- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Immune defenses are crucial for survival but costly to develop and maintain. Increased immune investment is therefore hypothesized to trade-off with other life-history traits. Here,
we examined innate and adaptive immune responses to environmental heterogeneity in wild Antarctic fur seals. In a fully crossed, repeated measures design, we sampled 100 pups and their
mothers from colonies of contrasting density during seasons of contrasting food availability. Biometric and cortisol data as well as blood for the analysis of 13 immune and oxidative status
markers were collected at two key life-history stages. We show that immune responses of pups are more responsive than adults to variation in food availability, but not population density,
and are modulated by cortisol and condition. Immune investment is associated with different oxidative status markers in pups and mothers. Our results suggest that early life stages show
greater sensitivity to extrinsic and intrinsic effectors, and that immunity may be a strong target for natural selection even in low-pathogen environments such as Antarctica. SIMILAR CONTENT
BEING VIEWED BY OTHERS PARENTAL MORPH COMBINATION DOES NOT INFLUENCE INNATE IMMUNE FUNCTION IN NESTLINGS OF A COLOUR-POLYMORPHIC AFRICAN RAPTOR Article Open access 26 May 2021 LITTLE
EVIDENCE OF INBREEDING DEPRESSION FOR BIRTH MASS, SURVIVAL AND GROWTH IN ANTARCTIC FUR SEAL PUPS Article Open access 01 June 2024 NEONATAL ANTIPREDATOR TACTICS SHAPE FEMALE MOVEMENT PATTERNS
IN LARGE HERBIVORES Article 04 December 2024 INTRODUCTION The immune system comprises multiple mechanisms to protect an individual against pathogens and parasites but can essentially be
split into two components, the innate and adaptive systems. The cellular and humoral effectors of the innate system are the body’s first line of defense, initiating a broad and rapid cascade
effect to clear foreign intruders1,2. The adaptive immune system, by contrast, is composed of a small number of cells and molecules generated de novo to target specific pathogens1. Although
they differ mechanistically, both systems are essential for an effective immune response, so untangling how individuals manage the allocation of innate and adaptive immunity is important
for understanding fitness variation and population dynamics. A central assumption in eco-immunology is that a trade-off exists between investment in immune defense and other functions or
activities, such as growth and reproduction3. This is particularly relevant for wild populations that experience variation in two of the most prominent environmental factors known to
influence immune function – food availability and population density. When allocating finite energy resources, reduced food availability can constrain immune responses4, while the higher
risk of pathogen transmission at higher population densities may induce density-dependent immune responses5. When investigating life-history trade-offs, another layer of complexity is added
when the performance of one activity has downstream consequences on another. For example, mounting an immune response, and in particular an inflammatory response, may lead to oxidative
stress6,7, which in turn may constrain investment into growth and reproduction8. To understand how environmental factors impact traits closely linked with survival, it is therefore important
to consider the complex network of mechanisms influencing life-history traits and their trade-offs. Antarctic fur seals (_Arctocephalus gazella_) offer a unique opportunity to investigate
these potential trade-offs in a wild mammalian population subjected to natural variation in food availability and population density. The availability of the fur seal’s staple diet,
Antarctic krill, has been steadily declining over the past four decades due to local warming and the loss of sea ice, but food availability also fluctuates from year to year9,10.
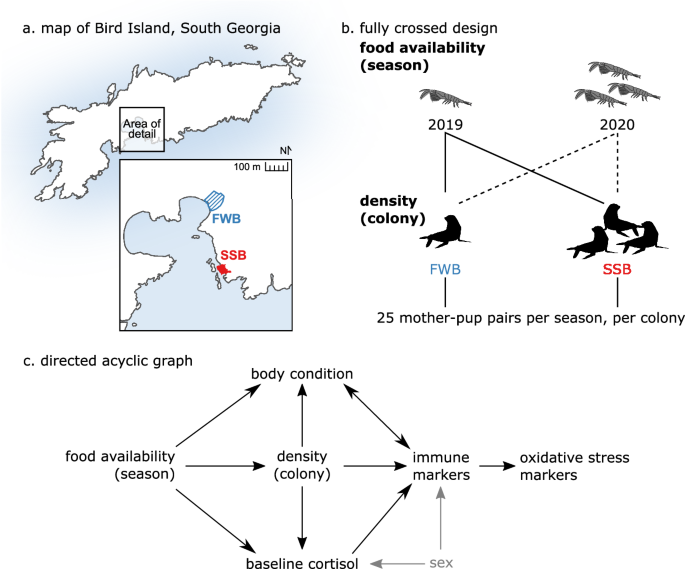
Furthermore, stable differences in population density exist among breeding colonies11. These characteristics are exemplified by our study population on the sub-Antarctic island of Bird
Island, South Georgia. During two consecutive breeding seasons, the first of which had one of the lowest food availabilities ever recorded, we tracked 100 Antarctic fur seal pups and their
mothers from two neighboring breeding colonies that experience comparable environmental conditions but which differ in population density (Fig. 1a, b)12,13. Focal pups and their mothers were
sampled at two key life-history stages: shortly after birth and again approximately 60 days later, when molting commences. The molt is a particularly important life history stage, as
molting is a costly process and signifies pups gaining nutritional and behavioral independence. At these two time points, we collected blood for the analysis of nine immune and four
oxidative status markers (Table 1) as well as biometric and baseline salivary cortisol data. This unique ‘natural experiment’ allowed us to investigate relationships among environmental
variation, trait variation, immunity, and oxidative stress. First, to provide a baseline, we characterized ontogenetic changes in the immune responses of pups in comparison to their mothers.
We then tested the following hypotheses: (i) investment into immunity depends on life history stage. Specifically, we hypothesized that there would be more variation in the immune responses
of pups, both because they have fewer resources available to buffer them from detrimental environmental conditions, and because they have not previously been exposed to parasites and
pathogens, whereas their mothers have immune memory; (ii) immune responses are influenced by environmental variation, with investment into immunity being negatively impacted by food stress
but positively impacted by population density due to the assumed higher prevalence of parasites and pathogens; (iii) immune investment trades off with other life-history traits.
Specifically, we hypothesized that elevated levels of the stress hormone cortisol and poorer body condition suppress immune defenses; and (iv) immune investment leads to oxidative stress
under the hypothesis that reactive oxygen species are generated during immune activation (e.g., oxidative burst) and that resources are limited and a trade-off exists between immune function
and antioxidant defenses (Fig. 1c). RESULTS We investigated the direct and total effects of food availability (season), density (colony), body condition, baseline cortisol, and sex on nine
immune markers, as well as the effect of these immune phenotypes on four markers of oxidative status in a wild population of Antarctic fur seals. Our final dataset comprised of 98 pups and
97 mothers randomly sampled from two neighboring breeding colonies of contrasting population density (Fig. 1a) over two consecutive breeding seasons of contrasting food availability (Fig.
1b). Repeated measures were successfully gathered from 67 pups and 80 mothers. The range, first and third quartiles, median, mean, and total number of missing values for all data can be
found in Supplementary Tables S1 and S2. The raw data are plotted in Supplementary Figs. S1–S18. Full summaries of the model outputs, including point estimates of the posterior mean and 95%
highest posterior density intervals (HPDIs), effective sample sizes, and _p_MCMC values for each predictor variable are provided in Supplementary Data Tables S1 (pups) and S2 (mothers).
REPEATABILITY Immune marker levels showed modest repeatability from birth to molt (i.e., point estimates for _R_ > 0.1) and repeatability was similar across all of the immune markers,
suggesting that individuals differ in their immune profiles. The 95% posterior intervals were correspondingly large (Supplementary Table S4). In pups, the mean variance explained by repeated
sampling of the same individual ranged from a high of 0.27 [95% HPDI: 0.12−0.42] for haptoglobin to a low of 0.14 [95% HPDI: 0.07−0.21] for hemolysis. In mothers, the range was 0.49 [95%
HPDI: 0.26−0.72] for neopterin to 0.13 [95% HPDI: 0.07−0.20] for BKA (_S. aureus_). Repeatability estimates from the null model were comparable and can be found in Supplementary Table S5.
DEVELOPMENT OF IMMUNITY AND OXIDATIVE STATUS OVER TIME BKA (_E. coli_), hemagglutination, hemolysis, IgG, and dROM levels increased significantly in pups from birth to molt (respective
Cohen’s _d_ = −0.62, −2.11, −2.18, −2.06, and −1.59), while lysozyme and neopterin concentrations decreased (respective Cohen’s _d_ = 0.64 and 1.19). In mothers, of the nine immune markers
assayed, only the BKA (_S. aureus_) and the innate/adaptive WBC ratio changed significantly between the sampling time points, both showing a decrease over the approximately 60 days between
giving birth and starting to wean their pup (Cohen’s _d_ = 1.14 and 0.77). SOD concentrations increased moderately between the sampling time points (Cohen’s _d_ = 0.53) (Supplementary Table
S6, Supplementary Fig. S1). At birth, differences between pup and mother immune and oxidative stress concentrations were moderate to large (Cohen’s _d_ > |0.5|) for all of the markers
except lysozyme and OXY. This difference between the two group means was notably reduced by the time pups started to molt and gain nutritional independence. BKA (_S. aureus_),
hemagglutination, hemolysis, lysozyme, IgG, the innate/adaptive WBC ratio, OXY, and dROM all showed a difference of less than 0.5 standard deviations. This suggests that immune and oxidative
status marker concentrations in pups converged on the adult phenotype before or around the time of increasing independence, as pups began to molt (Supplementary Table S6, Supplementary Fig.
S1). COMPARISON OF STRESS MARKERS There was no association between the neutrophil/lymphocyte ratio and baseline salivary cortisol in our full dataset of pups and mothers (_r_ = −0.05, 95%
CI = −0.22−0.12, _t_ = −0.62, df = 130, _p_ = 0.54) (Supplementary Fig. S19). Although the neutrophil-lymphocyte ratio has been cited as a potential indicator of chronic stress in multiple
mammals14, our results add to the growing body of evidence that these two metrics may not be interchangeable assessments of stress (reviewed in ref. 15). IMMUNITY IN PUPS The direct and
total effect of pup sex on immune marker levels was weak, with all of the point estimates being close to zero and the 95% HPDIs overlapping zero (Supplementary Data Table S1). The proportion
of BKA (_S. aureus_) and haptoglobin as well as the ratio of innate/adaptive WBCs were significantly lower in 2019 than 2020 and thus had lower values in the season of low food
availability. By contrast, neopterin concentrations were higher in 2019 (Fig. 2a). Population density had a total effect on BKA (_S. aureus_ and _E. coli_), with point estimates for both
assays being comparatively higher in individuals born at SSB, the high-density colony (Fig. 2b). We also found a positive correlation between baseline salivary cortisol and two immune
markers, neopterin and lysozyme, while cortisol was negatively correlated with four immune markers, BKA (_E. coli_), hemagglutination, hemolysis, and IgG (Fig. 2c). The exact opposite was
found for body condition in pups, which was positively correlated with BKA (_E. coli_), hemagglutination, hemolysis, haptoglobin, and IgG, and was negatively correlated with neopterin and
lysozyme (Fig. 2d). IMMUNITY IN MOTHERS The concentrations of BKA (_E. coli_), hemagglutination, hemolysis, and IgG were significantly higher in 2019, the year of low food availability (Fig.
3a). Population density had a total effect on BKA (_S. aureus_ and _E. coli_), with point estimates for both assays being higher in individuals breeding at SSB, the high-density colony
(Fig. 3b). We found a significant positive correlation between baseline cortisol and two immune markers, BKA (_S. aureus_) and the innate/adaptive WBC ratio. By contrast, negative
correlations were found between cortisol and both hemagglutination and hemolysis (Fig. 3c). Associations between maternal body condition and immune markers were weak, with all of the point
estimates being close to zero and the 95% HPDIs overlapping zero (Fig. 3d). OXIDATIVE STRESS In pups, neopterin was the only immune marker to correlate with OXY. None of the immune markers
correlated significantly with GPx or SOD. By contrast, the majority of immune markers were associated with dROM. Specifically, hemagglutination, hemolysis, haptoglobin, and IgG all
correlated positively with dROM levels, while neopterin and lysozyme were negatively correlated (Fig. 4). In mothers, the innate immune marker haptoglobin correlated positively with both OXY
and dROM, while the adaptive immune marker IgG correlated positively only with OXY. BKA (_E. coli_) correlated positively, and IgG correlated negatively with GPx. Lysozyme and the
innate/adaptive WBC ratio both correlated positively with SOD, while hemolysis showed a negative correlation (Fig. 5). DISCUSSION The trade-off between immune defense and other life-history
traits may be influenced by any number of extrinsic and intrinsic factors. Investigating broad, cross-scale links among environmental variation, trait variation, immunity, and oxidative
stress in wild populations is therefore essential for understanding the dynamics of immune activity and its influence on population dynamics. Here, we show that immune defense profiles are
strongly dependent on the physical and social environment, and vary with life-history stage in Antarctic fur seals. Developing offspring are especially sensitive to environmental variation
(i.e., food availability and population density) and invest differentially into immune defense depending on their cortisol levels and body condition. Furthermore, immune investment is
strongly correlated with oxidative stress in pups, but not in mothers. Together, these results suggest that offspring pay a greater cost of immune defense. By implication, immunity appears
to be a strong target for natural selection, even in polar environments which are believed to carry fewer pathogens16. Our findings lend support to calls for more long-term, longitudinal
research into the evolutionary trajectories of immune defenses in wild populations17. This will become increasingly important as anthropogenic climate change creates ever more extreme and
variable environmental conditions18. Previous work on the ontogeny of immunity in various pinniped species has found that antibody levels differ between pups and adults at birth, but
converge as pups approach their molt19,20,21,22,23,24. We obtained similar results, with pups and mothers differing significantly at nine of the eleven markers at birth, while only
haptoglobin and neopterin remained different as the pups approached nutritional independence. Both haptoglobin and neopterin represent induced innate immune responses with high energetic and
pathological costs but, while haptoglobin levels remained lower in pups relative to mothers throughout this study, neopterin levels were consistently higher. These patterns are indicative
of antibacterial preparedness and higher cellular immune activation during early development, a time when individuals are exposed to various novel pathogens, especially bacteria, which are
one of the main causes of death in this population24. In line with our hypothesis that individuals experiencing the highest energetic demands should down-regulate the costliest defenses25,
we found that Antarctic fur seal pups tended to invest less into immune responses under conditions of food stress. The only exception was neopterin, which had higher concentrations in pups
during the year of lower food availability. If food-limited individuals prioritize other energetically demanding processes in lieu of immunity, they may become more susceptible to
infections26. Given that neopterin is a byproduct of macrophage activity and an indicator of immune activation due to infection27, elevated levels of neopterin may indicate an increase in
susceptibility to infection when food is scarce. Contrary to our expectations, adult females showed increased investment into constitutive innate and adaptive immunity under food stress.
This is surprising because undernourished individuals should have fewer resources to mount a strong immune response4. One possible explanation for this finding could be that breeding females
were not necessarily under-nourished in the year of low food availability. This is supported by previous findings showing no differences in body condition between mothers in the two
consecutive years of our study12. Alternatively, our results are consistent with food-limited individuals prioritizing investment into those components of the immune system that carry the
lowest energetic and pathological costs (e.g., constitutive innate and antibody-mediated adaptive immunity). We found that both life history stages responded similarly to higher population
density, with pups and their mothers investing more into constitutive innate immunity. Individuals experiencing higher population densities may be more frequently exposed to parasites and
pathogens5,28 such that maintaining high levels of basal immunity can be beneficial, despite potential trade-offs with other life-history components29. In other words, the relative threat of
infection at higher density may be mirrored by the status of the immune system. However, despite this general pattern, we did not find an association between population density and IgG
concentration, an adaptive immune marker that reflects cumulative exposure to pathogens over time25 and which is therefore typically elevated at high density30,31. The lack of association in
our study could potentially be a reflection of the Antarctic setting, as a previous study of three Antarctic penguin species found that immunoglobulin levels increase northwards along a
latitudinal gradient32. Alternatively, it is possible that our study was too short to detect cumulative increases in IgG, especially as the mothers spent much of their time foraging at sea.
None of the immune markers varied between male and female pups. Although contrary to our initial expectations, sex differences in immunity may only become evident after individuals reach
sexual maturity and selection pressures on the sexes diverge33. This is supported by evidence from gray seal pups34 but contradicts previous findings in Galapagos sea lion pups23, implying
that sex-specific responses are species- and/or context-dependent. At both life history stages, we found null and negative correlations between baseline cortisol and the majority of immune
markers, in line with the known inhibitory effects of glucocorticoids on the immune system35. There were, however, two notable exceptions. First, neopterin was positively correlated with
baseline cortisol in pups. This is surprising given that neopterin is a byproduct of macrophage activity, and cortisol suppresses inflammation mediated by macrophages35. One possible
explanation is that our measure of cortisol from saliva reflects the short-term stress response, which can enhance the expression of immune responses36. Alternatively, chronically elevated
cortisol may increase susceptibility to infection and disease36 such that increased levels of neopterin indicate higher cellular immune activation under long-term stress. Second, BKA (_S.
aureus_) was positively correlated with baseline cortisol in adult females. This might again be related to a positive effect of short-term stress responses on the immune system36, translated
here as an upregulation of functionality (BKA, innate WBCs) and a downregulation of natural antibodies (agglutination). We further found that the relationship between immune response and
body condition differs between pups and mothers. In pups, most of the immune markers were positively correlated with body condition. This observation builds upon a large body of evidence
from wild populations37, including pinnipeds23,38,39, suggesting that well-nourished individuals can mount a stronger immune response. We also found that those immune markers that were
positively correlated with cortisol were negatively associated with body condition, and vice versa. This may indicate condition-dependent trade-offs between stress responses and immune
investment, both of which are energetically demanding processes. In contrast to pups, no association was found between body condition and immunity in mothers. This is likely due to selection
bias, as only those adult females that successfully carried a pup to term were included in our study, meaning that only mothers in relatively good condition were sampled. Consistent with
this explanation, we previously reported no differences in quality measures (body condition, span, girth, weight, length) between mothers from the two breeding colonies and between the two
years12. We found a strong relationship between immunity and oxidative status in the pups. In particular, diverse components of the immune system were found to correlate with dROM, including
both innate and adaptive immune markers. Reactive oxygen metabolites (ROMs) are by-products of the ROS-induced oxidation of organic substrates and their levels in the plasma often increase
during an immune response40. Our results are therefore consistent with previous research showing that oxidative stress increases when individuals mount an immune response6,7,8,40. However,
the opposite pattern was again observed for neopterin, suggesting that non-enzymatic antioxidant capacity (OXY) is increased and oxidative damage (dROM) is decreased in pups in response to
elevated macrophage activity. Moreover, levels of the two antioxidant enzymes glutathione peroxidase (GPx) and superoxide dismutase (SOD) did not correlate with any of the immune markers,
suggesting that these two enzymes were not upregulated in response to increased ROMs. The release of ROS by phagocytes is most commonly associated with bacterial killing. However, activated
macrophages usually produce far lower levels of ROS than neutrophils, and antioxidants play an important role in mitigating oxidative damage, such as ROMs41, which might explain the
associations observed in pups between ROMs and other immune markers. Thus, our results lend support to the notion that individuals invest more into innate cellular immune defense during
early development, when they first encounter parasites and/or pathogens. Contrary to our expectations, as well as our results for the pups, the majority of immune markers were not associated
with our marker of oxidative damage in mothers. By contrast, we found several significant correlations between immune markers and non-enzymatic antioxidant capacity, GPx, and SOD. This is
at odds with a recent meta-analysis, which found that the effect of the immune response on oxidative stress was similar in juveniles and adults7. Our results could potentially be explained
by the metabolic demands of growth, which have been shown to significantly increase oxidative damage8. Given that Antarctic fur seals grow most rapidly during the time from birth to
weaning42, this trade-off is expected to be more pronounced in pups than mothers. Another explanation might lie with the immaturity of the antioxidant system of pups, particularly in terms
of the expression of antioxidant enzymes, as previously described in hooded seals (_Cystophora cristata_) and in other vertebrates43,44. In our case, this was particularly evident for GPx
due to higher levels in adults than in pups. This is relevant because GPx detoxifies tissues from the accumulation of certain oxidized molecules quantified with our dROMs assay. Although the
lack of upregulation of antioxidant enzymes came at a cost in terms of oxidative damage, it might have been advantageous to mount an effective immune response. Indeed, the generation of ROS
during the oxidative burst in neutrophils is one important component of innate immunity. Both SOD and GPx remove certain ROS that are also generated during the oxidative burst. Thus, in
order to not compromise an already naïve immune response, pups appeared to make the best of a bad job keeping the expression of these two enzymes low. Our study exploited a unique natural
experiment in combination with an unusually large panel of immune and oxidative status markers. Our fully crossed, repeated measures design allowed us to investigate life-history tradeoffs
in the context of the social and physical environment, while our diverse panel of markers provided detailed insights into multiple components of the vertebrate immune system and their
relationship with oxidative stress. Arguably the strongest criticism of our work relates to the correlative nature of our results. However, our Bayesian network (Fig. 1c) was based on a
pre-defined set of causal hypotheses from the literature, such that likely causal relationships among the variables could be reasonably estimated. Furthermore, although antigen challenge
experiments would in principle be possible, they are not without drawbacks45, and experimental manipulations of wild populations have ethical implications46 as well as the potential to
disrupt natural host-parasite interactions and population dynamics47. These issues could be problematic for our study system, where long-term monitoring would be disrupted by invasive
experimentation. In conclusion, our results suggest that the immune profiles of developing offspring are more sensitive to both extrinsic and intrinsic factors than those of adults. Exposure
to novel parasites and pathogens during this early life stage likely imposes strong selective pressures, such that only those individuals capable of coping with exposure to diverse
pathogens survive to reproduce. Our results further suggest that a complex array of immune effectors control infections during early development. Consequently, natural selection appears to
have favored diversity in the detection, control, and elimination of pathogens. METHODS EXPERIMENTAL DESIGN This study was conducted at Bird Island, South Georgia (54°00′24.8ʺS,
38°03′04.1ʺW), a sub-Antarctic island located in the southern Atlantic Ocean. During the 2018–19 (hereafter 2019) and 2019–20 (hereafter 2020) breeding seasons (December to March), 25 unique
mother-pup pairs were sampled from two neighboring colonies, Freshwater Beach (FWB) and Special Study Beach (SSB) (Fig. 1a, b). Sampling was randomized with respect to pup sex, resulting in
a total of 51 male and 49 female focal pups. Pup mortality averaged 25.6% over the two seasons and colonies but was overall higher at FWB (32%) than SSB (12%)12. Mothers and pups were
sampled together twice, around 2–3 days after birth (denoted as age = 0 in the raw data and Supplementary Table S1) and approximately 60 days thereafter, as the pups began to molt and gain
nutritional independence. Given that the presence or absence of the mother could impact body condition and blood metabolites at the time of second sampling, mothers and pups were sampled
concurrently on the first day that the pup was seen together with its mother post-60 days of age. For sampling, we followed animal handling protocols established and refined by the British
Antarctic Survey over their 40-year Antarctic fur seal monitoring and survey program. In brief, adult females were captured with a noosing pole and immobilized on a restraint board. Pups
were captured with a slip noose or by hand and were restrained by hand. Individuals were released as close to their capture site as possible and the pups were reunited with their mothers.
When a focal individual was captured, weight and length measurements were taken, which were used to calculate body condition after48. Saliva was also collected to assess baseline cortisol
concentrations; for a more detailed description of our saliva sampling methods, cortisol concentration assessment, and analysis protocols, see ref. 49. For the analysis of immune and
oxidative status markers, we collected up to 2.5 ml of blood from the hind flipper using BD Discardit Eccentric Luer-Slip two-piece syringes and BD Microlance stainless steel needles (25 G,
0.5 × 25 mm). Blood was immediately transferred to Greiner Bio-One Plasma tubes coated with lithium heparin to prevent clotting. One drop of blood was used to prepare a smear on a glass
microscope slide (76 × 26 mm, cut edges, Menzel). The remaining blood was centrifuged at 1000 × _g_ for ten minutes to separate the plasma from the blood cells. Samples were stored in 200 µL
aliquots at −20 °C in the field and during transport, before being transferred to −80 °C in the laboratory. Although 18−20 gauge needles are recommended for blood collection in otariids50,
the use of smaller gauge needles did not hinder our analysis in terms of blood clotting or hemolysis. Hemolysis was noted in the field whenever detected (_n_ = 17 or 4% of the total samples)
and these samples were assessed during laboratory and data analysis for possible effects. IMMUNE AND OXIDATIVE STATUS ASSAYS Humoral and cellular components of the innate and adaptive
immune system (Table 1) were quantified using established assays that have been successfully applied to wild mammals e.g., refs. 51,52, including pinnipeds e.g., refs. 20,23,38,39,53,54,55.
Additionally, we measured one marker of plasma oxidative damage, one marker of plasma non-enzymatic antioxidant capacity, and plasma levels of two antioxidant enzymes (superoxide dismutase,
SOD, and glutathione peroxidase, GPx) using standard methods applied to a variety of wildlife species40,56. BACTERIAL KILLING ASSAYS We measured the overall function of the constitutive
innate immune system by assessing the in vitro bacterial killing activity of the plasma against the gram-negative _Escherichia coli_ (ATCC #8739) and gram-positive _Staphylococcus aureus_
(ATCC # 6538 P) using a liquid growth inhibition assay modified from57. The samples were diluted 1:2 with TSB before 5 µL of each diluted sample was added to a flat-bottomed 96-well plate.
The fresh bacterial cultures were diluted in TSB until the attenuance (_D_) at 595 nm was 0.01 and 45 µL was then added to each well. We mixed 45 µL of bacterial solution with 5 µL of TSB as
a positive control and used 50 µL of TSB only as a negative control. The plates were incubated at 37 °C for 20 h and the _D_ at 595 nm was read in a plate reader (Biotek) before and after
incubation. After subtracting the _D_ of samples at 0 h of incubation from the ones at 20 h of incubation, for each microbial strain the bacterial killing activity of was defined as the
percent of the killed bacteria, which was calculated as 1 – (_D_sample/_D_positive control). HEMOLYSIS–HEMAGGLUTINATION ASSAY Natural antibodies, measured as hemagglutination (HA) titers,
bind non-specifically to various antigens and have important roles in opsonization. Hemolysis (HL), mediated by complement, is part of the innate immune system and its activation results in
cell lysis, especially those previously opsonized. Levels of HA and HL were assessed using the hemolysis–hemagglutination assay as described by ref. 58 modified for mammals by using chicken
red blood cells as a target52,59. After pipetting 12.5 µL of plasma into the first two columns of a U-shaped 96-well microtitre plate, 12.5 µL sterile PBS were added to columns 2–12. The
content of the second column of wells was serially diluted (1:2) until the 11th column, resulting in a dilution series for each sample from 1/1 to 1/1024. The last column of the plate was
used as a negative control, containing only PBS. 12.5 µL of 1% chicken red blood cell suspension (supplied by Dunnlab) was added to all of the wells and incubated at 37 °C for 90 min. After
incubation, to increase the visualization of agglutination, the plates were tilted at a 45° angle at room temperature. Agglutination and lysis were recorded after 20 and 90 min,
respectively. Titers of HA and HL were given as the log2 of the reciprocal of the highest dilution of plasma showing complete hemagglutination or hemolysis, respectively52,58. LYSOZYME
CONCENTRATION Lysozyme is an enzyme with antibacterial activity, especially against gram-positive bacteria. To measure the concentration of lysozyme, we used the lysoplate assay method52,55.
4 µL of the sample was inoculated in the test holes of a 1% Noble agar gel (A5431, Sigma) containing 25 mg/100 ml lyophilized _Micrococcus lysodeikticus_ (M3770, Sigma), a bacterium that is
particularly sensitive to lysozyme concentration. Crystalline hen egg white lysozyme (L6876, Sigma) (concentration: 0.5, 0.8, 1, 2, 4, 8, 10, 20, 40 and 100 µg/ml) was used to prepare a
standard curve for each plate. Plates were incubated at 37 °C for 20 h. During incubation, a clear zone developed in the area of the gel surrounding the sample inoculation site corresponding
the bacterial lysis. The diameters of the cleared zones are proportional to the log of the lysozyme concentration. This area was measured three times digitally using the software ImageJ
(version 1.48, https://imagej.nih.gov/ij/) and the mean was converted to a semi-logarithmic plot into hen egg lysozyme equivalents (HEL equivalents, expressed in µg/ml) according to the
standard curve52. HAPTOGLOBIN CONCENTRATION Haptoglobin is an acute phase protein that usually occurs at low concentrations, but its production and secretion are increased in response to
acute infections and trauma. To measure the concentration of haptoglobin, the standard procedure of the commercial kit “PHASE”TM Haptoglobin Assay (Tridelta, Ireland) was followed, an assay
previously used in various pinniped species (e.g., refs. 39,53). After diluting the plasma samples (1:2) with PBS, hemoglobin was added. Haptoglobin binds to hemoglobin and maintains its
peroxidase activity at a low pH. The measured peroxidase activity of hemoglobin is directly proportional to the amount of haptoglobin in the sample. Haptoglobin concentrations were
calculated according to the standard curve on each plate and were expressed as mg/ml. If the values measured were higher than the highest standard, the out-of-range samples were diluted and
reanalyzed. NEOPTERIN CONCENTRATION Neopterin is synthesized primarily by activated macrophages and is considered a biomarker for the activation of the cellular Th1 immune response. To
measure the concentration of neopterin, we used a commercial neopterin ELISA kit (#RE59321; IBL International GmbH, Germany). Following the manufacturer’s protocol, 20 µL of standards,
commercial controls, and undiluted plasma samples were added in duplicate into wells of the microtiter plate coated with a goat anti-rabbit antibody. We added 100 µL of enzyme conjugate and
50 µL of neopterin antiserum to each well. We covered the microtiter plate with black adhesive foil and incubated it by gently vortexing the plate in the dark for 90 min. After incubation,
we removed the adhesive foil and washed the plate four times with 300 µL of diluted wash buffer. We then added 150 µL of substrate solution into each well and incubated the plate for 10 min.
We stopped the reaction by adding 150 µL of stop solution into each well. Absorbance was read at 450 nm (Biotek; µQuant Microplate Spectrophotometer). The final concentrations were
extrapolated from the standard curve for each plate and expressed in nmol/L. PROTEIN A ELISA TO MEASURE IMMUNOGLOBULIN G CONCENTRATIONS We measured the concentration of immunoglobulin G
(IgG), the most common antibody subtype, in plasma with a Protein A ELISA19,34. Briefly, high-binding 96-well plates were coated with 100 µl diluted plasma samples (1:20,000 in 50 mM NaHCO3,
pH 9.5) in duplicate and incubated for 1 h at 37 °C. Purified canine IgG (#0129-01, Southern Biontech) (concentration from 0.0625 µg/ml to 5 µg/ml) was used to prepare a standard curve for
each plate. After incubation, the plates were washed in Tris-buffered saline/Tween-20 (TBS-T), blocked with 1% gelatin (Merck) solution and incubated for 30 min at 37 °C. After washing, 100
µl of Protein A-horseradish peroxidase conjugate solution (Invitrogen, 1:2000 in TBS-T, pH 7.4) was added to each well. The wells were washed after 30 min incubation at room temperature and
submerged in 100 µl TMB 10% 3,3’, 5,5’-tetramethylbenzidine (SouthernBiotech) in DMSO (Sigma-Aldrich) diluted 1:100 in phosphate-citric buffer pH 5.0 and mixed with 30% H2O2 (Hedinger). The
reaction was stopped after 5 min with 1 M sulfuric acid and the absorbance was read immediately at 450 nm (Biotek; µQuant Microplate Spectrophotometer). The IgG concentration (µg/ml) was
calculated based on the standard curve. WHITE BLOOD CELL (WBC) COUNTS Blood smears were stained with May-Gruenwald’s solution (#T863.2, Carl Roth GmbH) and Giemsa (#T862.1, Carl Roth GmbH).
The total WBC count for each individual was obtained by counting the number of WBCs in ten randomly selected microscope fields under 100x magnification, as detailed in ref. 60. Differential
WBC counts were implemented by counting the relative numbers of neutrophils, basophils, eosinophils, monocytes, and lymphocytes among 100 leukocytes. Sixteen out of the 350 analyzed slides
had a total WBC count of zero and the basophil count was zero for 224 of the slides. We therefore used the ratio of innate to adaptive white blood cells (i.e., the differential count of
neutrophils + basophils + eosinophils + monocytes/lymphocytes) for further downstream analyses. This also equates to the ratio of myeloid to lymphoid cells and acts as a non-specific
indicator of acute inflammation. To assess the reliability of these data, we then repeated differential WBC counts for a random 5% of slides and calculated the interclass correlation
coefficient (ICC) using the R package psych version 2.2.961. All of the ICC values were above 0.5, indicative of moderate to excellent repeatability (ICC (3, _k_) for neutrophils = 0.91,
basophils = 0.57, eosinophils = 0.69, monocytes = 0.60, and lymphocytes = 0.84)62. Finally, we assessed the degree of association between the neutrophil-to-lymphocyte ratio, a frequently
used marker of stress in vertebrates, and salivary baseline cortisol concentration per individual with a Pearson’s _r_ using the R package stats version 4.2.1. Repeated measurements were
taken into consideration by calculating the correlation coefficient of the individual means63. DROM TEST ROMs (primary products of oxidative damage) were measured in plasma using the d-ROMs
test (Diacron International, Italy)8,64. We pipetted 4 µl of the sample plasma, reference standards (0.225 to 1.8 mM H2O2 equivalents), blanks, and quality controls in duplicate into 96-well
plates. We added to each well 200 µl of a solution containing acetic acid/sodium acetate buffer (0.01 M, pH 4.8) and the chromogen N,N-diethyl-p-phenylenediamine (ratio 100:1). The ROMs
that occur in the plasma react with the chromogen, generating a pink color whose intensity is proportional to the concentration. After an incubation at 37 °C for 75 min, the optical density
was measured at 505 nm and the resulting values were expressed as mM of H2O2 equivalents. OXY-ADSORBENT TEST The non-enzymatic antioxidant capacity of the plasma was measured using the
OXY-adsorbent test (Diacron International, Italy)8,64. This assay quantifies the in vitro reaction between non-enzymatic antioxidants (e.g., protein thiols, vitamins C and E) and
hypochlorous acid (HOCl; pro-oxidant generated endogenously by vertebrates). All plasma samples, reference standards (340 mM), and quality controls were initially diluted 1:100 with
distilled water. We then pipetted 5 µl of each one in duplicate into 96-well plates and immediately added 200 µl of HOCl. After incubation at 37 °C for 10 min, we added 2 µl of
N,N-diethyl-p-phenylenediamine to each well and mixed the solution. The N,N-diethyl-p-phenylenediamine reacts with the HOCl that did not react with the plasma antioxidants, generating a pink
color. We therefore measured the optical density at 505 nm and expressed the resulting OXY values as mM of HOCl neutralized. RANSEL TEST We measured the activity of glutathione peroxidase
(GPx) in plasma using the Ransel test (Randox Labs, UK). This assay quantifies the GPx relying on its catalytic activity of the oxidation of glutathione by cumune hydroperoxide. In the
presence of glutathione reductase and NADPH, the oxidized glutathione is converted to the reduced form with a concomitant oxidation of NADPH, resulting in a decrease in absorbance at 340 nm
with time. The kinetic reaction was followed for 3 min and a blank reaction was subtracted from the sample absorbance. The activity of GPx was expressed as units/l. RANSOD TEST We measured
the activity of superoxide dismutase (SOD) in plasma using the Ransod test (Randox Labs, UK). This assay employs xanthine and xanthine oxidase to generate superoxide radicals, which react
with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (INT) to form a red formazan dye; SOD is measured by the degree of inhibition of this reaction due to its role in the
dismutation of superoxide radicals. SOD activity was quantified using a calibration curve run for each assay. The activity of SOD was expressed as units/ml, where one unit of SOD causes a
50% inhibition. DEVELOPMENT OF IMMUNE AND OXIDATIVE STATUS MARKERS OVER TIME To investigate whether immune and oxidative status marker concentrations changed in pups and mothers during the
course the study, we respectively compared group means at birth and molt. We then compared the concentration of each marker in pups relative to mothers at the two measured time points to
determine whether pup concentrations had adjusted to adult levels by the time they began molting. We compared group means using ANOVA and Tukey’s HSD post-hoc tests in the R package multcomp
version 1.4-2065. Cohen’s d measure of effect size was calculated using the R package rstatix version 0.7.066. As proposed by ref. 67, |0.2| is considered a small, |0.5| a moderate, and
|0.8| a large effect size. STATISTICS AND REPRODUCIBILITY Generalized linear mixed models were fitted in a Bayesian framework using Markov chain Monte Carlo methods in the R package MCMCglmm
version 2.3468,69. We included season (food availability), colony (density), body condition, baseline cortisol, and sex as predictor variables of the immune markers (BKA (_E. coli_ and _S.
aureus_), hemagglutination, hemolysis, lysozyme, haptoglobin, neopterin, IgG, and the innate/adaptive WBC ratio). The nine immune markers were then independently fitted as predictor
variables of the four oxidative status markers OXY, dROM, GPx, and SOD. The hypothesized causal relationships among the predictor and response variables are depicted in Fig. 1c as a directed
acyclic graph (DAG). Sets of covariates for the unbiased estimation of direct and total causal effects70 were checked using the R package dagitty version 0.3.271. JUSTIFICATION FOR THE DAG
CASUAL STRUCTURE We hypothesized direct effects of season on density, body condition, and baseline cortisol. Yearly environmental changes on Bird Island are the main driver of variability in
food availability, with the first year of this study being among the worst on record in terms of food abundance. Consequently, significantly fewer individuals came ashore to breed and
population densities were overall lower in 2019 compared with 202012. Our previous research has further shown that both body condition12 and salivary cortisol levels49 vary by year. Finally,
lower food availability is expected to limit an individual’s ability to mount an immune response4, an effect that is likely to be stronger in pups, which have to invest in other
energetically demanding processes such as growth. We hypothesized direct effects of colony on body condition, baseline cortisol, and immune markers. We know from previous studies of the same
population that body condition12 and hair cortisol concentrations13 differ significantly between the two breeding colonies. This variability has been attributed to contrasting population
densities, with FWB having significantly fewer breeding females per square meter13 and a lower modal local density of pups throughout the breeding season12. In general, the transmission of
parasites and pathogens is also density dependent and consequently population density tends to correlate positively with immune cell profiles and the strength of immune responses72,73,74. We
hypothesized direct effects of three additional variables on immune marker levels: (i) Sex. Immune responses often differ between the sexes, although the direction of immunocompetence can
depend on a number of factors, such as sex-specific growth patterns and behaviors33. Sex has also been shown to influence cortisol levels in Antarctic fur seal pups49. (ii) Cortisol.
Glucocorticoids may mediate immune responses because elevated levels of the stress hormone have been strongly linked to immune defense suppression35. Reverse causality has, however, also
been suggested, whereby parasite infection may elicit an increase in plasma cortisol75. (iii) Body condition. Studies of wild populations provide strong evidence for a two-way relationship
between body condition and immunity37. In particular, poor condition may predispose an individual to infection, which will further reduce condition. Immune markers are included in our DAG as
response variables, and predictors of oxidative status marker concentrations. Assuming limited resources, there is a predicted trade-off between investment in immune function and
antioxidant defense76. Furthermore, increased innate immune activity may necessitate the downregulation of antioxidant enzymes, which would otherwise minimize inflammation and remove
reactive oxygen species purposefully generated to kill bacteria8. RANDOM EFFECTS STRUCTURE All of our models included individual identity (ID) as a random variable to control for repeated
measures. We also considered the possibility that the time of sampling could contribute towards some of the variation in our response variables given that components of the immune system are
known to oscillate along an active/rest cycle77. Previous research has shown that Antarctic fur seal pups are diurnal78 but less is known about adult female activity patterns. We therefore
fitted linear models to assess the proportion of variability in immune and oxidative status marker concentrations explained by the hour of sample collection. Models were fitted using the R
package lmer version 1.1-3079. None of the markers crossed a pre-defined threshold R-squared value of 5%, with the average proportion of variance in the dependent variable explained by the
hour of sampling being 0.48% (Supplementary Table S3). We therefore elected not to include the time of sampling as a random effect in the models. MODEL PARAMETERS All immune and oxidative
status markers were zero mean and unit standardized. Baseline cortisol, a continuous predictor variable, was measured in ng/ml. Binary predictor variables were coded as such (i.e., 0/1),
with 2019 as the reference category for season (a proxy of food availability), FWB as the reference category for colony (a proxy of density), and female as the reference category for sex.
Mothers and pups were analyzed separately. Sex was not included as a predictor variable in models for the mothers. We fitted models with a Gaussian distribution. For the R structure in our
model, we kept the flat, default prior for the mean (−106, 106) and specified a weak prior variance (V = 1, nu = 0.002). For the G structure (i.e., random effects variance components), we
specified a proper Cauchy prior (V = 2, nu = 1). Pilot runs (thinning = 10, burn-in samples = 3000, number of iterations = 13,000) indicated autocorrelation for some variance components. We
therefore increased the thinning interval, burn-in, and number of iterations (x 100) to guarantee the mean and variance effective sample sizes were least 600, but ideally ≥1000. We assessed
convergence of the chains visually and ensured that autocorrelation was less than 0.1. Our final check was to simulate new data given the model parameter values (variance/co-variance
structures) and plot them against our real data to ensure they overlapped. MODEL OUTPUTS For the binary response variables, the standardized mean difference was used as an index of effect
size80. For the continuous variables, the posterior was the concentration of probability around the estimate of the mean, such that positive values are indicative of a positive relationship
between the predictor and response. We considered a predictor variable to significantly correlate with the response when the _p_MCMC < 0.05, where _p_MCMC is equal to two times the
smaller of the MCMC posterior probability estimates that the parameter value is above or below zero. REPEATED MEASURES Repeatability, i.e., individual consistency in immune marker levels
over the 60 days from birth to the start of molt, was calculated using the full model according to ref. 81. Specifically, R = VID/(VID + VF + VR), where VID stands for between-individual
variance (here, also random effect variance), VF for the fixed effect variance, and VR for the residual variance. For comparison, as recommended by ref. 82, repeatability was also calculated
for the null model (i.e., without including the fixed effects), which provides an estimate of the variance observed in the raw data. A high repeatability (i.e., point estimates for R
approaching 1) would indicate that individual immune marker levels differ minimally between sampling time points, suggesting comparable investment into immune parameters at birth and molt.
Given that we collected two measurements per individual and have repeated measures from 67 pups and 80 mothers, the standard error of the within-subject standard deviation is 15%83. We ran
all analyses in R version 4.2.184. The raw data and code can be found on Zenodo85. REPORTING SUMMARY Further information on research design is available in the Nature Portfolio Reporting
Summary linked to this article. DATA AVAILABILITY Raw data including the numerical source data underlying the graphs and charts shown in the manuscript can be found on Zenodo under
https://doi.org/10.5281/zenodo.11208287. CODE AVAILABILITY We ran all analyses in R version 4.2.1. Code can be found on Zenodo under https://doi.org/10.5281/zenodo.11208287. REFERENCES *
Chaplin, D. D. Overview of the immune response. _J. Allergy Clin. Immunol._ 125, S3–S23 (2010). Article PubMed PubMed Central Google Scholar * Moser, M. & Leo, O. Key concepts in
immunology. _Vaccine_ 28S, C2–C13 (2010). Article Google Scholar * Sheldon, B. C. & Verhulst, S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology.
_Trends Ecol. Evol._ 11, 317–321 (1996). Article CAS PubMed Google Scholar * Forbes, K. M. et al. Food limitation constrains host immune responses to nematode infections. _Biol. Lett._
12, 20160471 (2016). Article PubMed PubMed Central Google Scholar * Côté, I. M. & Poulin, R. Parasitism and group size in social animals: a meta-analysis. _Behav. Ecol._ 6, 159–165
(1995). Article Google Scholar * Schneeberger, K., Czirják, G. Á. & Voigt, C. C. Inflammatory challenge increases measures of oxidative stress in a free-ranging, long-lived mammal. _J.
Exp. Biol._ 216, 4514–4519 (2013). CAS PubMed Google Scholar * Costantini, D. A meta-analysis of impacts of immune response and infection on oxidative status in vertebrates. _Conserv.
Physiol._ 10, coac018 (2022). Article CAS PubMed PubMed Central Google Scholar * Costantini, D. Understanding diversity in oxidative status and oxidative stress: the opportunities and
challenges ahead. _J. Exp. Biol._ 222, jeb194688 (2019). Article PubMed Google Scholar * Forcada, J. & Hoffman, J. I. Climate change selects for heterozygosity in a declining fur seal
population. _Nature_ 511, 462–465 (2014). Article CAS PubMed Google Scholar * Atkinson, A. et al. Krill (_Euphausia superba_) distribution contracts southward during rapid regional
warming. _Nat. Clim. Change_ 9, 142–147 (2019). Article Google Scholar * Cassini, M. H. The evolution of reproductive systems in pinnipeds. _Behav. Ecol._ 10, 612–616 (1999). Article
Google Scholar * Nagel, R. et al. Evidence for an Allee effect in a declining fur seal population. _Proc. R. Soc. B_ 288, 20202882 (2021). Article PubMed PubMed Central Google Scholar *
Meise, K., Von Engelhardt, N., Forcada, J. & Hoffman, J. I. Offspring hormones reflect the maternal prenatal social environment: potential for foetal programming? _PLoS One_ 11,
e0145352 (2016). Article PubMed PubMed Central Google Scholar * Swan, M. P. & Hickman, D. L. Evaluation of the neutrophil-lymphocyte ratio as a measure of distress in rats. _Lab.
Anim._ 43, 276–282 (2014). Article Google Scholar * Davis, A. K. & Maney, D. L. The use of glucocorticoid hormones or leucocyte profiles to measure stress in vertebrates: What’s the
difference? _Methods Ecol. Evol._ 9, 1556–1568 (2018). Article Google Scholar * Černý, J., Elsterová, J., Růžek, D. & Grubhoffer, L. Vertebrate viruses in polar ecosystems. in _Life in
Extreme Environments_ (eds. Di Prisco, G., Edwards, H. G. M., Elster, J. & Huiskes, A. H. L.) 126–148 (Cambridge University Press, 2020). * Peters, A., Delhey, K., Nakagawa, S.,
Aulsebrook, A. & Verhulst, S. Immunosenescence in wild animals: meta-analysis and outlook. _Ecol. Lett._ 22, 1709–1722 (2019). Article PubMed Google Scholar * Ummenhofer, C. C. &
Meehl, G. A. Extreme weather and climate events with ecological relevance: a review. _Philos. Trans. R. Soc. B_ 372, 20160135 (2017). Article Google Scholar * Ross, P. S., Pohajdak, B.,
Bowen, W. D. & Addison, R. F. Immune function in free-ranging harbor seal (_Phoca vitulina_) mothers and their pups during lactation. _J. Wildl. Dis._ 29, 21–29 (1993). Article CAS
PubMed Google Scholar * King, D. P. et al. Ontogeny of humoral immunity in northern elephant seal (_Mirounga angustirostris_) neonates. _Comp. Biochem. Physiol. Part B_ 121, 363–368
(1998). Article CAS Google Scholar * Ferreira, A. P. S. et al. Serum immunoglobulin G concentration in southern elephant seal, _Mirounga leonina_ (Linnaeus, 1758), from Elephant island
(Antarctica): Sexual and adrenal steroid hormones effects. _Vet. Immunol. Immunopathol._ 106, 239–245 (2005). Article CAS PubMed Google Scholar * Banuet-Martínez, M. et al. Climatic
anomaly affects the immune competence of California sea lions. _PLoS One_ 12, e0179359 (2017). Article PubMed PubMed Central Google Scholar * Brock, P. M., Hall, A. J., Goodman, S. J.,
Cruz, M. & Acevedo-Whitehouse, K. Immune activity, body condition and human-associated environmental impacts in a wild marine mammal. _PLoS One_ 8, e67132 (2013). Article CAS PubMed
PubMed Central Google Scholar * Baker, J. R. & Mccannt, T. S. Pathology and bacteriology of adult male Antarctic fur seals, _Arctocephalus gazella_, dying at Bird Island, South
Georgia. _Br. Vet. J._ 145, 263–275 (1989). Article CAS PubMed Google Scholar * Lee, K. A. Linking immune defenses and life history at the levels of the individual and the species.
_Integr. Comp. Biol._ 46, 1000–1015 (2006). Article CAS PubMed Google Scholar * Beldomenico, P. M. & Begon, M. Disease spread, susceptibility and infection intensity: vicious
circles? _Trends Ecol. Evol._ 25, 21–27 (2010). Article PubMed Google Scholar * Huber, C. et al. Immune response-associated production of neopterin. _J. Exp. Med._ 160, 310–316 (1984).
Article CAS PubMed Google Scholar * McCallum, H., Barlow, N. & Hone, J. How should pathogen transmission be modelled? _Trends Ecol. Evol._ 16, 295–300 (2001). Article CAS PubMed
Google Scholar * Schneeberger, K., Czirják, G. Á. & Voigt, C. C. Measures of the constitutive immune system are linked to diet and roosting habits of neotropical bats. _PLoS One_ 8,
e54023 (2013). Article CAS PubMed PubMed Central Google Scholar * Nelson, R. J. et al. Photoperiod reproductive and population density interact to affect and immune function in male
prairie voles. _Am. J. Physiol. Regulat. Integr. Comp. Physiol._ 270, R571–R577 (1996). Article CAS Google Scholar * Flies, A. S., Mansfield, L. S., Grant, C. K., Weldele, M. L. &
Holekamp, K. E. Markedly elevated antibody responses in wild versus captive spotted hyenas show that environmental and ecological factors are important modulators of immunity. _PLoS One_ 10,
e0137679 (2015). Article PubMed PubMed Central Google Scholar * Barbosa, A., Merino, S., Benzal, J., Martinez, J. & García-Fraile, S. Geographic variation in the immunoglobulin
levels in pygoscelid penguins. _Polar Biol._ 30, 219–225 (2007). Article Google Scholar * Stoehr, A. M. & Kokko, H. Sexual dimorphism in immunocompetence: what does life-history theory
predict? _Behav. Ecol._ 17, 751–756 (2006). Article Google Scholar * Hall, A. J., McConnell, B. J. & Barker, R. J. The effect of total immunoglobulin levels, mass and condition on the
first-year survival of grey seal pups. _Funct. Ecol._ 16, 462–474 (2002). Article Google Scholar * Shimba, A. & Ikuta, K. Control of immunity by glucocorticoids in health and disease.
_Semin. Immunopathol._ 42, 669–680 (2020). Article CAS PubMed Google Scholar * Dhabhar, F. S. Effects of stress on immune function: the good, the bad, and the beautiful. _Immunol. Res._
58, 193–210 (2014). Article CAS PubMed Google Scholar * Beldomenico, P. M. et al. Poor condition and infection: a vicious circle in natural populations. _Proc. R. Soc. B: Biol. Sci._
275, 1753–1759 (2008). Article Google Scholar * Vera-Massieu, C., Brock, P. M., Godínez-Reyes, C. & Acevedo-Whitehouse, K. Activation of an inflammatory response is context-dependent
during early development of the California sea lion. _R. Soc. Open Sci._ 2, 150108 (2015). Article PubMed PubMed Central Google Scholar * Peck, H. E., Costa, D. P. & Crocker, D. E.
Body reserves influence allocation to immune responses in capital breeding female northern elephant seals. _Funct. Ecol._ 30, 389–397 (2016). Article Google Scholar * Costantini, D.
Oxidative stress ecology and the d-ROMs test: facts, misfacts and an appraisal of a decade’s work. _Behav. Ecol. Sociobiol._ 70, 809–820 (2016). Article Google Scholar * Forman, H. J.
& Torres, M. Reactive oxygen species and cell signaling: Respiratory burst in macrophage signaling. _Am. J. Respiratory Crit. Care Med._ 166, S4–S8 (2002). Article Google Scholar *
Payne, M. R. Growth in the Antarctic fur seal _Arctocephalus gazella_. _J. Zool. _187, 1–20 (1979). Article Google Scholar * Vázquez-Medina, J. P., Soñanez-Organis, J. G., Burns, J. M.,
Zenteno-Savín, T. & Ortiz, R. M. Antioxidant capacity develops with maturation in the deep-diving hooded seal. _J. Exp. Biol._ 214, 2903–2910 (2011). Article PubMed PubMed Central
Google Scholar * Vázquez-Medina, J. P. et al. Maturation increases superoxide radical production without increasing oxidative damage in the skeletal muscle of hooded seals (_Cystophora
cristata_). _Can. J. Zool._ 89, 206–212 (2011). Article Google Scholar * Staszewski, V. & Boulinier, T. Vaccination: a way to address questions in behavioral and population ecology?
_Trends Parasitol._ 20, 17–22 (2004). Article PubMed Google Scholar * Richter, S. H. & Hintze, S. From the individual to the population – and back again? Emphasising the role of the
individual in animal welfare science. _Appl. Anim. Behav. Sci._ 212, 1–8 (2019). Article Google Scholar * Forbes, K. M. et al. Food provisioning alters infection dynamics in populations of
a wild rodent. _Proc. R. Soc. B_ 282, 20151939 (2015). Article PubMed PubMed Central Google Scholar * Peig, J. & Green, A. J. New perspectives for estimating body condition from
mass/length data: the scaled mass index as an alternative method. _Oikos_ 118, 1883–1891 (2009). Article Google Scholar * Nagel, R. et al. Low heritability and high phenotypic plasticity
of cortisol in response to environmental heterogeneity in fur seals. _Ecol. Evol._ 12, e8757 (2022). Article PubMed PubMed Central Google Scholar * Dierauf, L. & Gulland, F. M. D.
(eds). _CRC Handbook of Marine Mammal Medicine_ (2nd ed.) (CRC Press, 2001). * Demas, G. E., Zysling, D. A., Beechler, B. R., Muehlenbein, M. P. & French, S. S. Beyond
phytohaemagglutinin: assessing vertebrate immune function across ecological contexts. _J. Anim. Ecol._ 80, 710–730 (2011). Article PubMed Google Scholar * Heinrich, S. K. et al. Cheetahs
have a stronger constitutive innate immunity than leopards. _Sci. Rep._ 7, 44837 (2017). Article PubMed PubMed Central Google Scholar * Thomton, J. D. & Mellish, J.-A. E. Haptoglobin
concentrations in free-range and temporarily captive juvenile steller sea lions. _J. Wildl. Dis._ 43, 258–261 (2007). Article CAS PubMed Google Scholar * McGill, S., Burchmore, R. J.
S., Pomeroy, P. P. & Kennedy, M. W. Is a little enough? Paucity of immune proteins in serum of precocial neonates of a marine carnivoran—the Atlantic grey seal. _Front. Ecol. Evol._ 9,
802510 (2022). Article Google Scholar * Meza Cerda, M. I. et al. Developing immune profiles of endangered Australian sea lion (_Neophoca cinerea_) pups within the context of endemic
hookworm (_Uncinaria sanguinis_) infection. _Front. Vet. Sci._ 9, 824584 (2022). Article PubMed PubMed Central Google Scholar * Costantini, D., Czirják, G. Á., Melzheimer, J., Menges, V.
& Wachter, B. Sex and species differences of stress markers in sympatric cheetahs and leopards in Namibia. _Comp. Biochem. Physiol. Part A_ 227, 8–13 (2019). Article CAS Google
Scholar * Rowe, M., Czirják, G. Á., McGraw, K. J. & Giraudeau, M. Sexual ornamentation reflects antibacterial activity of ejaculates in mallards. _Biol. Lett._ 7, 740–742 (2011).
Article PubMed PubMed Central Google Scholar * Matson, K. D., Ricklefs, R. E. & Klasing, K. C. A hemolysis-hemagglutination assay for characterizing constitutive innate humoral
immunity in wild and domestic birds. _Dev. Comp. Immunol._ 29, 275–286 (2005). Article CAS PubMed Google Scholar * Gilot-Fromont, E. et al. Immune phenotype and body condition in roe
deer: individuals with high body condition have different, not stronger immunity. _PLoS One_ 7, e45576 (2012). Article CAS PubMed PubMed Central Google Scholar * Menéndez-Blázquez, J.
et al. Leukocyte counts in blood smears of Antarctic seals and penguins: a new less time-consuming method. _Polar Biol._ 44, 2195–2198 (2021). Article Google Scholar * Revelle, W. psych:
procedures for psychological, psychometric, and personality research. software https://CRAN.R-project.org/package=psych (2018). * Koo, T. K. & Li, M. Y. A guideline of selecting and
reporting intraclass correlation coefficients for reliability research. _J. Chiropr. Med._ 15, 155–163 (2016). Article PubMed PubMed Central Google Scholar * Bland, A. Calculating
correlation coefficients with repeated observations: Part 2 - correlation between subjects. _BMJ_ 310, 633 (1995). Article CAS PubMed PubMed Central Google Scholar * Costantini, D.,
Czirják, G. Á., Bustamante, P., Bumrungsri, S. & Voigt, C. C. Impacts of land use on an insectivorous tropical bat: the importance of mercury, physio-immunology and trophic position.
_Sci. Total Environ._ 671, 1077–1085 (2019). Article CAS Google Scholar * Hothorn, T., Bretz, F. & Westfall, P. Simultaneous inference in general parametric models. _Biometrical J._
50, 346–363 (2008). Article Google Scholar * Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. software
https://cran.r-project.org/web/packages/rstatix/index.html (2021). * Cohen, J. A Power Primer. _Psychol. Bull._ 112, 155–159 (1992). Article CAS PubMed Google Scholar * Hadfield, J. D.
MCMC methods for multi-response generalized linear mixed models: The MCMCglmm R package. _J. Stat. Softw._ 33, 1–22 (2010). Article Google Scholar * Hadfield, J. Markov chain Monte Carlo
generalised linear mixed models - Course Notes. https://rdrr.io/cran/MCMCglmm/man/MCMCglmm.html (2019). * Cinelli, C., Forney, A. & Pearl, J. A crash course in good and bad controls.
_Sociol. Methods Res._ https://doi.org/10.1177/00491241221099552 (2022). * Textor, J., van der Zander, B., Gilthorpe, M. S., Liśkiewicz, M. & Ellison, G. T. Robust causal inference using
directed acyclic graphs: the R package ‘dagitty’. _Int. J. Epidemiol._ 45, 1887–1894 (2016). PubMed Google Scholar * Van Lieshout, S. H. J. et al. Social effects on age-related and
sex-specific immune cell profiles in a wild mammal: Immune cell profiles in a wild mammal. _Biol. Lett._ 16, 20200234 (2020). Article PubMed PubMed Central Google Scholar * Møller, A.
P., Martín-Vivaldi, M., Merino, S. & Soler, J. J. Density-dependent and geographical variation in bird immune response. _Oikos_ 115, 463–474 (2006). Article Google Scholar * Lavigne,
D. M. & Schmitz, O. J. Global warming and increasing population densities: a prescription for seal plagues. _Mar. Pollut. Bull._ 21, 280–284 (1990). Article Google Scholar * Seguel,
M., Perez-Venegas, D., Gutierrez, J., Crocker, D. E. & DeRango, E. J. Parasitism elicits a stress response that allocates resources for immune function in South American fur seals
(_Arctocephalus australis_). _Physiol. Biochem. Zool._ 92, 326–338 (2019). Article PubMed Google Scholar * Eikenaar, C., Isaksson, C. & Hegemann, A. A hidden cost of migration? Innate
immune function versus antioxidant defense. _Ecol. Evol._ 8, 2721–2728 (2018). Article PubMed PubMed Central Google Scholar * McEachin, S., Drury, J. P., Anderson, C. N. & Grether,
G. F. Mechanisms of reduced interspecific interference between territorial species. _Behav. Ecol._ 33, 126-136 (2022). * Nagel, R. et al. Movement patterns and activity levels are shaped by
the neonatal environment in Antarctic fur seal pups. _Sci. Rep._ 11, 14323 (2021). Article CAS PubMed PubMed Central Google Scholar * Bates, D., Mächler, M., Bolker, B. & Walker, S.
Fitting linear mixed-effects models using lme4. _J. Stat. Softw._ 67, 1–48 (2015). Article Google Scholar * Durlak, J. A. How to select, calculate, and interpret effect sizes. _J.
Pediatr. Psychol._ 34, 917–928 (2009). Article PubMed Google Scholar * de Villemereuil, P., Morrissey, M. B., Nakagawa, S. & Schielzeth, H. Fixed-effect variance and the estimation of
repeatabilities and heritabilities: issues and solutions. _J. Evol. Biol._ 31, 621–632 (2018). Article PubMed Google Scholar * Wilson, A. J. Why h2 does not always equal VA/VP? _J. Evol.
Biol._ 21, 647–650 (2008). Article CAS PubMed Google Scholar * Bland, J. M. & Altman, D. G. Statistics notes: measurement error. _BMJ_ 313, 744 (1996). Article CAS PubMed PubMed
Central Google Scholar * R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing (2023). * Nagel, R. et al. Life-history stage
influences immune investment and oxidative stress in response to environmental heterogeneity in Antarctic fur seals. Data set. _Zenodo_ https://doi.org/10.5281/zenodo.11208287 (2024).
Download references ACKNOWLEDGEMENTS This research was funded by the German Research Foundation (DFG) as part of the SFB TRR 212 (NC3)—Project Numbers 316099922 and 396774617. It was also
supported by core funding from the Natural Environment Research Council to the British Antarctic Survey’s Ecosystems Program. This work was supported by a bursary from Antarctic Science Ltd.
Internal funds were provided from the Leibniz Institute for Zoo and Wildlife Research. Laboratory work was supported by an Erasmus+ internship. The authors would like to thank Sylvia Kaiser
for generating the cortisol data, and Vivienne Litzke for enlightening discussions about data visualization. We thank Ana Bertoldi Carneiro, Freya Blockley, Jamie Coleman, Alexandra Dodds,
Vicki Foster, Derren Fox, Iain Angus Gordon, Pauline Goulet, Rosie Hall, Cary Jackson, Adam Lowndes, Elizabeth Morgan, Rachael Orben, Jessica Ann Philips, David Reid, and Mark Whiffin for
assistance in the field. FUNDING Open Access funding enabled and organized by Projekt DEAL. AUTHOR INFORMATION Author notes * Rebecca Nagel Present address: School of Biology, University of
St Andrews, St Andrews, KY16 9TH, UK * Claire Stainfield Present address: Scotland’s Rural College, Craibstone Estate, Ferguson Building, Aberdeen, AB21 9YA, UK * These authors jointly
supervised this work: Gábor Á. Czirják, Jaume Forcada, Joseph I. Hoffman. AUTHORS AND AFFILIATIONS * Department of Evolutionary Population Genetics, Faculty of Biology, Bielefeld University,
33501, Bielefeld, Germany Rebecca Nagel, Iva Tuponja & Joseph I. Hoffman * Department of Animal Behaviour, Bielefeld University, 33501, Bielefeld, Germany Rebecca Nagel, Iva Tuponja
& Joseph I. Hoffman * Department of Wildlife Diseases, Leibniz Institute for Zoo and Wildlife Research, 10315, Berlin, Germany Katja Pohle, Lilla Jordán & Gábor Á. Czirják * British
Antarctic Survey, High Cross, Madingley Road, Cambridge, CB3 OET, UK Claire Stainfield, Camille Toscani, Cameron Fox‑Clarke, Jaume Forcada & Joseph I. Hoffman * Department of Ecological
and Biological Sciences, University of Tuscia, 01100, Viterbo, Italy David Costantini * Center for Biotechnology, Faculty of Biology, Bielefeld University, 33615, Bielefeld, Germany Joseph
I. Hoffman * Joint Institute for Individualisation in a Changing Environment, Bielefeld University and University of Münster, 33501, Bielefeld, Germany Joseph I. Hoffman Authors * Rebecca
Nagel View author publications You can also search for this author inPubMed Google Scholar * Katja Pohle View author publications You can also search for this author inPubMed Google Scholar
* Lilla Jordán View author publications You can also search for this author inPubMed Google Scholar * Iva Tuponja View author publications You can also search for this author inPubMed Google
Scholar * Claire Stainfield View author publications You can also search for this author inPubMed Google Scholar * Camille Toscani View author publications You can also search for this
author inPubMed Google Scholar * Cameron Fox‑Clarke View author publications You can also search for this author inPubMed Google Scholar * David Costantini View author publications You can
also search for this author inPubMed Google Scholar * Gábor Á. Czirják View author publications You can also search for this author inPubMed Google Scholar * Jaume Forcada View author
publications You can also search for this author inPubMed Google Scholar * Joseph I. Hoffman View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS R.N., C.S., C.F.-C., and C.T. collected the data. K.P., L.J., I.T., D.C., G.Á.C., and R.N. conducted the laboratory work. R.N. analyzed the full dataset and drafted the
manuscript. J.I.H., J.F., D.C., and G.Á.C. conceived of the study and contributed funding and materials. All of the authors commented on and approved the final manuscript. CORRESPONDING
AUTHOR Correspondence to Rebecca Nagel. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ETHICAL APPROVAL Sampling was carried out by the British Antarctic
Survey under permits from the Government of South Georgia and the South Sandwich Islands (Wildlife and Protected Areas Ordinance (2011), RAP permit numbers 2018/024 and 2019/032). The
samples were imported into the UK under permits from the Department for Environment, Food and Rural Affairs (Animal Health Act, import license number ITIMP18.1397) and from the Convention on
International Trade in Endangered Species of Wild Fauna and Flora (import numbers 578938/01-15 and 590196/01-18). All procedures used were approved by the British Antarctic Survey Animal
Welfare and Ethics Review Body (AWERB applications 2018/1050 and 2019/1058). The Nagoya protocol has not been extended to South Georgia & the South Sandwich Islands. We have complied
with all relevant ethical regulations for animal use. PEER REVIEW PEER REVIEW INFORMATION _Communications Biology_ thanks Mauricio Seguel and the other, anonymous, reviewer(s) for their
contribution to the peer review of this work. Primary Handling Editors: Gene Chong and Luke Grinham. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1
SUPPLEMENTARY DATA 2 REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use,
sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative
Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Nagel, R., Pohle, K., Jordán, L. _et al._ Life-history stage influences immune investment and oxidative stress in response to environmental
heterogeneity in Antarctic fur seals. _Commun Biol_ 7, 788 (2024). https://doi.org/10.1038/s42003-024-06499-6 Download citation * Received: 25 April 2023 * Accepted: 24 June 2024 *
Published: 29 June 2024 * DOI: https://doi.org/10.1038/s42003-024-06499-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative