- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Many species use chemical messengers to communicate a remarkable range of information. Mice appear to make particular use of chemical messengers, including effects on estrous cycling and
initiation, pregnancy, aggression, stress and of course attraction. Behavioral studies have helped identify several candidate messengers, or pheromones, that mediate attraction in mice. One
question is whether attractive chemical messengers induced a physical vaginal secretory response. The preparation hypothesis posits that increased vaginal secretion would lubricate and
protect the vagina in response to the prospect of imminent coitus, but this has been difficult to assess experimentally, particularly in mice. We developed a rapid, sensitive, minimally
invasive method of quantifying vaginal moisture in mice and used this model to test vaginal secretory responses to male bedding. We report that female mice experience an increase in vaginal
moisture after exposure to male, but not female, bedding. This response is induced by either physical or airborne exposure to male urine, to preputial gland extract, and to the preputial
gland-derived pheromone alpha/beta farnesenes. This vaginal response is diurnally regulated, seen only during their active phase. The response is sensitive to the estrous phase, with a clear
response during estrus but not during metestrus. We conclude that mice may serve as a model for aspects of vaginal function and that this assay will be readily applicable to other small
animals. The identification of a pheromone-mediated vaginal secretory response offers a window into the regulation of the vaginal environment and the neurobiology of sexual responses in
mice.
Many species use chemical messengers to communicate a wide range of information1. These messengers are generally thought to act through the olfactory system that is broadly divided into two
separate systems. Many of these messengers – sometimes also referred to as pheromones – are thought to act via the vomeronasal organ, a sensory epithelium that responds to non-volatile
messengers, and whose neurons project to the accessory olfactory bulb and from there to the rest of the brain2. However volatile messengers likely act via the main olfactory bulb via inputs
from olfactory neurons that line the sinus3. These messengers can derive from different sources: though many of these messengers are present in urine, released in part by preputial and
clitoral glands of male and female mice, there is also evidence for release of messengers through saliva, sweat, and even tears4,5. Taken as a whole, the range of signals communicated by
such chemical messengers and their consequent behavioral responses is remarkable. Some species such as mice appear to make particular use of these chemical messengers and several such
responses have already been characterized. For example, the “Whitten Effect” sees synchronized estrus in female mice after exposure to specific chemical messengers in male urine6,7. Other
effects have been described in mice, including the termination of pregnancy in response to the odor of an unfamiliar male, the so-called “Bruce effect”8, or the alteration of the duration of
estrous cycles in isolated group-housed females, the so-called “Lee-Boot effect”9. Typically these studies have made use of laboratory mice. Chemical messengers are of considerable interest
to the farming industry both as livestock ‘biostimulants’3,10 and to facilitate pest control (i.e. rodent attractants for traps)11. The role of chemical messengers in mediating female
attractive responses to males has been the subject of many studies. Several chemicals and proteins have been reported to serve as attractants for female mice, including the major urinary
protein darcin12, 3,4-dehydro-exo-brevicomin and 2-sec-butyl-4,5- dihydrothiazole (SBT)13, a mixture of alpha and beta farnesenes14 as well as hexadecanol and hexadecyl acetate15. Volatile
messengers such as alpha/beta farnesenes and hexadecanol/hexadecyl acetate are produced by the male preputial gland of the mouse14,15 and the rat16. Vaginal responses to attractive stimuli
have seen little study, despite the medical importance of vaginal health and function.
The vaginal environment is tightly regulated, while serving as a unique interface with the external world. Much remains unknown about how this vaginal environment is regulated. Our poor
understanding of the neurobiology of vaginal secretion is also reflected in the prevalence of and limited treatments for vaginal dryness, a condition that affects millions17. Frequently
painful, vaginal dryness affects quality of life, is accompanied by risk of infection, and, as a side effect of commonly prescribed drugs, is a contributor to patient non-compliance18,19,20.
The preparation hypothesis posits that animals experience an autonomic/involuntary increase in vaginal moisture when confronted with the prospect of imminent coitus in order to protect the
reproductive apparatus (discussed in21). However, this has been difficult to assess experimentally. Studies of chemoattractants have relied on behavioral preference tests that quantify time
spent investigating or loitering by a murine biochemical marker (e.g22). Vaginal secretion is under autonomic control (e.g23) and so offers a rapidly quantifiable physiological response, but
has seen limited study, particularly in small animals such as mice, due to the challenge of reliably quantifying small volumes of vaginal secretions.
The aim of this study was to develop a simple method for the quantification of vaginal moisture in female laboratory mice and to apply this method to test vaginal secretory responses to
attractive male stimuli. To accomplish this we adapted a long-established method from studies of lacrimation in mice24 to quantify vaginal secretion. We describe this rapid, minimally
invasive and readily repeatable assay including a method for the manufacture of a colorimetric thread. When initial studies indicated that exposure to male bedding increased vaginal
moisture, we examined the nature and origin of the chemical messenger, testing physical and airborne exposure to male urine, male glandular secretion, and the volatile chemical messengers
alpha/beta farnesenes that have previously been implicated in female attractive responses to males25. We additionally investigated the impact of the active/rest phase and estrous phases on
this vaginal moisture response.
Mice were group-housed in standard ventilated caging (cage floor dimensions: 33.5 × 18 cm, depth 14 cm), with corn cob-based bedding (Bed-o’combs laboratory animal bedding, The Andersons,
Maumee, OH). Mice were group-housed 3–4 mice per cage and were fed Inotiv Teklad 2918 irradiated rodent diet ad libitum. Because mice are nocturnal, we tested their responses during their
active phase, by maintaining them on a reverse light cycle, except to test for diurnal contributions as described below.
With one exception, C57BL/6 strain mice were tested. That exception was the study of diurnal regulation, for which CD1 strain mice were used. C57BL/6 mice were purchased from Jackson
Laboratory (Bar Harbor, ME). CD1 strain mice for studies of diurnal variation were bred in a colony in the same facility and kindly provided by Dr. Ken Mackie (Indiana University,
Bloomington IN). Mice were 3–6 months of age. All animal care and experimental procedures used in this study were approved by the Institutional Animal Care and Use Committee of Indiana
University and conform to the Guidelines of the National Institutes of Health on the Care and Use of Laboratory Animals. Experiments complied with ARRIVE guidelines.
Only adult female mice were tested for vaginal responses, but bedding and urine samples were obtained from both males and females and preputial glands were obtained from males. For study of
response to bedding the same group of females were exposed to male or female bedding with a 2 week ‘washout’ period. male bedding: 12 mice, female bedding: 11 mice.
For responses to olfactory stimuli numbers were as follows. Urine (contact): n = 19, Urine scent: n = 27, Preputial gland scent: n = 16, alpha/beta farnesenes scent: n = 13.
For tests of active vs. rest phase, 25 and 22 females, respectively.
Female mice were generally tested in two but no more than three experiments. This means that in the second or third experience had previous experience with male scents.
With the exception of one cohort of mice housed in standard light cycle, mice were housed in the same room but the males and females were sexually naïve, meaning that they had not had an
opportunity to physically interact with one another. As noted above, the C57BL/6 mice were purchased from Jackson laboratories, so the cage-mates were related but it is unknown whether the
mice in different cages were related. A given cage of CD1 strain females were siblings, but females from different cages in the diurnal variation study were from different dams.
The use of colorimetric threads is an established method for the measurement of small volumes of exocrine secretions such as tears in small animals including mice24,26. Fluids readily travel
along the threads, wetting/discoloring them for later quantification; more fluid results in a longer discolored portion of the thread and can therefore be taken as a measure of fluid
levels, though no claim is made that a given distance traveled corresponds to a specific volume of fluid.
Until recently a commercially produced version of these threads (ZoneQuick) was available (e.g26). These threads were impregnated with phenol red, pH sensitive colorimetric dye that shifts
between yellow at pH 6.8 to fuchsia at pH 8.2. The manufacturer ceased production of these threads in 2020, but the method is not difficult to replicate in-house and is described here. After
testing a variety of threads, we found that Coats & Clark 60:40 (cotton over a polyester core) 35wt had the best combination of stiffness and color absorption. This thread is readily
available from a variety of sewing supply vendors (e.g. www.joann.com). As a first step, thread is submerged in 100mL PBS (pH 7.4) for 10 min. After this, the thread is removed and allowed
to air-dry for 5–6 h. The rationale for this is to give the thread a consistent embedded pH of 7.4 so that even a small sample with a lower pH will still discolor the thread.
To prepare a phenol red/HEPES-buffered saline solution, we combine equal volumes of phenol red solution (1% in alcohol, Cat# UN1987, Sigma-Aldrich, St. Louis MO) and HBS (adjusted to pH 6.5
with NaOH) in a beaker. We generally prepare 10mL at a time.
Using forceps, dip the strings into the beaker for 2 s. The phenol red strings are placed between two paper towels to absorb excess dye mix. A fresh paper towel is placed over the threads
with a flat weight above to pin the thread between the paper towel sheets. Threads are allowed to dry for two days. Threads are initially red, but soon change to a golden yellow color as
they dry. Store the thread in a sealable plastic bag to minimize contact with air. These threads can be stored for several months. Threads are cut as needed on the day of experiments.
To allow for consistent placement in the vaginal cavity, a thread is inserted into a glass capillary (A-M Systems, 0.5 mm inner diameter, Cat#: 626000, fire-polished to prevent injury),
leaving 3 mm of thread outside the opening of the capillary. The capillary is then placed into the vaginal cavity of an unanesthetized mouse for 10 s. We have previously adapted these
threads for the measurement of salivation27 and Fig. 1 in that publication shows a sample thread positioned in a cannula as well as a sample discolored thread positioned next to a ruler for
measurement. See Supplementary Fig. S1 for a volume/thread length response curve. We pipetted up a given volume of phosphate buffered saline with 5% kolliphor as a wetting agent, then
expressed this volume at the tip and promptly touched the end of the thread to the tip of the pipette to allow for uptake of liquid by the thread. Based on uptake of 0.8, 1.0, 1.2 and 1.4
uL, a linear regression analysis yielded a slope of 23.9 ± 4.1 mm of distance along thread per microliter of saline. We did not include volumes below 0.8uL since these come with a risk of
pipetting error as well as adhesion of liquid to the outside of the pipette tip that may result in an exaggerated response. On a practical note, it is important to measure and record the
distance traveled within ~ 1 min since the sample gradually returns to a golden color as it dries. Moreover, a liquid sample with a lower pH will discolor the thread at the leading edge
(because of the embedded pH of the thread) so it is sometimes necessary to track the ‘leading edge’ of the discoloration. On a related note, we find that frequent prior handling of the mice
is important for these experiments, partly to reduce the stress of handling, but also because this greatly reduces the likelihood that mice will urinate during the procedure and so foul the
sample. The colorimetric threads are also an approximate pH sensor and the acidic urine28 overwhelms the embedded pH and so does not discolor the threads; this combined with the difference
in volume make it easy to determine when a sample is due to urination. Mice that see a urine response are excluded from a given experiment.
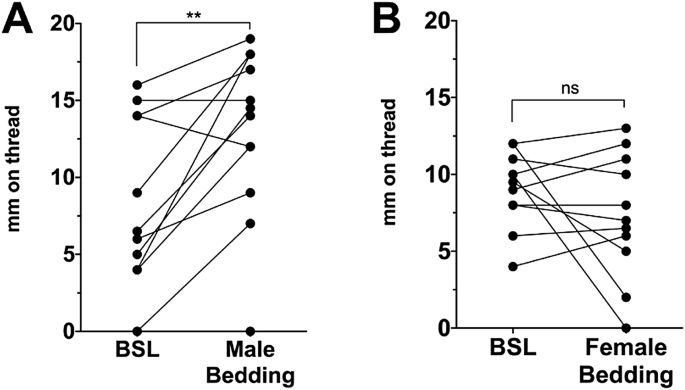
Bedding for a given experiment was obtained in coordination with the weekly animal facility cage-change. Bedding (from 6 days of habitation) was obtained from a cage of 3–4 male (or in one
experiment (Fig. 1B) female) mice. Bedding was used within 1 h. For a bedding exposure, males were relocated to a clean cage and females were moved into the soiled male cage and allowed to
roam for 1 h, after which the vaginal moisture measurement was obtained and females were moved back into their home cage. A given soiled male cage was used only once (for one cage of
females).
For the urine(contact) testing condition, urine is obtained from male mice by scruffing the animal and collecting, with a kim-wipe, freely secreted urine. Urine from 4 males is sufficient
for 3–4 females (i.e. one cage). Because not all males urinate ‘on demand’ the males originate from 1 to 2 cages. The kim-wipes are immediately mixed in with bedding of a fresh cage. Females
are placed into the cage and allowed to explore and as a rule they soon dig up the soiled tissue. After 1 h, after which the vaginal moisture measurement was obtained and females were moved
back into their home cage. A cage prepared in this manner was used only once (for one cage of females).
For urine-smell only condition, the urine is collected as above, but now the kim-wipes from two males are placed on a separator positioned on the wire caging, in a plastic weigh boat, but
below the cage filter top, so preventing physical contact with the mice while sealing in the scent.
Preputial glands were dissected and physically pressed using a spatula to release gland contents into a small plastic dish. Glands from two males (C57BL/6, age 3–4 months) were taken as a
single sample and the sample was used for 1 experiment. A total of 8 males were used for this experiment. Samples were used within 5 min of dissection then exposed as for urine-smell only
condition.
Implicated as a female attractant, farnesene is found in male urine at 5ppm and has been demonstrated to have attractant properties at 10ppm25. Farnesene occurs naturally as isomers several
of which have attractant properties25. For these experiments, farnesene was purchased from Sigma-Aldrich as a mixture of isomers (trans-β, cis-α, trans-α; cat: w383902). Farnesene was
diluted to 10 ppm (1:100 in ethanol, then 1:1000 in water, accounting for the density of the original stock) in a volume of 1mL and absorbed into paper towel and suspended in a plastic dish
as above.
To determine the estrous cycle phase of a given mouse we use the method described by Byers et al.29. We obtain a vaginal smear by inserting 10ul of sterile saline into the proximal vaginal
canal using a 20uL pipette. The liquid is then collected back into the pipette and is placed onto a slide and covered with a cover slip. The slide is then imaged within 3 h using a
camera-equipped phase-contrast microscope. The picture is saved for scoring. Evaluation of the images is done via a two-person consensus approach: each person scores the images; in the case
of disagreement, each makes a case for a given phase and the scorers arrive at a consensus. Occasionally scorers are uncertain about the phase because it exhibits characteristics of both.
This occurs most frequently for estrus vs. metestrus. In that case the sample is scored accordingly (e.g. estrus/metestrus) and the vaginal response data is excluded from analyses. These
ambiguous estrous typings represented ~ 10–15% of the total.
For odorant exposure experiments, several cages containing a group of C57BL/6 female mice (3–4/cage) are brought into an adjoining room separated by a closed door. Mice were tested in
late-morning, starting at 10AM to reduce the likelihood of unusual scents. All baseline vaginal moisture readings are obtained for each mouse as described under Measurement of Vaginal
Moisture above. This takes less than a minute per mouse so is accomplished in under half an hour. After this, the first cage of female mice are transferred into a scent-containing cage and
allowed to explore. Subsequent groups of mice are transferred at ~ 4–5 min intervals. After an hour of exploration, the first group of mice are tested, with vaginal moisture readings taken
for each female a second time to allow comparison to her own pre-exposure baseline. Subsequent cages were tested as they reached 1 h. Experimenters (both the experimenter obtaining a sample
and the individual quantifying the distance that the sample traveled along the thread) were aware of the experiment that was being conducted (i.e. they were not blinded). In principle, a
skilled observer could discern clues about the current state of estrus of a given mouse.
To test for the impact of diurnal rhythm, we tested responses to male urine (odor) in female mice maintained either standard or reverse light cycles. These responses were taken to represent
rest and active phases, respectively. Mice were tested in late-morning, starting at 10AM. C57BL/6 mice have been reported to be melatonin-deficient and to therefore not exhibit a standard
profile of diurnal/circadian responses30. We therefore instead tested CD1 strain mice.
To test for the role of estrous phase in vaginal responses, we tested the effect of exposure to urine (contact) as above in mice, then determined the estrus phase immediately after the
second (odor-response) vaginal moisture test. We compared mice in estrus or metestrus. Estrous phase was not routinely measured for the remaining experiments.
Analyses were done using Graphpad Prism. With one exception, experiments were analyzed using a two-tailed paired t-test comparing an experimental condition to the same-animal baseline. Our
analyses assumed that the data were normally distributed. A normality test of a sample baseline vaginal moisture data set (Smell-only, Fig. 2B, n = 27) using a D’Agostino & Pearson omnibus
normality test indicated that the data had a normal distribution (K2: 0.016, P value: 0.992). The coefficient of variance in the same data set was 47.2%. In Fig. 3, vaginal moisture data are
recorded by phase of estrus, and so a 1-way ANOVA was used, with a Bonferroni post-hoc test with a single pooled variance.
Female mice are housed as a group, generally 3–4 to a cage. This is in keeping with their nature as a social species; social isolation is considered a stressful condition. A given cage of
female mice are exposed to a scent as group for an hour. During that time, they are free to explore/investigate their environment as they see fit. They are then tested individually. This
experimental arrangement closely mirrors pharmacological treatments where group-housed mice are treated then returned to their home cage until the time of testing. Separating mice during
this treatment would add an additional variable (stress).
Vaginal responses to male but not female bedding. (A) Vaginal moisture before (baseline) and after exposure to male bedding shows that levels increase. (B) The same experiment, exposure to
female bedding does not elicit a response. A, n = 12; B, n = 11. **, p







