tunneling in the unimolecular decay of CH3CHOO Criegee intermediates to OH radical products. _J. Chem. Phys._ 145, 234308 (2016). Article PubMed CAS Google Scholar * Fang, Y.
et al. Communication: real time observation of unimolecular decay of Criegee intermediates to OH radical products. _J. Chem. Phys._ 144, 061102 (2016). Article PubMed CAS Google Scholar
* Chhantyal-Pun, R. et al. Direct measurements of unimolecular and bimolecular reaction kinetics of the Criegee intermediate (CH3)2COO. _J. Phys. Chem. A_ 121, 4–15 (2017). Article CAS
PubMed Google Scholar * Liu, F., Beames, J. M., Petit, A. S., McCoy, A. B. & Lester, M. I. Infrared-driven unimolecular reaction of CH3CHOO Criegee intermediates to OH radical
products. _Science_ 345, 1596 (2014). Article CAS PubMed Google Scholar * Vereecken, L., Novelli, A. & Taraborrelli, D. Unimolecular decay strongly limits the atmospheric impact of
Criegee intermediates. _Phys. Chem. Chem. Phys._ 19, 31599–31612 (2017). Article CAS PubMed Google Scholar * Barber, V. P. et al. Four-carbon Criegee intermediate from isoprene
ozonolysis: methyl vinyl ketone oxide synthesis, infrared spectrum, and OH production. _J. Am. Chem. Soc._ 140, 10866–10880 (2018). Article CAS PubMed Google Scholar * Vansco, M. F. et
al. Synthesis, electronic spectroscopy, and photochemistry of methacrolein oxide: a four-carbon unsaturated Criegee intermediate from isoprene ozonolysis. _J. Am. Chem. Soc._ 141,
15058–15069 (2019). Article CAS PubMed Google Scholar * Caravan, R. L. et al. Direct kinetic measurements and theoretical predictions of an isoprene-derived Criegee intermediate. _Proc.
Nat. Acad. Sci. USA_ 117, 9733–9740 (2020). Article CAS PubMed PubMed Central Google Scholar * Lin, Y.-H., Li, Y.-L., Chao, W., Takahashi, K. & Lin, J. J.-M. The role of the
iodine-atom adduct in the synthesis and kinetics of methyl vinyl ketone oxide—a resonance-stabilized Criegee intermediate. _Phys. Chem. Chem. Phys._ 22, 13603–13612 (2020). Article CAS
PubMed Google Scholar * Vansco, M. F., Marchetti, B. & Lester, M. I. Electronic spectroscopy of methyl vinyl ketone oxide: a four-carbon unsaturated Criegee intermediate from isoprene
ozonolysis. _J. Chem. Phys._ 149, 244309 (2018). Article PubMed CAS Google Scholar * Kuwata, K. T., Hermes, M. R., Carlson, M. J. & Zogg, C. K. Computational Studies of the
isomerization and hydration reactions of acetaldehyde oxide and methyl vinyl carbonyl oxide. _J. Phys. Chem. A_ 114, 9192–9204 (2010). Article CAS PubMed Google Scholar * Kuwata, K. T.
& Valin, L. C. Quantum chemical and RRKM/master equation studies of isoprene ozonolysis: methacrolein and methacrolein oxide. _Chem. Phys. Lett._ 451, 186–191 (2008). Article CAS
Google Scholar * Kuwata, K. T., Valin, L. C. & Converse, A. D. Quantum chemical and master equation studies of the methyl vinyl carbonyl oxides formed in isoprene ozonolysis. _J. Phys.
Chem. A_ 109, 10710–10725 (2005). Article CAS PubMed Google Scholar * Yin, C. & Takahashi, K. How does substitution affect the unimolecular reaction rates of Criegee intermediates?
_Phys. Chem. Chem. Phys._ 19, 12075–12084 (2017). Article CAS PubMed Google Scholar * Vansco, M. F. et al. Experimental evidence of dioxole unimolecular decay pathway for
isoprene-derived Criegee intermediates. _J. Phys. Chem. A_ 124, 3542–3554 (2020). Article CAS PubMed Google Scholar * Lin, Y.-H., Yang, C.-H., Takahashi, K. & Lin, J. J.-M. Kinetics
of unimolecular decay of methyl vinyl ketone oxide, an isoprene-derived Criegee intermediate, under atmospherically relevant conditions. _J. Phys. Chem. A_ 124, 9375–9381 (2020). Article
CAS PubMed Google Scholar * Anglada, J. M. & Solé, A. Impact of the water dimer on the atmospheric reactivity of carbonyl oxides. _Phys. Chem. Chem. Phys._ 18, 17698–17712 (2016).
Article CAS PubMed Google Scholar * Spietz, P., Gómez Martín, J. C. & Burrows, J. P. Spectroscopic studies of the I2/O3 photochemistry: Part 2. Improved spectra of iodine oxides and
analysis of the IO absorption spectrum. _J. Photochem. Photobiol. A_ 176, 50–67 (2005). Article CAS Google Scholar * Sander, S. P. et al. _Chemical Kinetics and Photochemical Data for Use
in Atmospheric Studies. Evaluation Number 17_. JPL Publication 10-6 (Jet Propulsion Laboratory, Pasadena, 2011). * Ting, W.-L., Chen, Y.-H., Chao, W., Smith, M. C. & Lin, J. J.-M. The
UV absorption spectrum of the simplest Criegee intermediate CH2OO. _Phys. Chem. Chem. Phys._ 16, 10438–10443 (2014). Article CAS PubMed Google Scholar * Foreman, E. S. et al. High
resolution absolute absorption cross sections of the \({\tilde{\mathrm{B}}}\)1A′–\({\tilde{\mathrm{B}}}\)1A′ transition of the CH2OO biradical. _Phys. Chem. Chem. Phys._ 17, 32539–32546
(2015). Article CAS PubMed Google Scholar * Lewis, T. R., Blitz, M. A., Heard, D. E. & Seakins, P. W. Direct evidence for a substantive reaction between the Criegee intermediate,
CH2OO, and the water vapour dimer. _Phys. Chem. Chem. Phys._ 17, 4859–4863 (2015). Article CAS PubMed Google Scholar * Smith, M. C. et al. UV absorption spectrum of the C2 Criegee
intermediate CH3CHOO. _J. Chem. Phys._ 141, 074302 (2014). Article PubMed CAS Google Scholar * Huang, H.-L., Chao, W. & Lin, J. J.-M. Kinetics of a Criegee intermediate that would
survive high humidity and may oxidize atmospheric SO2. _Proc. Nat. Acad. Sci. USA_ 112, 10857–10862 (2015). Article CAS PubMed PubMed Central Google Scholar * Chhantyal-Pun, R., Davey,
A., Shallcross, D. E., Percival, C. J. & Orr-Ewing, A. J. A kinetic study of the CH2OO Criegee intermediate self-reaction, reaction with SO2 and unimolecular reaction using cavity
ring-down spectroscopy. _Phys. Chem. Chem. Phys._ 17, 3617–3626 (2015). Article CAS PubMed Google Scholar * Anglada, J. M. et al. Atmospheric significance of water clusters and
ozone–water complexes. _J. Phys. Chem. A_ 117, 10381–10396 (2013). Article CAS PubMed Google Scholar * Yin, C. & Takahashi, K. Effect of unsaturated substituents in the reaction of
Criegee intermediates with water vapor. _Phys. Chem. Chem. Phys._ 20, 20217–20227 (2018). Article CAS PubMed Google Scholar * Pople, J. A., Head‐Gordon, M. & Raghavachari, K.
Quadratic configuration interaction. A general technique for determining electron correlation energies. _J. Chem. Phys._ 87, 5968–5975 (1987). Article CAS Google Scholar * Dunning, T. H.
Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. _J. Chem. Phys._ 90, 1007–1023 (1989). Article CAS Google Scholar *
Peterson, K. A., Woon, D. E. & Dunning, T. H. Benchmark calculations with correlated molecular wave functions. IV. The classical barrier height of the H+H2→H2+H reaction. _J. Chem.
Phys._ 100, 7410–7415 (1994). Article CAS Google Scholar * Becke, A. D. Density-functional thermochemistry. III. The rol e of exact exchange. _J. Chem. Phys._ 98, 5648–5652 (1993).
Article CAS Google Scholar * Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. _Phys. Rev. B_ 37,
785–789 (1988). Article CAS Google Scholar * Krishnan, R., Binkley, J. S., Seeger, R. & Pople, J. A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave
functions. _J. Chem. Phys._ 72, 650–654 (1980). Article CAS Google Scholar * Newland, M. J. et al. Atmospheric isoprene ozonolysis: impacts of stabilised Criegee intermediate reactions
with SO2, H2O and dimethyl sulfide. _Atmos. Chem. Phys._ 15, 9521–9536 (2015). Article CAS Google Scholar * Nguyen, T. L., Lee, H., Matthews, D. A., McCarthy, M. C. & Stanton, J. F.
Stabilization of the simplest Criegee intermediate from the reaction between ozone and ethylene: a high-level quantum chemical and kinetic analysis of ozonolysis. _J. Phys. Chem. A_ 119,
5524–5533 (2015). Article CAS PubMed Google Scholar * Kuo, M.-T., Takahashi, K. & Lin, J. J.-M. Reactions of Criegee intermediates are enhanced by hydrogen-atom relay through
molecular design. _Chem. Phys. Chem._ 21, 2056–2059 (2020). Article CAS PubMed Google Scholar * Li, Y.-L., Lin, C.-Y., Lin, Y.-H. & Lin, J. J.-M. Temperature-dependent kinetics of
the simplest Criegee intermediate reaction with dimethyl sulfoxide. _J. Chin. Chem. Soc._ 67, 1563–1570 (2020). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work
is supported by Academia Sinica (AS-CDA-106-M05) and Ministry of Science and Technology, Taiwan (MOST 109-2113-M-001-027-MY3 (JJML); MOST 109-2113-M-001-008 (KT); MOST
109-2639-M-009-001-ASP). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute of Atomic and Molecular Sciences, Academia Sinica, Taipei, Taiwan Yen-Hsiu Lin, Cangtao Yin, Kaito Takahashi
& Jim Jr-Min Lin * Department of Chemistry, National Taiwan University, Taipei, Taiwan Jim Jr-Min Lin Authors * Yen-Hsiu Lin View author publications You can also search for this author
inPubMed Google Scholar * Cangtao Yin View author publications You can also search for this author inPubMed Google Scholar * Kaito Takahashi View author publications You can also search for
this author inPubMed Google Scholar * Jim Jr-Min Lin View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.J.-M.L. conceived the experiment.
Y.-H.L. set up the experiment, performed the measurements, and analyzed the experimental data. C.Y. and K.T. performed the theoretical calculations. Y.-H.L., C.Y., K.T. and J.J.-M.L.
discussed the results. J.J.-M.L. wrote the paper. CORRESPONDING AUTHOR Correspondence to Jim Jr-Min Lin. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 PEER REVIEW FILE RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative
Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the
original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in
the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended
use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lin, YH., Yin, C., Takahashi, K. _et al._ Surprisingly long lifetime of
methacrolein oxide, an isoprene derived Criegee intermediate, under humid conditions. _Commun Chem_ 4, 12 (2021). https://doi.org/10.1038/s42004-021-00451-z Download citation * Received: 06
October 2020 * Accepted: 05 January 2021 * Published: 05 February 2021 * DOI: https://doi.org/10.1038/s42004-021-00451-z SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative