- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Electrocatalytic semihydrogenation of acetylene provides a clean pathway to the production of ethylene (C2H4), one of the most widely used petrochemical feedstocks. However, its
performance is still well below that of the thermocatalytic route, leaving the practical feasibility of this electrochemical process questionable. Here our techno-economic analysis shows
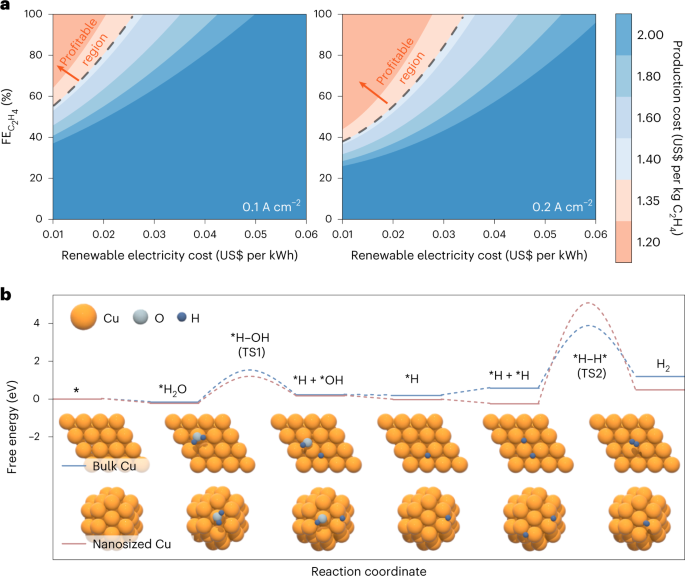
that this process becomes profitable if the Faraday efficiency exceeds 85% at a current density of 0.2 A cm−2. As a result, we design a Cu nanoparticle catalyst with coordinatively
unsaturated sites to steer the reaction towards these targets. Our electrocatalyst synthesized on gas diffusion layer coated carbon paper enables a high C2H4 yield rate of 70.15 mmol mg−1
h−1 and a Faraday efficiency of 97.7% at an industrially relevant current density of 0.5 A cm−2. Combined characterizations and calculations reveal that this performance can be attributed to
the favourable combination of a higher energy barrier for the coupling of active hydrogen atoms (H*) and weak absorption of *C2H4. The former suppresses the competitive hydrogen evolution
reaction, whereas the latter avoids overhydrogenation and C–C coupling. Further life cycle assessment evidences the economic feasibility and sustainability of the process. Our work suggests
a way towards rational design and manipulation of nanocatalysts that could find wider and greener catalytic applications. Access through your institution Buy or subscribe This is a preview
of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value
online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS HIGHLY EFFICIENT ETHYLENE PRODUCTION VIA
ELECTROCATALYTIC HYDROGENATION OF ACETYLENE UNDER MILD CONDITIONS Article Open access 06 December 2021 ROOM-TEMPERATURE ELECTROCHEMICAL ACETYLENE REDUCTION TO ETHYLENE WITH HIGH CONVERSION
AND SELECTIVITY Article 01 July 2021 SELECTIVE PRODUCTION OF ETHYLENE GLYCOL AT HIGH RATE VIA CASCADE CATALYSIS Article 26 June 2023 DATA AVAILABILITY The spreadsheets used for the cost
analyses and CO2 emissions and for Supplementary Tables 1–7 are available in Supplementary Data 1 and 2. Source data are provided with this paper. REFERENCES * Leow, W. R. et al.
Chloride-mediated selective electrosynthesis of ethylene and propylene oxides at high current density. _Science_ 368, 1228–1233 (2020). CAS Google Scholar * Jiang, K. et al. Metal ion
cycling of Cu foil for selective C–C coupling in electrochemical CO2 reduction. _Nat. Catal._ 1, 111–119 (2018). CAS Google Scholar * Chai, Y. et al. Control of zeolite pore interior for
chemoselective alkyne/olefin separations. _Science_ 368, 1002–1006 (2020). CAS Google Scholar * Bodke, A. S., Olschki, D. A., Schmidt, L. D. & Ranzi, E. High selectivities to ethylene
by partial oxidation of ethane. _Science_ 285, 712–715 (1999). CAS Google Scholar * Gomez, E., Yan, B., Kattel, S. & Chen, J. G. Carbon dioxide reduction in tandem with light-alkane
dehydrogenation. _Nat. Rev. Chem._ 3, 638–649 (2019). CAS Google Scholar * Gao, Y. et al. Recent advances in intensified ethylene production—a review. _ACS Catal._ 9, 8592–8621 (2019). CAS
Google Scholar * Zhao, B. et al. Unveiling the activity origin of iron nitride as catalytic material for efficient hydrogenation of CO2 to C2+ hydrocarbons. _Angew. Chem. Int. Ed._ 60,
4496–4500 (2021). CAS Google Scholar * Jiang, W. et al. Pd-modified ZnO–Au enabling alkoxy intermediates formation and dehydrogenation for photocatalytic conversion of methane to ethylene.
_J. Am. Chem. Soc._ 143, 269–278 (2021). CAS Google Scholar * Jiao, X. et al. Conversion of waste plastics into value-added carbonaceous fuels under mild conditions. _Adv. Mater._ 33,
2005192 (2021). CAS Google Scholar * Schobert, H. Production of acetylene and acetylene-based chemicals from coal. _Chem. Rev._ 114, 1743–1760 (2014). CAS Google Scholar * Ma, J. et al.
Pyrolysis of pulverized coal to acetylene in magnetically rotating hydrogen plasma reactor. _Fuel Process. Technol._ 167, 721–729 (2017). CAS Google Scholar * Bond, R. L., Galbraith, I.
F., Ladner, W. R. & McConnell, G. I. T. Production of acetylene from coal, using a plasma jet. _Nature_ 200, 1313–1314 (1963). CAS Google Scholar * Yan, B., Xu, P., Guo, C. Y., Jin, Y.
& Cheng, Y. Experimental study on coal pyrolysis to acetylene in thermal plasma reactors. _Chem. Eng. J._ 207–208, 109–116 (2012). Google Scholar * Zhang, L. et al. Efficient
electrocatalytic acetylene semihydrogenation by electron-rich metal sites in N-heterocyclic carbene metal complexes. _Nat. Commun._ 12, 6574 (2021). CAS Google Scholar * Studt, F. et al.
Identification of non-precious metal alloy catalysts for selective hydrogenation of acetylene. _Science_ 320, 1320–1322 (2008). CAS Google Scholar * Cao, Y. et al. Adsorption site
regulation to guide atomic design of Ni–Ga catalysts for acetylene semi‐hydrogenation. _Angew. Chem. Int. Ed._ 132, 11744–11749 (2020). Google Scholar * Wang, S. et al. Highly efficient
ethylene production via electrocatalytic hydrogenation of acetylene under mild conditions. _Nat. Commun._ 12, 7072 (2021). CAS Google Scholar * Bu, J. et al. Selective electrocatalytic
semihydrogenation of acetylene impurities for the production of polymer-grade ethylene. _Nat. Catal._ 4, 557–564 (2021). CAS Google Scholar * Shi, R. et al. Room-temperature
electrochemical acetylene reduction to ethylene with high conversion and selectivity. _Nat. Catal._ 4, 565–574 (2021). CAS Google Scholar * Vilé, G., Albani, D., Almora-Barrios, N., López,
N. & Pérez-Ramírez, J. Advances in the design of nanostructured catalysts for selective hydrogenation. _ChemCatChem_ 8, 21–33 (2016). Google Scholar * Vilé, G. et al. A stable
single-site palladium catalyst for hydrogenations. _Angew. Chem. Int. Ed._ 54, 11265–11269 (2015). Google Scholar * Omar, S. et al. Density functional theory analysis of dichloromethane and
hydrogen interaction with Pd clusters: first step to simulate catalytic hydrodechlorination. _J. Phys. Chem. C_ 115, 14180–14192 (2011). CAS Google Scholar * Huang, F. et al. Insight into
the activity of atomically dispersed Cu catalysts for semihydrogenation of acetylene: impact of coordination environments. _ACS Catal._ 12, 48–57 (2022). CAS Google Scholar * Semagina, N.
& Kiwi‐Minsker, L. Recent advances in the liquid‐phase synthesis of metal nanostructures with controlled shape and size for catalysis. _Catal. Rev._ 51, 147–217 (2009). CAS Google
Scholar * Xing, Z., Hu, L., Ripatti, D. S., Hu, X. & Feng, X. Enhancing carbon dioxide gas-diffusion electrolysis by creating a hydrophobic catalyst microenvironment. _Nat. Commun._ 12,
136 (2021). CAS Google Scholar * Nguyen, T. N. & Dinh, C.-T. Gas diffusion electrode design for electrochemical carbon dioxide reduction. _Chem. Soc. Rev._ 49, 7488–7504 (2020). CAS
Google Scholar * Yang, K., Kas, R., Smith, W. A. & Burdyny, T. Role of the carbon-based gas diffusion layer on flooding in a gas diffusion electrode cell for electrochemical CO2
reduction. _ACS Energy Lett._ 6, 33–40 (2021). CAS Google Scholar * Gu, Y. et al. Two-dimensional porous molybdenum phosphide/nitride heterojunction nanosheets for pH-universal hydrogen
evolution reaction. _Angew. Chem. Int. Ed._ 60, 6673–6681 (2021). CAS Google Scholar * Haase, F. T. et al. Size effects and active state formation of cobalt oxide nanoparticles during the
oxygen evolution reaction. _Nat. Energy_ 7, 765–773 (2022). CAS Google Scholar * Xie, L. et al. Molecular engineering of a 3D self-supported electrode for oxygen electrocatalysis in
neutral media. _Angew. Chem. Int. Ed._ 58, 18883–18887 (2019). CAS Google Scholar * Lum, Y. et al. Tuning OH binding energy enables selective electrochemical oxidation of ethylene to
ethylene glycol. _Nat. Catal._ 3, 14–22 (2020). CAS Google Scholar * Shin, H., Hansen, K. U. & Jiao, F. Techno-economic assessment of low-temperature carbon dioxide electrolysis. _Nat.
Sustain._ 4, 911–919 (2021). Google Scholar * Gao, Y. et al. Field-induced reagent concentration and sulfur adsorption enable efficient electrocatalytic semihydrogenation of alkynes. _Sci.
Adv._ 8, eabm9477 (2022). CAS Google Scholar * Durante, C. An electrochemical way to pure ethylene. _Nat. Catal._ 4, 537–538 (2021). CAS Google Scholar * Wu, Y., Liu, C., Wang, C., Lu,
S. & Zhang, B. Selective transfer semihydrogenation of alkynes with H2O (D2O) as the H (D) source over a Pd–P cathode. _Angew. Chem. Int. Ed._ 59, 21170–21175 (2020). CAS Google Scholar
* Li, H. et al. σ-alkynyl adsorption enables electrocatalytic semihydrogenation of terminal alkynes with easy-reducible/passivated groups over amorphous PdS_x_ nanocapsules. _J. Am. Chem.
Soc._ 144, 19456–19465 (2022). CAS Google Scholar * Higgins, D., Hahn, C., Xiang, C., Jaramillo, T. F. & Weber, A. Z. Gas-diffusion electrodes for carbon dioxide reduction: a new
paradigm. _ACS Energy Lett._ 4, 317–324 (2019). CAS Google Scholar * Cheng, W., Zhang, H., Luan, D. & Lou, X. W. Exposing unsaturated Cu1-O2 sites in nanoscale Cu-MOF for efficient
electrocatalytic hydrogen evolution. _Sci. Adv._ 7, eabg2580 (2021). CAS Google Scholar * Wu, Y. et al. Converting copper sulfide to copper with surface sulfur for electrocatalytic alkyne
semi-hydrogenation with water. _Nat. Commun._ 12, 3881 (2021). CAS Google Scholar * Han, J. et al. A reconstructed porous copper surface promotes selectivity and efficiency toward C2
products by electrocatalytic CO2 reduction. _Chem. Sci._ 11, 10698–10704 (2020). CAS Google Scholar * Tao, Z., Wu, Z., Wu, Y. & Wang, H. Activating copper for electrocatalytic CO2
reduction to formate via molecular interactions. _ACS Catal._ 10, 9271–9275 (2020). CAS Google Scholar * Lei, Q. et al. Investigating the origin of enhanced C2+ selectivity in
oxide-/hydroxide-derived copper electrodes during CO2 electroreduction. _J. Am. Chem. Soc._ 142, 4213–4222 (2020). CAS Google Scholar * Ma, C. Y. et al. Mesoporous Co3O4 and Au/Co3O4
catalysts for low-temperature oxidation of trace ethylene. _J. Am. Chem. Soc._ 132, 2608–2613 (2010). CAS Google Scholar * McKean, D. C. Individual CH bond strengths in simple organic
compounds: effects of conformation and substitution. _Chem. Soc. Rev._ 7, 399–422 (1978). CAS Google Scholar * Yamamoto, M. et al. Softened CH stretching vibration of a long-chain
_n_-alkane, _n_-C44H90, physisorbed on a Ag(111) surface: an infrared reflection absorption spectroscopic study. _J. Phys. Chem. B_ 104, 7370–7376 (2000). CAS Google Scholar * Sherbo, R.
S., Kurimoto, A., Brown, C. M. & Berlinguette, C. P. Efficient electrocatalytic hydrogenation with a palladium membrane reactor. _J. Am. Chem. Soc._ 141, 7815–7821 (2019). CAS Google
Scholar * De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? _Science_ 364, eaav3506 (2019). Google Scholar * Rojas Sánchez,
D., Khalilpour, K. & Hoadley, A. F. A. How sustainable is CO2 conversion to ethanol? A life cycle assessment of a new electrocatalytic carbon utilisation process. _Sustain. Energy Fuels_
5, 5866–5880 (2021). Google Scholar * Chang, X., Malkani, A., Yang, X. & Xu, B. Mechanistic insights into electroreductive C–C coupling between CO and acetaldehyde into multicarbon
products. _J. Am. Chem. Soc._ 142, 2975–2983 (2020). CAS Google Scholar * Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using
IFEFFIT. _J. Synchrotron Radiat._ 12, 537–541 (2005). CAS Google Scholar * Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a
plane-wave basis set. _Phys. Rev. B_ 54, 11169 (1996). CAS Google Scholar * Blöchl, P. E. Projector augmented-wave method. _Phys. Rev. B_ 50, 17953 (1994). Google Scholar * Perdew, J. P.,
Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. _Phys. Rev. Lett._ 77, 3865 (1996). CAS Google Scholar * Mathew, K., Sundararaman, R., Letchworth-Weaver, K.,
Arias, T. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. _J. Chem. Phys._ 140, 084106 (2014). Google Scholar *
Henkelman, G. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. _J. Chem. Phys._ 113, 9901–9904 (2000). CAS Google Scholar
Download references ACKNOWLEDGEMENTS We acknowledge the National Natural Science Foundation of China (grant no. 21871206 to B.Z.) and L. Zheng at the 1W1B beamline of the Beijing Synchrotron
Radiation Facility for supporting this project. We also thank Y. Liu for help with the ATR–FTIR measurements. AUTHOR INFORMATION Author notes * These authors contributed equally: Bo-Hang
Zhao, Fanpeng Chen, Mengke Wang. AUTHORS AND AFFILIATIONS * Department of Chemistry, Institute of Molecular Plus, School of Science, Tianjin University, Tianjin, China Bo-Hang Zhao, Fanpeng
Chen, Mengke Wang, Yongmeng Wu, Cuibo Liu, Yifu Yu & Bin Zhang * Institute of New Energy Materials, School of Materials Science and Engineering, Tianjin University, Tianjin, China
Chuanqi Cheng * Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Frontiers Science Center for Synthetic Biology (Ministry of Education), Tianjin University, Tianjin, China Yifu
Yu & Bin Zhang Authors * Bo-Hang Zhao View author publications You can also search for this author inPubMed Google Scholar * Fanpeng Chen View author publications You can also search for
this author inPubMed Google Scholar * Mengke Wang View author publications You can also search for this author inPubMed Google Scholar * Chuanqi Cheng View author publications You can also
search for this author inPubMed Google Scholar * Yongmeng Wu View author publications You can also search for this author inPubMed Google Scholar * Cuibo Liu View author publications You can
also search for this author inPubMed Google Scholar * Yifu Yu View author publications You can also search for this author inPubMed Google Scholar * Bin Zhang View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS B.Z. conceived the idea and directed the project. B.-H.Z. and B.Z. designed the experiments. B.-H.Z. and M.W. performed
the materials synthesis and electrochemical experiments. B.-H.Z., F.C. and M.W. carried out the in situ experiments. C.C. performed and analysed the DFT calculations. B.-H.Z., Y.W., C.L.,
Y.Y. and B.Z. analysed the experimental data. B.-H.Z. and F.C. wrote the paper. B.Z. revised the paper. All authors discussed the results and commented on the paper. CORRESPONDING AUTHOR
Correspondence to Bin Zhang. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Sustainability_ thanks Feng Jiao,
Christian Durante and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with
regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–41, Notes 1–30 and Tables 1–7.
REPORTING SUMMARY SUPPLEMENTARY VIDEO 1 Videos for the ESAE process driven by solar-derived electricity. SUPPLEMENTARY DATA 1 The spreadsheets used for the cost analyses and CO2 emissions.
SUPPLEMENTARY DATA 2 Datasets for Supplementary Tables 1–7. SOURCE DATA SOURCE DATA FIG. 1 The source data underlying Fig. 1. SOURCE DATA FIG. 2 The source data underlying Fig. 2. SOURCE
DATA FIG. 3 The source data underlying Fig. 3. SOURCE DATA FIG. 4 The source data underlying Fig. 4. SOURCE DATA FIG. 5 The source data underlying Fig. 5. RIGHTS AND PERMISSIONS Springer
Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author
self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Zhao, BH., Chen, F., Wang, M. _et al._ Economically viable electrocatalytic ethylene production with high yield and selectivity. _Nat Sustain_ 6, 827–837 (2023).
https://doi.org/10.1038/s41893-023-01084-x Download citation * Received: 27 April 2022 * Accepted: 09 February 2023 * Published: 09 March 2023 * Issue Date: July 2023 * DOI:
https://doi.org/10.1038/s41893-023-01084-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative









