- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Histone acetylation, a crucial epigenetic modification, is governed by histone acetyltransferases (HATs), that regulate many biological processes. Functions of HATs in insects are
not well understood. We identified 27 HATs and determined their functions using RNA interference (RNAi) in the model insect, _Tribolium castaneum_. Among HATs studied,
N-alpha-acetyltransferase 40 (_NAA40_) knockdown caused a severe phenotype of arrested larval development. The steroid hormone, ecdysone induced _NAA40_ expression through its receptor, EcR
(ecdysone receptor). Interestingly, ecdysone-induced _NAA40_ regulates _EcR_ expression. NAA40 acetylates histone H4 protein, associated with the promoters of ecdysone response genes: _EcR_,
_E74_, _E75_, and _HR3_, and causes an increase in their expression. In the absence of ecdysone and NAA40, histone H4 methylation by arginine methyltransferase 1 (ART1) suppressed the above
genes. However, elevated ecdysone levels at the end of the larval period induced _NAA40_, promoting histone H4 acetylation and increasing the expression of ecdysone response genes. NAA40 is
also required for EcR, and steroid-receptor co-activator (SRC) mediated induction of _E74_, _E75_, and _HR3_. These findings highlight the key role of ecdysone-induced _NAA40_-mediated
histone acetylation in the regulation of metamorphosis. SIMILAR CONTENT BEING VIEWED BY OTHERS 5MC MODIFICATION ORCHESTRATES CHORIOGENESIS AND FERTILIZATION BY PREVENTING PROLONGED _FTZ-F1_
EXPRESSION Article Open access 12 December 2023 HISTONE 4 LYSINE 5/12 ACETYLATION ENABLES DEVELOPMENTAL PLASTICITY OF _PRISTIONCHUS_ MOUTH FORM Article Open access 13 April 2023 MIR-276 AND
MIR-182013-5P MODULATE INSECT METAMORPHOSIS AND REPRODUCTION VIA DUALLY REGULATING JUVENILE HORMONE ACID METHYLTRANSFERASE Article Open access 02 December 2024 INTRODUCTION Epigenetic
modifications regulate many fundamental biological processes by tightly regulating chromatin structure, influencing promoter access, and modulating gene expression1,2. Among these
modifications, acetylation is one of the crucial regulatory mechanisms carried out by acetyltransferases. The diversity of acetyltransferases is remarkable, and they are categorized based on
their subcellular localization as well as structural and functional similarities of their catalytic domains. Based on subcellular localization, histone acetyltransferases are divided into
two classes: type A HATs located in the nucleus and type B HATs located in the cytoplasm. However, some HAT proteins function in multiple locations, making their classification challenging.
To address this ambiguity, acetyltransferases are further categorized into different families based on structural and functional similarities of their catalytic domains. For instance, lysine
acetyltransferases (KATs) are known for adding an acetyl moiety to the epsilon-amino group of lysine residues in histones and other proteins3,4. Within KATs families, GCN5-related
N-acetyltransferases (GNAT), MYST and p300/CBP families are extensively studied in mammals5,6. GNAT catalyze the transfer of an acetyl group from acetyl-coenzyme A (Ac-CoA) to various
primary amine substrates, including histones and these are the first identified KATs7. The MYST family KATs acetylates both histones and non-histone proteins, regulating diverse functions
including gene regulation, DNA repair, cell cycle, stem cell homeostasis and development8. The another KAT family member - CREB-binding protein (CBP) emerged as a key player in juvenile
hormone (JH) signaling, specifically by inducing the JH primary response gene, Krüppel homolog 1 (_Kr-h1_) in both Red flour beetle, _Tribolium castaneum_ and Yellow fever mosquito, _Aedes
aegypti_ in our previous studies9,10,11,12. Conversely, histone deacetylases (HDACs), especially HDAC1, HDAC3, and HDAC11, exhibited contrasting effects by removing acetyl groups from lysine
amino acids in histones and repressing JH signaling by suppressing the _Kr-h1_ gene13,14,15. These studies highlight the critical roles of histone acetylation and deacetylation in insect
hormone actions that regulate insect development. In the model insect, _Drosophila melanogaster_, several KATs have been identified and characterized, however, their functional
characterization in other insect species is lacking. Another class of acetyltransferases, N-terminal acetyltransferases (NATs) mediate widespread protein modification that is conserved from
yeast to humans. NATs transfer the acetyl group from Ac-CoA to the α-amino group in the N-terminal amino acid residue of histone proteins or peptides. NATs play crucial roles in multiple
biological processes, including protecting proteins from degradation, proteasome localization, cell survival, hormonal regulation, apoptosis and maintenance of organelle structure, and
function2,16,17,18,19,20,21. In humans, six NATs (NatA-NatF) have been identified, each with unique subunit composition, substrate preferences, and induced phenotypes2,22. However,
information on NATs functions in insects, except in _Drosophila_, is limited. This knowledge gap presents an opportunity to investigate the roles of acetyltransferases in other insects
beyond _Drosophila_. In this study, we identified various acetyltransferases potentially involved in the growth, development, and metamorphosis of the model insect, _T. castaneum_. To
determine the functions of these acetyltransferases, we performed RNA interference (RNAi) experiments to knockdown genes coding for KATs and NATs in _T. castaneum_. Among the
acetyltransferases studied, the knockdown of _NAA40_ (TC015921), a member of the NAT family showed severe phenotypes, including larval development arrest and mortality during metamorphosis.
This observation led us to prioritize NAA40 for further in-depth investigations to comprehend its role in regulating insect hormone action, development, and metamorphosis. RESULTS
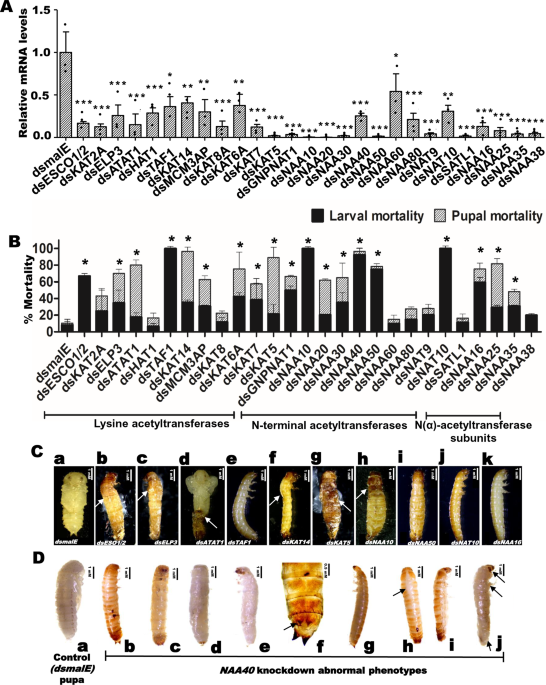
IDENTIFICATION AND DETERMINATION OF FUNCTIONS OF ACETYLTRANSFERASES IN THE RED FLOUR BEETLE, _T. CASTANEUM_ We identified 27 genes coding for acetyltransferases in _T. castaneum_ genome
using _D. melanogaster_ HAT protein sequences. These HATs were classified into Lysine acetyltransferases (KATs) and N-terminal acetyltransferases (NATs) based on the structural and
functional similarities of their catalytic domains. Among the 27 HATs identified, 12 belong to the KATs category (Supplementary Table 1). Within in the KATs category, KAT5, KAT6A, KAT7 and
KAT8 were identified to contain the MYST domain, known for its involvement in the acetylation of many nuclear proteins that regulate many biological functions, including gene regulation, DNA
repair, cell-cycle regulation, apoptosis, and development8. Previous studies have already identified two multifunctional KATs, CREB binding protein (CBP), and steroid receptor co-activator
(SRC), as important players in the hormonal regulation of development and metamorphosis in _T. castaneum_9,10,11,23. To characterize the function of identified acetyltransferases, we
knockdown all 27 acetyltransferases and evaluated knockdown efficiency in larvae injected with their respective cognate dsRNA. The mRNA levels of most of the target genes exhibited a
reduction of over 70% in respective dsRNA treatments when compared to their mRNA levels in control larvae injected with _dsmalE_ (Fig. 1A and Supplementary Data 2). Then we recorded the
phenotypic changes resulting from the knockdown of each acetyltransferase. Among the KATs studied, knockdown of Transcription initiation factor IID (TFIID) subunit 1 (_TAF1_) caused 100%
larval mortality by 10 days after dsRNA injection (Fig. 1B). Developmental growth defects and mortality were observed in larvae and pupae developed from larvae injected with _dsESCO1_/_2_,
_dsELP3_ (Elongator complex protein 3), _dsATAT1_ (Alpha-tubulin N-acetyltransferase 1), _dsKAT14_ (Atac2, Cysteine-rich protein 2-binding protein) and _dsKAT5_ (Tip60) (Fig. 1C). Moreover,
moderate to higher mortality was observed in larvae injected with _dsKAT2A_, _dsMCM3AP_, _dsKAT6A_, and _dsKAT7_ compared to the control (Fig. 1B). Eleven genes coding for N-terminal
acetyltransferases (NAT) and four genes coding for N (alpha)-acetyltransferase (NAA) subunits were identified in _T. castaneum_, respectively (Supplementary Tables 2 and 3). Among the NAA
subunit coding genes, knockdown of _NAA16_ arrested larval growth and caused 60% larval mortality, while the knockdown of _NAA25_ and _NAA35_ also caused developmental defects and mortality
(Fig. 1B, C). Among the NAT group, the knockdown of _NAA10_ (N-alpha-acetyltransferase 10), _NAA40_ (N-alpha-acetyltransferase 40), _NAA50_ (N-alpha-acetyltransferase 50), and _NAT10_ (RNA
cytidine acetyltransferase) caused severe developmental defects and higher larval mortality (Fig. 1B, C). Similarly, the knockdown of _GNPNAT1_ (Glucosamine 6-phosphate N-acetyltransferase),
_NAA20_ (N-alpha-acetyltransferase 20), _NAA30_ (N-alpha-acetyltransferase 30), and _SATL1_ (Diamine acetyltransferase 2) caused developmental defects, as well as larval and pupal
mortality. Notably, the most severe phenotypes such as larval growth arrest at the last instar larval stage, dorsal split failure, inability to pupate, and high larval mortality, were
observed in the _NAA40_ knockdown larvae. Given these compelling observations, our further studies were focused on understanding the precise role of NAA40 in regulating larval and pupal
growth, development, and metamorphosis in _T. castaneum_. NAA40 IS ESSENTIAL FOR LARVAL GROWTH, DEVELOPMENT, AND METAMORPHOSIS To investigate the role of NAA40 (NatD) in the development and
metamorphosis of _T. castaneum_, we injected 1 µg of _dsNAA40_ or _dsmalE_ (negative control) into newly molted last instar larvae and recorded phenotype changes. The results revealed that
_NAA40_ knockdown larvae remained struck in larval stage and failed to form complete pupae even after nine days, whereas the control larvae injected with _dsmalE_ pupated within seven days.
Notably, larvae did not develop a dorsal split, but a white larval cuticle was detected upon removing the old cuticle on the 9th day after _dsNAA40_ injection (Fig. 1D: b−e). Then these
larvae transformed into larval-pupal intermediates, displaying characteristics such as dorsal splitting and the presence of pupal and adult structures (Fig. 1D: h, i). We further verified
the phenotypes resulting from _NAA40_ knockdown by injecting the last instar larvae with various concentrations: 50, 100, 250, 500 ng and 1 µg of _dsNAA40_. Notably, the higher
concentrations (500 ng and 1 µg) of _dsNAA40_ led to higher knockdown of target gene, increased mortality, and severe developmental defects, including formation of larval-pupal intermediates
with compound eyes. Lower concentrations (50, 100 and 250 ng) of _dsNAA40_ injection resulted in moderate phenotypes compared to the 1 µg _dsNAA40_ treatment (Supplementary Fig. 1). To
understand the effects of _NAA40_ knockdown at different instars, we injected 1 µg of _dsNAA40_ into penultimate larvae. These larvae showed dorsal split after six days (Fig. 1D: h, i), and
some larvae molted into the last instar stage, then larval growth arrest occurred at 13 days after dsRNA injection. Also, pupal development was arrested when _dsNAA40_ was injected into the
newly formed pupae. Contrastingly, the control group consisting of newly molted last instar larvae, penultimate larvae, or pupae injected with _dsmalE_ displayed normal growth and
development, they pupated and later emerged as normal adults (Fig. 1C_a, D_a). ECDYSONE INDUCES _NAA40_ EXPRESSION To understand the role of NAA40, we determined the expression profile of
_NAA40_ during the larval and pupal developmental stages of _T. castaneum_. The results revealed that _NAA40_ mRNA levels gradually increased from the penultimate larval stage, reaching
maximum at 24 h after pupal ecdysis. Then they decreased and reached their lowest levels by the end of the pupal stage (Fig. 2A and Supplementary Data 2). The expression pattern of _NAA40_
exhibited a similar trend to the ecdysone titer levels during the penultimate and last instar larval and pupal stages of _T. castaneum_24. Intrigued by this similar pattern, we investigated
whether _NAA40_ is induced by ecdysone. In this regard, we exposed TcA cells (developed from _T. castaneum_) to 10 µM of 20-hydroxyecdysone (20E-most active form of ecdysone, hereafter
referred to as ecdysone) for 6 h and determined the _NAA40_ expression. The results showed that _NAA40_ mRNA levels increased in ecdysone-treated cells compared to control cells exposed to
Dimethyl sulfoxide (DMSO) (Fig. 2B). However, exposure to juvenile hormone III (JH III) did not induce the expression of the _NAA40_ gene. While, the JH primary response gene, _Kr-h1_ used
as a positive control was induced by JH III in these cells, demonstrating the activity of JH III used in these experiments (Fig. 2B). Moreover, ecdysone typically induces its target genes
through its receptor known as ecdysone receptor (EcR)25. To determine whether EcR is required for the ecdysone induction of _NAA40_, we treated TcA cells with _dsEcR_ or _dsmalE_ for 72 h
before exposure to ecdysone or DMSO. As expected, _NAA40_ mRNA levels increased in TcA cells treated with _dsmalE_ and exposed to ecdysone but not in _EcR_ knockdown cells exposed to
ecdysone (Fig. 2C). To confirm the effect of ecdysone on these cells, we examined the expression of _E75_, an ecdysone-induced transcription factor, serving as a positive control, which was
induced by ecdysone (Fig. 2C). KNOCKDOWN OF _NAA40_ DECREASES THE EXPRESSION OF ECDYSONE-RESPONSE GENES Next, to investigate the role of ecdysone-induced _NAA40_ in the modulation of
ecdysone-response genes, we knocked down _NAA40_ in _T. castaneum_ larvae by injecting _dsNAA40_ or _dsmalE_. RNA isolated from treated larvae at 72 h post-injection was used to prepare
RNA-sequencing libraries and which were then sequenced. After quality control, the mapping of sequencing reads indicated that 90% of the reads were mapped to the _T. castaneum_ reference
genome. Subsequent differential expression analysis of RNA-seq data identified 731 differentially expressed genes (DEGs) between _dsNAA40_ and _dsmalE_ treated larvae, with a cutoff value ≥
2-fold difference in the expression and false discovery rate (FDR) corrected _P_ ≤ 0.05 (Supplementary Data 1). Among these DEGs, 55% (401) genes were upregulated, and 45% (330) genes were
downregulated in _NAA40_ knockdown larvae (Fig. 3A, B and Supplementary Data 1). Web-based gene enrichment (WEGO) analysis of differentially expressed genes showed significant alterations in
GO terms related to circadian rhythm, anatomical structure development, response to external or endogenous stimuli, signal transducers, and ecdysone response genes (Supplementary Fig. 2).
Notably, genes involved in lipid biosynthesis, larval cuticle development and methylation related methyltransferases, such as arginine N-methyltransferase 1 (ART1) and methyltransferase-like
protein 23 (MTP23), were upregulated in the _NAA40_ knockdown samples (Fig. 3C). Interestingly, the expression levels of many ecdysone response genes, including _E74_, _E75_ and _HR3_, were
decreased in the _NAA40_ knockdown samples indicating that NAA40 is required for ecdysone signaling. To determine if NAA40 exerts its influence on ecdysteroid biosynthesis, we assessed the
expression levels of Shadow and Phantom genes involved in ecdysteroids biosynthesis in _NAA40_ knockdown larvae. Intriguingly, the expression levels of both _Shadow_ and _Phantom_ genes did
not change in the _NAA40_ knockdown last instar larvae compared to their expression levels in control larvae injected with _dsmalE_ (Supplementary Fig. 3). This result indicates that NAA40
exerts its influence on ecdysone signaling rather than influencing ecdysteroid biosynthesis. Ecdysone regulates the transcription of ecdysone response genes through ecdysone receptor (EcR)
complex. Therefore, to elucidate whether NAA40 is involved in ecdysone signaling, we compared _NAA40_ knockdown RNA-seq data with ecdysone receptor (_EcR_) knockdown data from
_Drosophila_25. Interestingly, knockdown of _TcNAA40_ or _DmEcR_ affected some common gene ontologies, such as cuticle development, lipid transport, gluconeogenesis, neuropeptide signaling,
and steroid hormone mediated signaling, etc. (Fig. 3D and Supplementary Table 4). Therefore, these results indicate a potential connection between NAA40 and ecdysone signaling. NAA40
ACETYLATES HISTONE H4 NAA40, an N-terminal acetyltransferase acetylates histone H2A/H2B and H4 to activate target genes26,27. To identify which histone proteins are acetylated by NAA40, we
knocked down _NAA40_ in TcA cells for 72 h. The nuclear proteins isolated from these cells were used to perform western blots with antibodies specific to individual acetylated histones.
Surprisingly, the histone H2A and H2B acetylation levels did not show significant changes in the _NAA40_ knockdown cells. However, a significant decrease was observed in the acetylation
levels of histone H4 in TcA cells after _NAA40_ knockdown (Fig. 4A and Supplementary Figs. 5, 11). This decrease was detected using the histone H4 specific Ac-Histone H4 antibody, which
detects acetylation of Ser1, Lys5, Lys8, and Lys12 residues in the histone H4 tail. To further validate these findings in vivo, we reconfirmed the reduction in histone H4 acetylation levels
in _T. castaneum_ larvae after _NAA40_ knockdown (Fig. 4B). These results together demonstrate that NAA40 acetylates histone H4. Further, to identify which residue of histone H4 is
acetylated by NAA40, we performed western blotting using the histone H4-residue specific antibodies that detect acetylation of Lys5 or Lys8 or Lys12 in the histone H4 tail. Results revealed
that the acetylation levels of Lys5, Lys8, and Lys12 residues of histone H4 were not significantly changed in the _NAA40_ knockdown cells compared to their levels in the control cells
treated with _dsmalE_ (Supplementary Fig. 4 and Supplementary Data 2). No antibodies are available commercially to detect the histone H4 serine 1 acetylation levels. Previous literature
demonstrated that NAA40 acetylates the first serine residue of histone H4 tail27,28. Therefore, based on these studies we presume that NAA40 may acetylate the first serine (Ser1) residue in
the N-terminal region of histone H4 to alter target gene expression. However, further studies are needed to confirm the specificity of NAA40 in acetylating serine 1 residue of histone H4. To
understand the influence of ecdysone on histone H4 acetylation levels, TcA cells were exposed to ecdysone for 24 h, then nuclear proteins were extracted for assessing histone H4 acetylation
levels by western blotting. Results revealed that the histone H4 acetylation levels increased in cells exposed to ecdysone compared to cells exposed to DMSO (Fig. 4C). To further delve into
the role of NAA40 in ecdysone-mediated histone H4 acetylation, we knockdown _NAA40_ in TcA cells and exposed them to ecdysone. Notably, the ecdysone induced histone H4 acetylation levels in
TCA cells treated with _dsmalE_ and exposed to 20-hydroxyecdysone (20E), compared to cells treated with _dsNAA40_ and exposed to 20E (Fig. 4D). This result indicates that ecdysone mediates
the induction of histone H4 acetylation levels through _NAA40_. To investigate the potential effect of decreased histone H4 acetylation levels in _NAA40_ knockdown cells on the accessibility
of target gene promoters (identified based on RNA-seq data), we performed the Chromatin Immunoprecipitation (ChIP) assay. Chromatin was extracted from _NAA40_ knockdown TcA cells and
immunoprecipitated using the Ac-Histone H4 specific antibody. Using DNA recovered from immunoprecipitation, we analyzed the enrichment levels of target ecdysone response gene promoters, as
well as the _HSP90_ (housekeeping gene – control) promoter in _NAA40_ knockdown cells. Knockdown of _NAA40_ resulted in reduced enrichment levels of _E74_, _E75_ and _HR3_ gene promoters,
compared with the corresponding promoter enrichment levels in the control cells treated with _dsmalE_ (Fig. 4E). We did not observe any significant enrichment of the _HSP90_ promoter in the
_NAA40_ knockdown cells. These results demonstrate that NAA40 acetylates histone H4 which may be associated with the promoters of _E74_, _E75_ and _HR3_ genes. NAA40, an N-terminal
acetyltransferase, contains a conserved acetyl-CoA binding motif responsible for transferring the acetyl group from the substrate, Acetyl-CoA, to histones. To determine whether
acetyltransferase activity of NAA40 is required for the expression of _E74_, _E75_ and _HR3_ genes, we produced a _NAA40_ mutant construct by deleting QRKGLG amino acids, located from 148 to
153 region, which constitutes the Acetyl-CoA binding motif of _TcNAA40_ (Supplementary Fig. 6). Both wildtype and mutant constructs were transfected into TcA cells, and RNA isolated from
these cells was used to quantify _E74_, _E75_ and _HR3_ mRNA levels. The results demonstrated a significant increase in _E74_, _E75_ and _HR3_ mRNA levels in TcA cells transfected with
wildtype _NAA40_ construct, compared to their levels in control cells transfected with either the vector control or _NAA40_ mutant (lacking acetyl-CoA binding motif) construct (Fig. 5A).
These results demonstrate that acetyltransferase activity of NAA40 is critical for inducing the expression of _E74_, _E75_ and _HR3_ genes. NAA40 LOCALIZES INTO THE NUCLEUS Next, we
investigated whether NAA40 has the ability to translocate into the nucleus to acetylate histone proteins linked to the promoters of target genes. To test this hypothesis, we predicted a
putative signal peptide sequence (MGRKSSAKSKEKRLKRKEEQ) at the N-terminal region of _NAA40_. Then generated both wildtype _NAA40_ (having the predicted signal peptide sequence) and mutant
_NAA40_ (lacking the predicted signal peptide sequence)-_EGFP_ (enhanced green fluorescent protein) fusion constructs. These constructs were transfected into TcA cells, and accumulation of
EGFP signal in the nucleus was assessed by confocal imaging. Interestingly, we observed a substantial amount of EGFP accumulation in the nucleus of TcA cells transfected with the wildtype
_NAA40_ (having the signal peptide)-EGFP fusion construct, compared to the EGFP accumulation in TcA cells transfected with the _NAA40_-EGFP fusion construct lacking the signal peptide which
showed more accumulation in the cytoplasm (Fig. 5B and Supplementary Fig. 7). Furthermore, we evaluated whether the lack of a signal peptide could result in the degradation of the NAA40
protein. To investigate this, we extracted proteins from TcA cells at 72 h post-transfections with NAA40 constructs containing or lacking a signal peptide and performed western blotting
using the GFP (D5.1) Rabbit monoclonal antibody. Notably, there were no significant differences observed between NAA40 proteins with and without a signal peptide (Supplementary Fig. 7 and
Supplementary Data 2). These data suggest that NAA40 localizes into the nucleus to acetylate target histones and may lead to the induction of the target genes. In addition to acetylating
histones, some HATs have been known to directly interact with nuclear hormone receptor complexes or promoters, thereby stimulating the transcription of target genes12. Considering this
possibility, we determined whether NAA40 directly interacts with the promoters of ecdysone response genes. We predicted the presence of ecdysone response elements (EcREs) within the
promoters of _E75_ and _HR3_ genes. The predicted EcRE regions from these gene promoters were cloned into the pGL3 vector containing the luciferase gene. Then, we tested the ecdysone
responsiveness of these constructs by transfecting them into TcA cells and exposing the cells to ecdysone. The luciferase activity significantly increased in cells transfected with the
EcRE-luciferase constructs and exposed to ecdysone, compared to those transfected with the empty vector and exposed to DMSO, confirming that these promoter elements are ecdysone responsive
(Supplementary Fig. 8). Next, we knocked down _NAA40_ in TcA cells and transfected these cells with the EcREs constructs, then assessed the luciferase activity. Surprisingly, no significant
difference in the luciferase activity levels was detected between the _NAA40_ knockdown and control cells treated with _dsmalE_ (Supplementary Fig. 8). These results suggest that NAA40 may
not directly interact with the EcREs located in the upstream region of _E75_ and _HR3_ genes. Though, NAA40 might still induce the expression of ecdysone response genes by acetylating the
histone H4 within the nucleus. NAA40 REGULATES ECDYSONE RECEPTOR _ECR-A_ GENE EXPRESSION THROUGH A POSITIVE-FEEDBACK LOOP Interestingly, our RNA-seq data revealed a decrease in ecdysone
receptor – _EcR_ mRNA levels in _NAA40_ knockdown _T. castaneum_ larvae (Fig. 3C). Similarly, TcA cells also showed reduced expression of _EcR-A_ after _NAA40_ knockdown (Fig. 6A). These
intriguing findings led us to explore the possibility of NAA40 involvement in a positive feedback loop, regulating its own activator, _EcR_ expression. To test this hypothesis, we
overexpressed _NAA40_ in TcA cells and assessed its impact on the expression of genes coding for ecdysone receptors. The mRNA levels of _EcR-A_ were significantly enhanced in cells
overexpressing the wild-type _NAA40_ (Fig. 6B and Supplementary Data 2). However, the acetyl-CoA binding motif lacking mutant _NAA40_ overexpression did not increase _EcR-A_ expression,
indicating that acetyltransferase activity of NAA40 is required for _EcR-A_ expression (Fig. 6B). Notably, the expression levels of other ecdysone receptors: _USP-A_ and _USP-B_ and _EcR-B_,
remained unaffected by the knockdown of _NAA40_ (Fig. 6A). This result suggests that NAA40 specifically regulates _EcR-A_ expression, perhaps through acetylating histones associated with
the promoter regions of this gene. To confirm this hypothesis, we performed the ChIP assay using the histone H4 specific Ac-Histone H4 antibody to assess the enrichment of _EcR_ promoter in
the _NAA40_ knockdown samples. Notably, the _EcR_ promoter enrichment levels were significantly reduced in the _NAA40_ knockdown cells compared to those in cells treated with _dsmalE_ (Fig.
4E). These results suggest that NAA40 is involved in the regulation of its own activator, _EcR-A_ expression, possibly by increasing the histone H4 acetylation associated with its promoter
region. NAA40 MAY ACT COOPERATIVELY WITH SRC/TAIMAN TO ACTIVATE ECDYSONE RESPONSE GENES Previous studies in _D. melanogaster_ showed that in the presence of ecdysone, the steroid receptor
co-activator (SRC)/taiman interacts with many nuclear receptors, including the ecdysone receptor, to facilitate the activation of ecdysone response genes, especially _E75_ and
_HR3_29,30,31,32. In the current study, _NAA40_ knockdown led to a reduction in the expression of _E75_ and _HR3_ genes (Fig. 7A Supplementary Data 2). To understand whether SRC and NAA40
cooperatively regulate these genes expression, we first knocked down _SRC_ in TcA cells. Results show a significant decrease in _E74_, _E75_ and _HR3_ mRNA levels in the _SRC_ knockdown
cells compared to their levels in the control cells, suggesting that SRC is required for the expression of these genes in TcA cells (Fig. 7A). Next, we examined the effects of simultaneous
knockdown of both _SRC_ and _NAA40_ on the expression of the above genes. Notably, the decrease in mRNA levels of _E74_, _E75_ and _HR3_ was similar between the cells with simultaneous
knockdown of both _SRC_ and _NAA40_ and cells with knockdown of _NAA40_ or _SRC_ alone (Fig. 7A). To validate these findings, we transfected TcA cells with expression constructs containing
complete open reading frames of wild-type _TcNAA40_ and _TcSRC_ and exposed these cells to ecdysone or DMSO. Simultaneous overexpression of _SRC_ and _NAA40_ induced _E74_, _E75_ and _HR3_
genes. However, the mRNA levels were not significantly different compared to their levels in cells overexpressing _NAA40_ or _SRC_ alone (Fig. 7B). To verify the cooperation between SRC and
NAA40, we knocked down _NAA40_ and overexpressed _SRC_. In the absence of _NAA40_, overexpression of _SRC_ did not lead to an increase in the expression of ecdysone response genes,
suggesting that NAA40 is required for ecdysone/SRC/EcR-mediated induction of _E74_, _E75_ and _HR3_ genes. ARGININE METHYLTRANSFERASE 1 (ART1) METHYLATES ARGININE 3 (ARG3) IN THE N-TERMINAL
REGION OF HISTONE H4 Interestingly, our RNA-seq data revealed an increase in Arginine methyltransferase 1 (ART1) mRNA levels in _NAA40_ knockdown _T. castaneum_ larvae (Fig. 3C). Arginine
methyltransferase 1 (ART1) methylates Arginine 3 (Arg3) in the N-terminal region of histone H433. Depletion of ART1-mediated methylation of Arg3 leads to an immediate acetylation of histone
H4 and increase in the expression of target genes33. To test the potential involvement of ART1 in the regulation of primary ecdysone response genes, we knocked down _ART1_ in both _T.
castaneum_ larvae and TcA cells. We observed an increase in the expression of _EcR-A_, _E74_, _E75_, and _HR3_ in both _T. castaneum_ larvae and TcA cells treated with _dsART1_, compared to
their levels in respective controls treated with _dsmalE_ (Fig. 8A, B and Supplementary Data 2). To further explore the intricate interplay between ART1 and NAA40, we knockdown _ART1_ and
overexpressed _NAA40_ in TcA cells. Results show an increase in the expression levels of _EcR-A_, _E74_, _E75_ and _HR3_ genes (Fig. 8B). To determine whether ART1-mediated methylation has a
role in the suppression of these genes, _ART1_ was knocked down in TcA cells. Nuclear proteins were extracted from these cells and performed western blotting using the Anti-Histone H4
asymmetric di-methyl Arg3 antibody to detect histone H4-Arg3 methylation levels. The histone H4 methylation levels decreased in the _ART1_ knockdown cells compared to the control cells
treated with _dsmalE_. However, _NAA40_ knockdown did not significantly alter the histone H4 methylation levels (Fig. 8C and Supplementary Figs. 5 and 12). Conversely, overexpression of
_NAA40_ led to slight reduction in the histone H4 methylation levels. While the knockdown of _ART1_ and overexpression of _NAA40_ further reduced the histone H4 methylation levels. To
explore whether ART1 methylated histone H4 is associated with _EcR_, _E74_, _E75_ and _HR3_ promoters, we knockdown _ART1_ in TcA cells and performed ChIP assays. Chromatin from treated
cells enriched using the Anti-Histone H4 asymmetric di-methyl Arg3 antibody. The enrichment levels of _EcR_, _E74_, _E75_ and _HR3_ promoters decreased in the _ART1_ knockdown cells compared
to control cells treated with _dsmalE_ (Fig. 8D). Interestingly, overexpression of _NAA40_ resulted in reduced enrichment levels of the _EcR_, _E74_, _E75_ and _HR3_ promoters in the
chromatin enriched using the Anti-Histone H4 asymmetric di-methyl Arg3 antibody (Fig. 8D). These results indicate that ART1 methylated histone H4-Arg3 mark is possibly associated with the
promoters of _EcR_, _E74_, _E75_ and _HR3_ genes and potentially plays a role in ART1-mediated suppression of these genes by methylating histone H4. DISCUSSION Epigenetic modifiers play a
crucial role in regulating gene expression during insect growth, development, and metamorphosis34. This study focuses on NAA40, a histone acetyltransferase, and its involvement in ecdysone
signaling during metamorphosis of _T. castaneum_. Through RNAi-mediated knockdown of various HATs, including _NAA40_, we identified key HATs that are essential for larval growth,
development, and metamorphosis of _T. castaneum_. Among the HATs studied, _NAA40_ knockdown resulted in severe phenotypes such as larval growth arrest, blocked larval-pupal metamorphosis,
and death at the prepupal stage, which are similar to _EcR_ knockdown effects in insects35. Based on these observations, we hypothesized that NAA40 may modulate ecdysone regulation of
growth, development, and metamorphosis of _T. castaneum_. Additionally, _NAA40_ developmental expression levels correlate with ecdysteroid titers reported previously24. Studies in TcA cells
verified this hypothesis, ecdysone through its receptor EcR, induces _NAA40_ expression. In contrast, histone deacetylases (HDACs) are suppressed by the anti-metamorphic hormone – juvenile
hormone (JH) to prevent precocious metamorphosis of _T. castaneum_ larvae13,14,15. Notably, ecdysone does not influence the expression of _HDACs_, and JH does not affect _NAA40_ expression.
These findings highlight the distinct roles of different epigenetic modifiers in JH and ecdysone action in regulation of gene expression during metamorphosis. Interestingly, _NAA40_
knockdown resulted in decreased expression of its own activator, _EcR-A_, while _NAA40_ overexpression increased _EcR-A_ mRNA levels, suggesting a positive feedback loop. Positive feedback
loops are crucial for amplifying signaling and achieving desired phenotypes36. In insects, ecdysone plays a key role in the larval-pupal developmental transition that relies on higher and
prolonged ecdysone signaling during metamorphosis. Our studies showed that NAA40 is perhaps involved in amplifying ecdysone signaling during _T. castaneum_ metamorphosis. During
metamorphosis, increased ecdysteroid titers facilitate the dimerization of EcR/USP receptor complex, that may bind to predicted EcRE elements (GGTTTGATGATCC) in the _NAA40_ promoter, thereby
inducing its expression. However, further investigations are necessary to confirm this interaction in the upstream region of NAA40. Interestingly, NAA40 further enhances _EcR_ expression to
amplify ecdysone signaling to ensure a successful larval-pupal transition. Notably, knockdown of _NAA40_ prevented a successful larval-pupal transition in _T. castaneum_, indicating that
NAA40-mediated positive feedback regulation may play a critical role in amplifying ecdysone signaling during metamorphosis. Ecdysone triggers the activation of various transcription factors,
including E74, E75, and HR3, to facilitate the transition from larval to pupal stages, with the help of NAA40. Previous studies demonstrated that the ecdysone receptor complex recruit
specific histone acetyltransferases (HATs) such as CBP, SRC, and nucleosome remodeling factor (NURF). And these complexes interact with ecdysone response elements (EcREs) present in the
promoters of ecdysone response genes, activating their expression37,38,39. Unlike other HATs, NAA40 lacks a DNA binding domain but contains a conserved acetyl-CoA binding motif, QRKGLG,
which perhaps responsible for transferring acetyl groups from acetyl-CoA to histones for N-terminal acetylation of histone H4 (Supplementary Fig. 6). Experiments involving transfection of
_NAA40_ mutant construct (lacking the above acetyl-CoA binding motif) failed to support ecdysone induction of its response genes. Additionally, transfection of TcA cells with EcREs of _E75_
and _HR3_ gene promoter constructs and knockdown of _NAA40_, did not exhibit any effect on ecdysone-induced luciferase activity. This data suggests that NAA40 does not directly interact with
the promoters of _E75_ and _HR3_ genes. Instead, NAA40 likely regulates the expression of ecdysone response genes by modulating the acetylation levels of histone H4 localized in their
promoters. NAA40 (NatD) distinguishes itself from other NATs as it independently functions without co-activators or auxiliary subunits2,40. A previous study in _D. melanogaster_, the steroid
receptor co-activator (SRC), a HAT, was found to be essential for the expression of _E75_ and _HR3_ genes30. Our data in _T. castaneum_ revealed that NAA40 is also required for the
expression of _E75_ and _HR3_ genes. Knockdown and overexpression experiments demonstrated that SRC alone could not support the expression of these genes in the absence of NAA40, suggesting
that both SRC and NAA40 are required for the expression of ecdysone response genes. These results are similar to previous study showed how SRC and CBP synergize to regulate estrogen receptor
expression41. SRC and CBP act as co-activators, interacting with ecdysone receptor complex in the presence of ecdysone, then bind to EcREs in the promoters of ecdysone response genes, and
inducing target gene expression37. However, our studies indicated that the luciferase gene regulated by EcREs from _E75_ and _HR3_ promoters was unaffected by _NAA40_ knockdown. This
suggests that NAA40 may not directly interact with the promoters to activate their expression. Instead, NAA40 may acetylate the histone H4 mark associated with the _E75_ and _HR3_ gene
promoters. This acetylation could potentially facilitate the recruitment of ecdysone receptor complexes and co-activators such as SRC and CBP to the promoters of ecdysone-response genes,
ultimately leading to their activation. However, additional investigations are necessary to validate this hypothesis further. Another intriguing finding of this study is the contrasting
actions of ART1-mediated histone H4 methylation, which suppresses ecdysone activity. Histone modifications can yield different effects on the same target, depending on the specific modifier
involved. For instance, histone H3 serine 10 phosphorylation stimulates histone H3 lysine 14 acetylation42. Similarly, knockdown of _ART1_ leads to increased histone H4 acetylation due to
the depletion of histone H4 arginine 3 methylation in erythroid cells43. In Hela cells, ART1-mediated methylation of histone H4 arginine 3 facilitates subsequent acetylation of histone H4
tails by acetyltransferases (HATs)43. Conversely, histone H4 acetylation prevents its methylation by ART133. Our data and other studies suggest that histone H4 acetylation by NAA40 may
potentially influence histone H4 arginine 3 methylation mediated by ART1. Additionally, the developmental expression patterns of _ART1_ and _NAA40_ during the penultimate and last instar
larval and pupal stages suggest that histone H4 methylation may be replaced by acetylation upon _NAA40_ expression in response to increased ecdysteroid levels (Supplementary Fig. 9). This
shift occurs before metamorphosis initiation, leading to the activation of ecdysone-induced transcription factors. In _D. melanogaster_, ART1 acts as a repressor of the ecdysone receptor,
EcR, by methylating histone H4 associated with the _EcR_ promoter44. Similarly, our findings suggest that ART1-mediated methylation of arginine 3 on histone H4 may be localized at the _EcR_
promoter, potentially leading to the repression of _EcR_ and other ecdysone response genes. These data suggest that the regulation of histone H4 methylation and acetylation levels plays a
vital role in modulating the ecdysone response. ART1 may function as an EcR repressor, while NAA40-induced acetylation could counteract ART1 repressive activity, thereby promoting _T.
castaneum_ metamorphosis. In conclusion, our study highlights the role of NAA40 in _T. castaneum_ metamorphosis. Ecdysone triggers significant changes in gene expression through chromatin
modifications mediated by epigenetic modifiers45. The _NAA40_ gene, induced by ecdysone, likely plays a crucial role in these chromatin modifications, involved in ecdysone regulation during
metamorphosis. Based on our findings, we propose a model for the epigenetic modulation of ecdysone action during metamorphosis (Supplementary Fig. 10). Ecdysone induces the _NAA40_ gene
expression, and subsequently, NAA40 acetylates histone H4, possibly localized at the promoters of key ecdysone response genes coding for _EcR, E74, E75, and HR3_. This acetylation
facilitates an increase in their expression and promotes larval-pupal metamorphosis. On the other hand, in the absence of ecdysone, ART1 methylates histone H4 associated with the promoters
of _EcR_, _E74_, _E75_, and _HR3_ genes, repressing their expression and inhibiting metamorphosis. The dynamic changes in histone acetylation and methylation levels at the promoters of
ecdysone response genes play pivotal roles in modulating ecdysone action and regulating insect metamorphosis. Our findings shed light on the intricate epigenetic regulation involved in
ecdysone-induced metamorphosis in insects. METHODS INSECT STRAINS The North American Georgia strain GA-146 of the red flour beetle _T. castaneum_ (Herbst) was used in experiments. The
beetles were reared as described previously13. CELL CULTURE _Tribolium castaneum_ cell line, BCIRL-TcA-CLG1 (TcA) established from a co-culturing adult, and pupal tissues were obtained from
Dr. Goodman47. These cells were maintained in EX-CELL 420 medium (Sigma-Aldrich, St. Louis, MO) supplemented with 10% FBS (Fetal Bovine Serum) (VWR Seradigm Fetal Bovine Serum, Radnor, PA)
and 1 µg/ml of Penicillin-Streptomycin antibiotic mix in 5 ml sterile flasks at 28°C. HORMONE TREATMENTS Technical grade 20-Hydroxyecdysone (20E, Catalog No: H5142, Sigma-Aldrich, St. Louis,
MO) and juvenile hormone III, (JH III, Catalog No: J2000, Sigma-Aldrich, St. Louis, MO) were dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO). Pre-seeded cells were
exposed to 10 μM of 20E or JH III for 6 h. The control cells were treated with the same volume of DMSO. GENE EXPRESSION ANALYSIS Total RNA was extracted from treated and control insects or
cells using the TRI reagent-RT (Catalog No: RT 111, Molecular Research Center Inc., Cincinnati, OH). Complementary DNA (cDNA) was synthesized from 2 μg of total RNA samples using the M-MLV
reverse transcriptase (Catalog No: 28-025-013, Invitrogen, USA) in a 20 μl reaction as per the manufacturer’s instruction. The expression of the target genes was assessed using the iTaq
Universal SYBR Green Supermix by following the manufacturer’s recommendations (Catalog No: 1725120, Bio-Rad, Hercules, CA). The relative mRNA levels were calculated as described previously48
after normalizing with a reference gene, Ribosomal protein 49 (_RP49_). KNOCKDOWN OF TARGET GENES Gene fragments ranging from 300 to 500 bp were amplified using gene-specific primers
(Supplementary Table 5). These primers were designed to incorporate T7 promoter sequences at the 5’ end. cDNA was used as a template for amplifying target genes. Double-stranded RNA (dsRNA)
was synthesized using the MEGAscript T7 kit (Catalog No: A57622, Invitrogen, USA) and the PCR product was purified following the manufacturer’s instructions. Newly molted last instar larvae
were microinjected with 1 μg of cognate dsRNA. While control larvae were injected with _dsmalE_: dsRNA targets the gene coding for a maltose-binding protein of _E. coli_. RNA-SEQUENCING AND
ANALYSIS In our previous publications, we have provided a detailed description of the method followed in this study9,49. Briefly, RNA-seq libraries were prepared using 2 µg of total RNA per
replicate. These libraries were size selected, pooled and sequenced using the Illumina Hiseq 4000 sequencer at Duke University Sequencing and Genomic Technologies (NC, USA). Raw reads after
quality control – demultiplexed, trimmed, and were mapped back to the _T. castaneum_ reference genome (assembly Tcas5.2). This mapping was performed using the CLC genomic workbench pipeline
(Version 11.0.1, Qiagen Bioinformatics, Valencia, CA) with pre-optimized parameters, such as unique exon mapping, mismatch cost = 2, insertion cost = 3, deletion cost = 3, length fraction =
0.8, similarity fraction = 0.8. Differential gene expression analysis was performed using the “Empirical analysis of DGE” (EDGE) tool within the CLC genomic workbench with uniquely mapped
reads. Transcripts exhibiting a fold change of ≥ 2 and a false discovery rate (FDR) corrected _P_-value cutoff of ≤ 0.05 were considered as differentially expressed genes. The K-mer
clustering tool in the CLC genomics workbench was utilized to group transcripts. Functional annotation of the selected transcripts was performed using the Blast2Go pro plugin within the CLC
genomics workbench. Gene ontology (GO) enrichment analysis was done using the Web Gene Ontology Annotation Plot (WEGO), by plotting of the GO information of the differentially expressed
genes against the GO terms of _T. castaneum_ genome50. The GO terms of _NAA40_ knockdown RNA-seq data compared with the GO terms of the _EcR_ knockdown data from _Drosophila_25 and generated
a Venn diagram to represent the common and unique GO terms enriched between the two datasets. WESTERN BLOT ANALYSIS AND CHROMATIN IMMUNOPRECIPITATION (CHIP) ASSAY Chromatin-bound nuclear
proteins were extracted and performed Western blotting by following previously established protocols9,12,13,51. Briefly, treated cells or larval samples were lysed using a lysis buffer
containing 50 mM tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% NP-40 and 0.1% cOmplete Mini Protease Inhibitor Cocktail (Cat No: C852A34, Roche Diagnostics, USA) on ice. Following a 10 min
incubation on ice, 1/10 volume of 5 M NaCl was added to release chromatin-bound proteins. Then nuclear proteins were precipitated by adding 2 volumes of ice-cold acetone and allowed to
precipitate overnight at −20 °C, followed by centrifugation at 3000 g for 15 min at 4 °C. The pellet was washed with ice-cold acetone and dissolved in 1% Sodium dodecyl sulfate (SDS). A
previously optimized concentration of 50 μg of total protein extracted from treated and control TcA cells or larvae were used for western blotting. Histone H4 acetylation modifications were
detected using 1:1000 diluted Ac-Histone H4 (E-5) mouse monoclonal antibody (mAb) (Catalog No: sc-377520, Santa Cruz Biotechnology, Inc. USA). This antibody detects Ser1, Lys5, Lys8, and
Lys12 acetylated residues in the histone H4 tail. The acetylation of individual amino acids, Lys5, Lys8 and Lys12 in the histone H4 tail were detected using the Acetyl-Histone H4 antibody
sampler kit that includes Acetyl-Histone H4 (Lys5) mAb, Acetyl-Histone H4 (Lys8) polyclonal antibody (pAb), Acetyl-Histone H4 (Lys12) mAb and Histone H4 mAb (Catalog No: 8346, Cell Signaling
Technology, USA). Histone H4-Arg3 methylation levels were determined using the 1:1000 diluted Anti-Histone H4 asymmetric di-methyl Arg3 antibody which detects methylation of Arginine 3 of
histone H4 (Catalog No. ab194683, Abcam, USA). The GFP (D5.1) Rabbit mAb (Catalog No: 2956, Cell signaling Technology, USA) was employed to detect signal peptide containing wildtype NAA40
protein and signal peptide lacking NAA40 protein. Mouse IgG kappa binding protein (m-IgGk BP) (Catalog No: sc-516102, Santa Cruz Biotechnology, Inc. USA) conjugated to Horseradish Peroxidase
(HRP) or Anti-rabbit IgG-HRP-linked Antibody (Catalog No: 7074, Cell Signaling Technology, USA) were used as secondary antibodies. The blots were developed by incubating them in a
chemiluminescence reagent, SupersignalTM West Femto Maximum Sensitivity Substrate (Catalog No: PI34095, ThermoFisher, USA). Band densities were quantified using Image-J software. The mean
band intensity of target protein acetylation/methylation among treatments and control was normalized using the loading control protein, β-Actin. Subsequently, normalized protein
acetylation/methylation levels were then represented as relative fold change compared to the control. ChIP assays were performed following previously described protocol12. Briefly, _NAA40_
was knocked down in TcA cells for 72 h. Then cells were fixed using 1% formaldehyde to cross-link DNA and associated proteins. The fixed cells were harvested, and the cross-linked chromatin
was immunoprecipitated using the 5 μg of Ac-Histone H4 (E-5) or Anti-Histone H4 asymmetric di-methyl Arg3 antibody or IgG (negative control) antibodies by following the manufacturer
protocol. The DNA recovered from the immunoprecipitated samples was used for promoter enrichment analysis using qPCR. Each immunoprecipitated sample was normalized with its input material
and promoter enrichments were represented as a percent input. GENERATION OF PLASMID CONSTRUCTS AND TRANSFECTION INTO TCA CELLS Polyubiquitin promoter was PCR amplified (Supplementary Table
5) from _T. castaneum_ genomic DNA and cloned into the pIEx-4 vector digested with _Nhe_ I and _Nco_ I restriction enzymes. This vector is designated as the PolyUbi-pIEx-4 vector. Next, PCR
amplified the full-length coding sequence of _T. castaneum NAA40_ gene and cloned it into the PolyUbi-pIEx-4 vector using _Nco_ I and _Hind_ III restriction enzymes. Created a mutant form of
_TcNAA40_ lacking acetyl-CoA binding motif (QRKGLG amino acids – essential for NAA40 acetyltransferase activity) by performing site-directed mutagenesis (Cat No: E0554S, New England
Biolabs, USA). To study the cooperativity between NAA40 and SRC, we used the TcSRC construct developed in our previous study31. For overexpression of recombinant proteins, TcA cells were
transfected with 500 ng of respective plasmid constructs using the X-tremeGENE™HP DNA transfection reagent (Catalog No: 6366244001, Roche Diagnostics, USA). After 72 h, the total RNA was
extracted from the transfected cells and used to assess the expression levels of target genes. Using the _B. mori_ ecdysone response elements (_EcREs_) consensus sequences, we predicted the
presence of ecdysone response elements (_EcREs_) in the upstream region of _TcE75_ and _TcHR3_ genes52. The predicted _EcREs_ were cloned into the pGL3 vector, which contains the luciferase
gene, to drive luciferase gene expression. We tested the ecdysone response of these constructs by transfecting them into TcA cells and exposing the cells to either 10 μM of 20E or DMSO. In
the next experiment, we treated TcA cells with _dsNAA40 or dsmalE_ and then transfected these cells with _EcRE_ constructs. After 48 h, the cells were exposed to 10 μM of 20E or DMSO for an
additional 24 h and assessed the luciferase activity. To study the localization of exogenously expressed _NAA40_, we predicted a putative signal peptide sequence at the N-terminal region of
_NAA40_. The complete ORF of _NAA40_ and _NAA40_ lacking signal peptide sequence were PCR amplified (Supplementary Table 5) and then fused with the EGFP complete ORF sequence and cloned into
the pIEx-4 vector containing the polyubiquitin promoter using the Gibson assembly kit, following the manufacturer’s protocol (Catalog No: A46627, ThermoFisher, USA). TcA cells were
transfected with the above (_EGFP_ + _NAA40_ with signal peptide or _EGFP_ + _NAA40_ lacking signal peptide) fusion constructs. After 72 h post-transfection, the cells were washed with 1X
phosphate buffer saline (PBS), fixed, and mounted using the Everbrite mounting medium containing DAPI (Sigma-Aldrich, St. Louis, MO). Cells were imaged using a Leica TCS SP8 DLS (Digital
LightSheet) confocal microscope at 63X magnification. The fluorescence intensities of DAPI and EGFP were quantified using Image J software and normalized against DAPI, following a method
described previously53. The mean relative fluorescence units (RFU) represented as a relative fold change. STATISTICS AND REPRODUCIBILITY The mortality data significance between treatments
and control was analyzed using one-way ANOVA with the GraphPad Prism v5.0 software. For multi-group comparisons, ANOVA with Post hoc Tukey’s honestly significant difference (HSD) test was
employed. Student’s _t_ test or One-way ANOVA was used to analyze the expression data between treatments and control. Statistical significance was defined as *_p_ < 0.05, **_p_ < 0.01,
***_p_ < 0.001; ns, not significant. All the experiments were conducted with a minimum of three repetitions, each consisting of four biological replicates. DATA AVAILABILITY The RNA-seq
data reported in this study have been deposited into the National Center for Biotechnology Information’s Sequence Read Archive (NCBI-SRA) database with the following accession number:
PRJNA612693. All data supporting the findings of the paper are present in the paper/Supplementary information/Data 1. Raw data used for plots are available as Supplementary Data 2.
REFERENCES * Javaid, N. & Choi, S. Acetylation- and methylation-related epigenetic proteins in the context of their targets. _Genes_ 8, 196 (2017). Article PubMed PubMed Central
Google Scholar * Starheim, K. K., Gevaert, K. & Arnesen, T. Protein N-terminal acetyltransferases: when the start matters. _Trends Biochem. Sci._ 37, 152–161 (2012). Article CAS
PubMed Google Scholar * Marmorstein, R. & Zhou, M. M. Writers and readers of histone acetylation: structure, mechanism, and inhibition. _Cold Spring Harb. Perspect. Biol._ 6, a018762
(2014). Article PubMed PubMed Central Google Scholar * Yang, X. J. & Seto, E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention.
_Oncogene_ 26, 5310–5318 (2007). Article CAS PubMed Google Scholar * Sheikh, B. N. & Akhtar, A. The many lives of KATs - detectors, integrators and modulators of the cellular
environment. _Nat. Rev. Genet._ 20, 7–23 (2019). Article CAS PubMed Google Scholar * Ali, I., Conrad, R. J., Verdin, E. & Ott, M. Lysine acetylation goes global: from epigenetics to
metabolism and therapeutics. _Chem. Rev._ 118, 1216–1252 (2018). Article CAS PubMed PubMed Central Google Scholar * Roth, S. Y., Denu, J. M. & Allis, C. D. Histone
acetyltransferases. _Annu Rev. Biochem._ 70, 81–120 (2001). Article CAS PubMed Google Scholar * Avvakumov, N. & Côté, J. The MYST family of histone acetyltransferases and their
intimate links to cancer. _Oncogene_ 26, 5395–5407 (2007). Article CAS PubMed Google Scholar * Roy, A., George, S. & Palli, S. R. Multiple functions of CREB-binding protein during
postembryonic development: identification of target genes. _BMC Genomics_ 18, 996 (2017). Article PubMed PubMed Central Google Scholar * Roy, A. & Palli, S. R. Epigenetic
modifications acetylation and deacetylation play important roles in juvenile hormone action. _BMC Genomics_ 19, 934 (2018). Article CAS PubMed PubMed Central Google Scholar * Xu, J.,
Roy, A. & Palli, S. R. CREB-binding protein plays key roles in juvenile hormone action in the red flour beetle, _Tribolium Castaneum_. _Sci. Rep._ 8, 1426 (2018). Article PubMed PubMed
Central Google Scholar * Gaddelapati, S. C., Dhandapani, R. K. & Palli, S. R. CREB-binding protein regulates metamorphosis and compound eye development in the yellow fever mosquito,
_Aedes aegypti_. _Biochim. et. Biophys. Acta (BBA) - Gene Regulat. Mech._ 1863, 194576 (2020). Article CAS Google Scholar * George, S., Gaddelapati, S. C. & Palli, S. R. Histone
deacetylase 1 suppresses Krüppel homolog 1 gene expression and influences juvenile hormone action in _Tribolium castaneum_. _Proc. Natl Acad. Sci. USA_ 116, 17759–17764 (2019). Article CAS
PubMed PubMed Central Google Scholar * George, S. & Palli, S. R. Histone Deacetylase 11 knockdown blocks larval development and metamorphosis in the red flour beetle, _Tribolium
castaneum_. _Front Genet_ 11, 683 (2020). Article CAS PubMed PubMed Central Google Scholar * George, S. & Palli, S. R. Histone deacetylase 3 is required for development and
metamorphosis in the red flour beetle, _Tribolium castaneum_. _BMC Genomics_ 21, 420 (2020). Article CAS PubMed PubMed Central Google Scholar * Drazic, A., Myklebust, L. M., Ree, R.
& Arnesen, T. The world of protein acetylation. _Biochim Biophys. Acta_ 1864, 1372–1401 (2016). Article CAS PubMed Google Scholar * Arnesen, T. et al. Proteomics analyses reveal the
evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. _Proc. Natl Acad. Sci. USA_ 106, 8157–8162 (2009). Article CAS PubMed PubMed Central
Google Scholar * Hwang, C. S., Shemorry, A. & Varshavsky, A. N-terminal acetylation of cellular proteins creates specific degradation signals. _Science_ 327, 973–977 (2010). Article
CAS PubMed PubMed Central Google Scholar * Pavlou, D. & Kirmizis, A. Depletion of histone N-terminal-acetyltransferase Naa40 induces p53-independent apoptosis in colorectal cancer
cells via the mitochondrial pathway. _Apoptosis_ 21, 298–311 (2016). Article CAS PubMed Google Scholar * Yi, C. H. et al. Metabolic regulation of protein N-alpha-acetylation by Bcl-xL
promotes cell survival. _Cell_ 146, 607–620 (2011). Article CAS PubMed PubMed Central Google Scholar * Goetze, S. et al. Identification and functional characterization of N-terminally
acetylated proteins in _Drosophila melanogaster_. _PLoS Biol._ 7, e1000236 (2009). Article PubMed PubMed Central Google Scholar * Van Damme, P., Arnesen, T. & Gevaert, K. Protein
alpha-N-acetylation studied by N-terminomics. _FEBS J._ 278, 3822–3834 (2011). Article PubMed Google Scholar * Zhang, Z. L., Xu, J. J., Sheng, Z. T., Sui, Y. P. & Palli, S. R. Steroid
receptor co-activator is required for juvenile hormone signal transduction through a bHLH-PAS transcription factor, Methoprene tolerant. _J. Biol. Chem._ 286, 8437–8447 (2011). Article CAS
PubMed Google Scholar * Parthasarathy, R., Tan, A., Bai, H. & Palli, S. R. Transcription factor broad suppresses precocious development of adult structures during larval-pupal
metamorphosis in the red flour beetle, _Tribolium castaneum_. _Mech. Dev._ 125, 299–313 (2008). Article CAS PubMed Google Scholar * Uyehara, C. M. & McKay, D. J. Direct and
widespread role for the nuclear receptor EcR in mediating the response to ecdysone in _Drosophila_. _Proc. Natl Acad. Sci. USA_ 116, 9893–9902 (2019). Article CAS PubMed PubMed Central
Google Scholar * Song, O. K., Wang, X., Waterborg, J. H. & Sternglanz, R. An Nalpha-acetyltransferase responsible for acetylation of the N-terminal residues of histones H4 and H2A. _J.
Biol. Chem._ 278, 38109–38112 (2003). Article CAS PubMed Google Scholar * Magin, R. S., Liszczak, G. P. & Marmorstein, R. The molecular basis for histone H4- and H2A-specific
amino-terminal acetylation by NatD. _Structure_ 23, 332–341 (2015). Article CAS PubMed PubMed Central Google Scholar * Ju, J. et al. NatD promotes lung cancer progression by preventing
histone H4 serine phosphorylation to activate Slug expression. _Nat. Commun._ 8, 928 (2017). Article PubMed PubMed Central Google Scholar * Bai, J., Uehara, Y. & Montell, D. J.
Regulation of invasive cell behavior by taiman, a _Drosophila_ protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. _Cell_ 103, 1047–1058 (2000). Article CAS
PubMed Google Scholar * Yamanaka, N., Rewitz, K. F. & O’Connor, M. B. Ecdysone control of developmental transitions: lessons from _Drosophila_ research. _Annu. Rev. Entomol._ 58,
497–516 (2013). Article CAS PubMed Google Scholar * Zhang, Z., Xu, J., Sheng, Z., Sui, Y. & Palli, S. R. Steroid receptor co-activator is required for juvenile hormone signal
transduction through a bHLH-PAS transcription factor, methoprene tolerant. _J. Biol. Chem._ 286, 8437–8447 (2011). Article CAS PubMed Google Scholar * Liu, P., Fu, X. & Zhu, J.
Juvenile hormone-regulated alternative splicing of the taiman gene primes the ecdysteroid response in adult mosquitoes. _Proc. Natl Acad. Sci. USA_ 115, E7738–e7747 (2018). CAS PubMed
PubMed Central Google Scholar * Huang, S., Litt, M. & Felsenfeld, G. Methylation of histone H4 by arginine methyltransferase PRMT1 is essential in vivo for many subsequent histone
modifications. _Genes Dev._ 19, 1885–1893 (2005). Article CAS PubMed PubMed Central Google Scholar * Palli, S. R. Epigenetic regulation of post-embryonic development. _Curr. Opin.
Insect Sci._ 43, 63–69 (2020). Article PubMed PubMed Central Google Scholar * Tan, A. & Palli, S. R. Ecdysone receptor isoforms play distinct roles in controlling molting and
metamorphosis in the red flour beetle, _Tribolium castaneum_. _Mol. Cell. Endocrinol._ 291, 42–49 (2008). Article CAS PubMed PubMed Central Google Scholar * Freeman, M. Feedback control
of intercellular signalling in development. _Nature_ 408, 313–319 (2000). Article CAS PubMed Google Scholar * Kirilly, D. et al. Intrinsic epigenetic factors cooperate with the steroid
hormone ecdysone to govern dendrite pruning in _Drosophila_. _Neuron_ 72, 86–100 (2011). Article CAS PubMed Google Scholar * Badenhorst, P. et al. The _Drosophila_ nucleosome remodeling
factor NURF is required for Ecdysteroid signaling and metamorphosis. _Genes Dev._ 19, 2540–2545 (2005). Article CAS PubMed PubMed Central Google Scholar * Zhu, J., Chen, L., Sun, G.
& Raikhel, A. The Competence Factor Ftz-F1 Potentiates Ecdysone Receptor Activity via Recruiting a p160/SRC Coactivator. _Mol. Cell. Biol._ 26, 9402–9412 (2007). Article Google Scholar
* Polevoda, B., Hoskins, J. & Sherman, F. Properties of Nat4, an N(alpha)-acetyltransferase of _Saccharomyces cerevisiae_ that modifies N termini of histones H2A and H4. _Mol. Cell
Biol._ 29, 2913–2924 (2009). Article CAS PubMed PubMed Central Google Scholar * Acevedo, M. L. & Kraus, W. L. Mediator and p300/CBP-steroid receptor coactivator complexes have
distinct roles, but function synergistically, during estrogen receptor alpha-dependent transcription with chromatin templates. _Mol. Cell Biol._ 23, 335–348 (2003). Article CAS PubMed
PubMed Central Google Scholar * Cheung, P. et al. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. _Mol. Cell_ 5,
905–915 (2000). Article CAS PubMed Google Scholar * Wang, H. et al. Methylation of histone H4 at arginine 3 facilitating transcriptional activation by nuclear hormone receptor. _Science_
293, 853–857 (2001). Article CAS PubMed Google Scholar * Kimura, S. et al. _Drosophila_ arginine methyltransferase 1 (DART1) is an ecdysone receptor co-repressor. _Biochem Biophys. Res
Commun._ 371, 889–893 (2008). Article CAS PubMed Google Scholar * Truman, J. W. & Riddiford, L. M. The evolution of insect metamorphosis: a developmental and endocrine view. _Philos.
Trans. R. Soc. B: Biol. Sci._ 374, 20190070 (2019). Article CAS Google Scholar * Haliscak, J. P. & Beeman, R. W. Status of malathion resistance in five genera of beetles infesting
farm-stored corn, wheat, and oats in the United States. _J. Econ. Entomol._ 76, 717–722 (1983). Article CAS Google Scholar * Goodman, C. L. et al. A cell line derived from the red flour
beetle _Tribolium castaneum_ (Coleoptera: Tenebrionidae). _Vitr. Cell Dev. Biol. Anim._ 48, 426–433 (2012). Article Google Scholar * Sharath Chandra, G., Asokan, R., Manamohan, M., Krishna
Kumar, N. K. & Sita, T. Evaluation of reference genes for quantitative real-time PCR normalization in cotton bollworm _Helicoverpa armigera_. _Mol. Biol._ 48, 813–822 (2014). Article
CAS Google Scholar * Kalsi, M. & Palli, S. R. Cap n collar transcription factor regulates multiple genes coding for proteins involved in insecticide detoxification in the red flour
beetle, _Tribolium castaneum_. _Insect Biochem Mol. Biol._ 90, 43–52 (2017). Article CAS PubMed Google Scholar * Ye, J. et al. WEGO: a web tool for plotting GO annotations. _Nucleic
Acids Res_ 34, W293–W297 (2006). Article CAS PubMed PubMed Central Google Scholar * Weinert, B. T. et al. Proteome-Wide Mapping of the _Drosophila_ Acetylome Demonstrates a High Degree
of Conservation of Lysine Acetylation. _Sci. Signal._ 4, ra48–ra48 (2011). Article CAS PubMed Google Scholar * Kayukawa, T., Jouraku, A., Ito, Y. & Shinoda, T. Molecular mechanism
underlying juvenile hormone-mediated repression of precocious larval–adult metamorphosis. _Proc. Natl Acad. Sci. USA_ 114, 1057–1062 (2017). Article CAS PubMed PubMed Central Google
Scholar * Yoon, J. S., Gurusamy, D. & Palli, S. R. Accumulation of dsRNA in endosomes contributes to inefficient RNA interference in the fall armyworm, _Spodoptera frugiperda_. _Insect
Biochem Mol. Biol._ 90, 53–60 (2017). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank Dr. Lynn Riddiford from the University of Washington for her helpful
comments on the manuscript. Research reported in this publication was supported by the National Institute Of General Medical Sciences of the National Institutes of Health under Award Number
R01GM070559 and the US Department of Agriculture (under HATCH Project 2353057000). The content is solely the responsibility of the authors and does not necessarily represent the official
views of the National Institutes of Health or the US Department of Agriculture. AUTHOR INFORMATION Author notes * Sharath Chandra Gaddelapati Present address: Donald Danforth Plant Science
Center, St. Louis, MO, 63132, USA AUTHORS AND AFFILIATIONS * Department of Entomology, College of Agriculture, Food and Environment, University of Kentucky, Lexington, KY, 40546, USA Sharath
Chandra Gaddelapati, Smitha George, Anilkumar Moola, Karthi Sengodan & Subba Reddy Palli Authors * Sharath Chandra Gaddelapati View author publications You can also search for this
author inPubMed Google Scholar * Smitha George View author publications You can also search for this author inPubMed Google Scholar * Anilkumar Moola View author publications You can also
search for this author inPubMed Google Scholar * Karthi Sengodan View author publications You can also search for this author inPubMed Google Scholar * Subba Reddy Palli View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.R.P. and S.C.G. Conceptualization and designed the experiments; S.C.G., S.G., A.M. and K.S. performed
the experiments; S.C.G., S.G and S.R.P. data analyzed; S.C.G., S.G and S.R.P. Wrote and edited the paper. CORRESPONDING AUTHOR Correspondence to Subba Reddy Palli. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Communications Biology_ thanks Chengjun Li and the other, anonymous, reviewer(s) for
their contribution to the peer review of this work. Primary Handling Editor: Johannes Stortz. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1
SUPPLEMENTARY DATA 2 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Gaddelapati, S.C., George, S., Moola, A. _et al._ N(alpha)-acetyltransferase 40-mediated histone acetylation plays an important role in ecdysone regulation of metamorphosis
in the red flour beetle, _Tribolium castaneum_. _Commun Biol_ 7, 521 (2024). https://doi.org/10.1038/s42003-024-06212-7 Download citation * Received: 28 July 2023 * Accepted: 18 April 2024
* Published: 03 May 2024 * DOI: https://doi.org/10.1038/s42003-024-06212-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative





:max_bytes(150000):strip_icc():focal(999x0:1001x2)/amanda-3-423f7071f53944c2aa8b21ce90ecf8d2.jpg)