- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
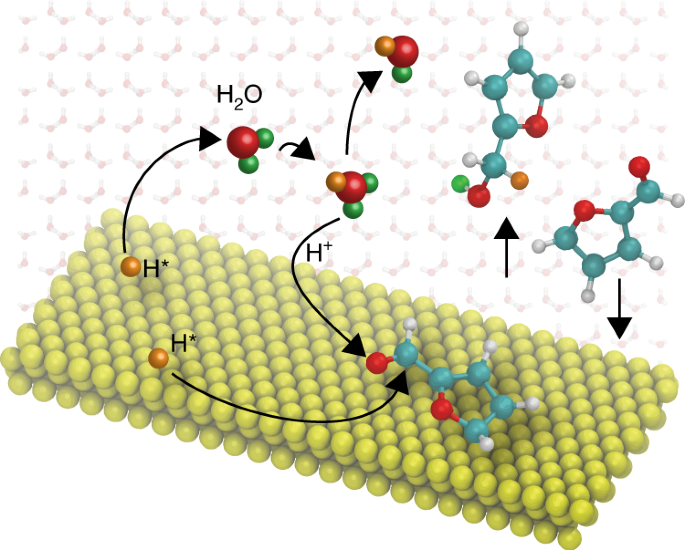
Compared to the vapour phase, liquid-phase heterogeneous catalysis provides additional degrees of freedom for reaction engineering, but the multifaceted solvent effects complicate analysis
of the reaction mechanism. Here, using furfural as an example, we reveal the important role of water-mediated protonation in a typical hydrogenation reaction over a supported Pd catalyst.
Depending on the solvent, we have observed different reaction orders with respect to the partial pressure of H2, as well as distinct selectivity towards hydrogenation of the conjugated C=O
and C=C double bonds. Free energy calculations show that H2O participates directly in the kinetically relevant reaction step and provides an additional channel for hydrogenation of the
aldehyde group, in which hydrogen bypasses the direct surface reaction via a hydrogen-bonded water network. This solution-mediated reaction pathway shows the potential role of the solvent
for tuning the selectivity of metal-catalysed hydrogenation when charge separation on the metal surface is feasible.
Any data that support the plots within this paper and other findings of the study are available from the corresponding author upon reasonable request. The following files are available in
the Supplementary Information: catalyst particle size calculations, FAL conversion and product yields in water at varying times and H2 pressures, H/D exchange experiment, derivation of rate
equations, AIMD calculations of FAL in water, atomic structures along the reaction pathway, free energy diagram for furanyl ring hydrogenation and maximum rate analysis data.
This work was supported by the US Department of Energy, Basic Energy Sciences (grant no. DE-SC0018284). The computational research used the supercomputer resources of the National Energy
Research Scientific Computing Centre (NERSC), the OU Supercomputing Centre for Education & Research (OSCER) at the University of Oklahoma and the Tandy Supercomputing Centre (TSC). The
authors thank T. Sooknoi (King Mongkut’s Institute of Technology Ladkrabang, Thailand) for valuable discussions.
These authors contributed equally: Zheng Zhao, Reda Bababrik, Wenhua Xue.
Center for Interfacial Reaction Engineering and School of Chemical, Biological and Materials Engineering, The University of Oklahoma, Norman, OK, USA
Zheng Zhao, Reda Bababrik, Nicholas M. Briggs, Dieu-Thy Nguyen, Umi Nguyen, Steven P. Crossley, Bin Wang & Daniel E. Resasco
Department of Physics and Engineering Physics, The University of Tulsa, Tulsa, OK, USA
Z.Z. conducted material synthesis, reaction tests and the H/D exchange experiment. R.B. completed the DFT calculations, the free energy calculations and the micro kinetic analysis. W.X.,
Y.L. and S.W. performed the DFT calculations. N.M.B. and S.P.C. conducted the catalyst characterization and analysed the data. D.-T.N. and U.N. performed the AIMD calculations. All authors
discussed the results and commented on the manuscript. B.W. and D.E.R supervised the project.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Figures 1–17, Supplementary Table 1, Supplementary Methods, Supplementary Notes 1–4, Supplementary References
Anyone you share the following link with will be able to read this content:





