- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
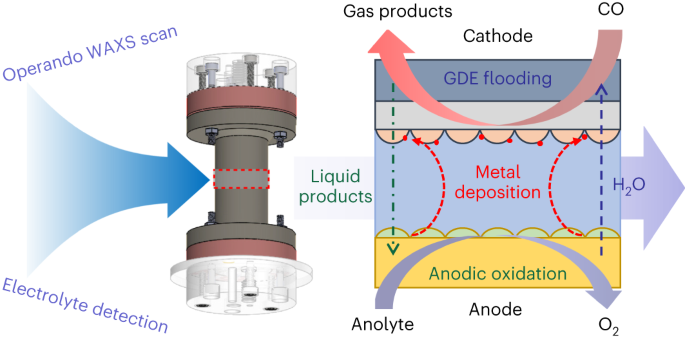
ABSTRACT CO electrolysis (COE) has emerged as an important alternative technology to couple with other sustainable techniques for transitioning towards a carbon-neutral future. A large
challenge for the deployment of high-rate COE is the limited durability of membrane-electrode assembly (MEA) devices. Here, by using an operando wide-angle X-ray scattering technique and
monitoring the change of electrolyte, we identified several degradation mechanisms of the MEA during high-rate COE. Cathodic gas-diffusion electrode (GDE) flooding and Ir contaminants
(crossover from anode) are two main issues causing excessive hydrogen evolution, which can be partly alleviated by increasing the polytetrafluoroethylene content in GDEs and using an
alkaline stable Ni-based anode. During long-term stability, the dynamic evolution of anolyte became the main issue: the pH would continuously drop due to cathodic acetate formation and
anodic ethanol oxidation. By compensating for this issue, we maintained a Faradaic efficiency of C2+ products at more than 70% for 136 hours. Access through your institution Buy or subscribe
This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our
best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only
$9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS UPSCALED PRODUCTION OF AN
ULTRAMICROPOROUS ANION-EXCHANGE MEMBRANE ENABLES LONG-TERM OPERATION IN ELECTROCHEMICAL ENERGY DEVICES Article Open access 12 May 2023 A SCALABLE MEMBRANE ELECTRODE ASSEMBLY ARCHITECTURE FOR
EFFICIENT ELECTROCHEMICAL CONVERSION OF CO2 TO FORMIC ACID Article Open access 22 November 2023 AQUEOUS ALTERNATING ELECTROLYSIS PROLONGS ELECTRODE LIFESPANS UNDER HARSH OPERATION
CONDITIONS Article Open access 23 July 2024 DATA AVAILABILITY The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary
Information. Raw X-ray data generated at the ESRF large-scale facility are available at https://doi.org/10.15151/ESRF-ES-703258873 from 2025. Alternatively, this data can be available from
the corresponding author upon request. Source data are provided with this paper. REFERENCES * Davis, S. J. et al. Net-zero emissions energy systems. _Science_ 360, eaas9793 (2018). Article
PubMed Google Scholar * Jouny, M., Hutchings, G. S. & Jiao, F. Carbon monoxide electroreduction as an emerging platform for carbon utilization. _Nat. Catal._ 2, 1062–1070 (2019).
Article CAS Google Scholar * Molitor, B. et al. Carbon recovery by fermentation of CO-rich off gases—turning steel mills into biorefineries. _Bioresour. Technol._ 215, 386–396 (2016). *
Ozden, A. et al. Carbon-efficient carbon dioxide electrolysers. _Nat. Sustain._ 5, 563–573 (2022). Article Google Scholar * Ma, M. et al. Local reaction environment for selective
electroreduction of carbon monoxide. _Energy Environ. Sci._ 15, 2470–2478 (2022). Article CAS Google Scholar * Xu, Q. et al. Enriching surface-accessible CO2 in the zero-gap
anion-exchange-membrane-based CO2 electrolyzer. _Angew. Chem. Int. Ed._ 62, e202214383 (2022). Article Google Scholar * Haas, T., Krause, R., Weber, R., Demler, M. & Schmid, G.
Technical photosynthesis involving CO2 electrolysis and fermentation. _Nat. Catal._ 1, 32–39 (2018). Article CAS Google Scholar * Song, Y., Zhang, X., Xie, K., Wang, G. & Bao, X.
High-temperature CO2 electrolysis in solid oxide electrolysis cells: developments, challenges, and prospects. _Adv. Mater._ 31, 1902033 (2019). Article CAS Google Scholar * Li, C. W.,
Ciston, J. & Kanan, M. W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. _Nature_ 508, 504–507 (2014). Article CAS PubMed Google Scholar
* Wang, L. et al. Electrochemically converting carbon monoxide to liquid fuels by directing selectivity with electrode surface area. _Nat. Catal._ 2, 702–708 (2019). Article CAS Google
Scholar * Wakerley, D. et al. Gas diffusion electrodes, reactor designs and key metrics of low-temperature CO2 electrolysers. _Nat. Energy_ 7, 130–143 (2022). Article CAS Google Scholar
* Rabiee, H. et al. Gas diffusion electrodes (GDEs) for electrochemical reduction of carbon dioxide, carbon monoxide, and dinitrogen to value-added products: a review. _Energy Environ. Sci._
14, 1959–2008 (2021). Article CAS Google Scholar * Jouny, M., Luc, W. & Jiao, F. High-rate electroreduction of carbon monoxide to multi-carbon products. _Nat. Catal._ 1, 748–755
(2018). Article CAS Google Scholar * Overa, S. et al. Enhancing acetate selectivity by coupling anodic oxidation to carbon monoxide electroreduction. _Nat. Catal._ 5, 738–745 (2022).
Article CAS Google Scholar * Ji, Y. et al. Selective CO-to-acetate electroreduction via intermediate adsorption tuning on ordered Cu–Pd sites. _Nat. Catal._ 5, 251–258 (2022). Article
CAS Google Scholar * Li, J. et al. Constraining CO coverage on copper promotes high-efficiency ethylene electroproduction. _Nat. Catal._ 2, 1124–1131 (2019). Article CAS Google Scholar
* Li, J. et al. Copper adparticle enabled selective electrosynthesis of n-propanol. _Nat. Commun._ 9, 4614 (2018). Article PubMed PubMed Central Google Scholar * Zhuang, T.-T. et al.
Copper nanocavities confine intermediates for efficient electrosynthesis of C3 alcohol fuels from carbon monoxide. _Nat. Catal._ 1, 946–951 (2018). Article CAS Google Scholar * Li, J. et
al. Effectively increased efficiency for electroreduction of carbon monoxide using supported polycrystalline copper powder electrocatalysts. _ACS Catal._ 9, 4709–4718 (2019). Article CAS
Google Scholar * Sullivan, I. et al. A hybrid catalyst-bonded membrane device for electrochemical carbon monoxide reduction at different relative humidities. _ACS Sustain. Chem. Eng._ 7,
16964–16970 (2019). Article CAS Google Scholar * Ripatti, D. S., Veltman, T. R. & Kanan, M. W. Carbon monoxide gas diffusion electrolysis that produces concentrated C2 products with
high single-pass conversion. _Joule_ 3, 240–256 (2019). Article CAS Google Scholar * Luc, W. et al. Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to
acetate. _Nat. Catal._ 2, 423–430 (2019). Article CAS Google Scholar * Pang, Y. et al. Efficient electrocatalytic conversion of carbon monoxide to propanol using fragmented copper. _Nat.
Catal._ 2, 251–258 (2019). Article CAS Google Scholar * Zhu, P. et al. Direct and continuous generation of pure acetic acid solutions via electrocatalytic carbon monoxide reduction.
_Porc. Natl Acad. Sci. USA_ 118, e2010868118 (2021). Article CAS Google Scholar * Yadegari, H. et al. Glycerol oxidation pairs with carbon monoxide reduction for low-voltage generation of
C2 and C3 product streams. _ACS Energy Lett._ 6, 3538–3544 (2021). Article CAS Google Scholar * Zhou, Y., Ganganahalli, R., Verma, S., Tan, H. R. & Yeo, B. S. Production of C3–C6
acetate esters via CO electroreduction in a membrane electrode assembly cell. _Angew. Chem. Int. Ed._ 61, e202202859 (2022). Article CAS Google Scholar * Yan, Y. et al. Interface
regulation promoting carbon monoxide gas diffusion electrolysis towards C2+ products. _Chem. Commun._ 58, 3645–3648 (2022). Article CAS Google Scholar * Wang, X. et al. Efficient
electrosynthesis of _n_-propanol from carbon monoxide using a Ag–Ru–Cu catalyst. _Nat. Energy_ 7, 170–176 (2022). Article Google Scholar * Ji, Y., Guan, A. & Zheng, G. Copper-based
catalysts for electrochemical carbon monoxide reduction. _Cell Rep. Phys. Sci._ 3, 101072 (2022). Article CAS Google Scholar * Kastlunger, G. et al. Using pH dependence to understand
mechanisms in electrochemical CO reduction. _ACS Catal._ 12, 4344–4357 (2022). Article CAS Google Scholar * Wang, L. et al. Electrochemical carbon monoxide reduction on polycrystalline
copper: effects of potential, pressure, and pH on selectivity toward multicarbon and oxygenated products. _ACS Catal._ 8, 7445–7454 (2018). Article CAS Google Scholar * Heenen, H. H. et
al. The mechanism for acetate formation in electrochemical CO(2) reduction on Cu: selectivity with potential, pH, and nanostructuring. _Energy Environ. Sci._ 15, 3978–3990 (2022). Article
CAS Google Scholar * Li, D. et al. Durability of anion exchange membrane water electrolyzers. _Energy Environ. Sci._ 14, 3393–3419 (2021). Article CAS Google Scholar * Xu, Q. et al.
Anion exchange membrane water electrolyzer: electrode design, lab-scaled testing system and performance evaluation. _EnergyChem._ 4, 100087 (2022). Article CAS Google Scholar * Moss, A.
B. et al. Versatile high energy X-ray transparent electrolysis cell for operando measurements. _J. Power Sources_ 562, 232754 (2023). Article CAS Google Scholar * Moss, A. B. et al. In
operando investigations of oscillatory water and carbonate effects in MEA-based CO2 electrolysis devices. _Joule_ 7, 350–365 (2023). Article CAS Google Scholar * Yang, K., Kas, R., Smith,
W. A. & Burdyny, T. Role of the carbon-based gas diffusion layer on flooding in a gas diffusion electrode cell for electrochemical CO2 reduction. _ACS Energy Lett._ 6, 33–40 (2021).
Article CAS Google Scholar * Wang, Z., Guo, X., Montoya, J. & Nørskov, J. K. Predicting aqueous stability of solid with computed Pourbaix diagram using SCAN functional. _NPJ Comput.
Mater._ 6, 160 (2020). Article Google Scholar * Disch, J. et al. High-resolution neutron imaging of salt precipitation and water transport in zero-gap CO2 electrolysis. _Nat. Commun._ 13,
6099 (2022). Article CAS PubMed PubMed Central Google Scholar * Xing, Z., Hu, L., Ripatti, D. S., Hu, X. & Feng, X. Enhancing carbon dioxide gas-diffusion electrolysis by creating a
hydrophobic catalyst microenvironment. _Nat. Commun._ 12, 136 (2021). Article CAS PubMed PubMed Central Google Scholar * Wu, Y. et al. Mitigating electrolyte flooding for
electrochemical CO2 reduction via infiltration of hydrophobic particles in a gas diffusion layer. _ACS Energy Lett._ 7, 2884–2892 (2022). Article CAS Google Scholar * Garg, S., Giron
Rodriguez, C. A., Rufford, T. E., Varcoe, J. R. & Seger, B. How membrane characteristics influence the performance of CO2 and CO electrolysis. _Energy Environ. Sci._ 15, 4440–4469
(2022). Article CAS Google Scholar * Vass, Á., Kormányos, A., Kószó, Z., Endrődi, B. & Janáky, C. Anode catalysts in CO2 electrolysis: challenges and untapped opportunities. _ACS
Catal._ 12, 1037–1051 (2022). Article CAS PubMed PubMed Central Google Scholar * Xu, Q. et al. Fluorination-enabled reconstruction of NiFe electrocatalysts for efficient water
oxidation. _Nano Lett._ 21, 492–499 (2021). Article CAS PubMed Google Scholar * Lai, S. C. S. et al. Effects of electrolyte pH and composition on the ethanol electro-oxidation reaction.
_Catal. Today_ 154, 92–104 (2010). Article CAS Google Scholar * Monyoncho, E. A., Woo, T. K. & Baranova, E. A. in _Electrochemistry_ Vol. 15 (eds Banks, C. & McIntosh, S.) 1–57
(The Royal Society of Chemistry, 2019). * Guo, J., Chen, R., Zhu, F.-C., Sun, S.-G. & Villullas, H. M. New understandings of ethanol oxidation reaction mechanism on Pd/C and Pd2Ru/C
catalysts in alkaline direct ethanol fuel cells. _Appl. Catal. B_ 224, 602–611 (2018). Article CAS Google Scholar * Hasa, B. et al. Benchmarking anion-exchange membranes for
electrocatalytic carbon monoxide reduction. _Chem. Catal._ 3, 100450 (2023). Article CAS Google Scholar * Larrazábal, G. O., Okatenko, V., Chorkendorff, I., Buonsanti, R. & Seger, B.
Investigation of ethylene and propylene production from CO2 reduction over copper nanocubes in an MEA-type electrolyzer. _ACS Appl. Mater. Interfaces_ 14, 7779–7787 (2022). Article PubMed
Google Scholar * Ashiotis, G. et al. The fast azimuthal integration Python library: pyFAI. _J. Appl. Crystallogr._ 48, 510–519 (2015). Article CAS PubMed PubMed Central Google Scholar
Download references ACKNOWLEDGEMENTS The research leading to these results has received funding from the ECOEthylene project from Innovation Fund Denmark (grant no. 8057-00018B), and the
Villum Center for the Science of Sustainable Fuels and Chemicals grant no. 9455. We thank the ESRF for providing the high-energy X-ray beam and ID31 beamline staff for experimental support.
AUTHOR INFORMATION Author notes * These authors contributed equally: Qiucheng Xu, Sahil Garg. AUTHORS AND AFFILIATIONS * Surface Physics and Catalysis (Surf Cat) Section, Department of
Physics, Technical University of Denmark, Lyngby, Denmark Qiucheng Xu, Sahil Garg, Asger B. Moss, Ib Chorkendorff & Brian Seger * Experimental Division, European Synchrotron Radiation
Facility, Grenoble, France Marta Mirolo & Jakub Drnec Authors * Qiucheng Xu View author publications You can also search for this author inPubMed Google Scholar * Sahil Garg View author
publications You can also search for this author inPubMed Google Scholar * Asger B. Moss View author publications You can also search for this author inPubMed Google Scholar * Marta Mirolo
View author publications You can also search for this author inPubMed Google Scholar * Ib Chorkendorff View author publications You can also search for this author inPubMed Google Scholar *
Jakub Drnec View author publications You can also search for this author inPubMed Google Scholar * Brian Seger View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS Q.X. and S.G. are the lead authors. Q.X. wrote the manuscript with input from all coauthors. S.G. helped revise the manuscript and participated in all results
discussions. Q.X., S.G. and A.B.M. carried out beamline electrochemical measurements and conducted X-ray data analysis. M.M. and J.D. assisted in performing in operando experiments at ESRF.
J.D., I.C. and B.S. guided the project and oversaw its development. B.S. set the overall direction of the project and helped edit the overall manuscript. CORRESPONDING AUTHOR Correspondence
to Brian Seger. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Catalysis_ thanks the anonymous reviewers for
their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–31, Note 1 and Tables 1 and 2. SOURCE DATA SOURCE DATA FIG. 3 Statistical source data.
SOURCE DATA FIG. 4 Statistical source data. SOURCE DATA FIG. 5 Statistical source data. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds
exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely
governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Xu, Q., Garg, S., Moss, A.B. _et al._ Identifying and
alleviating the durability challenges in membrane-electrode-assembly devices for high-rate CO electrolysis. _Nat Catal_ 6, 1042–1051 (2023). https://doi.org/10.1038/s41929-023-01034-y
Download citation * Received: 18 January 2023 * Accepted: 31 August 2023 * Published: 28 September 2023 * Issue Date: November 2023 * DOI: https://doi.org/10.1038/s41929-023-01034-y SHARE
THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative







:max_bytes(150000):strip_icc():focal(399x0:401x2)/donald-trump-01-800-9-480d525ba21d41ddafcfca8c72ca8be1.jpg)