- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT As global warming intensifies, heat stress has become a major environmental constraint threatening crop production and quality worldwide. Here, we characterize _Heat-induced long
intergenic noncoding RNA 1_ (_HILinc1_), a cytoplasm-enriched lincRNA that plays a key role in thermotolerance regulation of pear (_Pyrus_ spp.). _HILinc1 Target 1_ (_PbHILT1_) which is the
target transcript of _HILinc1_, was stabilized via complementary base pairing to upregulate its expression. PbHILT1 could bind to Heat shock transcription factor A1b (PbHSFA1b) to enhance
its transcriptional activity, leading to the upregulation of a major downstream transcriptional regulator, _Multiprotein bridging factor 1c_ (_PbMBF1c_), during heat response. Transient
overexpressing of either _HILinc1_ or _PbHILT1_ increases thermotolerance in pear, while transient silencing of _HILinc1_ or _PbHILT1_ makes pear plants more heat sensitive. These findings
provide evidences for a new regulatory mechanism by which _HILinc1_ facilitates PbHSFA1b activity and enhances pear thermotolerance through stabilizing _PbHILT1_ transcripts. SIMILAR CONTENT
BEING VIEWED BY OTHERS COMPARATIVE ANALYSIS OF LONG NONCODING RNAS IN ANGIOSPERMS AND CHARACTERIZATION OF LONG NONCODING RNAS IN RESPONSE TO HEAT STRESS IN CHINESE CABBAGE Article Open
access 01 March 2021 IN-FRAME EDITING OF TRANSCRIPTION FACTOR GENE _RDD1_ TO SUPPRESS MIR166 RECOGNITION INFLUENCES NUTRIENT UPTAKE, PHOTOSYNTHESIS, AND GRAIN QUALITY IN RICE Article Open
access 24 June 2022 THE LONG NONCODING RNA _LAL_ CONTRIBUTES TO SALINITY TOLERANCE BY MODULATING _LHCB1S’_ EXPRESSION IN _MEDICAGO TRUNCATULA_ Article Open access 08 March 2024 INTRODUCTION
Suitable temperature is one of the vital environmental conditions for plant growth and development. According to data collected from National Aeronautics and Space Administration, the
average global temperature on Earth has increased by around 0.8 °C since 18801. According to the data on Tianqihoubao website (http://www.tianqihoubao.com), the average maximum temperature
of major pear-producing areas in China (including Hebei, Anhui, Shandong, Henan, Shanxi and Zhejiang) was 38.8 °C in 2022, which increased about 2.3 °C compared with 2011. The extremely high
temperature events are becoming an increasingly challenging abiotic stress that causes great damage to plants including deciduous fruit trees such as pear, by inducing reactive oxygen
species (ROS) accumulation, damaging membrane structures, initiating protein misfolding, etc2,3. Consequently, plants experience decreased photosynthesis, sunburn, poor pollination and
fertilization, and low fruit-setting rates, resulting in a serious decline in agronomic yield and quality1,2,4,5,6,7. In recent years, large-scale genome-wide studies indicated that
thousands of RNAs lacking protein-coding capacity can be transcribed from plant genomes. In particular, long noncoding RNAs (lncRNAs), whose length is >200 nucleotides, have been revealed
to play key roles in plants in response to abiotic stress8,9. For example, overexpression of _npc536_ (_long non-protein coding536_), a natural antisense transcript of _AT1G67930_, resulted
in longer root lengths in _Arabidopsis thaliana_ under salt stress10. Nucleus-located _DROUGHT INDUCED lncRNA_ was upregulated by drought, salt, and abscisic acid treatments, promoting
_Arabidopsis_ tolerance to drought and salt stress11. Cold-induced _MADS AFFECTING FLOWERING4 Antisense RNA_ (_MAS_) was reported to interact with WD repeat domain 5a (WDR5a), one core
component of the COMPASS-like complexes, and positively regulate _MAF4_ (_MADS AFFECTING FLOWERING4_) expression by chromatin modification12. It has also been reported that some lncRNAs,
such as _induced by phosphate starvation 1_ (_IPS1_), _cis-NAT__AMT1.1_, and _TAS3_, take part in nutrient deficiency regulation13,14,15. It is therefore reasonable to explore whether
lncRNAs play important roles in heat stress–mediated biological processes. Indeed, there is growing support for a link between lncRNAs and plant thermotolerance. For example, in
_Arabidopsis_, _asHSFB2a_ (natural antisense transcript of _HSFB2a_) was found to be induced by heat stress and negatively regulate _HSFB2a_ expression16. _NAT398b/c_ (natural antisense
transcripts of _MIR398_ genes) have been proved to repress miR398b/c biogenesis by impairing the stability of pri-miR398b/c and interfering with its cleavage, thereby attenuating plant
thermotolerance17. In poplar (_Populus simonii_), TCONS_00202587 functioned as an RNA scaffold to interfere with target gene transcription, and enhanced _Arabidopsis_ thermotolerance through
overexpression9. To date, a number of heat-response lncRNAs have been identified through high-throughput sequencing;9,16 however, the regulatory mechanism of the lncRNAs in thermotolerance
is still largely unknown, especially for long intergenic noncoding RNAs (lincRNAs). Heat shock transcription factors (HSFs) play important roles in the response and acclimation of eukaryotes
under heat stress. Based on their basic structures and evolutionary relationships, HSFs are divided into three classes, HSFA, HSFB, and HSFC, among which the function of HSFA1s play master
transcriptional regulators of the heat shock-response (HSR) genes in plants2,18. Under room temperature, HEAT SHOCK PROTEIN (HSP)70 and HSP90 bind to HSFA1s to suppress their activities in
tomato (_Solanum lycopersicum_) and _Arabidopsis thaliana_19,20. As the temperature rising, HSFA1s are released from the inert complex and specifically bind to the heat shock element (HSE)
in the promoter region of HSR genes to regulate their expression2,18,20,21,22,23,24,25,26,27. HSFA1b directly binds to the promoter of _MBF1c_ (_Multiprotein bridging factor 1c_) and
stimulates its transcription in _Arabidopsis thaliana_28. The plants are survived by a complex regulatory cascade through HSR genes at high temperature by scavenging ROS and repairing cell
damage, which underlies the acquisition of thermotolerance29,30,31. Although the majority of HSR genes are modulated by HSFA1s, several HSFs are reported to be involved in the HSR in a
HSFA1s-independent manner, such as HSFA4s, HSFA5, and HSFA82,20,21,25,32,33,34,35. Whether those HSFA1s-independent HSFs could influence the functions of the HSFA1s under heat stress is yet
to be elucidated, however. Pear is a horticultural crop widely cultivated in the world, and its yield and quality are seriously affected by high temperature. To explore heat resistance
mechanism in pear, we conducted transcriptome analysis on ‘hongbaoshi’ pear under heat stress. Among differentially expressed genes (DEGs), we identified a heat-induced lincRNA, _HILinc1_,
in pear (_Pyrus_ spp.). _HILinc1_ is directly regulated by PbHSFA4b and stabilize _HILinc1 Target 1_ (_PbHILT1_) transcripts by complementarily base pairing, leading to the enhancement of
its expression level and accumulation of PbHILT1 protein in the nucleus. PbHILT1 functions as a transcriptional assistant to strengthen PbHSFA1b transcriptional activity, resulting in the
upregulation of its downstream HSR gene targets, such as _PbMBF1c_, which has a dominant-positive influence on heat tolerance in pear. RESULTS IDENTIFICATION OF HEAT-INDUCED LINCRNA
_HILINC1_ IN PEAR To investigate the influence of high temperature to the pear, ‘Conference’ (_Pyrus communis_), ‘Akizuki’ (_Pyrus pyrifolia_), ‘Zaojinsu’ (_Pyrus_ spp.), ‘Jinshuisu’
(_Pyrus_ spp.) and ‘Hongbaoshi’ (_Pyrus_ spp.) were subjected to 38 °C, and all of the five pear cultivars were damaged by heat (Supplementary Fig. 1). After 6 h treatment at 38 °C, the
expression of several HSR genes, like _PbMBF1c_, were induced to a high level in ‘Hongbaoshi’ (Supplementary Fig. 2). To investigate how lncRNAs respond to heat stress in pear, leaves of the
crossbreed ‘Hongbaoshi’ (_Pyrus_ spp.) with the strongest heat resistance were collected after 6 h treatment at 38 °C or 25 °C and subjected to high-throughput sequencing. Based on the
pipeline (Supplementary Fig. 3a), we found 370 differentially expressed polyadenylated lncRNAs (Supplementary Data 4). Among these, 234 were upregulated (Supplementary Fig. 3b), and were
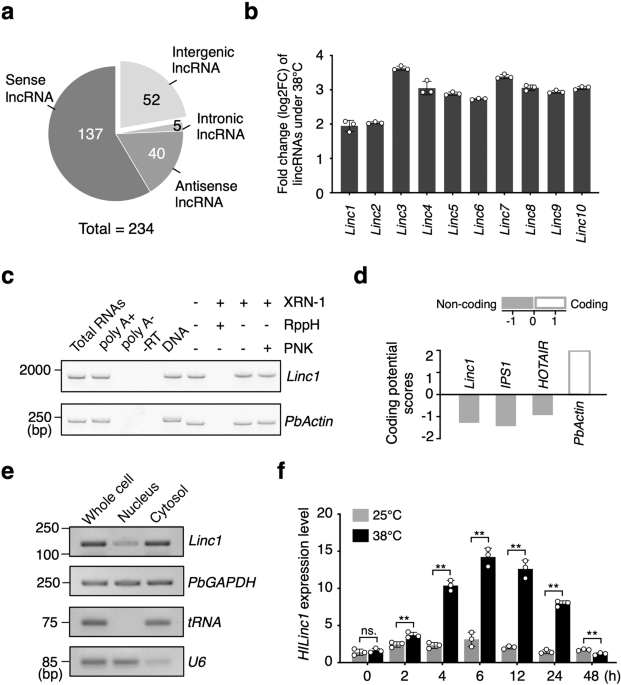
therefore considered as heat-induced lncRNAs, further classified into 137 overlapping, 52 intergenic, 40 natural antisense, and five intronic lncRNAs (Fig. 1a). Among all 52 long intergenic
noncoding RNAs, _Linc1_ was most abundant under 25 °C (Supplementary Data 4), and was substantially upregulated after the heat treatment (Fig. 1b). In addition, the upregulation of _Linc1_
under heat stress could be observed in majority of the pear cultivars (Supplementary Fig. 4a). In conclusion, _Linc1_ was a heat-inducible lncRNAs in pear. To further identify the
characteristic of _Linc1_, 5′ and 3′ rapid amplification of cDNA ends (RACE) was used in ‘Hongbaoshi’ (_Pyrus_ spp.), obtaining the full length of 1850 bp (Supplementary Fig. 4b, c). _Linc1_
is located on chromosome 5, and its transcript was modified with a poly(A)+ tail and a 5′ 7-methylguanylate cap (Fig. 1c). _Linc1_ is unlikely to encode a protein as its coding potential
score, calculated via CPC (http://cpc.cbi.pku.edu.cn/)36, was –1.28 (Fig. 1d), which was under −1, indicating no coding ability. A subcellular distribution analysis showed that _Linc1_ was
more abundant in the cytosolic fraction than the nuclear fraction (Fig. 1e). We next confirmed the temporal expression pattern of _Linc1_ under 38 °C. The results showed that _Linc1_ was
induced after heat treatment and its expression peaked after 6 h at a level about triple that of the control before decreasing (Fig. 1f). Taken together, we identified a lincRNA induced by
heat treatment in pear, named as _Heat-induced long intergenic noncoding RNA 1_ (_HILinc1_). _HILINC1_ POSITIVELY REGULATES PEAR THERMOTOLERANCE In order to investigate the function of
_HILinc1_ under heat stress, the expression was regulated using vacuum infiltration via _Agrobacterium tumefaciens_ in pear, which were then exposed to 38 °C. Conspicuous differences in heat
tolerance were observed between the control (transformed by an empty vector) and transgenic plants (Supplementary Figs. 1 and 5). Leaves of the control plants began wilting at 12 h post
heat treatment (HPHT) and started browning at 24 HPHT. By 48 HPHT, the brown area had expanded and the petioles had wilted, browned, and drooped (Fig. 2a). All _HILinc1-_overexpressing
plants exhibited strong heat tolerance and did not undergo leaf blade wilting until 48 HPHT, with no tissue browning. By contrast, the _HILinc1-_silenced plants started wilting (6 HPHT) and
browning (12 HPHT) earlier than the control. The browning rate of the _HILinc1_-silenced plants reached 75% accompanied by 25% death by 48 HPHT (Fig. 2b, c). Furthermore, the soil and plant
analyzer development (SPAD) value indicated that the chlorophyll content of _HILinc1-_overexpressing plant leaves was higher than that of the control leaves, while their electrolyte leakage
and MDA (malondialdehyde) content declined by 20% and 23%, respectively (Fig. 2d–f). _HILinc1-_silenced plants displayed the opposite changes (Fig. 2d–f). We next examined several
heat-regulated genes to elucidate the influence of _HILinc1_ in the heat-response signaling pathway. Overexpressing _HILinc1_ resulted in the upregulation of _PbMBF1c_, _PbPIP2A_
(_plasmamembrane intrinsic protein 2A_), _PbHSP15.7_, and _PbHSP16.9-I1_, while silencing _HILinc1_ suppressed the expression of those four genes (Supplementary Fig. 6). Taken together,
these results indicated that _HILinc1_ is involved in the fine-tuning of thermotolerance in pear. PBHSFA4B IS RESPONSIBLE FOR THE TRANSCRIPTION OF _HILINC1_ To explore the key transcription
factor (TF) controlling _HILinc1_ expression under heat stress, PlantTFDB (http://planttfdb.cbi.pku.edu.cn) was firstly employed to analyze the _cis_-acting elements on the promoter sequence
of _HILinc1_. A HSE was found in the promoter region from –1080 to –1057 bp upstream of _HILinc1_. In particular, there was also a predicted 342 bp open reading frame containing the HSE
domain locating from –1190 to –848 bp upstream of _HILinc1_ (Supplementary Fig. 7a). To investigate whether this ORF containing HSE was responsible for _HILinc1_ induction under heat stress,
_pHILinc1190::GUS_ and _pHILinc848::GUS_ (_GUS_ gene under the control of _HILinc1_ promoter with or without the ORF) were constructed and transformed into ‘Hongbaoshi’ leaves (Fig. 3a).
Both histochemical staining and expression analysis showed that the induction of GUS activity was much stronger in leaves expressing _pHILinc1190::GUS_ than those expressing
_pHILinc848::GUS_ after 38 °C treatment (Fig. 3b, c). Furthermore, mutation of the HSE site resulted in a significant decline in GUS activity (Fig. 3b, c), which supported the core role of
HSE on the _HILinc1_ promoter in heat response. Among the TF candidates predicted to bind to the HSE on _HILinc1_ promoter by PlantTFD, PbHSFA4b showed the highest binding score. To verify
the interaction between PbHSFA4b and the _HILinc1_ promoter region containing HSE (–1057 to –1080) in vitro, electrophoretic mobility shift assays (EMSAs) were employed. The results showed
that PbHSFA4b directly bound to the DNA probe, which was competed by the unlabeled probe (Fig. 3d). And PbHSFA4b failed to bind to the probe containing a mutated HSE site (Fig. 3a, d). Yeast
one-hybrid (Y1H) assays presented results consistent with the EMSA (Supplementary Fig. 7b). The result of chromatin immunoprecipitation (ChIP) showed that PbHSFA4b could bind to the
promoter of _HILinc1_ at 38 °C but not 25 °C in ‘Hongbaoshi’ (Fig. 3e). In addition, RT-qPCR experiments showed that _PbHSFA4b_ was upregulated after a 6 h 38 °C treatment (Supplementary
Fig. 7c). Compared with control plants, expression of _HILinc1_ was significantly induced in the leaves of _PbHSFA4b-_overexpressing plants under heat stress, while the opposite trend was
detected in _PbHSFA4b-_silenced plants (Fig. 3f and Supplementary Fig. 7d). In total, the above data revealed that PbHSFA4b positively regulates the transcription of _HILinc1_ in response to
heat stress by directly binding to the HSE on its promoter region. _HILINC1_ STABILIZES TRANSCRIPTS OF ITS TARGET GENE THROUGH COMPLEMENTARY BASE PAIRING It has been reported that lincRNAs
are able to regulate the expression of neighboring genes37. Therefore, 5000 bp both upstream and downstream of _HILinc1_ according to pear genome database were scanned for identifying
potential targets of _HILinc1_ from its neighboring genes. Two ORFs were located upstream and downstream of _HILinc1_, respectively (Fig. 4a). We found no conserved domains in the proteins
encoded by the two ORFs, according to CDD (Conserved Domain Database) (https://www.ncbi.nlm.nih.gov/cdd) and Pfam (http://pfam.xfam.org/), and tentatively named them _Pyrus bretschneideri
HILinc1 Target 1_ (_PbHILT1_) and _HILinc1 Target 2_ (_PbHILT2_) (Fig. 4a). In addition to having a similar tissue-specific expression pattern to _HILinc1_ in all five tested cultivars of
pear (Supplementary Fig. 8), _PbHILT1_ was induced in leaves overexpressing _HILinc1_ and downregulated when _HILinc1_ was silenced (Fig. 4b and Supplementary Fig. 9). By comparison, whether
at 25 °C or 38 °C, the expression level of _PbHILT2_ was barely influenced by _HILinc1_ (Supplementary Fig. 9). Furthermore, _PbHILT1_ expression was also increased in
_PbHSFA4b-_overexpressing leaves and reduced in _PbHSFA4b-_silenced leaves (Fig. 4c). These results showed that _PbHILT1_ was likely regulated by _HILinc1_ in responds to heat stress.
Unexpectedly, a fragment of _HILinc1_ (from 1348 to 1416 bp) was found to reverse-complement with _PbHILT1_ sequence from 21 to 93 bp (Fig. 4d). Northern blot analysis was conducted using
the complementary region probes of _HILinc1_ and _PbHILT1_, respectively. It was found that the two regions could hybridized into bands of different sizes, indicating that the complementary
region did not form double-stranded RNA that could induce RNA degradation (Supplementary Fig. 10). Combined with our previous findings that _HILinc1_ positively regulates _PbHILT1_, we
raised a hypothesis that _PbHILT1_ transcript might be stabilized by _HILinc1_ via RNA interaction. To verify this hypothesis, total RNA of 38 °C-treated pear leaves were digested with RNase
A/T1 mix and two sets of specific primers respectively against the complementary (set 1) and non-complementary (set 2) sequences were designed for RT-PCR detection. The results showed that
the complementary fragment (set 1) survived the degradation by RNase A/T1, whereas the non-complementary fragment (set 2) did not (Fig. 4d). Furthermore, the RNA decay rate of _PbHILT1_ was
measured in tissue-cultured pear treated with the transcriptional inhibitor actinomycin D. The decline rate of the _PbHILT1_ transcripts was slower in _HILinc1_-overexpressing plants than in
controls, while by contrast, _HILinc1_ silencing caused faster degradation of _PbHILT1_ transcripts (Fig. 4e). Deleting the reverse-complement fragment of _HILinc1_ destroyed its function
in regulating _PbHILT1_ (Fig. 4f), which reaffirmed that the regulatory mechanism was likely based on a double-stranded RNA intermediate formed between the _HILinc1_ and _PbHILT1_
transcripts. Additionally, _PbHILT1_ had no effect on _HILinc1_ (Supplementary Fig. 11). Taken together, these results suggest that _HILinc1_ forms an RNA duplex with _PbHILT1_ transcripts
through complementary base pairing, which stabilizes _PbHILT1_ transcripts. _PBHILT1_ POSITIVELY REGULATES PEAR THERMOTOLERANCE _PbHILT1_ expression increased after 38 °C treatment and
peaked at 6 HPHT, which was consistent with the expression change of _HILinc1_ (Fig. 1f and Supplementary Fig. 12a). In order to investigate the function of _PbHILT1_ under heat stress,
_PbHILT1_-overexpressing and -silenced plants were exposed to 38 °C. Obvious differences in heat tolerance were observed between the control and transgenic plants (Fig. 5 and Supplementary
Fig. 12c, d). Compared with the control plants, _PbHILT1-_overexpressing plants did not display leaf blade wilting until 48 HPHT, which indicated enhanced heat tolerance. By contrast,
_PbHILT1-_silenced plants were observed to be more heat sensitive, which wilted and browned earlier, and suffered great damage at 48 HPHT (Fig. 5A–C). The browning rate of the
_PbHILT1_-silenced plants reached 50% accompanied by 25% death by 48 HPHT (Fig. 5D). Correspondingly, leaves from _PbHILT1-_overexpressing plants showed higher SPAD values and lower
electrolyte leakage and MDA contents than control, while _PbHILT1-_silenced plants displayed the opposite changes (Fig. 5E–G). These results indicated that _PbHILT1_ participates in the
regulation of pear thermotolerance. To confirm whether _PbHILT1_ regulates downstream HSR genes, we detected the expression of _PbMBF1c_, _PbPIP2A_, _PbHSP15.7_, and _PbHSP16.9-I1_ in
_PbHILT1_-overexpressing and -silenced pears. RT-qPCR analysis showed that overexpression of _PbHILT1_ led to the upregulation of the four genes, while silencing of _PbHILT1_ caused their
suppression, which were similar with the effects of _HILinc1_ overexpression and silencing, respectively (Fig. 6A and Supplementary Figs. 13–17). Moreover, overexpression of _PbHILT1__mut_
had no influence on _PbMBF1c_, _PbPIP2A_, _PbHSP15.7_, and _PbHSP16.9-I1_ (Fig. 6A and Supplementary Fig. 13). Taken together, the results suggest that _PbHILT1_, the target gene of
_HILinc1_, can regulate the expression of _PbMBF1c_, _PbPIP2A_, _PbHSP15.7_, and _PbHSP16.9-I1_. PBHILT1 INTERACTS WITH PBHSFA1B AND ENHANCES ITS TRANSCRIPTIONAL ACTIVITY Based on our
previous findings, several HSR genes were positively regulated by _HILinc1_ and its target gene _PbHILT1_ (Fig. 6A and Supplementary Figs. 13–17). PbHILT1, despite lacking a nucleus location
signal and self-activation activity (Supplementary Fig. 12b), accumulated in the nucleus (Fig. 6B). Accordingly, we hypothesized that PbHILT1 might be carried into the nucleus by a TF to
act as a transcriptional assistant. To verify this conjecture, a semi-in vivo pulldown assay was employed for the identification of PbHILT1-associated TFs. Prokaryotic expressed PbHILT1-GST
was incubated with total proteins of heat treated ‘Hongbaoshi’ leaves. According to the mass spectrometry results, PbHSFA1b, which was previously reported to account for the transcription of
_PbMBF1c_28, attracted our attention (Supplementary Data 5). Overexpressing _PbHSFA1b_ resulted in increased expression of _PbMBF1c_, while its silencing downregulated _PbMBF1c_ (Fig. 6C).
Chromatin Immunoprecipitation (ChIP) assays presented that PbHSFA1b could directly bind to the promoter region of _PbMBF1c_ at 38 °C but not 25 °C in ‘Hongbaoshi’ (Fig. 6D). There are three
HSEs in the promoter region of _PbMBF1c_, and EMSA results showed that PbHSFA1b could directly bind to HSE1 and HSE2 (Supplementary Fig. 18a–c). Additionally, _PbHSFA1b_ showed an elevated
expression level in response to heat stress (Supplementary Fig. 18d). These results indicate that PbHSFA1b acts as the TF of _PbMBF1c_ in pear. To determine whether PbHILT1 is involved in
the regulation of _PbMBF1c_ transcription as an assistant with PbHSFA1b, we examined the interaction between PbHILT1 and PbHSFA1. Chromatin Immunoprecipitation (Co-IP), yeast two-hybrid
(Y2H), and split-luciferase assays were used to verify the interaction between PbHILT1 and PbHSFA1b, especially in nucleus by Bimolecular fluorescence complementation (BiFC) (Fig. 6E, F, and
Supplementary Fig. 19). EMSA assays showed that PbHILT1 had no effect on the binding of PbHSFA1b on _PbMBF1c_ promoter (Supplementary Fig. 20). To further inspect the influence of PbHILT1
on PbHSFA1b activity, _pPbMBF1c::LUC_ was co-expressed with _PbHILT1_ and/or _PbHSFA1b_. The strongest fluorescence intensity was observed when both _PbHILT1_ and _PbHSFA1b_ were expressed
with _pPbMBF1c::LUC_ (Fig. 6G), demonstrating that PbHILT1 could enhance the transcriptional activity of PbHSFA1b. In addition, overexpression of _PbHSFA4b_ upregulated _PbMBF1c_, while
silencing _PbHSFA4b_ resulted in decreased expression of _PbMBF1c_ (Supplementary Fig. 21a). However, _PbHSFA1b_ expression was not affected in either _PhHSFA4b_ overexpressing or silencing
line (Supplementary Fig. 21b). _HILinc1_ and _PbHILT1_ homologous genes are absent in _Arabidopsis thaliana_. In order to figure out whether _HILinc1_ and _PbHILT1_ would affect
thermotolerance of heterologous plants, we transformed _A. thaliana_ with _HILinc1_ and _PbHILT1_, and obtained five independent transformants. Compared with wild type, death rates of
_HILinc1_/_PbHILT1_ plants were significantly lower after 40 °C treatment for 4 days, followed by recovery under 21 °C for 7 days (Supplementary Fig. 22a, b), demonstrating that these
transgenic plants had acquired thermotolerance. Furthermore, expression level of _AtMBF1c_ also significantly increased in _HILinc1_/_PbHILT1_ plants (Supplementary Fig. 22c). Collectively,
our data suggest that lincRNA _HILinc1_ could promote PbHSFA1b activity and enhance _PbMBF1c_ transcription by regulating its target gene, _PbHILT1_, which is beneficial to plant
thermotolerance. DISCUSSION In this study, we demonstrated that _HILinc1_, a heat-induced lincRNA in pear, is directly regulated by PbHSFA4b and stabilizes the mRNA of its target gene,
_PbHILT1_, through complementary base pairing. PbHILT1 interacts with PbHSFA1b and enhances its transcriptional activity to upregulate _PbMBF1c_, helping to improve thermotolerance in pear
(Fig. 7). It was previously shown that HSFA1b, a member of HSF family, binds to _MBF1c_ promoter to increase its expression and activates a series of downstream HSR genes, improving plant
thermotolerance2,18,20,25,28. HSFA4b is a member of class A HSFs; and the molecular pathway underlying its role in the response to heat stress remains unclear32. Based on our data, both
_PbHSFA4b_ and _PbHSFA1b_ respond to heat stress in pear leaves (Supplementary Figs. 7c and 17d), which is consistent with the findings in other species2,32,38. Overexpression or silencing
_PbHSFA4b_ had no effect on _PbHSFA1b_ expression (Supplementary Fig. 21b), and the regulation of _PbHSFA1b_ expression also did not influence _PbHSFA4b_ (Supplementary Fig. 21c), implying
that there is no transcriptional regulation existed between _PbHSFA1b_ and _PbHSFA4b_. Nonetheless, _PbHSFA4b_ could regulate the expression of _PbMBF1c_ (Fig. 6A), a direct target of
_PbHSFA1b_, for which there are two possible explanations. One is that PbHSFA4b binds to _PbMBF1c_ promoter and directly regulates its transcription. The second is that PbHSFA4b takes
control of _PbMBF1c_ indirectly through its TFs, such as PbHSFA1b. Further studies found that the fluorescence signal was barely observed when _pPbMBF1c::LUC_ was co-expressed with
_PbHSFA4b_ (Supplementary Fig. 21d). It is, therefore, reasonable to speculate that _PbHSFA4b_ modulates _PbMBF1c_ via another pathway, comprising more regulatory factors, rather than
directly activating the transcription of _PbMBF1c_. In the current study, _HILinc1_, a heat-induced lincRNA in pear, was identified through high-throughput sequencing. The expression of
_HILinc1_ was directly regulated by PbHSFA4b. _PbHILT1_ is located upstream of _HILinc1_, and is the target gene of this lincRNA. Notably, overexpressing or silencing _HILinc1_ or _PbHILT1_
led to an expression change in _PbMBF1c_. Given the findings above, we conjectured that _PbMBF1c_ was regulated by _PbHSFA4b_ via the _HILinc1–PbHILT1_ regulatory module. Although PbHILT1
was shown to accumulate in the nucleus under heat stress, it showed no transcriptional auto-activation capability, which indicated that it was unable to activate the transcription of
_PbMBF1c_ independently. Further investigation demonstrated that PbHILT1 was able to interact with PbHSFA1b and enhance its transcriptional activity, resulting in the increased expression of
_PbMBF1c_ (Fig. 7). PbHILT1, first characterized in this study, thus functions to activate PbHSFA1b activity, which is different from the HSF-binding protein (AtHSBP), a negative regulator
of HSFA1b previously reported in Arabidopsis39. Overexpression of _PbHILT1_ improved the thermotolerance of ‘Hongbaoshi’, while _PbHILT1_-silenced plants showed more serious injury under
heat stress. This illustrated that _PbHILT1_ plays a dominant role in positively regulating pear thermostolerance. We have also performed analysis in other species, such as apple, tobacco
and _Arabidopsis_ etc., and found that only apple has homologous _HILinc1_, while lacking of homologous of target gene PbHILT1. Thus, the _HILinc1_-PbHILT1 regulatory pathway is unique in
pears. These findings reveal a new heat-response signaling pathway between PbHSFA4b and PbHSFA1b. PbHSFA4b–HILinc1_–_PbHILT1_–_PbHSFA1b is likely to be a crucial regulatory module regulating
PbHSFA1b and heat tolerance special in pear. Based on genome location and context, lncRNAs can be classified as overlapping lncRNAs, natural antisense transcripts, lincRNAs, and intronic
noncoding RNAs12,40. _HILinc1_ belongs to lincRNA. Natural antisense transcripts are the most widely studied lncRNAs in plants, which usually function through regulating their corresponding
sense transcripts;12,17,41,42,43 however, studies on the regulatory mechanisms of lincRNAs are limited due to the uncertainty of their target genes. In general, there are three approaches to
predict the target genes of lincRNAs. First, lincRNAs are likely to regulate neighboring genes37, so these can be explored as potential targets. The second way is to identify a specific
association with the sequences of protein-coding genes, such as the existence of complementary base fragments44. The third is to examine correlations in expression patterns between lincRNAs
and protein-coding genes45,46. To reveal the potential target gene of _HILinc1_, we analyzed its neighboring genes and found two ORFs, one located upstream and one downstream of _HILinc1_
(Fig. 4). _PbHILT1_ exhibited similar tissue expression specificity to _HILinc1_ and was positively regulated by the lincRNA, which was confirmed in several pear cultivars. _HILinc1_
contained a fragment that reverse-complemented partial sequences of _PbHILT1_, which was shown to be responsible for forming RNA duplexes with _PbHILT1_ transcripts to stabilize them. This
type of regulatory mechanism has never been identified in plants; however, similar examples have been reported in animals and microbes, such as _BACE1-AS_ and _PTENpg1 asRNA β_ in human
cells47,48, and _NfiS_ in _Pseudomonas stutzeri_49,50. The accumulation of PbHILT1 proteins was observed in _HILinc1_-overexpressing plants, which might be explained by two possibilities. On
one hand, _HILinc1_ increased _PbHILT1_ expression by stabilizing its mRNA, raising the efficiency of ribosome binding and translation. On the other hand, _HILinc1_ might not only regulate
the transcript level of _PbHILT1_, but also affect the translation efficiency of _PbHILT1_ mRNA, similar to the function of _NfiS_;50 however, this hypothesis requires further exploration.
Furthermore, research in mammals showed that nuclear-localized lncRNAs can interact with DNA, RNAs, and proteins to modulate nucleosome incorporation, chromatin structure, and gene
transcription, while cytoplasmic lncRNAs are more likely to function in posttranscriptional gene regulation, such as mRNA degradation and translation, or signaling transduction51. _HILinc1_
was found mainly in the cytoplasm (Fig. 1e), and was shown to participate in heat-responsive signaling pathway by stabilizing the transcripts of its target gene, _PbHILT1_ (Fig. 7), which is
consistent with the findings in mammals. In addition, there may be other proteins involved in the formation or unwinding of the RNA duplex between _HILinc1_ and _PbHILT1_ transcripts, as
their bond appears much stronger than general hydrogen bonding. Further investigations are needed to explore the binding and unwinding mechanisms of this special RNA duplex. Like
protein-coding genes, the transcription of lncRNAs is under the control of their promoters. _PbHILT1_ was found to be located upstream of _HILinc1_, overlapping with the crucial fragment in
the _HILinc1_ promoter required for heat responsiveness. PbHSFA4b bound to the HSE contained in the key fragment and enhanced the transcription of _HILinc1_ in response to heat stress (Fig.
3). It is very rarely reported in plants that a DNA fragment can be transcribed as a protein-coding gene and simultaneously act as a promoter to control the transcription of downstream
genes. A previous study revealed that the expression of the lincRNA _ELENA1_ was induced by both elf18 and flg22 in Arabidopsis, with the region containing the CBL6-coding locus in the
_ELENA1_ promoter being responsible for elf18 and flg22 responsiveness52, which bore a resemblance to our study. Pear belongs to perennial woody fruit tree, and its genetic transformation
has been reported only a few times in the ‘Conforence’ variety with low transformation efficiency53. In this study, _Agrobacterium tumetobacter_ vacuum infiltration method was used to
overexpress or silence related genes for functional research, and it was found that the transformation efficiency of this instantaneous transformation method could reach about 80%, which
was54. However, we also admit that used transformation system was not stable and lasted for a short time. Therefore, in this study, the stable transformation system of _Arabidopsis_ was used
for further verification, and the same conclusion was obtained as that in pears. In summary, we identified a heat-responsive lincRNA, _HILinc1_, which was directly regulated by _PbHSFA4b_
and could promote PbHSFA1b activity through its target gene, _PbHILT1_; however, there are still some mysteries to be investigated. First, it cannot be excluded that other TFs might also
take control of _HILinc1_ expression under heat stress. Second, there is a high probability that several regulators may be involved in the formation and unwinding of the RNA duplex between
_HILinc1_ and _PbHILT1_ transcripts. Third, it is unknown whether PbHILT1 can activate TFs other than PbHSFA1b. All these unknown aspects are worth further exploration. METHODS PLANT
MATERIALS AND GROWTH CONDITIONS All pears used in this study, including crossbreeds ‘Hongbaoshi’ (_Pyrus_ spp.), ‘Zaojinsu’ (_Pyrus_ spp.) and ‘Jinshuisu’ (_Pyrus_ spp.), ‘Akizuki’ (_Pyrus
pyrifolia_), and ‘Conference’ (_Pyrus communis_), were tissue-cultured on Murashige and Skoog (MS) medium containing 6-benzylaminopurine (0.8 mg/L) and 1-naphthylacetic acid (0.1 mg/L) at 24
± 1 °C under long-day conditions (16 h light/8 h dark). The plantlets were transferred to fresh medium every 40 d. HEAT TREATMENT AND THERMOTOLERANCE ASSAY Pear plantlets subcultured for 40
d were transferred to 38 °C (heat treatment) or 25 °C (controls). After being treated for different time periods (0, 2, 4, 6, 12, 24, or 48 h), the plant was observed and the leaves were
harvested for RNA isolation. Physiological indexes were measured at 24 HPHT. The relative chlorophyll contents of leaves were examined using a SPAD 502 device (Konica Minolta, Osaka, Japan).
Electrolyte leakage and MDA contents of the leaves were measured as reported previously54,55. After growing at 21 °C under long-day conditions (16 h light/8 h dark) for 4 weeks, wild type
(Columbia) and transgenic _Arabidopsis thaliana_ were treated at 38 °C (heat treatment) or 21 °C (controls) for 4 d, followed by 7-d-recovery at 21 °C, and the death rates were calculated.
SEQUENCING AND ANALYSIS FOR THE IDENTIFICATION OF LNCRNAS An EASYspin RNA Rapid Plant Kit (Biomed Gene Technology, Beijing, China) was used to isolate total RNAs from the leaves of
‘Hongbaoshi’ at 6 HPHT, and were then treated with DNase I (Biomed Gene Technology, Beijing, China). Samples grown at 25°C served as controls. High-purity and high-integrity RNA samples were
sent to Gooal Gene Corporation (Wuhan, China) for the RNA library construction and sequencing on an Illumina HiSeq 2500 sequencing platform (Illumina Inc., San Diego, CA, USA). Three
biological repeats were performed. The low-quality bases and adapter sequences were discarded from the raw sequencing reads, and the remaining clean reads were mapped to the pear (_Pyrus
bretschneideri_) reference genome (http://peargenome.njau.edu.cn/default.asp?d=4&m=2) using STAR version 2.5.3 with default parameters. The pipeline in Supplementary Fig. 3a was employed
to identify heat-responsive lncRNA candidates in pear, based on a previous report12. The transcripts with a low abundance (FPKM ≤ 10), short length (length < 200 nt), or those that
overlap with known mRNAs were removed. Moreover, the remaining transcripts were subjected to a coding potential calculation using the Coding Potential Assessment Tool (CPAT, version 1.2.2,
http://lilab.research.bcm.edu/cpat)56 and the coding–noncoding index (CNCI)57. Only transcripts with both negative CPAT and CNCI scores were annotated as lncRNAs and used for a further
expression analysis. 5′ AND 3′ RACE RNA samples were isolated from pear leaves after 6 h treatment at 38 °C. The 5′ RACE was performed with a 5′-Full RACE Kit (Takara Bio, Shiga, Japan) and
the 3′ RACE was carried out with a 3′-Full RACE Core Set using PrimeScriptTM RTase (Takara Bio). The 5′ and 3′ PCR products were amplified using gene-specific primers (listed in
Supplementary Data 1) and cloned into the pMD18-T vector for sequencing. RNA ISOLATION AND DIGESTION Poly(A)+ and Poly(A)− RNAs were isolated from the total RNAs of heat-treated pear leaves
using a polyA SpinTM mRNA Isolation Kit (New England Biolabs, Ipswich, MA, USA). T4 polynucleotide kinase (New England Biolabs), RNA 5′ pyrophosphohydrolase (New England Biolabs), and 5′–3′
exoribonuclease (New England Biolabs) were used for the RNA digestion, according to a previous study58. After digestion, the RNAs were purified using the modified cetyltrimenthyl ammonium
bromide (CTAB) method59 and subjected to RT-PCR. Primer sequences are provided in Supplementary Data 1. NUCLEAR AND CYTOSOLIC FRACTIONATION The fractionation of nuclear and cytosolic
components was performed as previously reported12. Leaves of pear plantlets were ground to a fine powder after a 6 h heat treatment and mixed with 2 volumes of lysis buffer (250 mM sucrose,
20 mM Tris–HCl [pH 7.4], 20 mM KCl, 2.5 mM MgCl2, 2 mM EDTA, 5 mM DTT, 25% glycerol, and 40 U/mL RNase inhibitor). A double layer of Miracloth (Merck, Darmstadt, Germany) was used to filter
the homogenate. After centrifugation at 13,000 _g_ for 10 min at 4 °C, the supernatant was collected as the cytoplasmic fraction. The pellet was washed with nuclear resuspension buffer (20
mM Tris–HCl [pH 7.4], 2.5 mM MgCl2, 5 mM DTT, 25% glycerol, 2% Triton X-100, and 160 U/mL RNase inhibitor) and resuspended in 500 μL Extraction Buffer II (250 mM sucrose, 10 mM MgCl2, 10 mM
Tris–HCl [pH 8.0], 5 mM β-mercaptoethanol, 1% Triton X-100, 350 U/mL RNase inhibitor, and 1× protease inhibitor) after centrifugation at 1500 _g_ for 2 min at 4 °C. The suspension was then
overlaid on top of 500 μL Extraction Buffer III (1.7 M sucrose, 2 mM MgCl2, 10 mM Tris–HCl [pH 8.0], 5 mM β-mercaptoethanol, 0.15% Triton X-100, 350 U/mL RNase inhibitor, and 1× protease
inhibitor) and centrifuged at 13,000 _g_ for 20 min at 4 °C. The pure nuclear pellet was resuspended in lysis buffer. RNAs in the cytosolic and nuclear fractions were obtained using the
modified CTAB method59 and subjected to RT-PCR analyses. U6 and tRNA were used as nuclear and cytosolic RNA markers, respectively. The primers used in RT-PCR were shown in Supplementary Data
1. The isolation of nuclear and cytosolic proteins was performed using the Plant Nuclear/Cytosolic Protein Extraction Kit (Bestbio, Shanghai, China), according to the manufacturer’s
protocol. TRANSIENT TRANSFORMATION ASSAY To evaluate the influences of _HILinc1_ and _PbHILT1_ in pear thermotolerance, they were cloned into pFGC5941. For their overexpression, the intron
region of pFGC5941 was replaced by the full-length sequence of _HILinc1_ or _PbHILT1_. For their silencing, the specific fragments of _HILinc1_ or _PbHILT1_ were cloned into the two flanks
of the intron in pFGC5941 in sense and antisense orientations. The empty vector of pFGC5941 was used as the control. _Agrobacterium tumefaciens_ cells were transformed with the different
constructs. After being cultivated overnight in selection medium, the cells were resuspended in injection buffer (10 mM MgCl2, 10 mM MES-KOH [pH 5.2], 100 μM acetosyringone). The 40-day-old
tissue-cultured pear was completely immersed in the infection solution for infiltrating under a vacuum of 65 kPa for 20 min. The transformed plantlets were cultivated under 25 °C for 3 d
then exposed to 38 °C for different time periods, with plants continuously grown at 25 °C used as controls. RNASE PROTECTION ASSAY Pear leaves were collected for RNA extraction at 6 HPHT.
The RNA was treated with RNase A/T1 mix (Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C for 30 min, followed by digestion with proteinase K. The RNA was purified using the modified
CTAB method59, and cDNA was synthesized for RT-PCR. The primer sequences are provided in Supplementary Data 1. RNA DECAY ASSAY After a 6 h treatment at 38 °C, the plantlets were
vacuum-infiltrated for 20 min at 65 kPa in a solution containing 20 μg/mL actinomycin D (Merck). Leaves were harvested before (0 h) and 4, 8, and 16 h after the treatment, and were used for
RNA extraction and RT-qPCR assays. The primer sequences are provided in Supplementary Data 1. TOTAL RNA EXTRACTION AND NORTHERN BLOT ANALYSIS Total RNA was isolated from ‘Hongbaoshi’ leaves
using a modified cetyltrimeth- ylammonium bromide (CTAB) method60 and treated with DNase I (Invitrogen) to remove DNA contamination. RNA integrity was verified by electrophoresis on a 1.2%
agar gel, and the concentration was measured using an ND-1000 NanoDrop spectrophotometer (Thermo Fisher Scientific). RNA gel blot analysis was performed using a Digoxin Hybridization
Detection Kit following the manufacturer’s instructions (Mylab; DIGD-120). Approximately 60 μg of RNA was separated in a 15% polyacrylamide gel and electrically transferred to Hybond-N +
membranes (GE Healthcare). _HILinc1_ and _PbHILT1_ probes, including antisense and sense probes, were synthetized with a DIG RNA Labeling Kit (SP6/T7) (Roche) using the primers in
Supplementary Data 1. ELECTROPHORETIC MOBILITY SHIFT ASSAY _PbHSFA4b_ was cloned into pET-30a to produce the His-PbHSFA4b fusion protein, while _PbHSFA1b_ was cloned into pGEX-4T for
GST-PbHSFA1b purification. Complementary pairs of 5′ biotin-labeled and unlabeled oligonucleotides (sequences shown in Supplementary Data 1) were annealed in 10× buffer solution (100 mM
Tris–HCl [pH 7.5], 10 mM EDTA, and 1 M NaCl) at 75 °C for 30 min and used as probes. The EMSAs were performed using a LightShiftTM chemiluminescent EMSA Kit (Thermo Fisher Scientific). The
reaction mixture was mixed with loading buffer and subjected to gel electrophoresis on a 6% polyacrylamide gel at 100 V for 1 h, then transferred to a Hybond-N+ membrane (GE Healthcare,
Chicago, IL, USA). After being UV cross-linked, the signal on the membrane was detected according to the manufacturer’s protocol. YEAST ONE- AND TWO-HYBRID ASSAY For the Y1H assays, DNA
fragments from the _HILinc1_ promoter containing the HSE were amplified and cloned into the pHIS2 vector, serving as the bait construct. For the prey construct, the coding region of
_PbHSFA4b_ was introduced into the pGADT7 vector. The constructs were transformed into yeast strain Y187 using the LiAc/SSDNA/PEG method61. The transformants were grown on synthetic defined
(SD)/–Trp–Leu medium and then spotted onto SD/–Trp–Leu–His plates supplemented with 30 mM 3-amino-1,2,4-triazole (3-AT) for high-stringency screening. For the Y2H assays, _PbHSFA1b_ and
_PbHILT1_ were cloned into pGADT7 and pGBKT7, respectively, and transformed into yeast strain AH109. The transformation method and screening strategy were the same as those used in the Y1H
assays. GUS STAINING Fragments of different lengths from the _HILinc1_ promoter (−1 to −848 or −1190 bp) were cloned into pCAMBIA1305.1 to drive the expression of the β-glucuronidase (GUS)
reporter. The _pHILinc848::GUS_ and _pHILinc1190::GUS_ constructs were transformed into the leaves of 40-day-old ‘Hongbaoshi’ plantlets via _Agrobacterium_. Three days after infiltration,
the plants were exposed to 38 °C for 6 h. Leaves were collected before and after the treatment for histochemical GUS staining and an expression analysis by RT-qPCR. The GUS staining was
performed as previously described62. Briefly, the leaves were incubated with X-gluc solution followed by decoloration using 75% ethanol. The primers used in the RT-qPCR are listed in
Supplementary Data 1. SPLIT-LUCIFERASE ASSAY The _PbMBF1c_ promoter (1.5 kb upstream of the translation start site) was cloned into pGreenII 0080-LUC to drive the expression of the firefly
luciferase reporter. _PbHSFA1b_ and _PbHSFA4b_ were under the control of the _35_ _S_ promoter in pFGC5941. For the interaction analysis between PbHILT1 and PbHSFA1b, pCAMBIA1300-nLUC and
pCAMBIA1300-cLUC were employed. The constructs were transformed into _Agrobacterium_ and transiently expressed in _Nicotiana benthamiana_ leaves by co-infiltration. Two days later,
split-luciferase assays were carried out as previously described63. The fluorescence signal was detected on a Tanon 5200 Multi system (Tanon Science and Technology, Shanghai, China).
BIMOLECULAR FLUORESCENCE COMPLEMENTATION A BiFC was carried out using pCAMBIA1300-YFPn and pCAMBIA1300-YFPc to confirm the interaction between PbHILT1 and PbHSFA1b. Yellow fluorescent
protein signals in transformed tobacco leaves were observed using confocal laser microscopy on the Leica TCS SP8 device (Leica Microsystems, Wetzlar, Germany) 2 d after infiltration. SEMI-IN
VIVO PULLDOWN ASSAY _PbHILT1_ was cloned into pGEX4T-1, and PbHILT1-GST was purified in a prokaryotic system. Before the elution, the recombinant protein was incubated with total protein
extracted from ‘Hongbaoshi’ leaves at 6 HPHT using a Plant Protein Extraction Kit (Huaxingbio, Beijing, China). The final eluent was collected and sent to the QLBio Corporation (Beijing,
China) for mass spectrometry. STATISTICS AND REPRODUCIBILITY Statistical analyses of data other than transcriptome data were performed with GraphPad Prism 9 software. The number of samples
per independent experiment (N) and the specific statistical hypothesis testing method (_t_-test) are described in the legends of the corresponding figures. _P_ < 0.05 was considered
statistically significant for these comparisons. Data are expressed as mean ± standard deviation (s.d.) values. REPORTING SUMMARY Further information on research design is available in the
Nature Research Reporting Summary linked to this article. DATA AVAILABILITY The RNA-seq data generated in this study are available in the NCBI SRA under accession PRJNA702636. The ID of
newly generated plasmids in Addgene were available in Supplementary Data 1. Source data are provided in Supplementary Data 2, 3 and uncropped blots are shown at the end of Supplementary data
information. Any other data associated with the findings of this study are available from the corresponding author upon request. REFERENCES * Janni, M. et al. Molecular and genetic bases of
heat stress responses in crop plants and breeding for increased resilience and productivity. _J. Exp. Bot._ 71, 3780–3802 (2020). Article CAS PubMed PubMed Central Google Scholar * Li,
B. et al. Molecular mechanisms governing plant responses to high temperatures. _J. Integr. Plant Biol._ 60, 757–779 (2018). Article PubMed Google Scholar * Liu, G. T. et al. Differential
proteomic analysis of grapevine leaves by iTRAQ reveals responses to heat stress and subsequent recovery. _BMC Plant Biol._ 14, 110 (2014). Article PubMed PubMed Central Google Scholar
* Xie, W. et al. Decreases in global beer supply due to extreme drought and heat. _Nat. Plants_ 4, 964–973 (2018). Article PubMed Google Scholar * Anderson, W. B. et al. Synchronous crop
failures and climate-forced production variability. _Sci. Adv._ 5, eaaw1976 (2019). Article CAS PubMed PubMed Central Google Scholar * Chen, C. et al. Global warming and shifts in
cropping systems together reduce China’s rice production. _Global Food Security_ 24, 100359 (2020). * Sage, T. L. et al. The effect of high temperature stress on male and female reproduction
in plants. _Field Crops Res._ 182, 30–42 (2015). Article Google Scholar * Zhao, J. et al. Regulation of Non-coding RNAs in Heat Stress Responses of Plants. _Front Plant Sci._ 7, 1213
(2016). Article PubMed PubMed Central Google Scholar * Song, Y. et al. High-Temperature-Responsive Poplar lncRNAs Modulate Target Gene Expression via RNA Interference and Act as RNA
Scaffolds to Enhance Heat Tolerance. _Int. J. Mol. Sci_. 21, 6808 (2020). * Ben Amor, B. et al. Novel long non-protein coding RNAs involved in Arabidopsis differentiation and stress
responses. _Genome Res._ 19, 57–69 (2009). Article PubMed PubMed Central Google Scholar * Qin, T. et al. A Nucleus-Localized Long Non-Coding RNA Enhances Drought and Salt Stress
Tolerance. _Plant Physiol._ 175, 1321–1336 (2017). Article CAS PubMed PubMed Central Google Scholar * Zhao, X. et al. Global identification of Arabidopsis lncRNAs reveals the regulation
of MAF4 by a natural antisense RNA. _Nat. Commun._ 9, 5056 (2018). Article PubMed PubMed Central Google Scholar * Franco-Zorrilla, J. M. et al. Target mimicry provides a new mechanism
for regulation of microRNA activity. _Nat. Genet._ 39, 1033–1037 (2007). Article CAS PubMed Google Scholar * Shin, S. Y. et al. Transcriptomic analyses of rice (Oryza sativa) genes and
non-coding RNAs under nitrogen starvation using multiple omics technologies. _BMC Genom._ 19, 532 (2018). Article Google Scholar * Fukuda, M. et al. Genome-Wide Analysis of Long Intergenic
Noncoding RNAs Responding to Low-Nutrient Conditions in Arabidopsis thaliana: Possible Involvement of Trans-Acting siRNA3 in Response to Low Nitrogen. _Plant Cell Physiol._ 60, 1961–1973
(2019). Article CAS PubMed Google Scholar * Wunderlich, M. et al. Heat shock factor HSFB2a involved in gametophyte development of Arabidopsis thaliana and its expression is controlled by
a heat-inducible long non-coding antisense RNA. _Plant Mol. Biol._ 85, 541–550 (2014). Article CAS PubMed PubMed Central Google Scholar * Li, Y. et al. Natural antisense transcripts of
MIR398 genes suppress microR398 processing and attenuate plant thermotolerance. _Nat. Commun._ 11, 5351 (2020). Article CAS PubMed PubMed Central Google Scholar * Scharf, K. D. et al.
The plant heat stress transcription factor (Hsf) family: structure, function and evolution. _Biochim. Biophys. Acta_ 1819, 104–119 (2012). Article CAS PubMed Google Scholar * Hahn, A. et
al. Crosstalk between Hsp90 and Hsp70 Chaperones and Heat Stress Transcription Factors in Tomato. _Plant Cell_ 23, 741–55 (2011). * Ohama, N. et al. Transcriptional Regulatory Network of
Plant Heat Stress Response. _Trends Plant Sci._ 22, 53–65 (2017). Article CAS PubMed Google Scholar * Liu, H. C. et al. The role of class A1 heat shock factors (HSFA1s) in response to
heat and other stresses in Arabidopsis. _Plant Cell Environ._ 34, 738–751 (2011). Article CAS PubMed Google Scholar * Yoshida, T. et al. Arabidopsis HsfA1 transcription factors function
as the main positive regulators in heat shock-responsive gene expression. _Mol. Genet Genom._ 286, 321–332 (2011). Article CAS Google Scholar * Baniwal, S. K. et al. Heat stress response
in plants: a complex game with chaperones and more than twenty heat stress transcription factors. _J. Biosci._ 29, 471–487 (2004). Article CAS PubMed Google Scholar * Suzuki, N. et al.
Identification of the MBF1 heat-response regulon of Arabidopsis thaliana. _Plant J._ 66, 844–851 (2011). Article CAS PubMed PubMed Central Google Scholar * Ding, Y. et al. Molecular
Regulation of Plant Responses to Environmental Temperatures. _Mol. Plant_ 13, 544–564 (2020). Article CAS PubMed Google Scholar * Jingyu, Z. et al. Crop Improvement Through Temperature
Resilience. _Ann. Rev. Plant Biol._ 70, 753–780 (2019). * Liu, H. T. et al. The calmodulin-binding protein kinase 3 is part of heat-shock signal transduction in Arabidopsis thaliana. _Plant
J._ 55, 760 (2010). Article Google Scholar * Ulrike, B. et al. Arabidopsis HEAT SHOCK TRANSCRIPTION FACTORA1b overexpression enhances water productivity, resistance to drought, and
infection. _J. Exp. Bot._ 64, 3467–3481 (2013). Article Google Scholar * Suzuki, N. et al. The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis
thaliana. _J. Biol. Chem._ 283, 9269–9275 (2008). Article CAS PubMed Google Scholar * Suzuki, N. et al. Enhanced tolerance to environmental stress in transgenic plants expressing the
transcriptional coactivator multiprotein bridging factor 1c. _Plant Physiol._ 139, 1313–1322 (2005). Article CAS PubMed PubMed Central Google Scholar * Qin, D. et al. Overexpression of
heat stress-responsive TaMBF1c, a wheat (Triticum aestivum L.) Multiprotein Bridging Factor, confers heat tolerance in both yeast and rice. _Plant Mol. Biol._ 87, 31–45 (2015). Article CAS
PubMed Google Scholar * Busch, W. et al. Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana. _Plant J._ 41, 1–14 (2005). Article
CAS PubMed Google Scholar * Baniwal, S. K. et al. Role of heat stress transcription factor HsfA5 as specific repressor of HsfA4. _J. Biol. Chem._ 282, 3605–3613 (2007). Article CAS
PubMed Google Scholar * Andrasi, N. et al. The mitogen-activated protein kinase 4-phosphorylated heat shock factor A4A regulates responses to combined salt and heat stresses. _J. Exp.
Bot._ 70, 4903–4918 (2019). Article CAS PubMed PubMed Central Google Scholar * Giesguth, M. et al. Redox-dependent translocation of the heat shock transcription factor AtHSFA8 from the
cytosol to the nucleus in Arabidopsis thaliana. _FEBS Lett._ 589, 718–725 (2015). Article CAS PubMed Google Scholar * Kong, L. et al. CPC: assess the protein-coding potential of
transcripts using sequence features and support vector machine. _Nucleic Acids Res._ 35, W345–W349 (2007). Article PubMed PubMed Central Google Scholar * Guttman, M. et al. Modular
regulatory principles of large non-coding RNAs. _Nature_ 482, 339–346 (2012). Article CAS PubMed PubMed Central Google Scholar * Albihlal, W. S. et al. Arabidopsis HEAT SHOCK
TRANSCRIPTION FACTORA1b regulates multiple developmental genes under benign and stress conditions. _J. Exp. Bot._ 69, 2847–2862 (2018). Article CAS PubMed PubMed Central Google Scholar
* Hsu, S. F. et al. Cytosol-Localized Heat Shock Factor-Binding Protein, AtHSBP, Functions as a Negative Regulator of Heat Shock Response by Translocation to the Nucleus and Is Required for
Seed Development in Arabidopsis. _Plant Physiol._ 153, 773–784 (2010). Article CAS PubMed PubMed Central Google Scholar * Iyer, M. K. et al. The landscape of long noncoding RNAs in the
human transcriptome. _Nat. Genet._ 47, 199–208 (2015). Article CAS PubMed PubMed Central Google Scholar * Henriques, R. et al. The antiphasic regulatory module comprising CDF5 and its
antisense RNA FLORE links the circadian clock to photoperiodic flowering. _N. Phytol._ 216, 854–867 (2017). Article CAS Google Scholar * Swiezewski, S. et al. Cold-induced silencing by
long antisense transcripts of an Arabidopsis Polycomb target. _Nature_ 462, 799–802 (2009). Article CAS PubMed Google Scholar * Liu, X. et al. A novel antisense long noncoding RNA,
TWISTED LEAF, maintains leaf blade flattening by regulating its associated sense R2R3-MYB gene in rice. _N. Phytol._ 218, 774–788 (2018). Article CAS Google Scholar * Deforges, J. et al.
Prediction of regulatory long intergenic non-coding RNAs acting in trans through base-pairing interactions. _Bmc Genom._ 20, 601 (2019). Article Google Scholar * Xu, J. et al.
Third-Generation Sequencing Reveals LncRNA-Regulated HSP Genes in the Populus x canadensis Moench Heat Stress Response. _Front. Genet._ 11, 249 (2020). Article CAS PubMed PubMed Central
Google Scholar * Song, X.M. et al. Temperature expression patterns of genes and their coexpression with LncRNAs revealed by RNA-Seq in non-heading Chinese cabbage. _Bmc Genom._ 17, 297
(2016). * Faghihi, M. A. et al. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. _Nat. Med._ 14, 723–730 (2008).
Article CAS PubMed PubMed Central Google Scholar * Johnsson, P. et al. A pseudogene long-noncoding-RNA network regulates PTEN transcription and translation in human cells. _Nat. Struct.
Mol. Biol._ 20, 440–446 (2013). Article CAS PubMed PubMed Central Google Scholar * Zhang, H. et al. The Pseudomonas stutzeri-Specific Regulatory Noncoding RNA NfiS Targets katB mRNA
Encoding a Catalase Essential for Optimal Oxidative Resistance and Nitrogenase Activity. _J. Bacteriol_. 201, e00334–19 (2019). * Zhan, Y. et al. The novel regulatory ncRNA, NfiS, optimizes
nitrogen fixation via base pairing with the nitrogenase gene nifK mRNA in Pseudomonas stutzeri A1501. _Proc. Natl Acad. Sci. USA_ 113, E4348–A4356 (2016). Article CAS PubMed PubMed
Central Google Scholar * Statello, L. et al. Gene regulation by long non-coding RNAs and its biological functions. _Nat. Rev. Mol Cell Biol._ 22, 96–118 (2020). * Seo, J. S. et al.
ELF18-INDUCED LONG-NONCODING RNA Associates with Mediator to Enhance Expression of Innate Immune Response Genes in Arabidopsis. _Plant Cell_ 29, 1024–1038 (2017). Article CAS PubMed
PubMed Central Google Scholar * Charrier, A. et al. Efficient Targeted Mutagenesis in Apple and First Time Edition of Pear Using the CRISPR-Cas9 System. _Front. Plant Sci._ 10, 40 (2019).
Article PubMed PubMed Central Google Scholar * Hao, L. et al. A constitutive and drought-responsive mRNA undergoes long-distance transport in pear (Pyrus betulaefolia) phloem. _Plant
Sci._ 293, 110419 (2020). Article CAS PubMed Google Scholar * Zhang, H. et al. Enhanced Vitamin C Production Mediated by an ABA-Induced PTP-like Nucleotidase Improves Plant Drought
Tolerance in Arabidopsis and Maize. _Mol. Plant_ 13, 760–776 (2020). Article CAS PubMed Google Scholar * Wang, L. et al. CPAT: Coding-Potential Assessment Tool using an alignment-free
logistic regression model. _Nucleic Acids Res._ 41, e74 (2013). Article CAS PubMed PubMed Central Google Scholar * Luo, H. et al. De novo approach to classify protein-coding and
noncoding transcripts based on sequence composition. _Methods Mol. Biol._ 1182, 203–207 (2014). Article PubMed Google Scholar * Zhu, B. et al. RNA sequencing and functional analysis
implicate the regulatory role of long non-coding RNAs in tomato fruit ripening. _J. Exp. Bot._ 66, 4483–4495 (2015). Article CAS PubMed PubMed Central Google Scholar * Gambino, G. et
al. A Rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. _Phytochem Anal._ 19, 520–525 (2008). Article CAS PubMed Google Scholar *
Li, M. F. et al. Molecular analysis of two Chinese pear (Pyrus bretschneideri Rehd.) spontaneous self-compatible mutants, Yan Zhuang and Jin Zhui. _Plant Biol. (Stuttg.)_ 11, 774–783 (2009).
Article CAS Google Scholar * Ye, Y. et al. Docking of acetyl-CoA carboxylase to the plastid envelope membrane attenuates fatty acid production in plants. _Nat. Commun._ 11, 6191 (2020).
Article CAS PubMed PubMed Central Google Scholar * Mudunkothge, J. S. et al. The GUS reporter system in flower development studies. _Methods Mol. Biol._ 1110, 295–304 (2014). Article
CAS PubMed Google Scholar * Chen, H. et al. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. _Plant Physiol._ 146, 368–376 (2008). Article CAS
PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to Dr. Lijun Wang and Hongliang Zhu for their suggestion to improve the work. This work was
supported by the National Key Research and Development Program of China (2018YFD1000103), Natural Science Foundation of China (32272659), the Earmarked Fund for the China Agriculture
Research System (CARS-28-08), the 2115 Talent Development Program of China Agricultural University and the Construction of Beijing Science and Technology Innovation and Service Capacity in
Top Subjects (CEFF-PXM2019_014207_000032). AUTHOR INFORMATION Author notes * These authors contributed equally: Yi Zhang, Shengnan Wang. AUTHORS AND AFFILIATIONS * Collage of Horticulture,
China Agricultural University, 100193, Beijing, China Yi Zhang, Shengnan Wang, Wei Li, Shengyuan Wang, Li Hao, Chaoran Xu, Yunfei Yu, Ling Xiang, Tianzhong Li & Feng Jiang Authors * Yi
Zhang View author publications You can also search for this author inPubMed Google Scholar * Shengnan Wang View author publications You can also search for this author inPubMed Google
Scholar * Wei Li View author publications You can also search for this author inPubMed Google Scholar * Shengyuan Wang View author publications You can also search for this author inPubMed
Google Scholar * Li Hao View author publications You can also search for this author inPubMed Google Scholar * Chaoran Xu View author publications You can also search for this author
inPubMed Google Scholar * Yunfei Yu View author publications You can also search for this author inPubMed Google Scholar * Ling Xiang View author publications You can also search for this
author inPubMed Google Scholar * Tianzhong Li View author publications You can also search for this author inPubMed Google Scholar * Feng Jiang View author publications You can also search
for this author inPubMed Google Scholar CONTRIBUTIONS Y.Z. and T.L. conceived the project; Y.Z., S.N.W., and L.H. designed the experiments; Y.Z., S.N.W., and S.Y.W. performed the
experiments; C.X., Y.Y, C.Z., and L.X. analyzed the data; Yi Y.Z., F.J., and W.L. wrote and modified the paper. CORRESPONDING AUTHORS Correspondence to Tianzhong Li or Feng Jiang. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Communications Biology_ thanks Biao Jin, Andrzej Pacak and the other,
anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Gracjan Michlewski and Eve Rogers. Peer reviewer reports are available. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION PEER REVIEW FILE
SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2 SUPPLEMENTARY DATA 3 SUPPLEMENTARY DATA 4 SUPPLEMENTARY DATA 5 REPORTING
SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes
were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Zhang, Y., Wang, S., Li, W. _et al._ A long noncoding RNA _HILinc1_ enhances pear thermotolerance by stabilizing _PbHILT1_ transcripts through complementary base pairing. _Commun
Biol_ 5, 1134 (2022). https://doi.org/10.1038/s42003-022-04010-7 Download citation * Received: 15 November 2021 * Accepted: 20 September 2022 * Published: 26 October 2022 * DOI:
https://doi.org/10.1038/s42003-022-04010-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative