- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Combination antiretroviral therapy (ART) has improved outcomes for human immunodeficiency virus (HIV) associated non-Hodgkin lymphoma. This is an analysis of 127 patients with HIV
with Burkitt lymphoma (HIV-BL) and diffuse large B-cell lymphoma (HIV-DLBCL) treated at the Zhongnan Hospital of Wuhan University over a 17-year period during the ART and rituximab era. The
median CD4 count for the cohorts was 0.141 × 109/L (range, 0.001-0.861 × 109/L). DA-EPOCH ± R (54%) were most commonly used in HIV-BL. CHOP± R (42%) was most commonly used to treat
HIV-DLBCL. The complete response rate after first-line curative therapy was 10/28 (36%) in HIV-BL and 25/57 (44%) in HIV-DLBCL. The 2-year progression-free survival (PFS) and overall
survival (OS) for the HIV-BL cohort was 50% and 41% respectively. The 2-year PFS and OS for the HIV-DLBCL cohort was 55% and 47% respectively. Current China practice favours the treatment of
HIV-BL and HIV-DLBCL similarly to the HIV-negative population with the use of concurrent ART. However, due to the extremely low percentage of patients receiving ART prior to the lymphoma
diagnosis, the high percentage of patients with poor performance status, and the advanced stage at diagnosis, the treatment of HIV-related lymphoma remains the major challenge in China.
SIMILAR CONTENT BEING VIEWED BY OTHERS OUTCOMES IN PATIENTS WITH CLASSIC HODGKIN LYMPHOMA REFRACTORY OR INTOLERANT TO BRENTUXIMAB VEDOTIN AND ANTI-PD-1 THERAPY: A REAL WORLD ANALYSIS FROM 15
U.S. ACADEMIC CENTERS Article Open access 26 March 2025 CLINICAL FEATURES AND OUTCOMES OF NEWLY DIAGNOSED CLASSICAL HODGKIN LYMPHOMA PATIENTS IN SAUDI ARABIA: A MULTICENTER COHORT STUDY
Article Open access 26 May 2025 LONG-TERM SURVIVAL BENEFIT OF ANTI-PD-1 THERAPY IN PATIENTS WITH RELAPSED OR REFRACTORY CLASSICAL HODGKIN LYMPHOMA Article Open access 20 September 2023
INTRODUCTION In China, 110,000 people were newly infected with HIV in 2020, reaching 1.04 million HIV-positive people1. People living with HIV (PLWH) have a greater risk of developing cancer
than the general population. Cancers that develop in the setting of HIV have traditionally been categorized into two categories: cancers that, when present, provide an AIDS diagnosis
(AIDS-defining malignancies, or ADMs) and cancers that do not necessarily signal AIDS (non-AIDS-defining malignancies, or NADMs)2. The most common subtypes of HIV-associated non-Hodgkin
lymphoma (NHL) are diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma (BL), which are both ADMs3. Before the development of effective combination antiretroviral therapy (ART), the
relative risk for NHL was estimated as 60- to 200-fold compared with the general population4. Since the introduction of ART in 1996, the incidence of various ADMs has decreased by 75–80%5.
However, the risk remains significantly elevated for each AIDS-defining NHL subtype, specifically DLBCL standardized incidence ratio (SIR 10), and BL (SIR 20)6,7. HIV-DLBCL, which accounts
for 45% of all HIV-related NHL subtypes, is linked to higher-risk prognostic characteristics8. HIV-DLBCL patients had a median survival duration of only 5 to 8 months prior to the advent of
ART. This is because opportunistic infections can quickly complicate patients, which raises mortality9. In the ART era, individuals with HIV-DLBCL may experience a 5-year overall survival
(OS) of up to 55% when ART is combined with intense chemotherapy10. The preferred regimen varies by region and institution, but one retrospective series suggests that DA-EPOCH-R (etoposide,
prednisolone, vincristine, cyclophosphamide, doxorubicin, rituximab) may be associated with superior OS in immunocompetent patients with HIV-DLBCL. Currently, the first-line regimens used
are R-CHOP-based regimens (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone) and DA-EPOCH-R11,12. HIV-BL, which accounts for 35% of NHL subtypes, is more prevalent in PLWH
with a comparatively elevated CD4 cell count13. The incidence of BL has not decreased throughout the ART era, in contrast to other subtypes. One of the suggested theories is that elevated
CD4 cell numbers could trigger more B cell activation, which would speed up the production of lymphomagenic molecular events like the _c-myc_ immunoglobulin translocation event in BL14. As
of right now, R-CODOX-M/IVAC (rituximab, cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate, ifosfamide, etoposide, high-dose cytarabine), R-Hyper-CVAD (rituximab,
cyclophosphamide, doxorubicin, vincristine, methotrexate, high-dose cytarabine), and DA-EPOCH-R are the preferred chemotherapy regimens currently used to treat BL in the general
population15,16. In the general population, rituximab is an essential part of B-cell NHL therapy; nevertheless, preliminary phase 2 studies raised concerns about safety in HIV17. A phase 3
trial revealed that rituximab enhanced toxicity in patients with CD4 counts < 50 cells/µL and was unable to show any additional benefits when added to CHOP18. Rituximab, however, seems to
be safe in all other individuals according to a pooled analysis of AMC studies. Particularly, patients with a CD4 count < 50 cells/µL had a treatment-related mortality (TRM) of 37%,
while the remaining patients had a TRM of only 6% (_p_ < 0.01)11. Non-comparison trials have also contributed to the broad view that rituximab should be used while infection is being
monitored, as well as preventive hematopoietic growth factors in all patients and antibiotic prophylaxis in those at greatest risk19. Studies summarizing the Chinese experience treating
NHL-associated HIV are currently lacking. In the present study, we report on 17 years of retrospective China data on HIV-BL and HIV-DLBCL, including diagnosis, therapy, and lymphoma- related
outcomes. METHODS AND MATERIALS STUDY POPULATION Consecutive patients with HIV-associated, biopsy confirmed, BL and DLBCL diagnosed between 1 January 2005 and 31 December 2022 at the
Department of Infectious Diseases, Zhongnan Hospital of Wuhan University through electronic database were included for this single center retrospective analysis. Other histological subtypes
such as Hodgkin lymphoma, T-cell lymphoma, primary effusion lymphoma, primary central nervous system (CNS) lymphoma and plasmablastic lymphoma were excluded. This study was approved by the
Medical Ethics Committee of Zhongnan Hospital of Wuhan University (No. 2023266 K). It was performed in accordance with the principles of the Declaration of Helsinki. All patients provided
written informed consent. Data on patient demographics, HIV-related characteristics at the time of lymphoma diagnosis (e.g., CD4 count, ART regimen, concurrent hepatitis B/C co-infection),
lymphoma-related characteristics (e.g., cell-of-origin subtype for DLBCL, presence of MYC proto-oncogene, bHLH transcription factor (_MYC_), BCL2 apoptosis regulator (_BCL2_) or BCL6
transcription repressor (_BCL6_) rearrangements, lactate dehydrogenase (LDH), Ann Arbor stage, extranodal sites, Eastern Cooperative Oncology Group Performance Status (ECOG PS) score,
International Prognostic Index (IPI) score), and treatment-related information (e.g., first-line and subsequent therapy regimens including radiotherapy, the use of rituximab, CNS
prophylaxis, the use of concurrent ART) were collected and analysed. TREATMENT AND SUPPORTIVE CARE Before 2018, HIV patients received free anti HIV treatment provided by the Chinese
government, which included two nucleoside/nucleotide reverse transcriptase inhibitors (zidovudine + lamivudine, or lamivudine + tenofovir disoproxil) plus one non-nucleoside reverse
transcriptase inhibitor (efaviren, or nevirapine) or protease inhibitor (lopinavir, or ritonavir capsules), and after 2018, due to the availability of drugs, the ART regimen was adjusted to
either bictegravar soudium, emtricitanine and tenofovir alafenamid fumarite tablets or dolutegravir sodium and lamivudine tablets. Most oncologists continue ART during chemotherapy. In early
ART days concerns arouse regarding drug-drug interactions and increased toxicities. With new ART medications available, it is now possible to avoid specific ART components to minimize
drug-drug interaction20. Cotrimoxazole was given to prevent pneumocystis jirovecii pneumonia. The chemotherapy cohort included patients who received any of the following regimens: CHOP;
R-CHOP; EPOCH; R-EPOCH. OUTCOME ASSESSMENT Treatment outcome assessments were performed either positron emission tomography (PET) ± computed tomography ± bone marrow (BM) biopsy. They were
assessed according to the standard response criteria at the time of assessment; either response evaluation criteria in solid tumors (RECIST) 2000 or Lugano 201421,22. Response outcomes were
defined as either complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD). Date of HIV-lymphoma diagnosis, death, progression/relapse, and last
follow-up was collected for survival analysis. Additional information collected include the number of cycles of chemotherapy delivered, evidence of serious treatment-related complications,
the HIV viral load and CD4 cell counts were tested at diagnosis. STATISTICAL ANALYSIS The program used was GraphPad Prism version 10.0.0 (GraphPad Software Inc. www.graphpad.com). Baseline
characteristics were presented using mean (standard deviation) or median (interquartile range (IQR)) for continuous variables and count (percentage) for categorical variables. Baseline
demographic, clinical and pathological variables were compared between BL and DLBCL groups using Pearson’s chi-square test and the Mann-Whitney _U_-test. Progression-free survival (PFS) was
defined as the time from lymphoma diagnosis to disease progression, relapse or death from any cause. OS was defined as the time from lymphoma diagnosis to last follow-up or death from any
cause. Kaplan-Meier curves were used for survival analysis (OS and PFS) and comparisons between groups were analysed using the log-rank (Mantel-Cox) test and univariate Cox proportional
hazards regression model. Changes over time in CD4 counts was presented using median and IQR. A _p_ < 0.05 was considered statistically significant. Missing data was handled using the
pairwise deletion statistical method. RESULTS PARTICIPANT CHARACTERISTICS A total of 127 patients were included in the study. There were 86 (68%) patients with HIV-DLBCL and 41 (32%) with
HIV-BL. The median (range) follow-up was 0.4 (0.1–14.0) years. Baseline characteristics for each cohort are summarised in Table 1. The median (range) age of the cohort was 47.1 (11–71) years
and 114 (90%) were male. Of the 127 patients, 89 (70%) were concurrently diagnosed with NHL and HIV. The median CD4 count for the cohorts was 0.141 × 109/L (range, 0.001–0.861 × 109/L), of
whom 79 (62%) had a CD4 cell count below 0.200 × 109/L at diagnosis. 11 patients had concurrent hepatitis B surface antigen positivity and 9 patients had active hepatitis C infection at
diagnosis. Before 2018, there were limited HIV virus load testing institutions and equipment in China, mainly concentrated in provincial and above disease prevention and control centers for
testing. Although there have been more HIV testing institutions since 2018, they have not yet become widespread, and only some patients have information on their virus load at the time of
initial diagnosis. There were 25 patients (25/127, 13 with DLBCL, 12 with BL) with HIV viral loads available at first diagnosis, 52% of patients had a HIV viral load of < 50 copies/mL.
The majority of patients (_n_ = 91 (72%), HIV-BL (98%) and HIV-DLBCL (59%)) were diagnosed with high-risk disease based on their disease-specific IPI (DLBCL R-IPI by Sehn et al. and BL-IPI
by Olszewski et al.23,24). In the HIV-BL cohort, 32 (78%) patients had an ECOG PS of ≥ 2, 35 (85%) with stage III/IV disease, 32 (78%) with two or more extranodal sites, 36 (88%) with an
elevated LDH, 24 (59%) with BM involvement, 12 (29%) with CNS/leptomeningeal involvement at diagnosis and 17 (41%) with B symptoms. In the HIV-DLBCL cohort, 50 (58%) patients had an ECOG PS
of ≥ 2, 60 (70%) with stage III/IV disease, 31 (36%) with two or more extranodal sites, 50 (58%) with an elevated LDH, 33 (38%) with BM involvement, 20 (23%) with CNS/leptomeningeal
involvement and 37 (43%) with B symptoms. There were 82 (65%) patients had an ECOG PS of ≥ 2 with a higher proportion seen in the HIV-BL cohort (78% vs. 58%, _p_ = 0.028). There were 63
(50%) patients with two or more extranodal sites with a higher proportion seen in the HIV-BL cohort (78% vs. 36%, _p_ = 0.000). There were 86 (68%) patients with an elevated LDH with a
higher proportion seen in the HIV-BL cohort (88% vs. 58%, _p_ = 0.001). There were 57 (45%) patients with BM involvement with a higher proportion seen in the HIV-BL cohort (59% vs. 38%, _p_
= 0.033). There were 75 (59%) patients with bulky disease with a higher proportion seen in the HIV-BL cohort (78% vs. 50%, _p_ = 0.003). LYMPHOMA TREATMENT A total of 85/127 (67%) patients
received therapy with curative intent, where 23/85 (27%), 60/85 (71%), and 2/85 (2%) were in the 1–3, 4–6, and > 6 cycles chemotherapy cohort, respectively. The patients had less than
four cycles of antilymphoma therapy due to poor PS and advanced stage at diagnosis, most of them died due to the rapid progression of the disease. 1/127 patients with HIV-BL and 6/127 with
HIV-DLBCL receiving palliative therapy. 35/127 (28%) patients received no anti-lymphoma therapy because of fear of discrimination and poor financial situation. Patients with HIV-BL were most
commonly treated with dose-intensive regimens such as R-DA-EPOCH or DA-EPOCH (54%), followed by R-CHOP (12%). CHOP-based chemotherapy was most commonly used to treat HIV-DLBCL, with 36
patients receiving this regimen (42%). There were 21 (24%) patients with HIV- DLBCL managed with more intensive regimens DA-EPOCH ± R. Of the patients receiving curative chemotherapy, 45
(35%) patients received rituximab (21 (51%) with HIV-BL and 24 (28%) with HIV-DLBCL). In all, 89 (70%) patients received ART with chemo-immunotherapy concurrently. Only nearly half of the
patients (62/127 (49%)) completed their intended chemotherapy regimen. There were 6 (5%) patients who had treatment truncated due to infective complications (5 (4%) patients), pulmonary
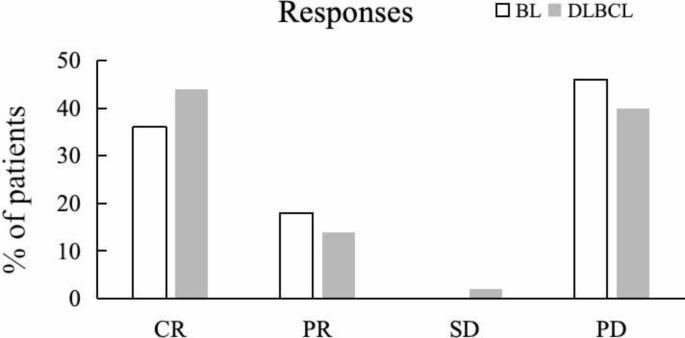
embolism (one (0.8%) patient). The computed tomography or PET-defined CR rate after first-line curative therapy was 10/28 (36%) in HIV-BL and 25/57 (44%) in HIV-DLBCL (Fig. 1). In HIV-BL,
best tumor responses included CR (6 R-DA-EPOCH, 2 DA-EPOCH, 2 R-CHOP, 10 patients), PR (2 R-DA-EPOCH, 2 DA-EPOCH, 1 R-CHOP, 5 patients), 0 patient for SD, and PD (8 R-DA-EPOCH, 2 DA-EPOCH, 2
R-CHOP, 1 CHOP, 13 patients). In HIV-DLBCL, best tumor responses included CR (5 R-DA-EPOCH, 3 DA-EPOCH, 6 R-CHOP, 11 CHOP, 25 patients), PR (2 R-DA-EPOCH, 2 DA-EPOCH, 3 R-CHOP, 1 CHOP, 8
patients), SD (1 R-CHOP, 1 patients), and PD (4 R-DA-EPOCH, 5 DA-EPOCH, 3 R-CHOP, 11 CHOP, 23 patients). Febrile neutropenia (Common Terminology Criteria for Adverse Events Grade ≥ 3) was
higher in the HIV-BL cohort compared to the HIV-DLBCL cohort (68% vs. 47% respectively, _p_ = 0.075). It was also higher for DA-EPOCH ± R when compared to CHOP ± R (57% vs. 42%, _p_ =
0.259). There were two treatment related deaths, one from pulmonary embolism, the other from tracheoesophageal fistula in the HIV-BL group. There were four treatment related deaths, three
from infections (one intestinal infection, two pulmonary infection), one from gastrointestinal bleeding in the HIV-DLBCL group. SURVIVAL OUTCOMES 92 (72%) patients received anti-tumor
therapy (85 patients received curative therapy, 7 patients received palliative therapy), and those 35 patients did not receive chemotherapy have been excluded from the survival analyses.
After a median (range) follow-up of 0.4 (0.1–14.0) years, 53 (58%) patients had died; 45 (49%) from disease, two from severe pneumonias, one from pulmonary embolism, one from severe diarrhea
leading to water electrolyte disorders, one from sepsis, one from infection caused by esophagotracheal fistula, one from gastric cancer, and one from valvular heart disease. There were no
HIV-related deaths. The 2-year OS rate for the entire cohort was 45% (95% confidence interval (CI) 35-55%). The 2-year OS for the HIV-BL cohort was 41% (95% CI 24%-58%) and 47% (95% CI
34%-59%) for HIV-DLBCL cohort. The 2-year PFS for the HIV-BL cohort was 50% (95% CI 30%-66%) and for the HIV-DLBCL cohort was 55% (95% CI 41%-67%) (Fig. 2). An exploratory univariate
analysis was performed on selected lymphoma variables (histological subtype, IPI > 2, BM involvement, CNS involvement, elevated LDH, bulky disease, B symptoms, rituximab use) and CD4
count < 0.100 × 109/L with the recognition that this study was not powered to draw definitive conclusions (Table 2). In our analysis, no variables impacted on OS. CNS involvement
negatively impacted PFS (HR 3.23, 95% CI 1.38–7.53, _p_ = 0.007). In HIV-BL, B symptoms negatively impacted OS (HR 10.07, 95% CI 1.92–52.72, _p_ = 0.006) and PFS (HR 8.73, 95% CI 1.43–53.35,
_p_ = 0.019), and CD4 count < 0.100 × 109/L negatively impacted OS (HR 12.49, 95% CI 2.44–63.80, _p_ = 0.002) and PFS (HR 8.23, 95% CI 1.92–35.36, _p_ = 0.005) (Table 3). In HIV-DLBCL,
CNS involvement negatively impacted PFS (HR 4.59, 95% CI 1.20–17.52, _p_ = 0.026) (Table 4). DISCUSSION Understanding the clinical features and prognostic variables of HIV-associated
lymphoma is limited since it is less common in China than HIV-negative lymphoma10,25. As the biggest cohort described in China to date, we performed a description of the clinical features
and prognosis of patients with HIV-DLBCL and BL across a 17-year period at a single academic medical center. The initial symptoms of HIV-associated lymphoma are more diverse than those of
HIV-negative lymphoma; more cases of this disease are diagnosed at an advanced stage, frequently with B symptoms present, and exhibit more extranodal involvement3. In the present study, the
majority of patients presented with advanced stage (85% in HIV-BL and 70% in HIV-DLBCL) and high-risk disease based on disease-specific IPI scoring systems (HIV-BL 98%, HIV-DLBCL 59%).
Patients had a high frequency of B symptoms (HIV-BL 41%, HIV-DLBCL 43%), extranodal involvement ≥ 2 (HIV-BL 78%, HIV-DLBCL 36%), and bulky disease (HIV-BL 78%, HIV-DLBCL 50%). According to
these findings, HIV-associated lymphoma may be more aggressive. On the other hand, compared to their HIV-negative counterparts, HIV positivity has been demonstrated to have no effect on
treatment deliverability, response, and patient survival in ART-treated patients15,26. A German group of patients with HIV-lymphoma who were diagnosed between 2005 and 2012 had a median CD4
count of 0.200 × 109/L, and 37% of them were on ART at the time of diagnosis27. In a French study, 79% of patients with an HIV-DLBCL cohort had already started ART, with a median CD4 count
of 0.233 × 109/L28. The bulk of patients in our study (70%) were on ART at the time of their lymphoma diagnosis, which probably played a role in the relatively low baseline mean and median
CD4 counts (0.182 and 0.141 × 109/L) observed in our real-world cohort when compared to developed countries27,28. This suggests that HIV screening should be a priority for patients with
newly diagnosed BL and DLBCL. According to research by Lim et al., patients with HIV-DLBCL had a 2-year PFS of 77% and an OS of 81%, while those with HIV-BL had a 2-year PFS of 67% and an OS
of 67%13, Coutinho et al. showed a 5-year OS of 78% versus 64% in HIV-positive and HIV-negative DLBCL respectively29, in patients with HIV-DLBCL, Besson et al. reported a 2-year PFS and OS
of 75% and 75%, respectively28. Regardless of HIV status, a US retrospective research for HIV-BL reported a 3-year PFS and OS rate of 64% and 70%, respectively30. A follow-up collaborative
study between the USA and the UK revealed that the 3-year PFS and OS for 249 HIV-BL patients treated between 2008 and 2019 were 61% and 66%, respectively15. Our study findings of a 2-year
PFS of 55% and OS of 47% in patients with HIV-DLBCL, and a 2-year PFS of 50% and OS of 41% in patients with HIV-BL, were lower than the published international data. Even though the
comparison is still in its preliminary stages, there are a few reasons why our study’s PFS and OS were lower and they merit additional research: (1) CD4 counts: The fact that 70% of the
patients in our study started ART concurrently to the lymphoma diagnosis suggests that the baseline mean and median CD4 counts were not particularly high. These patients are easily
complicated by opportunistic infections, resulting in an increase in mortality. (2) baseline clinicopathological characteristics: Due to poor PS and an advanced stage upon diagnosis, 27% of
patients received less than four cycles of antilymphoma therapy; the majority of these patients passed away as a result of the disease’s quick progression. According to the CALL trial,
patients with HIV- associated DLBCL who had 4–6 cycles of chemotherapy had considerably better outcomes; individuals who received fewer than 4 cycles of chemotherapy had an independent risk
factor for a poor prognosis (HR 0.520, 95% CI 0.424–0.637; _p_ < 0.001)9. (3) Rituximab: Rituximab usage is linked to improved PFS and OS in patients with a CD4 count ≥ 50 cells/µL and
positive expression of CD20. However, because infections are more common in patients with a CD4 count < 50 cells/µL, the benefit of rituximab is less evident31. 47 patients in our study
received rituximab, 11 of them had a CD4 count < 50 cells/µL, and 6 of the 11 patients (55%) died. Our results showed that treatment of HIV-related lymphoma remains the major challenge in
China. It is currently unknown if the prognostic risk of aggressive B-cell NHL associated with HIV infection may be evaluated using the same model as individuals without HIV infection. The
AIDS-related lymphoma IPI (ARL-IPI), developed by Barta SK et al., is a new prognostic index that patients patients into low-, intermediate-, and high-risk groups32. The ARL-IPI based on the
baseline characteristics of ECOG PS, LDH level, stage, number of involved extranodal sites and an HIV score that incorporates base-line CD4 count, HIV viral load and prior history of AIDS.
Barta SK et al. found that the ARL-IPI performed significantly better in predicting OS than the age-adjusted IPI32. Our analysis showed that CNS involvement (HR 3.23, 95% CI 1.38–7.53, _p_ =
0.007) was independent risk factors for adverse prognosis based on PFS for HIV-DLBCL and HIV-BL patients. In HIV-BL patients, B symptoms and CD4 count < 0.100 × 109/L were independent
risk factors for adverse prognosis. Numerous studies have shown that CODOX-M/IVAC chemotherapy, in addition to rituximab, is a feasible and efficient treatment for HIV-BL33. It has also been
discovered that DA-EPOCH-R is a good substitute with similar survival rates31. When there are obvious brain metastases, this regimen cannot be administered since the cytotoxic drugs in
R-EPOCH do not penetrate the blood-brain barrier; instead, regimens that include CNS therapy are better in this circumstance. None of the 22 patients in our cohort who received DA-EPOCH
treatment experienced CNS relapse, despite a previous retrospective research indicating a greater risk of CNS recurrence when this treatment was used15. The most widely used front-line
regimens in HIV-DLBCL were CHOP-based regimens (42%), while a sizable minority (25% DA-EPOCH ± R) received higher intensity regimens. This was probably caused by findings indicating that, in
HIV-DLBCL, DA-EPOCH-R compared favorably to R-CHOP11,12. The objective response rate was 58% vs. 57% in our trial, which did not show any significant treatment response between CHOP ± R and
DA-EPOCH ± R. However, DA-EPOCH ± R compared favorably to CHOP ± R in terms of survival rate (57% vs. 44%). LIMITATIONS OF THE STUDY One of the study’s shortcomings is its retrospective
design, which has biases by nature. Furthermore, this real-world study comprised low- and middle-income populations with lower popularization of rituximab, partly owing to the treatment
resources, supportive care, and patient affordability in developing countries. Other nations and areas with inadequate or unequal resources face the same problem, which may be a reflection
of differences in treatment philosophies and socioeconomic circumstances. CONCLUSIONS In conclusion, this study examining real-world Chinese data on HIV-BL and DLBCL showed the majority of
patients were on ART concurrently to the lymphoma diagnosis. Due to the extremely low percentage of patients receiving ART prior to the lymphoma diagnosis, the high percentage of patients
with poor PS, and the advanced stage at diagnosis, our study’s survival results were worse than those of industrialized nations. Furthermore, our research indicated that B symptoms and a low
CD4 count predicted poor OS and PFS in HIV-BL patients, and CNS involvement predicted poor PFS in HIV-DLBCL and BL patients. DATA AVAILABILITY Data are available upon request from the
[email protected]. REFERENCES * Cao, W., Hsieh, E. & Li, T. Optimizing treatment for adults with HIV/AIDS in China: Successes over two decades and remaining challenges. _Curr. HIV/AIDS
Rep._ 17 (1), 26–34 (2020). Article PubMed PubMed Central Google Scholar * Yarchoan, R. & Uldrick, T. S. HIV-associated cancers and related diseases. _N Engl. J. Med._ 378 (11),
1029–1041 (2018). Article PubMed PubMed Central Google Scholar * Carbone, A., Vaccher, E. & Gloghini, A. Hematologic cancers in individuals infected by HIV. _Blood_ 139 (7), 995–1012
(2022). Article CAS PubMed Google Scholar * Aboulafia, D. M. Non-Hodgkin lymphoma in people with HIV. _Lancet HIV_. 6 (4), e209–e210 (2019). Article PubMed Google Scholar * Borges,
Á. H. Combination antiretroviral therapy and cancer risk. _Curr. Opin. HIV AIDS_. 12 (1), 12–19 (2017). Article CAS PubMed PubMed Central Google Scholar * Hernández-Ramírez, R. U.,
Shiels, M. S., Dubrow, R. & Engels, E. A. Cancer risk in HIV-infected people in the USA from 1996 to 2012: A population-based, registry-linkage study. _Lancet HIV_. 4 (11), e495–e504
(2017). Article PubMed PubMed Central Google Scholar * AIDS-defining Cancer Project Working Group of IeDEA, COHERE in EuroCoord. Non-hodgkin lymphoma risk in adults living with HIV
across five continents. _AIDS_ 32 (18), 2777–2786 (2018). Article Google Scholar * Wang, C., Liu, J. & Liu, Y. Progress in the treatment of HIV-associated lymphoma when combined with
the antiretroviral therapies. _Front. Oncol._ 11, 798008 (2022). Article PubMed PubMed Central Google Scholar * Wang, C. et al. Impact of initial chemotherapy cycles and clinical
characteristics on outcomes for HIV-associated diffuse large B cell lymphoma patients: The Central and Western China AIDS Lymphoma League 001 study (CALL-001 study). _Front. Immunol._ 14,
1153790 (2023). Article CAS PubMed PubMed Central Google Scholar * Wang, C. et al. Clinical characteristics and outcomes of newly diagnosed patients with HIV-associated aggressive
B-cell NHL in China. _J. Cell. Mol. Med._ 26 (19), 5067–5077 (2022). Article CAS PubMed PubMed Central Google Scholar * Barta, S. K., Lee, J. Y., Kaplan, L. D., Noy, A. & Sparano,
J. A. Pooled analysis of AIDS malignancy consortium trials evaluating rituximab plus CHOP or infusional EPOCH chemotherapy in HIV-associated non-Hodgkin lymphoma. _Cancer_ 118 (16),
3977–3983 (2012). Article CAS PubMed Google Scholar * Barta, S. K. et al. Treatment factors affecting outcomes in HIV-associated non-hodgkin lymphomas: A pooled analysis of 1546
patients. _Blood_ 122 (19), 3251–3262 (2013). Article CAS PubMed PubMed Central Google Scholar * Lim, K. J. C. et al. Outcomes of human immunodeficiency virus-associated Burkitt
lymphoma and diffuse large B-cell lymphoma treated in Australia: A report from the Australasian Lymphoma Alliance. _Br. J. Haematol._ 201 (5), 865–873 (2023). Article CAS PubMed Google
Scholar * Opie, J., Antel, K., Koller, A. & Novitzky, N. In the South African setting, HIV-associated Burkitt lymphoma is associated with frequent leukaemic presentation, complex
cytogenetic karyotypes, and adverse clinical outcomes. _Ann. Hematol._ 99 (3), 571–578 (2020). Article CAS PubMed PubMed Central Google Scholar * Alderuccio, J. P. et al. HIV-associated
Burkitt lymphoma: Outcomes from a US-UK collaborative analysis. _Blood Adv._ 5 (14), 2852–2862 (2021). Article CAS PubMed PubMed Central Google Scholar * Roschewski, M. et al.
Multicenter study of risk-adapted therapy with dose-adjusted EPOCH-R in adults with untreated Burkitt lymphoma. _J. Clin. Oncol._ 38 (22), 2519–2529 (2020). Article CAS PubMed PubMed
Central Google Scholar * Spina, M. et al. Rituximab plus infusional cyclophosphamide, doxorubicin, and etoposide in HIV-associated non-Hodgkin lymphoma: Pooled results from 3 phase 2
trials. _Blood_ 105 (5), 1891–1897 (2005). Article CAS PubMed Google Scholar * Kaplan, L. D. et al. Rituximab does not improve clinical outcome in a randomized phase 3 trial of CHOP with
or without rituximab in patients with HIV-associated non-hodgkin lymphoma: AIDS-Malignancies Consortium Trial 010. _Blood_ 106 (5), 1538–1543 (2005). Article CAS PubMed PubMed Central
Google Scholar * Wei, K. C. et al. Pneumocystis Jirovecii pneumonia in HIV-uninfected, rituximab treated non-Hodgkin lymphoma patients. _Sci. Rep._ 8 (1), 8321 (2018). Article ADS
MathSciNet PubMed PubMed Central Google Scholar * Noy, A. Optimizing treatment of HIV-associated lymphoma. _Blood_ 134 (17), 1385–1394 (2019). Article PubMed PubMed Central Google
Scholar * Therasse, P. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of
the United States, National Cancer Institute of Canada. _J. Natl. Cancer Inst._ 92 (3), 205–216 (2000). Article CAS PubMed Google Scholar * Cheson, B. D. et al. Recommendations for
initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. _J. Clin. Oncol._ 32 (27), 3059–3067 (2014) (European Mantle Cell
Lymphoma Consortium; Italian Lymphoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español de Médula Ósea; German High-Grade
Lymphoma Study GroupNCIC Clinical Trials Group; Nordic Lymphoma Study Group). * Sehn, L. H. et al. The revised international prognostic index (R-IPI) is a better predictor of outcome than
the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. _Blood_ 109 (5), 1857–1861 (2007). Article CAS PubMed Google Scholar * Olszewski, A. J. et al.
Burkitt lymphoma international prognostic index. _J. Clin. Oncol._ 39 (10), 1129–1138 (2021). Article CAS PubMed PubMed Central Google Scholar * Xiong, Y. et al. The safety and efficacy
of PD-1 inhibitors in patients with advanced cancers and HIV/AIDS in China. _Front. Oncol._ 13, 1248790 (2023). Article CAS PubMed PubMed Central Google Scholar * Magnusson, T. et al.
Clinical features, treatments and outcomes of HIV associated diffuse large B-cell lymphoma: A single-center experience. _Blood_ 136, 20 (2020). Article Google Scholar * Schommers, P. et
al. Survival of AIDS-related diffuse large B-cell lymphoma, Burkitt lymphoma, and plasmablastic lymphoma in the German HIV lymphoma cohort. _Br. J. Haematol._ 168 (6), 806–810 (2015).
Article PubMed Google Scholar * Besson, C. et al. ANRS-CO16 LYMPHOVIR Cohort. Outcomes for HIV-associated diffuse large B-cell lymphoma in the modern combined antiretroviral therapy era.
_AIDS_ 31 (18), 2493–2501 (2017). Article PubMed Google Scholar * Coutinho, R. et al. HIV status does not impair the outcome of patients diagnosed with diffuse large B-cell lymphoma
treated with R-CHOP in the cART era. _AIDS_ 28 (5), 689–697 (2014). Article CAS PubMed Google Scholar * Evens, A. M. et al. Burkitt lymphoma in the modern era: real-world outcomes and
prognostication across 30 US cancer centers. _Blood_ 137 (3), 374–386 (2021). Article CAS PubMed PubMed Central Google Scholar * Sparano, J. A. et al. Response-adapted therapy with
infusional EPOCH chemotherapy plus rituximab in HIV-associated, B-cell non-Hodgkin’s lymphoma. _Haematologica_ 106 (3), 730–735 (2021). Article CAS PubMed Google Scholar * Barta, S. K.
et al. A new prognostic score for AIDS-related lymphomas in the rituximab-era. _Haematologica_ 99 (11), 1731–1737 (2014). Article PubMed PubMed Central Google Scholar * Atallah-Yunes, S.
A., Murphy, D. J. & Noy, A. HIV-associated Burkitt lymphoma. _Lancet Haematol._ 7 (8), e594–e600 (2020). Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS The
authors acknowledge the the patients, investigators, and staff at the Zhongnan Hospital of Wuhan University for their support. FUNDING This study was funded by the Center for AIDS Research,
Wuhan University (PTPP2023002). AUTHOR INFORMATION Author notes * Yu Xiong, Weicheng Liu and Xiaoping Chen contributed equally to this work. AUTHORS AND AFFILIATIONS * Department of
Radiation and Medical Oncology for Esophageal Mediastinal and Lymphatic Tumors, Zhongnan Hospital of Wuhan University, Wuhan, 430071, China Yu Xiong * Hubei Key Laboratory of Tumor
Biological Behaviors, Zhongnan Hospital of Wuhan University, Wuhan, 430071, China Yu Xiong * Hubei Clinical Cancer Study Center, Zhongnan Hospital of Wuhan University, Wuhan, 430071, China
Yu Xiong * Department of Colorectal and Anal Surgery (Clinical Center for Pelvic Floor Surgery), Zhongnan Hospital of Wuhan University, Wuhan, 430071, China Weicheng Liu * Hubei Key
Laboratory of Intestinal and Colorectal Diseases, Zhongnan Hospital of Wuhan University, Wuhan, 430071, China Weicheng Liu * Clinical Center of Constipation and Pelvic Floor Disease of
Wuhan, Wuhan, 430071, China Weicheng Liu * Clinical Center of Intestinal and Colorectal Diseases of Hubei Province, Wuhan, 430071, China Weicheng Liu * Department of Infectious Diseases,
Zhongnan Hospital of Wuhan University, 169 Donghu Road, Wuhan, 430071, China Xiaoping Chen, Pingzheng Mo, Yong Xiong, Liping Deng & Yongxi Zhang * Centre of AIDS Prevention and Cure,
Zhongnan Hospital of Wuhan University, Wuhan, 430071, China Xiaoping Chen, Pingzheng Mo & Yongxi Zhang Authors * Yu Xiong View author publications You can also search for this author
inPubMed Google Scholar * Weicheng Liu View author publications You can also search for this author inPubMed Google Scholar * Xiaoping Chen View author publications You can also search for
this author inPubMed Google Scholar * Pingzheng Mo View author publications You can also search for this author inPubMed Google Scholar * Yong Xiong View author publications You can also
search for this author inPubMed Google Scholar * Liping Deng View author publications You can also search for this author inPubMed Google Scholar * Yongxi Zhang View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors contributed to the study conception and design. Material preparation were by Yu.X., W.L., X.C., data
collection were by Yu.X., P.M., and analysis were performed by W.L., L.D., Yo.X. The first draft of the manuscript were written by L.D., Y.Z. and all authors commented on previous versions
of the manuscript. All authors read and approved the final manuscript. CORRESPONDING AUTHORS Correspondence to Liping Deng or Yongxi Zhang. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any
non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of
it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material
is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission
directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Xiong, Y., Liu, W., Chen, X. _et al._ Survival of HIV associated diffuse large B-cell lymphoma and Burkitt lymphoma in China. _Sci Rep_ 14, 30397 (2024).
https://doi.org/10.1038/s41598-024-80749-9 Download citation * Received: 16 May 2024 * Accepted: 21 November 2024 * Published: 05 December 2024 * DOI:
https://doi.org/10.1038/s41598-024-80749-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative







