- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The objective of this study was to investigate the relation between the electric field distribution within the cochlea during cochlear implant stimulation and the electrical
vestibular co-stimulation measured by vestibular evoked myogenic potentials (e-VEMPs). Measurements were done in adult Nucleus cochlear implant (CI) users with perimodiolar electrode arrays.
The electric field distribution within the cochlea was determined by Transimpedance Matrices recorded for all participants with a pulse width of 25 µs and a current level of 110 CL. Study
measurements were conducted in 25 ears of 24 participants. In 10 participants, e-VEMPs could be elicited (40%). The occurrence of e-VEMPs stimulated by the cochlear implant was correlated
with the magnitude of a corrected transimpedance at the most basal electrode. Since the results also suggest that there are patients with vestibular co-stimulation already present at their
everyday CI stimulation level, this needs to be taken into account by audiologists creating programming maps for CIs, e.g. by deactivation of basal electrode contacts, if dizziness occurs
during CI stimulation. SIMILAR CONTENT BEING VIEWED BY OTHERS INFLUENCE OF BONE CONDUCTION TRANSDUCER TYPE AND PLACEMENT ON OCULAR AND CERVICAL VESTIBULAR EVOKED MYOGENIC POTENTIALS Article
Open access 19 April 2021 ELECTRICALLY EVOKED MISMATCH NEGATIVITY RESPONSES TO LOUDNESS AND PITCH CUES IN COCHLEAR IMPLANT USERS Article Open access 10 February 2023 BONE CONDUCTION
STIMULATION EFFICIENCY AT COUPLING LOCATIONS CLOSER TO THE COCHLEA Article Open access 02 December 2024 INTRODUCTION There are several studies investigating the electrical stimulation of the
vestibular system1,2,3. In 1982 Eisenberg et al.4 already investigated the improvement of postural stability through the activation of cochlear implants. The effect of cochlear implantation
on vestibular evoked myogenic potentials is described in different studies5,6,7,8. Additionally, co-stimulation during cochlear implant (CI) stimulation has been described in this
context9,10. A recent study by Fröhlich et al.11 investigated the influence of CI stimulation parameters on the occurrence of e-VEMPs (electrically vestibular evoked myogenic potentials) in
Nucleus CI users. It was shown that high stimulation levels and monopolar stimulation at basal CI electrode contacts increased the probability of evoking e-VEMPs and thus vestibular
co-stimulation. The stimulation of a CI electrode contact does not lead to a focused excitation of neurons but rather results in a spread of current within the cochlea. This can be measured
with different tools. Spread of neural excitation (SOE) measurements have shown co-stimulation of neurons associated with other frequency areas and electrode contacts12,13. Spread of the
electric field – without information about neural excitation – can be assessed by using the transimpedance matrix (TIM) measurement for Nucleus cochlear implants (Cochlear Ltd., Sydney,
Australia). Other manufacturers refer to this measurement of spread of electric field as “electric field imaging” (EFI – Advanced Bionics, Stäfa, Switzerland, e.g14). or voltage matrix of
the impedance and field telemetry (Med-El, Innsbruck, Austria, e.g15). A general and incorporating name for these tools is “Stimulation-Current-Induced Non-Stimulating Electrode Voltage
recordings” (SCINSEVs)16. Here, we focus on TIM which shows transimpedances for 21 electrodes per each stimulating electrode in the cochlea. The stimulation current at one electrode is
constant and the voltage at each other electrode on the array within the cochlea can be determined using telemetry (for a detailed description see17). For CI stimulation, it is possible to
reference the active electrode to all other passively connected electrodes inside the cochlea (common ground mode). However, a monopolar stimulation using extracochlear electrodes is widely
used. For Nucleus cochlear implants, two extracochlear reference electrodes can be used. One contact is at the end of an extracochlear wire which is usually placed below the musculus
temporalis18 during surgery. Another contact is positioned on the implant housing. Since they are positioned outside the cochlea, it can be assumed that also other structures besides the
cochlear nerve might be electrically stimulated. One example is facial nerve co-stimulation as a side effect that can be seen during electric stimulation in some CI users19,20. Studies have
shown that vestibular co-stimulation in CI users can be detected by the recording of electrically elicited vestibular evoked myogenic potentials (e-VEMPs)2,3,21. However, it is unknown which
vestibular structures exactly are stimulated by the CI, i.e., the vestibular receptors (utricule and saccule) or the vestibular nerve, when e-VEMPs are elicited. The short e-VEMP latencies
reported by Fröhlich et al.11 suggest direct stimulation of the vestibular nerve. Despite the unknown target structures the source of the electric current is the CI itself with the electric
currents travelling along the cochlea. Thus, it can be assumed that the magnitude of the voltage, which is correlated to the magnitude of the transimpedance at the basal turn of the cochlea,
could be a predictor for the occurrence of vestibular co-stimulation and the occurrence of e-VEMPS. In order to test this hypothesis, we investigated the relation between the TIM and
presence of e-VEMPs in this study. Ramos de Miguel et al. investigated co-stimulation of the vestibular organ by TIM measurements in four patients using a research implant consisting of an
intracochlear and an intravestibular electrode array22. The authors concluded that there was no cross-stimulation from the cochlea to the vestibule or from the vestibular electrodes to the
cochlea in TIM recordings. Here, we used standard CIs with intracochlear electrode arrays and analysed TIMs in the basal part of the cochlea, hypothesizing that interindividual differences
in the presence or absence of e-VEMPs are most pronounced at locations with a small distance to the vestibular receptors. MATERIALS AND METHODS STUDY DESIGN AND PARTICIPANTS A prospective
explorative study was conducted between June 2020 and December 2021 at a single tertiary referral centre. Inclusion criteria were being between 18 and 65 years old and having a Nucleus
cochlear implant with a perimodiolar electrode array (Cochlear Ltd., Sydney, Australia) and full insertion. Patients with known vestibular disorders (e.g. vestibulopathy, Menière’s disease,
vestibular migraine), cochleovestibular schwannoma, cochlear malformations, electrode displacement on postoperative imaging, and cochlear fibrosis or sclerosis were excluded. The study
protocol was reviewed and approved by the ethics committee of the Martin Luther University Halle-Wittenberg (approval number: 2020-22) and performed in accordance with the relevant
guidelines and regulations. Written informed consent was obtained from all participants. EXPERIMENTAL SETUP AND PROCEDURES All measurements were conducted in a soundproof and
electromagnetically shielded booth suitable for audiological and electrophysiological measurements. CI stimulation was controlled using Custom Sound EP software (version 6.0, Cochlear Ltd.,
Sydney, Australia). Transimpedance measurements were conducted in the respective module using biphasic pulses with a pulse width of 25 µs and a current level (CL) of 110. For stimulation,
monopolar mode (MP1) was used and MP2 mode was used for recording. The time of recording was at the end of the first half of the biphasic pulse (T06). A stimulation at higher levels, which
were used for e-VEMP stimulation, was not tolerated by all patients. For the e-VEMP recordings, electric pulses were generated in the eABR module of Custom Sound EP software (version 6.0,
Cochlear Ltd., Sydney, Australia). An external trigger signal was generated in the module and sent to the Eclipse (Interacoustics, Middlefart, Denmark) recording system via the CI
programming pod. For all participants the same test audio processor (CP910, Cochlear, Sydney, Australia) was used for stimulus transmission to the implant. Electric tone bursts were composed
of biphasic pulses (25 µs pulse duration, 7 µs interphase gap) with a burst/stimulation rate of 1000 Hz and burst duration of 3.057 ms (= 4 pulses at 1000 Hz) and presented at 8 Hz. The
stimulation was done in monopolar mode between the housing electrode and the basal intracochlear electrode E3. For all participants, the maximum tolerable stimulation level (MTSL) at
stimulating electrode E3 was determined by subjective loudness scaling. The stimulation for e-VEMP recording started at that level. The specific setup and procedure for e-VEMP recordings can
be found in Fröhlich et al.11. DATA ANALYSIS The VEMP data were analysed according to the procedure described by Fröhlich et al.11. For this study, participants were divided into two groups
– one group of participants with recordable e-oVEMPs and/or e-cVEMPs (e-VEMP) and one group of participants without recordable e-VEMPs within the MTSL (no e-VEMP). The TIM data for all
patients were exported and plotted with python (version 3.8) as single matrix plots. The maximum for plotting was set to 2 kOhm. The recorded transimpedance line plot for stimulation at
electrode E3 was plotted for both groups. The transimpedances at basal electrodes E2 and E1 for stimulation at electrode E3 were further analysed and distributions are shown as boxplots.
_T_-tests were calculated for the comparison of group means and Bonferroni correction was applied for multiple comparisons. As the results by Fröhlich et al.11 showed a strong effect of the
maximum tolerable stimulation level on the occurrence of e-VEMPs, further analysis was performed to consider these individual tolerable levels of patients. The maximum tolerable comfort
level (MCL) was measured by subjective loudness scaling, i.e., asking the patient when the stimulus used for e-VEMP measurements could not be tolerated to be any louder. For one patient, the
TIM measurement was repeated with different stimulation levels (110 CL to 150 CL in 10 CL steps) to examine the change of transimpedance as a function of the stimulation level. Line graphs
for all stimulation levels were plotted. A correction factor was derived from the increase of the transimpedance for an increase of stimulation by 10 CL. The correction was then used to
calculate an estimated transimpedance at the maximum tolerable stimulation level for each patient. Corrected group means were plotted again as line graphs to compare the two groups with
respect to the maximum tolerable stimulation level. RESULTS The study included recordings in 25 CIs of 24 patients between 23 and 63 years of age. The mean age was 50.8 years (SD: 12.2
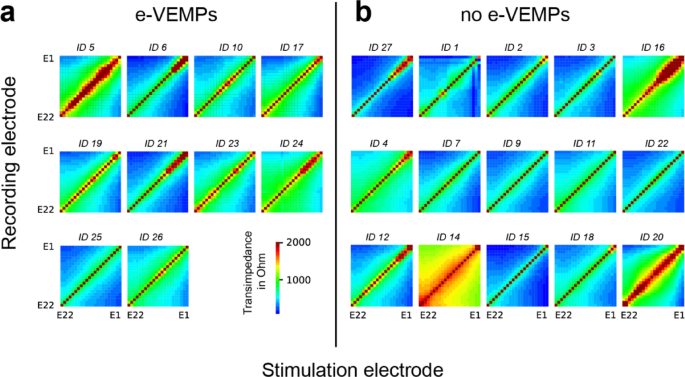
years). Further information about the study sample can be found in Table 1. All individual TIMs are shown in Fig. 1. Visual comparison revealed no obvious difference concerning the width (in
respect to the diagonal) or shape of the electric field between the two groups. There were wide (e.g. ID 5, 24, 14, 16, 20) and narrow (e.g. ID 6, 25, 27, 3, 15) distributions of the
electric field in both groups. Figure 2A shows the transimpedances for stimulation at electrode 3 and increasing stimulation level for one participant. An increase of 10 CL led to an
increase in transimpedance for a single electrode contact of 10 to 20 Ohm. In Fig. 2B the relation between increasing stimulation level and increasing transimpedance is shown. A regression
analysis showed a linear relation with a slope of 2.03 Ohms per CL (SD: 0.16; 95% CI: 1.59 to 2.46) at electrode 1 and 1.97 Ohms per CL (SD: 0.22; 95% CI: 1.36 to 2.57) at electrode 2. Thus,
a correction of 2 Ohm per 1 CL was applied for further analyses. Figure 3A shows the uncorrected line plots of the transimpedance at electrode 3 for both groups with all TIMs measured at
110 CL. Higher transimpedances in the basal part of the cochlea were observed for the e-VEMP group compared to the no e-VEMP group. In the e-VEMP group, the transimpedances were 1530 ± 500
Ohm at electrode 2 and 952 ± 190 Ohm at electrode 1. In the no e-VEMP group the transimpedances were 1308 ± 640 Ohm and 840 ± 355 Ohm, respectively. The differences were not statistically
significant (both _p_s = 0.37, Fig. 3). Figure 3B shows the line plots of the TIM corrected for individual maximum tolerable stimulation level by 2 Ohm / 1 CL. After the correction of TIM
values, the differences in transimpedance values of electrode 1 between the two groups were larger. At electrode 1, transimpedance in the e-VEMP group was 3052 ± 300 Ohm compared to 2620 ±
597 Ohm (_p_ = 0.046) in the no e-VEMP group. At electrode 2, the difference between e-VEMP and no e-VEMP group was not statistically significant (_p_ = 0.076). The individual data for the
transimpedance at electrode 1 and 2 are shown in Fig. 4. DISCUSSION This study is the first that looked at the occurrence of vestibular co-stimulation in CI users as indicated by the
presence of e-VEMPs and that analysed the intracochlear electric field distribution in these patients to investigate the role of current spread. None of the participating patients reported
any vestibular problems. In this study, visual inspection of all single TIMs (Fig. 1) did not show a difference in width or shape between the e-VEMP group, in which patients with o- and
c-VEMPs are combined, and the non e-VEMPs group. A more detailed look at the stimulation of electrode E3, which was the electrode contact that evoked the e-VEMPS in this study, revealed
differences between transimpedances in the basal cochlear region of trial subjects in the e-VEMP and the no e-VEMP group. The mean transimpedance, i.e., voltage, was higher in the e-VEMP
group than in the no e-VEMP group. However, the trend was not statistically significant, possibly due to the small sample size and standard stimulation current of 110 CL used for TIM
recordings. One of the major challenges in this study was to consider the influence of interindividual differences of maximum tolerable stimulation levels. The question arose whether an
increase of stimulation level, if tolerated, can lead to a vestibular co-stimulation and whether this was also reflected by the electric field distribution. E-VEMP response rates increased
with increasing stimulation level according to Fröhlich et al.11. Thus, some patients may have been classified into the no e-VEMP group because of insufficient stimulation level, even though
the electric field distribution could be rather large, which would facilitate co-stimulation. The measurement of the transimpedance was originally done in this study using a fixed
stimulation level of 110 CL to keep it comparable and to possibly find a systematic difference in field distribution between groups. Analysing the effect of increasing the current level (CL)
on the transimpedance measurements in a single subject, we found an increase of 2 Ohm per 1 CL (Fig. 2). An increase in stimulation level only resulted in a shift in the transimpedance but
not in a change of the distribution of the electric field. The application of this correction of 2 Ohm per CL to the TIM data revealed a significantly higher transimpedance, i.e. voltage, at
basal electrode contact E1 in the e-VEMP group as compared to the no e-VEMP group. Thus, a larger spread of electric field towards basal electrodes was associated with the occurrence of
e-VEMPs, which supports the theory that electric current spread is responsible for co-stimulation. Our data are consistent with the results of Ramos de Miguel et al.22 although we came to a
different conclusion. We would also interpret their data in Fig. 1 of19 as a current flow between vestibular electrodes and basal cochlear electrodes in some cases (except the right bottom
one). At least electrode 4, which is the most basal one in their implant, shows crosstalk. This is in line with our results. Different factors might have an influence on the distribution of
the electric field and need to be considered. The influence of the surgical technique and the implant type were not analysed in this study. A cochleostomy was performed 3 times in the e-VEMP
group and only once in the no e-VEMP group (Table 1). The mean duration of use was longer and thus the implant model was “older” in the e-VEMP group (Table 1). This inhomogeneity is a
limitation of the study and needs further investigation in future studies. Since the intracochlear electric field depends on many factors which are not fully understood and are different in
every CI patient, the risk for unintended vestibular co-stimulation is an individual risk. Among other factors, especially stimulation level, individual current spread may play a role. In
this study sample, one patient’s e-VEMP threshold was even within the everyday current level range (cochlear stimulation). In clinical routine CI fitting, it is therefore necessary to keep
in mind that vestibular co-stimulation is possible and might have clinical relevance. Deactivation of basal electrode contacts could help in cases when dizziness due to CI use is reported by
patients. At that point it is not possible to use the TIM measure as a predictor for e-VEMP occurrence but we were able to show a correlation between the technical measure and the
vestibular co-stimulation on group level. We are of the opinion that it is valuable to investigate this correlation further and analyse the effect of more influencing factors in further
studies to develop a predictor in the future. DATA AVAILABILITY The data of this research project are available on reasonable request from the corresponding author via mail contact
([email protected]). REFERENCES * Sluydts, M. et al. Electrical vestibular stimulation in humans: A narrative review. _Audiol. Neurootol_. 25, 6–24 (2020). Article PubMed Google
Scholar * Basta, D., Todt, I., Goepel, F. & Ernst, A. Loss of saccular function after cochlear implantation: the diagnostic impact of intracochlear electrically elicited vestibular
evoked myogenic potentials. _Audiol. Neurootol_. 13, 187–192 (2008). Article CAS PubMed Google Scholar * Parkes, W. J. et al. Vestibular evoked myogenic potential testing as an objective
measure of vestibular stimulation with cochlear implants. _Laryngoscope_ 127, E75–E81 (2017). Article PubMed Google Scholar * Eisenberg, L. S., Nelson, J. R. & House, W. F. Effects
of the single-electrode cochlear implant on the vestibular system of the profoundly deaf adult. _Ann. Otol. Rhinol. Laryngol. Suppl._ 91, 47–54 (1982). CAS PubMed Google Scholar * Miwa,
T., Minoda, R., Matsuyoshi, H. & Takeda, H. The effect of cochlear implants on vestibular-evoked myogenic potential responses and postural stability. _Auris nasus Larynx_. 46, 50–57
(2019). Article PubMed Google Scholar * Jin, Y. et al. Vestibular evoked myogenic potentials evoked by multichannel cochlear implant - influence of C levels. _Acta Otolaryngol._ 128,
284–290 (2008). Article PubMed Google Scholar * Ibrahim, I., Da Silva, S. D., Segal, B. & Zeitouni, A. Effect of cochlear implant surgery on vestibular function: meta-analysis study.
_J. Otolaryngol. - head Neck Surg. = Le J. d’oto-rhino-laryngologie Et De Chirurgie Cervicofac._ 46, 44 (2017). Article Google Scholar * Psillas, G. et al. Vestibular evoked myogenic
potentials in children after cochlear implantation. _Auris nasus Larynx_. 41, 432–435 (2014). Article PubMed Google Scholar * Coordes, A. et al. Sound-induced vertigo after cochlear
implantation. _Otol Neurotol_. 33, 335–342 (2012). Article PubMed Google Scholar * Imai, T. et al. Effects of cochlear implants on otolith function as evaluated by vestibulo-ocular reflex
and vestibular evoked myogenic potentials. _Auris nasus Larynx_. 46, 836–843 (2019). Article PubMed Google Scholar * Fröhlich, L., Plontke, S. K., Löffler, L. B., Manthey, A. &
Rahne, T. Stimulation conditions leading to electrical vestibular co-stimulation in cochlear implant users. _Laryngoscope Invest. Otolaryngol._ https://doi.org/10.1002/lio2.70011 (2024).
Article Google Scholar * Cohen, L. T., Richardson, L. M., Saunders, E. & Cowan, R. S. C. Spatial spread of neural excitation in cochlear implant recipients: comparison of improved ECAP
method and psychophysical forward masking. _Hear. Res._ 179, 72–87 (2003). Article PubMed Google Scholar * Goffi-Gomez, M. V. S. et al. Is the spread of excitation different between
adults and children cochlear implants users? _Eur. Arch. Otorhinolaryngol._ https://doi.org/10.1007/s00405-024-08451-0 (2024). Article PubMed Google Scholar * Saoji, A. A. et al.
Detection of extracochlear electrodes using electrical field imaging (EFI). _Otol Neurotol_. 46, e51–e55 (2025). Article PubMed Google Scholar * Franke-Trieger, A. et al. Voltage matrix
algorithm for intraoperative detection of cochlear implant electrode misplacement. _Audiol. Neurootol_, 1–17 (2024). * de Rijk, S. R., Tam, Y. C., Carlyon, R. P. & Bance, M. L. Detection
of extracochlear electrodes in cochlear implants with electric field imaging/transimpedance measurements: A human cadaver study. _Ear Hear._ 41, 1196–1207 (2020). Article PubMed PubMed
Central Google Scholar * Kopsch, A. C., Rahne, T., Plontke, S. K. & Wagner, L. Influence of the spread of the electric field on speech recognition in cochlear implant users. _Otol
Neurotol_. 45, e221–e227 (2024). Article PubMed Google Scholar * Zeng, F. G. Trends in cochlear implants. _Trends Amplif._ 8, 1–34 (2004). Article PubMed PubMed Central Google Scholar
* Cushing, S. L., Papsin, B. C., Strantzas, S. & Gordon, K. A. Facial nerve electromyography: a useful tool in detecting nonauditory side effects of cochlear implantation. _J.
Otolaryngol. - head Neck Surg. = Le J. d’oto-rhino-laryngologie Et De Chirurgie Cervicofac._ 38, 157–165 (2009). Google Scholar * Saoji, A. A. et al. Pathophysiology of facial nerve
stimulation and its implications for electrical stimulation in cochlear implants. _Otol Neurotol_. 45, e84–e90 (2024). Article PubMed Google Scholar * Rodriguez Montesdeoca, I. et al.
Differences in Vestibular-Evoked myogenic potential responses by using cochlear implant and otolith organ direct stimulation. _Front. Neurol._ 12, 663803 (2021). Article PubMed PubMed
Central Google Scholar * Ramos de Miguel, Á. et al. Stimulation crosstalk between cochlear and vestibular spaces during cochlear electrical stimulation. _Laryngoscope_
https://doi.org/10.1002/lary.31174 (2023). Article Google Scholar Download references ACKNOWLEDGEMENTS We thank Lea B. Löffler and Antonia Manthey for assistance in collecting the data.
FUNDING Open Access funding enabled and organized by Projekt DEAL. AUTHOR INFORMATION Author notes * Laura Fröhlich Present address: Department of Otolaryngology, Head and Neck Surgery,
University Hospital Bonn, Bonn, Germany AUTHORS AND AFFILIATIONS * Department of Otorhinolaryngology, Head & Neck Surgery, Martin Luther University Halle-Wittenberg, University Medicine
Halle (Saale), Halle (Saale), Germany Luise Wagner, Torsten Rahne & Stefan K. Plontke Authors * Luise Wagner View author publications You can also search for this author inPubMed Google
Scholar * Torsten Rahne View author publications You can also search for this author inPubMed Google Scholar * Stefan K. Plontke View author publications You can also search for this author
inPubMed Google Scholar * Laura Fröhlich View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conception and design of work: LF, TR, LWData
Collection: LFData analysis and interpretation: LW, LFDrafting the article: LWCritical revision of the article: LF, TR, SP. CORRESPONDING AUTHOR Correspondence to Luise Wagner. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use,
sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative
Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated
otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wagner, L., Rahne, T., Plontke, S.K. _et al._ A case series shows a relation between intracochlear electric field distribution and
vestibular co-stimulation with cochlear implants. _Sci Rep_ 15, 15976 (2025). https://doi.org/10.1038/s41598-025-00887-6 Download citation * Received: 09 September 2024 * Accepted: 02 May
2025 * Published: 08 May 2025 * DOI: https://doi.org/10.1038/s41598-025-00887-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable
link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * e-VEMPs * Cochlear
implant * Transimpedance matrix * Co-stimulation
:max_bytes(150000):strip_icc():focal(979x299:981x301)/robert-e.lee_-9f2799d8cf124b81a16649fe4cad86e5.jpg)







