- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
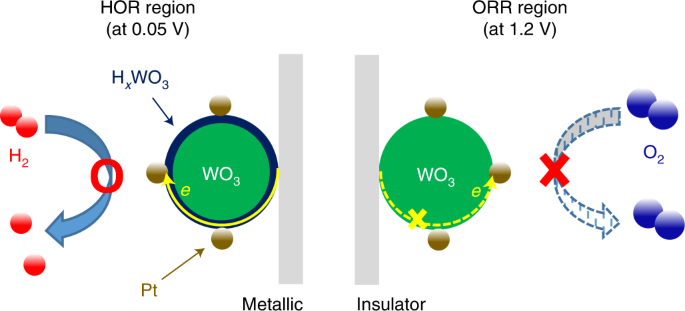
ABSTRACT Repetitive start-up and shut-down events in polymer electrolyte membrane fuel cells for automotive applications lead to serious corrosion of the cathode due to an instantaneous
potential jump that results from unintended air leakage into the anodic flow field followed by a parasitic oxygen reduction reaction (ORR) on the anode. Here we report a solution to the
cathode corrosion issue during the start-up/shut-down events whereby intelligent catalyst design is used to selectively promote the hydrogen oxidation reaction (HOR) while concomitantly
suppressing the ORR on the anode. Platinum thin layers supported on hydrogen tungsten bronze (Pt/H_x_WO3) suppressed the ORR by converting themselves into an insulator following exposure to
oxygen, while selectively promoting the HOR by regaining metallic conductivity following subsequent exposure to hydrogen. The HOR-selective electrocatalysis imparted by a metal–insulator
transition in Pt/H_x_WO3 demonstrated a remarkably enhanced durability of membrane electrode assemblies compared to those with commercial Pt/C catalysts. Access through your institution Buy
or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get
Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per
year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during
checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS EMBEDDED OXIDE
CLUSTERS STABILIZE SUB-2 NM PT NANOPARTICLES FOR HIGHLY DURABLE FUEL CELLS Article 01 July 2024 CHALLENGES IN APPLYING HIGHLY ACTIVE PT-BASED NANOSTRUCTURED CATALYSTS FOR OXYGEN REDUCTION
REACTIONS TO FUEL CELL VEHICLES Article 21 January 2021 REVEALING IN-PLANE MOVEMENT OF PLATINUM IN POLYMER ELECTROLYTE FUEL CELLS AFTER HEAVY-DUTY VEHICLE LIFETIME Article 27 July 2023 DATA
AVAILABILITY The data that support the plots in this paper and other findings of this study are available from the corresponding author on reasonable request. CHANGE HISTORY * _ 17 FEBRUARY
2021 A Correction to this paper has been published: https://doi.org/10.1038/s41929-020-00501-0 _ REFERENCES * Yu, P. T. et al. The impact of carbon stability on PEM fuel cell startup and
shutdown voltage degradation. _ECS Trans._ 3, 797–809 (2006). Article CAS Google Scholar * Borup, R. et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation.
_Chem. Rev._ 107, 3904–3951 (2007). Article CAS PubMed Google Scholar * de Bruijn, F. A., Dam, V. A. T. & Janssen, G. J. M. Review: durability and degradation issues of PEM fuel
cell components. _Fuel Cells_ 8, 3–22 (2008). Article CAS Google Scholar * Kaspar, R. B. et al. Reverse-current decay in hydroxide exchange membrane fuel cells. _J. Electrochem. Soc._
163, F377–F383 (2016). Article CAS Google Scholar * Reiser, C. A. et al. A reverse-current decay mechanism for fuel cells. _Electrochem. Solid-State Lett._ 8, A273–A276 (2005). Article
CAS Google Scholar * Tang, H. et al. PEM fuel cell cathode carbon corrosion due to the formation of air/fuel boundary at the anode. _J. Power Sources_ 158, 1306–1312 (2006). Article CAS
Google Scholar * Baumgartner, W. R. et al. Polarization study of a PEMFC with four reference electrodes at hydrogen starvation conditions. _J. Power Sources_ 182, 413–421 (2008). Article
CAS Google Scholar * Patterson, T. W. & Darling, R. M. Damage to the cathode catalyst of a PEM fuel cell caused by localized fuel starvation. _Electrochem. Solid-State Lett._ 9,
A183–A185 (2006). Article CAS Google Scholar * Yousfi-Steiner, N., Moçotéguy, P. H., Candusso, D. & Hissel, D. A review on polymer electrolyte membrane fuel cell catalyst degradation
and starvation issues: causes, consequences and diagnostic for mitigation. _J. Power Sources_ 194, 130–145 (2009). Article CAS Google Scholar * Ishigami, Y. et al. Corrosion of carbon
supports at cathode during hydrogen/air replacement at anode studied by visualization of oxygen partial pressures in a PEFC—start-up/shut-down simulation. _J. Power Sources_ 196, 3003–3008
(2011). Article CAS Google Scholar * Linse, N. et al. Quantitative analysis of carbon corrosion during fuel cell start-up and shut-down by anode purging. _J. Power Sources_ 219, 240–248
(2012). Article CAS Google Scholar * Chan, S. H. et al. Gas purging effect on the degradation characteristic of a proton exchange membrane fuel cell with dead-ended mode operation II.
Under different operation pressures. _Energy_ 131, 50–57 (2017). Article CAS Google Scholar * Yu, Y. et al. Effect of gas shutoff sequences on the degradation of proton exchange membrane
fuel cells with dummy load during startup and shutdown cycles. _Electrochim. Acta_ 71, 181–193 (2012). Article CAS Google Scholar * Chen, Y.-S. et al. A purge strategy for proton exchange
membrane fuel cells under varying-load operations. _Int. J. Hydrog. Energy_ 41, 12369–12376 (2016). Article CAS Google Scholar * Selvaganesh, S. V. et al. Graphitic carbon as durable
cathode-catalyst support for PEFCs. _Fuel Cells_ 11, 372–384 (2011). Article CAS Google Scholar * Wang et al. Durability investigation of carbon nanotube as catalyst support for proton
exchange membrane fuel cell. _J. Power Sources_ 158, 154–159 (2006). Article CAS Google Scholar * Watanabe, M. et al. Characterization of Pt catalysts on Nb-doped and Sb-doped SnO2–_δ_
support materials with aggregated structure by rotating disk electrode and fuel cell measurements. _Electrochim. Acta_ 110, 316–324 (2013). Article CAS Google Scholar * Uchida, M. et al.
Novel strategy to mitigate cathode catalyst degradation during air/air startup cycling via the atmospheric resistive switching mechanism of a hydrogen anode with a platinum catalyst
supported on tantalum-doped titanium dioxide. _J. Power Sources_ 294, 292–298 (2015). Article CAS Google Scholar * Hara, M. et al. Electrochemical and Raman spectroscopic evaluation of
Pt/graphitized carbon black catalyst durability for the start/stop operating condition of polymer electrolyte fuel cells. _Electrochim. Acta_ 70, 171–181 (2012). Article CAS Google Scholar
* Uchida, M. et al. Durability of Pt catalysts supported on graphitized carbon-black during gas-exchange start-up operation similar to that used for fuel cell vehicles. _J. Electrochem.
Soc._ 163, F644–F650 (2016). Article CAS Google Scholar * Dubau, L. et al. A review of PEM fuel cell durability: materials degradation, local heterogeneities of aging and possible
mitigation strategies. _WIREs Energy Eviron._ 3, 540–560 (2014). Article CAS Google Scholar * Srivastava, R. & Strasser, P. Oxygen evolution co-catalysts at fuel cell cathodes for
degradation mitigation during simulated start-up shut-down cycles. _ECS Trans._ 25, 565–571 (2009). Article CAS Google Scholar * Jang, S.-E. & Kim, H. Effect of water electrolysis
catalysts on carbon corrosion in polymer electrolyte membrane fuel cells. _J. Am. Chem. Soc._ 132, 14700–14701 (2010). Article CAS PubMed Google Scholar * Genorio, B. et al. Selective
catalysts for the hydrogen oxidation and oxygen reduction reactions by patterning of platinum with calix[4]arene molecules. _Nat. Mater._ 9, 998–1003 (2010). Article CAS PubMed Google
Scholar * Genorio, B. et al. Tailoring the selectivity and stability of chemically modified platinum nanocatalysts to design highly durable anodes for PEM fuel cells. _Angew. Chem. Int. Ed.
Engl._ 50, 5468–5472 (2011). Article CAS PubMed Google Scholar * Kim, Y.-T. et al. Hydrogen oxidation-selective electrocatalysis by fine tuning of Pt ensemble sites to enhance the
durability of automotive fuel cells. _ChemSusChem_ 10, 489–493 (2017). Article PubMed CAS Google Scholar * Ogi, T. et al. Electrospun Pt/SnO2 nanofibers as an excellent electrocatalysts
for hydrogen oxidation reaction with ORR-blocking characteristic. _Catal. Commun._ 33, 11–14 (2013). Article CAS Google Scholar * Romano-Rodriguez, A. et al. Insight into the role of
oxygen diffusion in the sensing mechanisms of SnO2 nanowires. _Adv. Funct. Mater._ 18, 2990–2994 (2008). Article CAS Google Scholar * Granqvist, C. G. Electrochromic tungsten oxide films:
review of progress 1993–1998. _Sol. Energy Mater. Sol. Cells_ 60, 201–262 (2000). Article CAS Google Scholar * Georg, A., Graf, W., Neumann, R. & Wittwer, V. The role of water in
gasochromic WO films. _Thin Solid Films_ 384, 269–275 (2001). Article CAS Google Scholar * Tseung, A. C. C. & Chen, K. Y. Hydrogen spill-over effect on Pt/WO3 anode catalysts. _Catal.
Today_ 38, 439–443 (2014). Article Google Scholar * Xi, Y. et al. Mechanism of hydrogen spillover on WO3(001) and formation of H_x_WO3 (_x_ = 0.125, 0.25, 0.375, and 0.5). _J. Phys. Chem.
C._ 118, 494–501 (2014). Article CAS Google Scholar * Shi, J. et al. Electrochemical catalytic activity for the hydrogen oxidation of mesoporous WO3 and WO3/C composites. _J. Mater.
Chem._ 18, 3575–3580 (2008). Article Google Scholar * Kawazoe, H. et al. Formation of hydrogen tungsten bronze by proton implantation. _Mater. Res. Bull._ 34, 115–121 (1999). Article
Google Scholar * Kamal, H. et al. Influence of proton insertion on the conductivity, structural and optical properties of amorphous and crystalline electrochromic WO3 films. _Phys. B:
Condens. Matter_ 349, 192–205 (2004). Article CAS Google Scholar * Adzic, R. R. et al. Palladium monolayer and palladium alloy electrocatalysts for oxygen reduction. _Langmuir_ 22,
10409–10415 (2006). Article PubMed CAS Google Scholar * Tseung, A. C. C. et al. High performance, platinum activated tungsten oxide fuel cell electrodes. _Nature_ 222, 556–558 (1969).
Article Google Scholar * Fabregat-Santiago, F. et al. Dynamic processes in the coloration of WO3 by lithium insertion. _J. Electrochem. Soc._ 148, E302–E309 (2001). Article CAS Google
Scholar * Bisquert, J. & Vikhrenko, V. S. Analysis of the kinetics of ion intercalation. Two state model describing the coupling of solid state ion diffusion and ion binding processes.
_Electrochim. Acta_ 47, 3977–3988 (2002). Article CAS Google Scholar * Kim, D.-J. et al. A study on the hydrogen intercalation into rf-magnetron sputtered amorphous WO3 film using cyclic
voltammetry combined with electrochemical quartz crystal microbalance technique. _Solid State Ion._ 109, 81–87 (1998). Article CAS Google Scholar * Lee, S.-H. et al. Crystalline WO3
nanoparticles for highly improved electrochromic applications. _Adv. Mater._ 18, 763–766 (2006). Article CAS Google Scholar * Wen, R.-T. et al. Eliminating degradation and uncovering
ion-trapping dynamics in electrochromic WO3 thin films. _Nat. Mater._ 14, 996–1001 (2015). Article CAS PubMed PubMed Central Google Scholar * Wen, R.-T. et al. Sustainable rejuvenation
of electrochromic WO3 films. _ACS Appl. Mater. Interfaces_ 7, 28100–28104 (2015). Article CAS PubMed Google Scholar * Mortimer, R. J. Electrochromic material. _Annu. Rev. Mater. Res._
41, 241–268 (2011). Article CAS Google Scholar * Reich, S. et al. A possible 2D H_x_WO3 Superconductor with a _T_c of 120 K. _J. Supercond. Nov. Magn._ 22, 343–346 (2009). Article CAS
Google Scholar * Tong, Q. et al. Rhenium-promoted Pt/WO3/ZrO2: an efficient catalyst for aqueous glycerol hydrogenolysis under reduced H2 pressure. _RSC Adv._ 6, 86663–86672 (2016). Article
CAS Google Scholar * Li, Haizeng et al. Self-seeded growth of nest-like hydrated tungsten trioxide film directly on FTO substrate for highly enhanced electrochromic performance. _J.
Mater. Chem. A_ 2, 11305–11310 (2014). Article CAS Google Scholar * Hsu, W.-C. et al. Hydrogen sensing characteristics of an electrodeposited WO3 thin film gasochromic sensor activated by
Pt catalyst. _Thin Solid Films_ 516, 407–411 (2007). Article CAS Google Scholar * Nørskov, J. K. et al. Changing the activity of electrocatalysts for oxygen reduction by tuning the
surface electronic structure. _Angew. Chem._ 118, 2963–2967 (2006). Article Google Scholar * Nørskov, J. K. et al. Modeling the electrochemical hydrogen oxidation and evolution reactions
on the basis of density functional theory calculations. _J. Phys. Chem. C_ 114, 18182–18197 (2010). Article CAS Google Scholar * Bard, A. J. et al. in _Electrochemical Methods:
Fundamentals and Applications_ 351 (Wiley, 1980). * Garsany, Y. et al. Experimental methods for quantifying the activity of platinum electrocatalysts for the oxygen reduction reaction.
_Anal. Chem._ 82, 6321–6328 (2010). Article CAS PubMed Google Scholar * Kresse, G. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set.
_Phys. Rev. B_ 54, 169 (1996). Article Google Scholar * Blöchl, P. E. Projector augmented-wave method. _Phys. Rev. B_ 50, 17953–17979 (1994). Google Scholar * Kresse, G. & Joubert, D.
From ultrasoft pseudopotentials to the projector augmented-wave method. _Phys. Rev. B_ 59, 1758 (1999). CAS Google Scholar * Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized
gradient approximation made simple. _Phys. Rev. Lett._ 77, 3865 (1996). Article CAS PubMed Google Scholar * Hammer, B., Hansen, L. B. & Nørskov, J. K. Improved adsorption energetics
within density-fuctional theory using revised Perdew–Burke–Ernzerhof functionals. _Phys. Rev. B_ 59, 7413–7421 (1999). Article Google Scholar Download references ACKNOWLEDGEMENTS This work
was supported by the National Research Foundation (NRF) of Korea grants (nos. 2019M3D1A1079306, 2019M3E6A1064521). V.S. and N.M.M. were supported by the U.S. Department of Energy, Office of
Energy Efficiency and Renewable Energy, Hydrogen and Fuel Cells Technologies Office. AUTHOR INFORMATION Author notes * These author contributed equally: Sang-Mun Jung, Su-Won Yun, Jun-Hyuk
Kim. AUTHORS AND AFFILIATIONS * Department of Materials Science and Engineering, Pohang University of Science and Technology, Gyeongbuk, Republic of Korea Sang-Mun Jung, Sang-Hoon You,
Jinheon Park, Junwoo Son & Yong-Tae Kim * Department of Energy System, Pusan National University, Busan, Republic of Korea Su-Won Yun & Jun-Hyuk Kim * Department of Chemical
Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Republic of Korea Seonggyu Lee, Yousung Jung & Jinwoo Lee * Department of Physics, Chung-Ang University, Seoul,
Republic of Korea Seo Hyoung Chang * Department of Physics Education, Seoul National University, Seoul, Republic of Korea Seung Chul Chae * Department of Chemistry, Ulsan National Institute
of Science and Technology (UNIST), Ulsan, Republic of Korea Sang Hoon Joo * Department of Chemical and Biological Engineering, Drexel University, Drexel, PA, USA Joshua Snyder * Materials
Science Division, Argonne National Laboratory, Lemont, IL, USA Vojislav Stamenkovic & Nenad M. Markovic Authors * Sang-Mun Jung View author publications You can also search for this
author inPubMed Google Scholar * Su-Won Yun View author publications You can also search for this author inPubMed Google Scholar * Jun-Hyuk Kim View author publications You can also search
for this author inPubMed Google Scholar * Sang-Hoon You View author publications You can also search for this author inPubMed Google Scholar * Jinheon Park View author publications You can
also search for this author inPubMed Google Scholar * Seonggyu Lee View author publications You can also search for this author inPubMed Google Scholar * Seo Hyoung Chang View author
publications You can also search for this author inPubMed Google Scholar * Seung Chul Chae View author publications You can also search for this author inPubMed Google Scholar * Sang Hoon
Joo View author publications You can also search for this author inPubMed Google Scholar * Yousung Jung View author publications You can also search for this author inPubMed Google Scholar *
Jinwoo Lee View author publications You can also search for this author inPubMed Google Scholar * Junwoo Son View author publications You can also search for this author inPubMed Google
Scholar * Joshua Snyder View author publications You can also search for this author inPubMed Google Scholar * Vojislav Stamenkovic View author publications You can also search for this
author inPubMed Google Scholar * Nenad M. Markovic View author publications You can also search for this author inPubMed Google Scholar * Yong-Tae Kim View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS Y.-T.K. conceived and designed the experiments. S.-M.J., S.-W.Y., J.-H.K., S.-H.Y, J.P., S.L., S.H.C. and S.C.C. performed the
experiments. Y.-T.K., S.H.J., J.L., Y.J., J. Son, V.S. and N.M.M. analysed the data. Y.-T.K., J. Snyder and N.M.M. co-wrote the paper. CORRESPONDING AUTHOR Correspondence to Yong-Tae Kim.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs 1–30 and Tables 1–3. RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jung, SM., Yun, SW., Kim, JH. _et al._ Selective electrocatalysis imparted by metal–insulator transition for durability enhancement of
automotive fuel cells. _Nat Catal_ 3, 639–648 (2020). https://doi.org/10.1038/s41929-020-0475-4 Download citation * Received: 26 August 2019 * Accepted: 26 May 2020 * Published: 29 June 2020
* Issue Date: August 2020 * DOI: https://doi.org/10.1038/s41929-020-0475-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative







:max_bytes(150000):strip_icc():focal(749x0:751x2)/darion-thomas-mugshot-031224-2560d45274a74897a39aa20718528075.jpg)