- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Cholangiocarcinoma (CCA), the bile duct cancer, is associated with a high burden and poor prognosis. This is due to the lack of early diagnostic tools and effective chemotherapy.
Molecular networking is a promising tool for investigating the molecular mechanisms of drugs or candidate molecules for various diseases. This study investigated molecular targets and
signaling pathways of the three components (atractylodin, beta-eudesmol, and hinesol) of _Atractylodes lancea_ Thunb. (DC.) (AL), the promising candidate for patients with advanced-stage
intrahepatic CCA (iCCA). The independent-sample T-test or Mann-Whitney U test was used to identify significant gene targets in (i) patients with advanced-stage iCCA who received AL treatment
and those who received palliative care alone, and (ii) patients with progressive and non-progressive diseases. A molecular network was constructed using Cytoscape to identify AL signaling
action pathways. Fifty-two genes were identified as the essential targeted genes in patients with advanced-stage iCCA. The most critical gene hubs were TNFα (1st rank), NRAS (2nd rank), and
PI3KCA (3rd rank). The false discovery rate (FDR) identified PI3K/AKT, NK cell-mediated cytotoxicity, and apoptosis as the top three significant pathways. Hinesol showed the highest binding
affinity compared with other components of AL and the standard anti-CCA drugs gemcitabine and 5-FU. Molecular networking is a valuable tool for investigating molecular signaling networks of
herbal medicine with multiple active and non-active ingredients. With multi-signaling targets linked to all tumor development and progression stages, the study supports AL as a promising
candidate for patients with advanced-stage iCCA. SIMILAR CONTENT BEING VIEWED BY OTHERS TANSHINONE IIA AFFECTS THE MALIGNANT GROWTH OF CHOLANGIOCARCINOMA CELLS BY INHIBITING THE
PI3K-AKT-MTOR PATHWAY Article Open access 29 September 2021 INVESTIGATING THE PI3K/AKT/MTOR AXIS IN BUZHONG YIQI DECOCTION’S ANTI-COLORECTAL CANCER ACTIVITY Article Open access 10 March 2025
NETWORK PHARMACOLOGY AND EXPERIMENTAL VERIFICATION BASED RESEARCH INTO THE EFFECT AND MECHANISM OF AUCKLANDIAE RADIX–AMOMI FRUCTUS AGAINST GASTRIC CANCER Article Open access 07 June 2022
INTRODUCTION Cholangiocarcinoma (CCA) is an aggressive malignancy often diagnosed in elderly patients who may not tolerate traditional chemotherapy due to adverse reactions and
comorbidities. This highlights the need for alternative treatments. Atractylodes lancea (Thunb.) DC (AL), a traditional herb used in East Asia, shows promise as a novel CCA therapy. Active
components like atractylodin and beta-eudesmol exhibit antiproliferative, anti-angiogenic, and anti-metastatic effects in preclinical studies1,2. Recent research highlights synergistic
interactions between AL’s bioactive constituents, such as beta-eudesmol, atractylodin, and hinesol, with favorable fractional inhibitory concentration (FIC) values3. These compounds induce
G1 cell cycle arrest and apoptosis in CCA cells by modulating p21 expression4. Beta-eudesmol also sensitizes chemotherapy-resistant CCA cells to agents like 5-FU and doxorubicin by altering
the Bax/Bcl-2 ratio and suppressing NQO1 expression5. It inhibits CCA cell migration by suppressing the epithelial-mesenchymal transition (EMT) pathway and downregulates the PI3K-AKT
signaling pathway6. In zebrafish models, beta-eudesmol shows anti-angiogenic effects by inhibiting Vegfa and Vegfr27. In vivo studies in a CCA hamster model further support these findings,
showing modulation of apoptosis-related genes8. Molecular networking, used in exploring drug and herbal interactions, has been applied in various diseases like rheumatoid arthritis9,
hepatocellular carcinoma10, and colorectal cancer11. While clinical trials have confirmed AL’s efficacy and safety in CCA treatment6, the detailed molecular mechanisms remain unclear. This
study aims to apply molecular networking to identify gene targets and signaling pathways modulated by AL in advanced-stage intrahepatic CCA (iCCA). It will focus on: (i) constructing a gene
ontology based on statistical analysis, (ii) identifying key gene targets through molecular network and functional enrichment analysis, and (iii) evaluating the binding affinity of AL’s
active compounds using in silico docking simulations. This approach will enhance understanding of AL’s mechanisms and its potential as a CCA therapeutic agent. METHODS STUDY DESIGN,
PATIENTS, AND STUDY PROCEDURES This study is part of a phase IIA open-label, randomized controlled trial assessing the dose, pharmacokinetics, safety, and efficacy of _Atractylodes
lancea_(AL) in patients with advanced-stage intrahepatic cholangiocarcinoma (iCCA)12 [01/03/2021; TCTR20210129007; ICTRP Search Portal (who.int)]. The protocol was approved by the Ethics
Committee of Sakol Nakorn Hospital (No. 049/2021) and conducted in compliance with the Helsinki Declaration. Informed consent was obtained from all participants. The AL treatment regimen
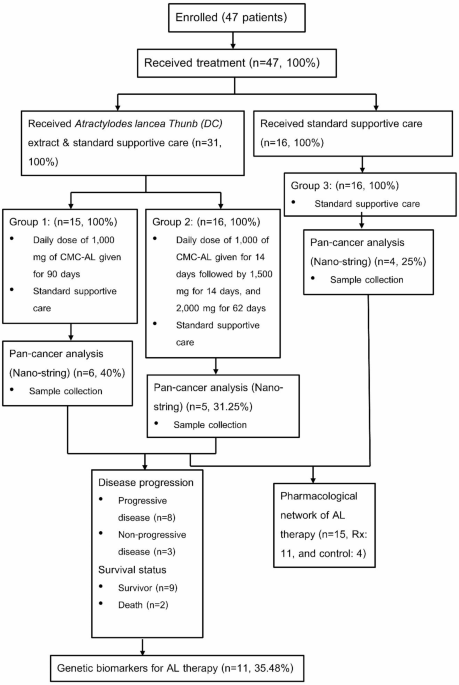
included: Group 1 (_n_ = 6): 1,000 mg daily of standardized AL extract for 90 days with standard care; 5 with non-progressive disease, 1 with progressive disease; Group 2 (_n_ = 5): Dose
escalation from 1,000 mg to 2,000 mg over 90 days with standard care; 3 with non-progressive disease, 2 with progressive disease; and Group 3 (_n_ = 4): Standard care alone; 3 with
progressive disease, 1 with non-progressive disease. Blood samples (3 mL) were collected on days 1 and 28. Plasma was isolated and stored at −80 °C for analysis. RNA was extracted from whole
blood, and gene expression was analyzed using the nCounter PanCancer Pathways Panel (NanoString Technologies, Seattle, US), which covered 730 genes linked to 13 cancer-related pathways. The
ratios of gene expression between day 28 and day 1 were analyzed to identify significant signaling pathways and differences between progressive and non-progressive disease, as well as
survivors and non-survivors. ANALYSIS OF THE ASSOCIATION BETWEEN GENE EXPRESSION AND AL TREATMENT Gene expression analysis in response to AL treatment involved a comprehensive network
analysis using functional enrichment through Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways, and Reactome. The comparisons included: (i) AL treatment (Groups 1
and 2) vs. non-treatment (Group 3), (ii) disease progression (progressive vs. non-progressive disease after AL treatment), and (iii) survival (survived vs. non-survived patients
post-treatment). Data normality was assessed using the Shapiro-Wilk test, with the independent-sample T-test for normally distributed variables and the Mann-Whitney U test for non-normally
distributed variables. The exact p-values were reported using SPSS version 23. Genes with significant expression changes were selected for molecular network analysis. The biological process
was the primary focus of molecular network analysis, as it captures the coordinated actions of molecular functions that collectively achieve biological objectives. This approach provides a
deeper understanding of the molecular mechanisms at play compared to examining individual molecular functions in isolation. Protein-protein interaction (PPI) networks were constructed using
Cytoscape, following these steps: (i) identification of significant gene expression variations between the AL-treated and control groups; (ii) access to the STRING database, selecting Homo
sapiens and full STRING networks with a confidence score cut-off of 0.4; (iii) importing gene expression data, including gene types, ratios, and p-values; (iv) functional enrichment analysis
through the cytoNCA plugin, which calculates betweenness, closeness, and degree centralities; and (v) iltering results to select KEGG pathways, Reactome pathways, and GO biological
processes. In the network, “degree” indicates a node’s direct connections, highlighting its role as a central hub. “Betweenness centrality” measures how often a node lies on the shortest
path between pairs of nodes, while “closeness centrality” indicates how efficiently a node connects to others in the network. GO and pathway enrichment analyses were performed using
Cytoscape 3.1.0 to visualize and annotate the results. Fisher’s exact test was used to select pathways involving at least three candidate genes, with statistical significance set at _p_ <
0.05 and a false discovery rate (FDR) cut-off of _p_ < 0.05. The PPI network and pathway enrichment analysis leveraged STRING confidence scores and FDR values to identify significant
protein interactions and associated pathways. THE ASSOCIATIONS BETWEEN BIOMARKERS AND THE FOLD CHANGES OF GENE EXPRESSIONS IN PATIENTS WITH DISEASE PROGRESSION (PROGRESSIVE AND
NON-PROGRESSIVE DISEASES) OR SURVIVAL STATUS (SURVIVORS AND NON-SURVIVORS) Independent t-test (for normally distributed data) and Mann Whitney U test (for non-normally distributed data) were
used to compare the differences in biomarkers (fold changes in biomarker levels and mRNA expression) between the groups of patients with progressive and non-progressive diseases, as well as
between those who survived and did not survive. Pearson correlation test (for normally distributed data) and Spearman’s correlation test (for non-normally distributed data) were used to
determine the correlation between biomarkers (fold changes in biomarker levels and mRNA expression) in the groups of patients with progressive and non-progressive diseases, as well as in
those who survived and did not survive. The statistical significance level was set at α = 0.05 for all tests. ROC ANALYSIS, CUT-OFF VALUE, AND MODEL PERFORMANCE Receive Operating Curve (ROC)
was applied to determine the cut-off levels for significant levels of mRNA expressions between the groups of patients with progressive and non-progressive disease and those who survived and
did not survive. Area Under Curve (AUC) of ≥ 0.7 was the predefined criterion for identifying substantial mRNA expressions between progressive and non-progressive diseases and survivors and
non-survivors. A p-value of ≤ 0.05 was set as a statistically significant level. The Youden’s index was employed to determine the optimal cut-off value. Sensitivity, specificity, accuracy
index, Negative predictive value (NPV), and positive predictive value (PPV) were computed to indicate the model’s performance. KAPLAN-MEIER ANALYSIS The designated cut-off value for each
important gene was used to categorize the significant genes into two groups, i.e., ratios above or below the threshold. The Kaplan Meier assessed the impact of mRNA expression (disease
progression and survival status) above or below the threshold on progression-free survival time (PFS) and overall survival (OS). A log-rank test was applied to determine the significant
differences between ratios (above or below threshold) on PFS and OS with the predefined p-value of ≤ 0.05.Pathway cross-talk analysis. The Jaccard index was used to assess the reliability of
cross-talk analysis. The inclusion criterion was a pathway with _≥_2 candidate genes (to exclude genes with inadequate biological information) and the exclusion criterion was the pair of
pathways with < 2 shared candidate genes. The selected pair of pathways were prioritized based on the Jaccard index of > 0.25 and > 0.6 for pathway-pathway interaction and pathway
redundancy, respectively13. IN SILICO DOCKING SIMULATION OF GENE TARGETS In silico docking simulations were conducted using AutoDock software to evaluate the binding affinities of
Atractylodes lancea (AL) compounds (atractylodin, beta-eudesmol, and hinesol), standard anti-CCA drugs (5-FU and gemcitabine), and reference compounds to ten significant target proteins:
JAK1, PI3KCA, HDAC2, ILR2A, ILR2B, TNF-α, SMAD2, NRAS, SOS1, ERRB2, and DMT3A. The crystallographic structures of the AL target proteins were retrieved from the RCSB Protein Data Bank, and
the active compound structures were obtained from the PubChem database in SDF format, then converted to PDB files using PyMOL or OpenBabel. A free energy force field was calculated using a
Lamarckian Genetic Algorithm to predict the binding affinity based on the Gibbs free energy. Docking was performed with the catalytic domain of each receptor to interact with the small
ligands (Table S1). The optimal docking conformation was selected based on the ligand conformation with the lowest root-mean-square deviation (RMSD) using a tolerance of 2.0 Å. A binding
affinity of ≥ 6 kcal/mol was considered acceptable. 5-FU and gemcitabine were chosen for docking simulations as they are first-line treatments for intrahepatic cholangiocarcinoma (iCCA).
RESULTS BASELINE CHARACTERISTICS The diagrammatic workflow in this study is shown in Fig. 1. Table 1 summarizes the baseline characteristics of the patients with advanced-stage iCCA in each
treatment group (group 1 and group 2) and the control group (group 3). No significant differences between AL-treated patients with progressive and non-progressive diseases were observed with
age (_p_ = 0.843), body weight (_p_ = 0.278), height (_p_ = 0.768), white blood cell count (_p_ = 0.212), platelet count (_p_ = 0.125), hemoglobin (_p_ = 0.105), INR (_p_ = 0.914), PT (_p_
= 0.753), PTT (_p_ = 0.694), creatinine clearance (_p_ = 0.471), AST (_p_ = 0.053), ALT (_p_ = 0.078), ALP (_p_ = 0.809), CPK (_p_ = 0.103), LDH (_p_ = 1.00), CA19-9 (_p_ = 0.661), CEA (_p_
= 0.177), and IL-6 (_p_ = 0.343) between the treatment and control groups. Subgroup analysis revealed no significant changes in baseline AST (_p_ = 0.842), ALT (_p_ = 0.630), ALP (_p_ =
0.352), LDH (_p_ = 0.376), CPK (_p_ = 0.09), CA19-9 (_p_ = 0.497), CEA (_p_ = 1), and IL6 (_p_ = 0.683) levels. The comparable values between survivors and non-survivors in the AL-treated
group were not substantially different [(AST, _p_ = 0.145), (ALT, _p_ = 0.352), (ALP, _p_ = 0.886), (LDH, _p_ = 1.00), (CPK, _p_ = 0.327), (CA19-9, _p_ = 1.00), (CEA, _p_ = 0.727), and (IL6,
_p_ = 0.183). ASSOCIATION BETWEEN GENE EXPRESSION AND AL TREATMENT Out of 730 genes analyzed, 52 showed significant changes in gene expression levels following AL treatment (Fig. 2).
PRKAR2A, SPOP, RXRG, FGF1, PAX8, TNFRSF10A, and TCFL1 genes showed high gene expression levels, while the remaining showed relatively low expression levels. Further analysis revealed a
marked difference in the expression levels of 16 gene targets in patients with progressive and non-progressive disease following AL therapy (Group 1 and 2). BDNF, ERBB2, TTK, and XPA showed
higher expression levels, while the remaining showed lower expression levels. Seven genes were identified with marked differences between survivors and non-survivors. HSPA1A, FOS, CBL, and
MMP9 were the top four upregulated genes. Tables S2, S3 and S4 summarize significant gene expressions (fold-change) between AL-treated and control group, and patients with progressive and
non-progressive diseases, and survivors and non-survivors, respectively. PROPOSED MOLECULAR SIGNALING PATHWAYS OF AL ACTION IN ADVANCED-STAGE ICCA A comprehensive network analysis in
AL-treated (Groups 1 and 2) and control (Group 3) identified 44 potential target genes. Thirty genes were identified as core hubs (Table 2). TNF-α was the network’s most significant hub (PPI
= 22), followed by PIK3CA (PPI = 15), NRAS (PPI = 14), JAK1 (PPI = 11), IL2RB (PPI = 11), and IL2RA (PPI = 11). Subsequent network interaction analysis revealed 44 nodes and 115 edges (PPI
= 5.18). Based on pathway enrichment analysis, AL action (specific condition or disease) was significantly linked with various signaling pathways, i.e., PI3K-AKT, NK cell-mediated
cytotoxicity, cellular apoptosis, MAPK, JAK-STAT and NF-kB in cancer, EGFR-resistance, focal adhesion, Fc epsilon RI, T cell receptor, cell senescence, regulation of actin cytoskeleton,
transcription misregulation, B cell receptor, mTOR, ERBB, Ras, VEGFR, PDL-1, RIG-I-like receptor, platinum drug resistance, and P53 signaling pathways (Fig. 3). Comprehensive network
analysis using Reactome database identified 38 multiple signaling pathways, i.e., signal transduction, IL2, IL3, IL4, IL5, IL13, GM-CSF, ILR SHC, extra-nuclear estrogen, FGFR3 in disease,
downstream signaling of activated FGFR1-4, DAP12, death receptor, FLT3, PDGFRA, and EGFR-v-III. These pathways were linked with 180 biochemical pathways. Functional enrichment analysis of
the 20 selected biological processes based on the set criterion, i.e., KEGG, biological processes, and Reactome are shown in Figs. 3 and 4, and 5, respectively. The set criterion included
the three shared genes with a p-value of ≤ 0.05 between signaling pathways for representing the KEGG, biological, and Reactome processes. A pharmacological mechanism of AL is shown in Fig.
6. THE ASSOCIATIONS BETWEEN BIOMARKERS AND THE FOLD CHANGES OF MRNA EXPRESSIONS IN PATIENTS WITH DISEASE PROGRESSION AND SURVIVAL STATUS, KAPLAN-MEIER ANALYSIS, AND COX-PROPORTIONAL
REGRESSION ANALYSIS DISEASE PROGRESSION The study assessed the relationship between proposed biomarkers and mRNA expression changes in patients experiencing disease progression and survival
status through Kaplan-Meier and Cox-proportional regression analyses. A significant difference in ALP ratios was observed between patients with progressive and non-progressive disease, with
a p-value of 0.048. Other biomarkers (AST, ALT, LDH, CPK, CA19-9, CEA, and IL-6) did not show significant associations (Table S5). The ROC analysis indicated that the cut-off value for ALP
ratios to distinguish between disease progression was ≥ 1.023 folds (AUC = 0.917, 95% CI = 0.739–1.00, _p_ = 0.04; Youden’s index = 0.875) (Fig S1). This yielded a sensitivity of 87.5%,
specificity of 100%, positive predictive value (PPV) of 1, negative predictive value (NPV) of 0.75, and accuracy index (AI) of 0.9. However, Kaplan-Meier analysis revealed significant no
differences in progression-free survival (PFS) based on these ALP ratios (_p_ = 0.104) (Fig S2). No significant difference following cox-proportional regression analysis was found disease
progression and ALP ratios (HR: 5.27, 95%CI: 0.89–100.6, _p_ = 0.1265) (high vs. low) (Fig S3). The ROC analysis for various genes indicated significant differences in their expression
between progressive and non-progressive disease: APC (≥ 1.49 folds, AUC = 0.917, _p_ < 0.001), BAX (≥ 0.82 folds, AUC = 0.917, _p_ < 0.001), DNMT3A (≥ 0.805 folds, AUC = 1, _p_ <
0.001). Others included ETS2, GNA11, IGF1, IRAK2, PLCG2, POLB, RAF1, THEM4, BDNF, CDKN2A, ERBB2, TTK, and XPA, with respective AUC values indicating strong predictive capability (AUCs ≥
0.917) (Fig S4 ,5). Significant associations with PFS were noted for DMNT3A, GNA11, IGF1, IRAK2, POLB, RAF1, THEM4, and XPA (Table S6, Fig S6-21). RAF1 and XPA demonstrated significant
hazard ratios 9.316 (_p_ = 0.042) and 0.174 (Reciprocal HR: 5.732) (_p_ = 0.037) (Fig S22-36, Table S7), respectively, indicating a strong association with disease progression. A correlation
analysis revealed positive correlations between ALP ratios and BAX, PLCG2, and RAF1, while BDNF and TTK showed negative correlations (Table S8). Notably, in patients with non-progressive
disease, a strong positive correlation (_r_ = 0.998, _p_ = 0.037) between ALP and GNA11 ratios was found, alongside a significant negative correlation with TTK (_r_=−1.00, _p_ = 0.009) in
patients responding to AL therapy (Table S9). SURVIVAL STATUS No significant differences in known biomarkers were observed between survivors and non-survivors receiving AL therapy (p-values
ranged from 0.145 to 1.00) (Table S10). ROC analysis revealed significant differences for AR and EYA1 ratios, both demonstrating AUC values of 1 (_p_ = 0.034), with cut-off values of ≥ 1.895
and ≥ 1.67 folds, respectively (Fig S37, Table S11). Significant differences in overall survival (OS) were observed for AR and EYA1 (_p_ = 0.008) (Fig S38, 39, Table S12). The Cox hazard
ratios did not demonstrate significant predictive value (_p_ = 0.504) (Fig S40, 41, Table S13). Strong positive correlations were found between AR and CA19-9 ratios (Table S14), and between
EYA1 and CA19-9 ratios (Table S15), indicating a potential association of these biomarkers with survival outcomes. In addition, negative correlations were reported between AR and ALT ratios
(Table S14), and between EYA1 and CPK ratios (Table S15, indicating an association of these biomarkers with survival outcomes. Logistic regression highlighted RAF1 and XPA as significant
predictors for disease progression, with acceptable McFadden’s scores indicating model reliability. Multi-logistic regression suggested that AR and EYA1 were significantly associated with
OS, but the high McFadden’s score indicated potential model inadequacy. Probability risks for disease progression were stratified into four levels based on RAF1 and XPA ratios, suggesting
tailored approaches for patient management based on risk assessment. ADVERSE DRUG REACTIONS The incidence rates of grade 2 and 3 adverse events were consistent across groups, with no
significant differences in blood parameters or liver function tests, suggesting minimal AL-related adverse effects. CROSS-TALK AMONG POTENTIAL TARGET GENES AND SIGNALING PATHWAYS Twenty-four
enriched pathways were investigated for the interplay between the two functional interaction pathways. The cross-talk signaling pathways associated with tumor growth/proliferation/ survival
of cancer, angiogenesis, and metastasis were categorized into three groups according to their abilities to depict the mechanisms involved in tumor formation and invasion. The major
cross-talk module consisted of various cellular processes, including cellular response to external stimuli, cell communication, regulation of cell death, signal transduction, and immune
response. Table 3 summarizes the cross-talk signaling pathways associated with tumor growth, proliferation, progression, survival, angiogenesis, and metastasis in patients with
advanced-stage iCCA following AL therapy. _Tumor growth/proliferation/survival pathway_ The PI3K-AKT and JAK-STAT pathways demonstrated the highest similarity in tumor growth, proliferation,
and survival, with a Jaccard value of 0.54, indicating significant overlap in the shared genes between the two pathways. The second-highest ranked pathways were MAPK-T cell receptor,
NK-cell mediated toxicity-B cell receptor, Fc epsilon RI-proteoglycan, proteoglycan-T cell receptors, proteoglycan-NK-cell mediated cytotoxicity, PDL-1-Ras, PDL-1, and TNF-α signaling
pathways. _Angiogenesis_ The highest-ranked pathways for angiogenesis included EGFR resistance-mTOR, EGFR resistance-Ras, EGFR resistance-PDL1, EGFR resistance-ErBb, ErBb-PDL1, ErBb-Ras, and
ErBb-proteoglycan signaling, all showing the same level of similarity with a Jaccard index of 0.5, indicating significant cross-linking in their involvement. _Metastasis_ The regulation of
actin cytoskeleton-Fc epsilon RI pathway had the highest Jaccard index for metastasis. CANDIDATE GENE TARGETS OF AL GENE TARGETS ASSOCIATED WITH DISEASE PROGRESSION Eleven gene targets and
18 edges, with an average degree of 3.27 [PPI], were used to build a comprehensive pharmacology-based network analysis in patients with progressive compared with non-progressive diseases.
ERBB2 (1st rank), CDKN2A (2nd rank), BDNF (3rd rank), and IGF-1 (4th rank), DNMT3A (4th rank), Raf (4th rank) and GNA11 (4th rank) showed degrees of 8, 6, 4, 3, 3, 3, and 3, respectively,
indicating their potential central hubs in regulating iCCA progression. Three different analyses for regulating iCCA progression following functional analysis based on the eleven gene
targets including KEGG pathway, biological processes, and Reactome pathway, were determined as follows: i) KEGG analysis of the modulatory effects of AL on various signaling pathways such as
the EGFR tyrosine kinase inhibitor, micro-RNA in cancer, Ras, TP53, platinum-drug resistance, MAPK, PI3K-AKT, ERBB2, and HIF-signaling pathways, II) signaling transduction of various
biological processes, e.g., regulation of the cellular metabolic process and protein phosphorylation, and intracellular signal transduction, III) Reactome analysis of platelet activation and
aggregation signaling pathways associated with GNA11, RAF1, IGF1, and PLCG2 target genes based on protein-protein interaction analysis. GENE TARGETS ASSOCIATED WITH SURVIVAL A comprehensive
pharmacology-based network analysis was performed in survivors and non-survivors using seven gene targets and five edges, with an average degree [PPI] of 1.43. AR, FOS, HSPA1A, and MMP9 had
degrees of 3 (1st rank), 3 (1st rank), 2 (2nd rank), and 2 (2nd rank). Functional enrichment analysis suggests that AL modulates toll-like receptor cascades, IL12 family signaling,
regulation of mRNA stability, autophagy, signaling by NOTCH, regulation of TP53 activity, signaling by TGF-beta receptor complex, and MAPK family signaling cascades. BINDING AFFINITY OF AL
TO CANDIDATE GENE TARGETS Table 4 summarizes the binding affinities of the three components of AL (atractylodin, beta-eudesmol, and atractylodin), standard drugs for iCCA (gemcitabine and
5-FU), and reference drugs/compounds (Ruxolitinib, idelalisib, panobinostat, SM16, GTP, BI-3406, Afatinib, and guadecitabine) to eleven candidate molecular targets of AL in iCCA. All
reference drugs showed the highest binding affinities to all targets. Among the three components of AL, hinesol showed the highest binding affinities to all targets. The binding affinity of
beta-eudesmol was relatively lower. Atractylodin weakly binds to most targets. For standard drugs, gemcitabine showed a high binding affinity comparable to hinesol. Residue proteins and root
mean square deviation (RMSD) for each drug and target (binding affinity ≥ 6 kcal mol−1) are shown in Supplementary figures S42-77. DISCUSSION ASSOCIATIONS BETWEEN BIOMARKERS AND DISEASE
PROGRESSION This study identified an ALP ratio increase (≥ 1.024) as a potential predictor of iCCA progression following AL treatment. However, Kaplan-Meier analysis showed no significant
correlation between ALP changes and progression-free survival (PFS), suggesting ALP ratios alone do not independently predict treatment response. Elevated ALP levels are linked to various
hepatobiliary and metastatic conditions, including CCA, where high ALP correlates with rapid progression after gemcitabine-cisplatin treatment (_p_= 0.01)14. Additionally, high platelet
counts and lower hemoglobin levels were associated with a worse prognosis, with hemoglobin showing a hazard ratio (HR) of 0.90 (95% CI = 0.83–0.97, _p_= 0.009)15. Regarding mRNA expression,
11 of 16 genes demonstrated predictive potential for AL response, with an area under the curve (AUC) > 0.9 (_p_ < 0.001). Patients with RAF1 ratios ≥ 0.5 had a 9.32-fold higher risk of
disease progression, while those with XPA levels < 1.34 had a 5.73-fold increased risk, emphasizing their role as early prognostic markers for AL therapy continuation. Elevated RAF1
ratios indicate heightened progression risk, whereas lower XPA levels suggest impaired DNA repair, contributing to disease advancement. Unlike targeted mRNA/protein-based therapies,
conventional chemotherapy (gemcitabine/cisplatin) lacks specific molecular targets. However, overexpression of ATP-binding cassette (ABC) transporters, such as ABCC1 (MRP1), reduces
intracellular drug concentrations, contributing to 5-FU resistance16. Similarly, altered orotate phosphoribosyl transferase activity weakens FdUMP activation, impairing 5-FU efficacy, while
OCT4 and MRP1 upregulation have been implicated in gemcitabine resistance17. Furthermore, FGFR2 alterations impact AKT/mTOR and STAT3 pathways, linking them to gemcitabine resistance,
reinforcing the need for FGFR2 testing before treatment18. For targeted therapy, risk-stratified gene expression analysis identified HACL1, LAMA4, GMNN, C1RL, PCBD1, and FXYD2 as prognostic
markers. The low-risk group responded better to bosutinib, gefitinib, gemcitabine, and paclitaxel, whereas the high-risk group showed improved responses to axitinib, cisplatin, and
imatinib19. Additionally, PD-L1 expression serves as a prognostic indicator for immune checkpoint inhibitor (ICI) efficacy in iCCA patients. Notably, there was no overlap in the genetic
markers associated with AL treatment and those linked to conventional or targeted therapies, suggesting distinct molecular pathways drive progressive disease under different therapeutic
regimens. CORRELATION ANALYSIS AND PROGNOSTIC MARKERS PLCG2 showed the strongest positive correlation with ALP (_r_ = 0.736, _p_ = 0.01), linking its activation of the ERK pathway to iCCA
proliferation and apoptotic resistance. Patients with elevated PLCG2 levels experienced worse disease progression (1.40 vs. 0.41). Conversely, TTK, a promoter of tumor survival in gastric
and hepatocellular cancers, exhibited a moderate inverse correlation with ALP (_r_=−0.727, _p_ = 0.01). These findings suggest that genetic signatures play a role in cancer biomarker
expression and disease progression. RAF1 ratios correlated with increased progression risk, while lower XPA ratios indicated reduced risk. Raf-1 inhibition has been shown to suppress CCA
metastasis by modulating MMP9/MMP4 expression and inhibiting EGF-induced cell growth20. Clinical studies reported an 18.1% partial response rate for Ras inhibitors across multiple cancers,
including CCA21. Re-challenging Raf-1 inhibitors in BRAF-1 aberrant patients also yielded clinical responses, supporting its therapeutic potential. XPA, crucial for nucleotide excision
repair (NER), mediates platinum-based chemotherapy resistance22. In progressive iCCA cases, mRNA expression of XPA was elevated; however, XPA ratios < 1 suggest AL may suppress its
expression, potentially reversing platinum resistance. Combining AL with gemcitabine-cisplatin may enhance disease control rates (DCR) in patients with platinum-resistant iCCA compared to
chemotherapy alone. SURVIVAL ANALYSIS This study found no significant differences in liver enzymes, CA19-9, CEA, or IL-6 between AL-treated iCCA patients and controls, suggesting these
biomarkers may not predict survival in this cohort. However, the small sample size, with only two deaths recorded, may limit statistical power. Previous studies have shown that ALP levels ≥
138 IU/L were associated with shorter overall survival (HR = 1.654, 95% CI = 1.170–2.38, _p_= 0.004) following chemotherapy, compared to ALP < 138 IU/L (11.6 vs. 4.47 months)23.
Similarly, CA19-9 < 1,000 IU/mL correlated with improved one-year survival23. While both gemcitabine and 5-FU influenced ALP levels, only gemcitabine-based treatment showed significant
changes23. Jiang et al. reported that iCCA patients with ALP ≤ 147 IU/L had longer one-year and three-year survival (23 vs. 10.4 months) and a 1.64-fold higher probability of survival (95%
CI = 1.20–2.22, _p_= 0.002) than those with ALP > 147 IU/L24. Higher CA19-9 levels were linked to better one-year survival than ALP ≤ 37 µg/mL (29.2 vs. 14.1 months), with a 1.80-fold
increased survival probability (95% CI = 1.337–2.445, _p_< 0.001) in those with ALP ≤ 37 µg/mL25. These findings highlight the prognostic value of ALP and CA19-9 in iCCA, though their
relevance in AL-treated patients remains unclear. PROMISING CANDIDATE GENE TARGETS FOR AL AL therapy targets critical pathways involved in iCCA progression, including PI3K-AKT, JAK-STAT, and
Ras/MAPK/mTOR, which regulate tumor growth, survival, and immune evasion26,27,28,29,30,31,32,33,34. AL enhances immune response by stimulating NK, T-, and B-cell activity through genes such
as GZMB, TNFRSF10A, PRKCG, PIK3CA, and SYK, which are linked to NK cell-mediated cytotoxicity and adaptive immunity35,36,37,38,39,40. Downregulation of tumor-promoting genes, including
GNA11 (3.5-fold), IGF1 (7.2-fold), THEM4 (13.15-fold), PLCG2 (8.2-fold), POLB (7.14-fold), and RAF1 (17.35-fold), correlates with reduced tumor proliferation, differentiation, invasion, and
metastasis41,42,43,44,45. Suppressing RAF1 may limit EGFR resistance and enhance the efficacy of EGFR/PI3K inhibitors, potentially overcoming drug resistance in iCCA46,47,48,49,50,51.
Additionally, suppression of TSLP (7-fold), COL5A2, FLNC, ITGA3, and PLAU may improve overall survival by reducing tumor progression, angiogenesis, and immune suppression52,53,54,55,56,57.
AL therapy appears to shift gene expression toward tumor suppression, supporting its potential as an adjunct treatment for advanced iCCA by inhibiting proliferation, reversing resistance,
and enhancing immune response. PROPOSED MOLECULAR TARGETS OF AL ACTION KEGG pathway enrichment analysis identified six critical pathways involved in iCCA progression following AL therapy:
tumor proliferation, growth, survival, angiogenesis, invasion, and metastasis. The PI3K/AKT pathway (FDR = 2.26E-23) was the most significantly altered, impacting tumor progression through
mTOR, Raf-1, NF-κB, MDM2, PDK1, p21/p27, Caspase 3/7/9, FOXO, eNOS, and
MMP-2/9/1317,18,34,41,42,43,44,45,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82. AL therapy modulated 15 key genes linked to the PI3K/AKT pathway,
including OSM, SOS1, IL2RA, IL2RB, NRAS, NOS3, EIF4EBP1, JAK1, SYK, BCL-2, FGF1, IKBKG, ITGA3, GNGT1, and PIK3CA. Notably, JAK1 (33-fold downregulated) regulates tumor growth and immune
response, and its suppression may control iCCA progression and reduce immunosuppressive effects17,31,54,70,71,72,73,74. OSM (downregulated), associated with lymphatic and distant metastasis,
correlates with better overall survival (OS) and recurrence-free survival (RFS)54. NOS3 (downregulated) suppresses apoptosis and promotes angiogenesis via VEGF; its downregulation may
inhibit metastasis43,76. EIF4EBP1 (downregulated) is linked to poor differentiation and lymph node metastasis in hilar CCA, and suppression may reduce tumor invasion28. SOS1 (22-fold
downregulated) regulates PI3K/AKT, EMT, and MAPK signaling, and its suppression could inhibit EGFR resistance, EMT, and metastasis78,79,80. ITGA3 (8-fold downregulated) activates PI3K
through focal adhesion; downregulation correlates with reduced tumor size, lymph node metastasis, and advanced TNM stage56. BCL-2 (7-fold downregulated) is linked to chemoresistance, and its
suppression may enhance apoptosis and treatment response46,75. FGF1 (14-fold upregulated) potentially activates angiogenesis via PI3K/AKT, although its impact may be minimal due to low
FGFR1 overexpression in iCCA83,84,85. Downregulation of key oncogenic genes in the PI3K/AKT pathway suggests that AL therapy suppresses iCCA proliferation, growth, angiogenesis, and
metastasis. The 6-fold downregulation of PIK3CA indicates a significant reduction in pathway activation, supporting the observed improvements in OS and progression-free survival (PFS) in
AL-treated patients compared to palliative care. These findings position AL as a promising adjunct therapy in advanced-stage iCCA. NK CELL-MEDIATED CYTOTOXICITY The NK cell-mediated
cytotoxicity pathway was the second most altered pathway following AL therapy, involving key immune response regulators like GZMB, TNFRSF10A, TNF-α, PKCG, and PIK3CA84. GZMB (downregulated)
is essential for apoptosis via caspase-3/7 activation, and its reduction may impair NK and T-cell cytotoxicity, affecting immunosuppressive functions52. TNFRSF10A (10-fold upregulated), also
known as TRAIL-1, promotes apoptosis in iCCA cells through DR4/5 activation. Its increase may compensate for GZMB downregulation, enhancing tumor cell death29. TNF-α (downregulated) plays a
role in NK cell proliferation and cytotoxicity, but its reduction did not significantly affect CDKN2A expression, indicating stable NK cell function80,81. AL therapy modulates NK
cell-mediated immune responses, potentially suppressing granzyme-mediated apoptosis while enhancing TRAIL-dependent apoptosis, supporting its anti-tumor effects in iCCA. Additionally,
downregulation of key oncogenic genes in the PI3K/AKT pathway suggests AL therapy inhibits iCCA proliferation, growth, angiogenesis, and metastasis. Notably, the 6-fold downregulation of
PIK3CA indicates a significant reduction in pathway activation, supporting the observed improvements in OS and PFS in AL-treated patients compared to palliative care. These findings position
AL as a promising adjunct therapy for advanced-stage iCCA. APOPTOSIS PATHWAY The apoptosis pathway ranked third among the pathways linked to AL action, involving genes such as GZMB,
TNFRSF10A, PI3KCA, BCL-2A1, MAP3K5, NRAS, BCL-2, TNF, and IKBKG. BCL-2A1, an anti-apoptotic protein, promotes survival in various cancers, with higher overexpression in lymphoma and leukemia
patients compared to those with solid tumors86. In advanced-stage solid tumors, BCL-2A1 upregulation is linked to chemoresistance86. The downregulation of both BCL-2A1 and BCL-2 after AL
therapy may increase sensitivity to chemotherapy-resistant iCCA. IN SILICO DOCKING SIMULATION In silico docking results revealed high binding affinities of hinesol and beta-eudesmol to the
candidate molecular targets, supporting AL’s clinical efficacy in a phase IIA trial12. While hinesol showed low cytotoxic potency against iCCA cells87, it had high binding affinity to all
targets except HDAC2, NRAS, and SOS1. Notably, hinesol combined with beta-eudesmol or atractylodin exhibited synergistic cytotoxic effects across all iCCA cell lines87. These findings
suggest that the compounds effectively bind to their molecular targets, preventing tumor development and inducing cell death. Gemcitabine demonstrated higher binding affinity than 5-FU to
all targets, likely due to the differing molecular targets of the two drugs—5-FU targets thymidylate synthase, while gemcitabine targets DNA replication components88. Patients treated with
gemcitabine plus cisplatin89showed superior tumor control, time-to-progression, disease-free survival, and overall survival compared to those treated with 5-FU, leucovorin, and cisplatin89.
5-FU and gemcitabine were selected for docking simulation as first-line treatments for intrahepatic cholangiocarcinoma, although their mechanisms of action are limited to targeting
AL-related molecules such as JAK1, PI3KCA, HDAC2, and IL2RA. Further preclinical studies are needed to confirm the efficacy of gemcitabine and 5-FU on these potential targets. POTENTIAL
ERRORS AND BIAS Potential errors in ligand-receptor interactions affecting ligand binding affinity can be categorized into four sources as follows: _Protein structure_ Missing residues or
domains may alter the binding pocket geometry. Incorrect rotamer assignments within the binding pocket can misalign interactions with the ligand. Protein flexibility is often overlooked.
_Ligand_ Inadequate sampling of ligand conformations may underestimate the optimal binding pose. Incorrect protonation states of functional groups can affect receptor interactions.
Inaccurate force field parameters can lead to incorrect energy calculations. _Docking algorithm_ Search algorithms may fail to sufficiently sample potential binding modes, especially in
complex interactions. Improperly defined binding sites may lead to irrelevant biological targets. _Environmental factors_ Not accounting for water molecules around the binding site can
distort protein-ligand interaction. EVIDENCE BEFORE THIS STUDY Beta-eudesmol, atractylodin, and hinesol --key active compounds of _Atractylodes lancea_(Thunb) DC exhibit antiproliferative,
apoptotic, anti-metastatic, and anti-angiogenic effects through multiple mechanisms: (i) promotion of cell cycle arrest through modulation of p16, CDK1, CDK4, and p218; (ii) apoptosis
activation through increasing Bax/Bcl2 ratio, TNFRSF6, cytochrome C, and caspase activation (caspase-3, −8, −9, Apaf1, Bax-3)8,90; (iii) immunostimulation through enhancing CDKN2B
expression, promoting immune control of iCCA90; (iv) PI3K-AKT pathway suppression through downregulating phosphorylated PI3K, AKT, p-p38MAPK, STAT1/3, HO1, and NF-κB6,91; (v)
anti-angiogenesis through downregulating Vegfa and Vegfr2 expression7; and (vi) anti-metastatic effects through downregulating MMP9, N-Cadherin, and TGF-beta expression8. These findings stem
from in vitro and in vivo studies; however, clinical validation in humans remains lacking. ADDED VALUE OF THIS STUDY This is the first study to provide a network analysis of AL and its
bioactive constituents—beta-eudesmol, atractylodin, and hinesol—in patients with advanced intrahepatic cholangiocarcinoma (iCCA). Our findings demonstrate that AL inhibits iCCA growth
through multiple mechanisms. AL promotes apoptosis via Bcl-2, Bcl-2A1, ASK, and SMAD2, enhances NK cell-mediated cytotoxicity through FAS-TRAIL, TNFRSF10A, PKCG, and TNF-alpha, and
suppresses CCA metastasis by targeting ITGA3 and EIF4EBP1. It also affects angiogenesis by interacting with NOS3, ERBB2, and FGF1/FGFR1. Additionally, AL sensitizes CCA to
gemcitabine/5-FU-induced chemoresistance by modulating the Bcl-2/Bcl-2A1 pathway. Docking simulations confirm that AL bioactives bind to key targets, including JAK-1, HDAC2, IL2RA, SMAD2,
NRAS, SOS1, ERBB2, and DNMT3A, which are involved in metastasis and angiogenesis. Notably, RAF1 and XPA were identified as potential new targets for CCA therapies. IMPLICATIONS OF ALL THE
AVAILABLE EVIDENCE Our data, consistent with previous studies, show that AL and its bioactive compounds significantly control cholangiocarcinoma (CCA) through multiple mechanisms, including
enhancing apoptosis, inhibiting cellular growth, boosting NK cell-mediated cytotoxicity, preventing angiogenesis, and inhibiting invasion. We also identify new targeted proteins for CCA
therapy. Previous research highlights AL’s antiproliferative effects, mainly via the PI3K/AKT pathway, and its role in caspase-dependent and independent apoptosis, anti-angiogenesis, and
tumor invasion prevention. Through network analysis, we identified new targets and confirmed existing ones. Notably, patients treated with AL and exhibiting non-progressive disease (with
lower RAF1 and higher XPA expressions) provide valuable insights for developing new drugs and treatment strategies for intrahepatic cholangiocarcinoma (iCCA). IMPACTS OF THE STUDY Combining
AL with a first-line regimen (gemcitabine plus cisplatin) may improve the disease control rate (DCR) in patients with platinum-based chemotherapy resistance. This study’s results, using a
probability approach, serve as early prognostic markers, aiding in predicting the DCR, minimizing unnecessary treatments, and maximizing efficacy in clinical settings. LIMITATIONS The
statistical analysis of multiple comparisons may be limited by a false positive rate, as Bonferroni correction was not applied. Additionally, the molecular network relies on static
protein-protein interactions (PPI), while PPIs are dynamic, and gene expression can change over time. mRNA expression may not always correlate with protein translation, and further
validation through protein expression, proteomics, and functional analysis, including western blot and kinetic binding assays, is needed to confirm these results. While docking simulations
provide useful insights into protein-ligand binding affinity, their accuracy is influenced by the quality of input data (protein structure, ligand quality, docking algorithm, and
environmental factors). Neglecting protein flexibility, as seen with AutoDock Vina, may lead to inaccurate binding affinity calculations. Further assays, including western blot analysis and
kinetic binding studies, are necessary to validate the interactions between AL bioactives and their targets. The Lamarckian Genetic Algorithm (LGA) in AutoDock accounts for flexible ligand
poses, helping predict binding affinity for flexible proteins in biological contexts. However, limitations like atom type constraints and memory issues can result in long computational
times. These limitations can be minimized by using well-refined protein structures (from X-ray crystallography or NMR with ≤ 3 Å resolution), selecting complete protein residues, defining
binding pockets based on existing literature, and considering the role of surrounding water molecules. CONCLUSION A molecular networking study confirmed AL as a potential alternative
treatment for iCCA by targeting key pathways such as PI3K/AKT, NK cell-mediated cytotoxicity, and apoptosis. These pathways regulate iCCA development, progression, angiogenesis, and
invasion. This study highlights the utility of molecular networking in identifying signaling targets of herbal medicines, which contain both active and non-active components. DATA
AVAILABILITY The authors confirm that the data supporting the findings of this study are available within the article and/or its Supplementary Materials and Methods. The datasets used and/or
analysed during the current study are available from the corresponding author upon reasonable request. REFERENCES * Banales, J. M. et al. Cholangiocarcinoma 2020: The next horizon in
mechanisms and management. _Nat. Rev. Gastroenterol. Hepatol._ 17:9, 557–588. https://doi.org/10.1038/s41575-020-0310-z (2020). Epub 2020 Jun 30. Article Google Scholar * Na-Bangchang, K.,
Plengsuriyakarn, T. & Karbwang, J. Research and development of atractylodes lancea (Thunb) DC. as a promising candidate for cholangiocarcinoma chemotherapeutics. _Evid. Based
Complement. Alternat Med._ 2017, 5929234. https://doi.org/10.1155/2017/5929234 (2017). Article PubMed PubMed Central Google Scholar * Martviset, P., Chaijaroenkul, W., Muhamad, P. &
Na-Bangchang, K. Bioactive constituents isolated from _Atractylodes lancea_ (Thunb.) DC. rhizome exhibit synergistic effect against cholangiocarcinoma cell. _J. Exp. Pharmacol._ 10, 59–64
(2018). Article PubMed PubMed Central CAS Google Scholar * Kotawong, K., Chaijaroenkul, W., Muhamad, P. & Na-Bangchang, K. Cytotoxic activities and effects of atractylodin and
β-eudesmol on the cell cycle arrest and apoptosis on cholangiocarcinoma cell line. _J. Pharmacol. Sci._ 136(2), 51–56. https://doi.org/10.1016/j.jphs.2017.09.033 (2018). Article PubMed CAS
Google Scholar * Srijiwangsa, P., Ponnikorn, S. & Na-Bangchang, K. Effect of β-eudesmol on NQO1 suppression-enhanced sensitivity of cholangiocarcinoma cells to chemotherapeutic
agents. _BMC Pharmacol. Toxicol._ 19(1), 32. https://doi.org/10.1186/s40360-018-0223-4 (2018). Article PubMed PubMed Central CAS Google Scholar * Acharya, B., Chajaroenkul, W. &
Na-Bangchang, K. β-Eudesmol inhibits the migration of cholangiocarcinoma cells by suppressing epithelial-mesenchymal transition via PI3K/AKT and p38MAPK modulation. _Asian Pac. J. Cancer
Prev._ 23 (8), 2573–2581. https://doi.org/10.31557/APJCP.2022.23.8.2573 (2022). Article PubMed PubMed Central CAS Google Scholar * Tshering, G., Pimtong, W., Plengsuriyakarn, T. &
Na-Bangchang, K. Anti-angiogenic effects of beta-eudesmol and atractylodin in developing zebrafish embryos. _Comp. Biochem. Physiol. C Toxicol. Pharmacol._ 243, 108980.
https://doi.org/10.1016/j.cbpc.2021.108980 (2021). Article PubMed CAS Google Scholar * Sonsomnuek, P., Tarasuk, M., Plengsuriyakarn, T., Boonprasert, K. & Na-Bangchang, K. Apoptotic
and anti-metastatic effects of _Atractylodes lancea_ (Thunb.) DC. in a Hamster model of cholangiocarcinoma. _Asian Pac. J. Cancer Prev._ 23 (9), 3093–3101.
https://doi.org/10.31557/APJCP.2022.23.9.3093 (2022). Article PubMed PubMed Central CAS Google Scholar * Zuo, J. et al. Integrating molecular network and metabolomics study on
anti-rheumatic mechanisms and antagonistic effects against methotrexate-induced toxicity of Qing-Luo-Yin. _Front. Pharmacol._ 9, 1472. https://doi.org/10.3389/fphar.2018.01472 (2018). [old:
5]. Article PubMed PubMed Central CAS Google Scholar * Liu, Z., Ma, H. & Lai, Z. Revealing the potential mechanism of _Astragalus membranaceus_ improving prognosis of hepatocellular
carcinoma by combining transcriptomics and molecular network. _BMC Complement. Med. Ther._ 21(1), 263. https://doi.org/10.1186/s12906-021-03425-9 (2021). Article PubMed PubMed Central
CAS Google Scholar * Lu, Y., Dong, K., Yang, M. & Liu, J. Molecular network-based strategy to investigate the bioactive ingredients and molecular mechanism of _Evodia rutaecarpa_ in
colorectal cancer. _BMC Complement. Med. Ther._ 23, 1433. https://doi.org/10.1186/s12906-023-04254-8 (2023). Article CAS Google Scholar * Na-Bangchang, K. et al. Phase IIa clinical trial
to evaluate safety and efficacy of capsule formulation of the standardized extract of _Atractylodes lancea_ (Thunb) DC in patients with advanced-stage intrahepatic cholangiocarcinoma. _J.
Tradit Complement. Med._ (2023). (In press). * Odongo, R., Demiroglu-Zergeroglu, A. & Çakır, T. A systems pharmacology approach based on oncogenic signalling pathways to determine the
mechanisms of action of natural products in breast cancer from transcriptome data. _BMC Complement. Med. Ther._ 21(1), 181. https://doi.org/10.1186/s12906-021-03340-z (2021). Article PubMed
PubMed Central CAS Google Scholar * Zhou, Z. et al. Tumor-associated neutrophils and macrophages interaction contributes to intrahepatic cholangiocarcinoma progression by activating
STAT3. _J. Immunother Cancer_ 9(3), e001946. https://doi.org/10.1136/jitc-2020-001946 (2021). Article PubMed PubMed Central Google Scholar * Yothaisong, S. et al. Increased activation of
PI3K/AKT signaling pathway is associated with cholangiocarcinoma metastasis and PI3K/mTOR Inhibition presents a possible therapeutic strategy. _Tumour Biol._ 34(6), 3637–3648.
https://doi.org/10.1007/s13277-013-0945-2 (2013). Article PubMed CAS Google Scholar * Fang, Z., Lu, L., Tian, Z. & Luo, K. Overexpression of phosphorylated 4E-binding protein 1
predicts lymph node metastasis and poor prognosis of Chinese patients with hilar cholangiocarcinoma. _Med. Oncol._ 31, 5940. https://doi.org/10.1007/s12032-014-0940-5 (2014). Article CAS
Google Scholar * Liao, W., Lin, J. X. & Leonard, W. J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. _Immunity_ 38(1), 13–25.
https://doi.org/10.1016/j.immuni.2013.01.004 (2013). Article PubMed PubMed Central CAS Google Scholar * Biswas, P. et al. Analysis of SYK gene as a prognostic biomarker and suggested
potential bioactive phytochemicals as an alternative therapeutic option for colorectal cancer: an in-silico pharmaco-Informatics investigation. _J. Pers. Med._ 11 (9), 888.
https://doi.org/10.3390/jpm11090888 (2021). Article PubMed PubMed Central Google Scholar * Kang, Q. et al. Characterization and prognostic significance of mortalin, Bcl-2 and Bax in
intrahepatic cholangiocarcinoma. _Oncol. Lett._ 15, 2161–2168. https://doi.org/10.3892/ol.2017.7570 (2018). Article PubMed CAS Google Scholar * Chen, L. & Tiannqianng, S. Multi-omics
characterization of cholangiocarcinoma and association with prognostic and therapeutic molecular subtypes (abstract). _ASCO_ Supplemennt_1.60 (2023) * Xia, T. et al. Immune cell atlas of
cholangiocarcinomas reveals distinct tumor microenvironments and associated prognoses. _J. Hematol. Oncol._ 15(1), 37. https://doi.org/10.1186/s13045-022-01253-z (2022). Article PubMed
PubMed Central CAS Google Scholar * Taniai, M. et al. Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. _Cancer Res._
64(10), 3517–3524. https://doi.org/10.1158/0008-5472.CAN-03-2770 (2004). Article PubMed CAS Google Scholar * Kobayashi, S., Werneburg, N. W., Bronk, S. F., Kaufmann, S. H. & Gores,
G. J. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. _Gastroenterology_ 128(7), 2054–2065.
https://doi.org/10.1053/j.gastro.2005.03.010 (2005). Article PubMed CAS Google Scholar * Roy, S., Glaser, S. & Chakraborty, S. Inflammation and progression of cholangiocarcinoma:
Role of angiogenic and lymphangiogenic mechanisms. _Front. Med. (Lausanne)_ 6, 293. https://doi.org/10.3389/fmed.2019.00293 (2019). Article PubMed Google Scholar * Ma, L. et al.
Single-cell atlas of tumor cell evolution in response to therapy in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. _J. Hepatol._ 75, 1397–1408.
https://doi.org/10.1016/j.jhep.2021.06.028 (2021). Article PubMed PubMed Central CAS Google Scholar * Vogler, M. BCL2A1: The underdog in the BCL2 family. _Cell. Death Differ._ 19,
67–74. https://doi.org/10.1038/cdd.2011.158 (2012). Article PubMed CAS Google Scholar * Pan, J. et al. Reactive oxygen species-activated akt/ask1/p38 signaling pathway in nickel
compound-induced apoptosis in Beas 2b cells. _Chem. Res. Toxicol._ 23, 568–557 (2010). Article ADS PubMed PubMed Central CAS Google Scholar * Donato, N. J. & Perez, M. Tumor
necrosis factor-induced apoptosis stimulates p53 accumulation and p21WAF1 proteolysis in ME-180 cells. _J. Biol. Chem._ 273(9), 5067–5072. https://doi.org/10.1074/jbc.273.9.5067 (1998).
Article PubMed CAS Google Scholar * Gámez-García, A. et al. ERK5 Inhibition induces autophagy-mediated cancer cell death by activating ER stress. _Front. Cell. Dev. Biol._ 9, 742049.
https://doi.org/10.3389/fcell.2021.742049 (2021). Article PubMed PubMed Central Google Scholar * Stoyanova, T., Roy, N., Kopanja, D., Bagchi, S. & Raychaudhuri, P. DDB2 decides cell
fate following DNA damage. _Proc. Natl. Acad. Sci. U S A_. 106(26), 10690–10695. https://doi.org/10.1073/pnas.0812254106 (2009). Article ADS PubMed PubMed Central Google Scholar *
Prager, I. & Watzl, C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. _J. Leukoc. Biol._ 105(6), 1319–1329. https://doi.org/10.1002/JLB.MR0718-269R (2019). Article
PubMed CAS Google Scholar * Moon, T. D., Morley, J. E., Vessella, R. L. & Lange, P. H. The role of calmodulin in human natural killer cell activity. _Scand. J. Immunol._ 18(3),
255–258. https://doi.org/10.1111/j.1365-3083.1983.tb00865.x (1983). Article PubMed CAS Google Scholar * Yan, Y., Gao, Z., Han, H., Zhao, Y. & Zhang, Y. NRAS expression is associated
with prognosis and tumor immune microenvironment in lung adenocarcinoma. _J. Cancer Res. Clin. Oncol._ 148(3), 565–575. https://doi.org/10.1007/s00432-021-03842- (2022). Article PubMed CAS
Google Scholar * Poltorak, M., Meinert, I., Stone, J. C., Schraven, B. & Simeoni, L. Sos1 regulates sustained TCR-mediated Erk activation. _Eur. J. Immunol._ 44(5), 1535–1540.
https://doi.org/10.1002/eji.201344046 (2014). Article PubMed CAS Google Scholar * Vaquero, J., Lobe, C. & Fouassier, L. Unveiling resistance mechanisms to EGFR inhibitors in
cholangiocarcinoma. _Oncotarget_ 9, 37274–37275. https://doi.org/10.18632/oncotarget.26403 (2018). Article PubMed PubMed Central Google Scholar * Peraldo-Neia, C. et al. Prognostic and
predictive role of EGFR pathway alterations in biliary cancer patients treated with chemotherapy and anti-EGFR. _PLoS One_ 13(1), e0191593. https://doi.org/10.1371/journal.pone.0191593
(2018). Article PubMed PubMed Central CAS Google Scholar * Yoon, H., Min, J. K., Lee, J. W., Kim, D. G. & Hong, H. J. Acquisition of chemoresistance in intrahepatic
cholangiocarcinoma cells by activation of AKT and extracellular signal-regulated kinase (ERK)1/2. _Biochem. Biophys. Res. Commun._ 405, 3. https://doi.org/10.1016/j.bbrc.2010.11.130 (2011).
Article CAS Google Scholar * De, S., Dermawan, J. K. & Stark, G. R. EGF receptor uses SOS1 to drive constitutive activation of NFκB in cancer cells. _Proc. Natl. Acad. Sci. U S A_.
111(32), 11721–11726. https://doi.org/10.1073/pnas.1412390111 (2014). Article ADS PubMed PubMed Central CAS Google Scholar * Loilome, W. et al. PRKAR1A overexpression is associated
with increased ECPKA autoantibody in liver fluke-associated cholangiocarcinoma: application for assessment of the risk group. _Tumour Biol._ 33(6), 2289–2298.
https://doi.org/10.1007/s13277-012-0491-3 (2012). Article PubMed CAS Google Scholar * Zheng, Y. et al. Specific genomic alterations and prognostic analysis of perihilar
cholangiocarcinoma and distal cholangiocarcinoma. _J. Gastrointest. Oncol._ 12(6), 2631–2642. https://doi.org/10.21037/jgo-21-776 (2021). Article PubMed PubMed Central Google Scholar *
Ma, D., Lian, F. & Wang, X. PLCG2 promotes hepatocyte proliferation in vitro via NF-κB and ERK pathway by targeting bcl2, Myc and ccnd1. _Artif. Cells Nanomed. Biotechnol._ 47,
1:3786–3792. https://doi.org/10.1080/21691401.2019.1669616 (2019). Article PubMed CAS Google Scholar * Alvaro, D. et al. Estrogens and insulin-like growth factor 1 modulate neoplastic
cell growth in human cholangiocarcinoma. _Am. J. Pathol._ 169(3), 877–888. https://doi.org/10.2353/ajpath.2006.050464 (2006). Article PubMed PubMed Central CAS Google Scholar * Vaquero,
J. et al. The IGF2/IR/IGF1R pathway in tumor cells and myofibroblasts mediates resistance to EGFR Inhibition in cholangiocarcinoma. _Clin. Cancer Res._ 24(17), 4282–4296.
https://doi.org/10.1158/1078-0432.CCR-17-3725 (2018). Article PubMed CAS Google Scholar * Matallanas, D. et al. Raf family kinases: old dogs have learned new tricks. _Genes Cancer_ 2(3),
232–260. https://doi.org/10.1177/1947601911407323 (2011). Article PubMed PubMed Central CAS Google Scholar * West, N. R., Owens, B. M. J. & Hegazy, A. N. The oncostatin M-stromal
cell axis in health and disease. _Scand. J. Immunol._ 88(3), e12694. https://doi.org/10.1111/sji.12694 (2018). Article PubMed CAS Google Scholar * Dai, J. et al. SPOP regulates the
expression profiles and alternative splicing events in human hepatocytes. _Open. Life Sci._ 18(1), 20220755. https://doi.org/10.1515/biol-2022-0755 (2023). Article PubMed PubMed Central
CAS Google Scholar * Ali, A., Nimisha, Sharma, A. K., Mishra, P. K. & Saluja, S. S. Clinical significance of SPOP and APC gene alterations in colorectal cancer in Indian population.
_Mol. Genet. Genomics_ 298(5), 1087–1105. https://doi.org/10.1007/s00438-023-02029-x (2023). Article PubMed CAS Google Scholar * Xu, J. et al. Properties and clinical relevance of
speckle-type POZ protein in human colorectal cancer. _J. Gastrointest. Surg._ 19, 1484–1496. https://doi.org/10.1007/s11605-015-2767-6 (2015). Article PubMed Google Scholar * Zhao, W.,
Zhou, J., Deng, Z., Gao, Y. & Cheng, Y. SPOP promotes tumor progression via activation of beta-catenin/TCF4 complex in clear cell renal cell carcinoma. _Int. J. Oncol._ 49, 1001–1008.
https://doi.org/10.3892/ijo.2016.3609 (2016). Article PubMed CAS Google Scholar * Wan, D. et al. PRKAR2A-derived circular RNAs promote the malignant transformation of colitis and
distinguish patients with colitis-associated colorectal cancer. _Clin. Transl Med._ 12 (2), e683. https://doi.org/10.1002/ctm2.683 (2022). Article PubMed PubMed Central CAS Google
Scholar * Duan, T., Zhou, D., Yao, Y. & Shao, X. The association of aberrant expression of FGF1 and mTOR-S6K1 in colorectal Cancer. _Front. Oncol._ 11, 706838.
https://doi.org/10.3389/fonc.2021.706838 (2021). Erratum in: Front Oncol. 2021;11:792453. Article PubMed PubMed Central CAS Google Scholar * Mehta, A. K., Gracias, D. T. & Croft, M.
TNF activity and T cells. _Cytokine_ 101, 14–18. https://doi.org/10.1016/j.cyto.2016.08.003 (2018). * Uramoto, H., Akyürek, L. M. & Hanagiri, T. A positive relationship between filamin
and VEGF in patients with lung cancer. _Anticancer Res._ 30, 10:3939–3944 (2010). PubMed Google Scholar * Huang, Y. et al. High expression of ITGA3 promotes proliferation and cell cycle
progression and indicates poor prognosis in intrahepatic cholangiocarcinoma. _Biomed. Res. Int._ 2018, 2352139. https://doi.org/10.1155/2018/2352139 (2018). Article PubMed PubMed Central
CAS Google Scholar * Ning, P. et al. PLAU plays a functional role in driving lung squamous cell carcinoma metastasis. _Genes Dis._ 11(2), 554–557.
https://doi.org/10.1016/j.gendis.2023.04.010 (2023). Article PubMed PubMed Central CAS Google Scholar * Wen, S. C. et al. ets-2 regulates cdc2 kinase activity in mammalian cells:
coordinated expression of cdc2 and Cyclin A. _Exp. Cell. Res._ 217(1), 8–14. https://doi.org/10.1006/excr.1995.1057 (1995). Article PubMed CAS Google Scholar * Zabuawala, T. et al. An
ets2-driven transcriptional program in tumor-associated macrophages promotes tumor metastasis. _Cancer Res._ 70(4), 1323–1333. https://doi.org/10.1158/0008-5472.CAN-09-1474 (2010). Article
PubMed PubMed Central CAS Google Scholar * Szymczyk, J. et al. FGF1 protects FGFR1-overexpressing cancer cells against drugs targeting tubulin polymerization by activating AKT via two
independent mechanisms. _Front. Oncol._ 12, 1011762. https://doi.org/10.3389/fonc.2022.1011762 (2022). Article PubMed PubMed Central CAS Google Scholar * Hong, J. et al. Expression of
variant isoforms of the tyrosine kinase SYK determines the prognosis of hepatocellular carcinoma. _Cancer Res._ 74, 6:1845–1856. https://doi.org/10.1158/0008-5472.CAN-13-2104 (2014). Article
PubMed PubMed Central CAS Google Scholar * Oba, M. et al. CCR7 mediates cell invasion and migration in extrahepatic cholangiocarcinoma by inducing epithelial-mesenchymal transition.
_Cancers (Basel)_ 15, 61878. https://doi.org/10.3390/cancers15061878 (2023). Article CAS Google Scholar * Roy, S., Glaser, S. & Chakraborty, S. Inflammation and progression of
cholangiocarcinoma: role of angiogenic and lymphangiogenic mechanisms. _Front. Med. (Lausanne)_ 6, 293. https://doi.org/10.3389/fmed.2019.00293 * Taniai, M. et al. Mcl-1 mediates tumor
necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. _Cancer Res._ 64, 10 (2004). Article Google Scholar * Ma, B. et al. Distinct clinical and
prognostic implication of IDH1/2 mutation and other most frequent mutations in large duct and small duct subtypes of intrahepatic cholangiocarcinoma. _BMC Cancer_ 20(1), 318.
https://doi.org/10.1186/s12885-020-06804-6 (2020). Article PubMed PubMed Central CAS Google Scholar * Mehta, A. K., Gracias, D. T. & Croft, M. TNF activity and T cells. _Cytokine_
101, 14–18. https://doi.org/10.1016/j.cyto.2016.08.003 (2018). Article PubMed CAS Google Scholar * Ran., G. H. et al. Natural killer cell homing and trafficking in tissues and tumors:
From biology to application. _Signal. Transduct. Target. Ther._ 7(1), 205. https://doi.org/10.1038/s41392-022-01058-z (2022). Article PubMed PubMed Central CAS Google Scholar * Wang,
J., Jiang, Y. H., Yang, P. Y., Liu, F. & Increased Collagen Type, V. α2 (COL5A2) in colorectal Cancer is associated with poor prognosis and tumor progression. _Onco Targets Ther._ 14,
2991–3002. https://doi.org/10.2147/OTT.S288422 (2021). Article PubMed PubMed Central Google Scholar * Liu, Q. et al. Oncostatin M expression and TP53 mutation status regulate
tumor-infiltration of immune cells and survival outcomes in cholangiocarcinoma. _Aging (Albany NY)_ 12(21), 21518–21543. https://doi.org/10.18632/aging.103936 (2020). Article PubMed CAS
Google Scholar * Chen, W. et al. Unraveling the heterogeneity of cholangiocarcinoma and identifying biomarkers and therapeutic strategies with single-cell sequencing technology. _Biomed.
Pharmacother._ 162, 114697. https://doi.org/10.1016/j.biopha.2023.114697 (2023). Article PubMed CAS Google Scholar * Lin, P. et al. Survival analysis of genome-wide profiles coupled with
connectivity map database mining to identify potential therapeutic targets for cholangiocarcinoma. _Oncol. Rep._ 40(6), 3189–3198. https://doi.org/10.3892/or.2018.6710 (2018). Article
PubMed PubMed Central CAS Google Scholar * Abell, K. & Watson, C. J. The Jak/Stat pathway: A novel way to regulate PI3K activity. _Cell. Cycle_ 4, 897–900.
https://doi.org/10.4161/cc.4.7.1837 (2005). Article PubMed CAS Google Scholar * Tang, S. et al. Association analyses of the JAK/STAT signaling pathway with the progression and prognosis
of colon cancer. _Oncol. Lett._ 17(1), 159–164. https://doi.org/10.3892/ol.2018.9569 (2019). Article PubMed CAS Google Scholar * Chen, B. et al. JAK1 as a prognostic marker and its
correlation with immune infiltrates in breast cancer. _Aging (Albany NY)_. 11(23), 11124–11135. https://doi.org/10.18632/aging.102514 (2019). Article PubMed CAS Google Scholar * Pei, Y.,
Cui, X. & Wang, Y. Regulation of IL-10 expression and function by JAK-STAT in CD8 + T cells. _Int. Immunopharmacol._ 128, 111563. https://doi.org/10.1016/j.intimp.2024.111563 (2024).
Article PubMed CAS Google Scholar * Shen, K. et al. The expression landscape of JAK1 and its potential as a biomarker for prognosis and immune infiltrates in NSCLC. _BMC Bioinform._ 22,
1471. https://doi.org/10.1186/s12859-021-04379-y (2021). Article CAS Google Scholar * Suksawat, M. et al. Upregulation of endothelial nitric oxide synthase (eNOS) and its upstream
regulators in _Opisthorchis viverrini_ associated cholangiocarcinoma and its clinical significance. _Parasitol. Int._ 66, 4:486–493. https://doi.org/10.1016/j.parint.2016.04.008 (2017).
Article PubMed CAS Google Scholar * Alwithenani, A. I. & Althubiti, M. A. Systematic analysis of spleen tyrosine kinase expression and its clinical outcomes in various cancers.
_Saudi J. Med. Med. Sci._ 8(2), 95–104. https://doi.org/10.4103/sjmms.sjmms_300_19 (2020). Article PubMed PubMed Central Google Scholar * Guittard, G. et al. Absence of both Sos-1 and
Sos-2 in peripheral CD4(+) T cells leads to PI3K pathway activation and defects in migration. _Eur. J. Immunol._ 45, 8. https://doi.org/10.1002/eji.201445226 (2015). Article CAS Google
Scholar * Alem, D. et al. Translational relevance of SOS1 targeting for KRAS-mutant colorectal cancer. _Mol. Carcinog._ 62(7), 1025–1037. https://doi.org/10.1002/mc.23543 (2023). Article
PubMed PubMed Central CAS Google Scholar * Li, Y., Yin, Y., He, Y., He, K. & Li, J. SOS1 regulates HCC cell epithelial-mesenchymal transition via the PI3K/AKT/mTOR pathway. _Biochem.
Biophys. Res. Commun._ 637, 161–169. https://doi.org/10.1016/j.bbrc.2022.11.015 (2022). Article PubMed CAS Google Scholar * Li, Q. et al. High integrin Α3 expression is associated with
poor prognosis in patients with non-small cell lung cancer. _Transl Lung Cancer Res._ 9(4), 1361–1378. https://doi.org/10.21037/tlcr-19-633 (2020). Article PubMed PubMed Central CAS
Google Scholar * Wu, A. et al. Integrated analysis of prognostic and immune associated integrin family in ovarian cancer. _Front. Genet._ 11, 705. https://doi.org/10.3389/fgene.2020.00705
(2020). Article PubMed PubMed Central CAS Google Scholar * Zheng, Q., Zhang, B., Li, C. & Zhang, X. Overcome drug resistance in cholangiocarcinoma: New insight into mechanisms and
refining the preclinical experiment models. _Front. Oncol._ 12, 850732. https://doi.org/10.3389/fonc.2022.850732 (2022). Article PubMed PubMed Central CAS Google Scholar * Sridharan, V.
et al. FGFR mRNA expression in cholangiocarcinoma and its correlation with FGFR2 fusion status and immune signatures. _Clin. Cancer Res._ 28(24), 5431–5439.
https://doi.org/10.1158/1078-0432.CCR-22-1244 (2022). Article PubMed PubMed Central CAS Google Scholar * Krook, M. A. et al. Efficacy of FGFR inhibitors and combination therapies for
acquired resistance in FGFR2-fusion cholangiocarcinoma. _Mol. Cancer Ther._ 19(3), 847–857. https://doi.org/10.1158/1535-7163.MCT-19-0631 (2020). Article PubMed PubMed Central CAS Google
Scholar * Zamai, L. et al. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and fas ligand by immature and mature primary human NK cells. _J. Exp. Med._ 188(12),
2375–2380. https://doi.org/10.1084/jem.188.12.2375 (1998). Article PubMed PubMed Central CAS Google Scholar * Matsuzawa, A. et al. ROS-dependent activation of the TRAF6-ASK1-p38 pathway
is selectively required for TLR4-mediated innate immunity. _Nat. Immunol._ 6(6), 587–592. https://doi.org/10.1038/ni1200 (2005). Article PubMed CAS Google Scholar * Ghafouri-Fard, S. et
al. 5-Fluorouracil: A narrative review on the role of regulatory mechanisms in driving resistance to this chemotherapeutic agent. _Front. Oncol._ 11, 658636 (2021). Article PubMed PubMed
Central CAS Google Scholar * Taïeb, J. et al. Optimization of 5-fluorouracil (5-FU)/cisplatin combination chemotherapy with a new schedule of leucovorin, 5-FU and cisplatin (LV5FU2-P
regimen) in patients with biliary tract carcinoma. _Ann. Oncol._ 13(8), 1192–1196 (2002). Article PubMed Google Scholar * Siebenhüner, A. R. et al. Adjuvant treatment of resectable
biliary tract cancer with cisplatin plus gemcitabine: A prospective single center phase II study. _BMC Cancer_18(1), 72 (2018). Article PubMed PubMed Central Google Scholar * Kotawong,
K., Chajaroenkul, W., Roytrakul, S., Phaonakrop, N. & Na-Bangchang, K. The proteomics and metabolomics analysis for screening the molecular targets of action of beta-eudesmol in
cholangiocarcinoma. _Asian Pac. J. Cancer Prev._ 22, 3:909–918. https://doi.org/10.31557/APJCP.2021.22.3.909 (2021). Article PubMed PubMed Central CAS Google Scholar * Mathema, V. B.,
Chaijaroenkul, W., Karbwang, J. & Na-Bangchang, K. Growth inhibitory effect of β-eudesmol on cholangiocarcinoma cells and its potential suppressive effect on heme oxygenase-1 production,
STAT1/3 activation, and NF-κB downregulation. _Clin. Exp. Pharmacol. Physiol._ 44(11), 1145–1154. https://doi.org/10.1111/1440-1681.12818 (2017). Article PubMed CAS Google Scholar
Download references FUNDING This research project was supported by the Thailand Science Research and Innovation Fundamental Fund Fiscal Year 2023 and Thammasat University (Chulabhorn
International College of Medicine, Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma). Kesara Na-Bangchang is funded by the National Research
Council of Thailand (NRCT): Contract number N42A671041. All funders have no roles for publication. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Centre of Excellence in Pharmacology and
Molecular Biology of Malaria and Cholangiocarcinoma, Chulabhorn International College of Medicine, Thammasat University (Rangsit Campus), 99 moo 18, Phaholyothin Road, Klong Luang District,
Pathumthani, 12121, Thailand Teerachat Saeheng, Wanna Chaijaorenkul & Kesara Na-Bangchang * Gradulate Program in Bioclinical Science, Chulabhorn International College of Medicine,
Thammasat University (Rangsit Campus), Klong Luang District, Pathumtanee province, Thailand Teerachat Saeheng, Ethan Vindvamara, Wanna Chaijaorenkul & Kesara Na-Bangchang * Sakol Nakorn
Hospital, Sakol Nakorn, Sakol Nakorn Province, Thailand Nisit Tongsiri & Kesara Na-Bangchang Authors * Teerachat Saeheng View author publications You can also search for this author
inPubMed Google Scholar * Ethan Vindvamara View author publications You can also search for this author inPubMed Google Scholar * Wanna Chaijaorenkul View author publications You can also
search for this author inPubMed Google Scholar * Nisit Tongsiri View author publications You can also search for this author inPubMed Google Scholar * Kesara Na-Bangchang View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS T.S.: Conceptualization, data curation, validation, formal analysis, methodology, writing-original
draft, interpretation. E.V.: Experimental study, W.C.: Experimental study. N.T.: Experimental study. K.N.: Conceptualization, data curation, funding acquisition, supervision, project
administration, writing—review, and editing. All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Kesara Na-Bangchang. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing interests. ETHICAL APPROVAL AND CONSENT TO PARTICIPATE This project has approval from the Human Research Ethics Committee of Sakol Nakorn hospital (No.
049/2563). All participants signed written informed consent forms prior to enrollment. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL Below is the link to the electronic supplementary material. SUPPLEMENTARY MATERIAL 1. RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and
indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third
party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Saeheng, T., Vindvamara, E.,
Chaijaorenkul, W. _et al._ A molecular network analysis and in silico docking of beta-eudesmol, atractylodin and hinesol in patients with advance stage intrahepatic cholangiocarcinoma. _Sci
Rep_ 15, 16279 (2025). https://doi.org/10.1038/s41598-025-91968-z Download citation * Received: 10 May 2024 * Accepted: 24 February 2025 * Published: 10 May 2025 * DOI:
https://doi.org/10.1038/s41598-025-91968-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Molecular network * _Atractylodes lancea_ Thunb (DC.) *
Traditional medicine * Cholangiocarcinoma









:max_bytes(150000):strip_icc():focal(216x0:218x2)/teresa-giudice-a-435-17a16a56fb0741a591597dab2ccb02c5.jpg)