- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Chronic histiocytic intervillositis (CHI) is a recurrent placental lesion where maternal macrophages infiltrate the intervillous space. Its cause is unknown, though due to
similarities to rejected allografts one hypothesis is that CHI represents maternal–fetal rejection. Here, virtual crossmatching was applied to healthy pregnancies and those with a history of
CHI. Anti-HLA antibodies, measured by Luminex, were present in slightly more controls than CHI (8/17 (47.1%) vs 5/14 (35.7%)), but there was no significant difference in levels of
sensitisation or fetal specific antibodies. Quantification of immunohistochemical staining for HLA-Class II was increased in syncytiotrophoblast of placentas with CHI (Grade 0.44 [IQR
0.1–0.7]) compared to healthy controls (0.06 [IQR 0–0.2]) and subsequent pregnancies (0.13 [IQR 0–0.3]) (_P_ = 0.0004_)._ HLA-Class II expression was positively related both to the severity
of CHI (r = 0.67) and C4d deposition (r = 0.48). There was no difference in overall C4d and HLA-Class I immunostaining. Though increased anti-HLA antibodies were not evident in CHI,
increased expression of HLA-Class II at the maternal–fetal interface suggests that they may be relevant in its pathogenesis. Further investigation of antibodies immediately after diagnosis
is warranted in a larger cohort of CHI cases to better understand the role of HLA in its pathophysiology. SIMILAR CONTENT BEING VIEWED BY OTHERS INFLAMMASOME-TARGETED THERAPY MIGHT PREVENT
ADVERSE PERINATAL OUTCOMES OF RECURRENT CHRONIC INTERVILLOSITIS OF UNKNOWN ETIOLOGY Article Open access 30 October 2024 THE ENRICHMENT OF NEUTROPHIL EXTRACELLULAR TRAPS IMPAIR THE PLACENTAS
OF SYSTEMIC LUPUS ERYTHEMATOSUS THROUGH ACCUMULATING DECIDUAL NK CELLS Article Open access 25 March 2021 SPATIAL PROTEOMICS REVEALS PHENOTYPIC AND FUNCTIONAL DIFFERENCES IN T CELL AND
MACROPHAGE SUBSETS DURING VILLITIS OF UNKNOWN ETIOLOGY Article Open access 09 January 2024 INTRODUCTION Throughout healthy human pregnancy the fetus is tolerated by the maternal immune
system despite possessing genetically foreign paternal human leukocyte antigen (HLA). This ability of the semi-allogeneic fetus to evade mounting an inflammatory response has led the
conceptus to be coined as the ‘most successful graft’1. In pregnancy, there are several mechanisms which promote maternal–fetal tolerance, including specialised placental HLA expression and
maternal immune cell adaptations2,3,4,5. The failure of these tolerogenic processes has been linked to the pathogenesis of multiple inflammatory placental disorders resulting in adverse
pregnancy outcomes such as preterm birth, miscarriage and stillbirth6,7,8. One example of a placental lesion with an immune etiology is chronic histiocytic intervillositis (CHI), in which
maternal macrophages infiltrate the intervillous space9. CHI is associated with fetal growth restriction (FGR), and in severe cases can result in miscarriage or stillbirth10. Though the
cause of CHI is unknown, previous case reports and series have proposed an antibody-mediated component, given its increased incidence in women with autoimmune disease11,12, high recurrence
rate13,14, evidence of increased anti-HLA antibodies15,16, and in select cases deposition of complement split product C4d15,17. In addition, some cases of CHI also exhibit accompanying
fibrin deposition10 and anti-paternal T cells have been detected in several women with the disorder16. As many of these pathological features are also common to rejected allografts, CHI is
hypothesised to represent maternal–fetal rejection and is consequently treated with immunosuppressive medication12,18,19,20. Despite suggestions of antibody involvement from case reports,
CHI’s rarity (~ 0.17% of pregnancies10) has made conducting larger scale studies into its pathophysiology difficult. Research into the condition is further complicated by the fact its
diagnosis can only be made after delivery of the placenta, in most cases when the health or survival of the fetus has already been compromised. For decades, predicting risk of rejection in
recipients of solid organ transplants has been possible via laboratory crossmatching techniques which informs clinical management and facilitates maintenance of a foreign graft for a
sustained period. In this study, we aimed to apply virtual crossmatching techniques to cases of CHI to investigate the possibility of placental rejection and characterise the HLA expression
profile in inflamed placentas. In doing so, we hypothesised that affected women would exhibit increased evidence of humoral involvement including high titre anti-HLA antibodies and
deposition of C4d, similar to that observed in the rejection of a transplanted organ. MATERIALS AND METHODS PARTICIPANT RECRUITMENT AND SAMPLE COLLECTION Participants were retrospectively
identified by searching medical records at Saint Mary’s Hospital, Manchester, UK, for women with a diagnosis of CHI in a previous pregnancy. Cases of CHI with archived placental tissue
available for analysis from the Paediatric and Perinatal Histopathology Department were included. Placental tissue from cases of CHI and healthy controls were received as formalin-fixed
paraffin embedded (FFPE) blocks, and accompanying hospital records collected where available, detailing maternal demographic data and obstetric history. For certain participants,
accompanying medical history could not be retrieved for the study as they had delivered at another centre or records were unavailable at the time of retrieval. These cases were excluded from
statistical analysis of demographic characteristics and pregnancy outcome, and analysis was restricted to blood and/or placental tissue. An initial subset of FFPE placental tissue from
healthy pregnancies was provided for the study by the Paediatric and Perinatal Histopathology Department, for which matched maternal blood was not collected. Pregnancy outcomes consisted of
livebirth, miscarriage (fetal death < 24 weeks’ gestation), stillbirth (fetal death ≥ 24 weeks’ gestation) and termination of pregnancy (TOP). Individualised birthweight centiles were
calculated using the GROW centile calculator for pregnancies ≥ 20 weeks’ gestation21. Blood was collected prospectively both from women in their second or third trimester attending Tommy’s
Rainbow Clinic for care in a subsequent pregnancy following a previous diagnosis of CHI, and healthy control pregnancies on the date of delivery at Saint Mary’s Hospital. Vacutainer™ EDTA
samples were obtained and centrifuged at 2000_ g_ for 10 min at 4 °C, and plasma supernatant retrieved and stored as aliquots at − 80 °C. The buffy coat layer was also retrieved at the red
cell/plasma interface for maternal DNA analysis and stored in the same manner. Where participants were giving birth at Saint Mary’s Hospital, placental tissue was collected and fixed in
formalin before embedding in paraffin wax to produce FFPE blocks. Umbilical cord tissue was also sampled, rinsed and frozen at − 80 °C for later use. Informed and written consent was
obtained from all study participants. For archived tissue from Paediatric and Perinatal Histopathology, ethical approval was granted by NRES Committee London-City & East (REC ref:
14/LO/1352). For samples gathered before July 2018, approval was granted by NRES Committee Northwest-Greater Manchester West (14/NW/1149). Between February and July 2018, the NRES Committee
South East Coast-Surrey Research Ethics Committee (16/LO/1666), and samples collected after this period were obtained under the Tommy’s Project Ethics (15/NW/0829). All methods were
performed in accordance with ethical committee guidelines and the Declaration of Helsinki. IMMUNOHISTOCHEMISTRY AND SEMI-QUANTITATIVE ANALYSIS Immunostaining for C4d in healthy control
placentas and those from index cases of CHI and subsequent pregnancies was carried out by the Department of Adult Histopathology, Manchester University NHS Foundation Trust. 5 µm sections of
FFPE tissue were cut from the centre and edge of the placenta, mounted onto SuperFrost slides (ThermoFisher Scientific) and dried overnight at 38 °C before long-term storage at room
temperature. A tissue biopsy section from a rejected kidney with confirmed antibody-mediated rejection was provided by Manchester Royal Infirmary Transplantation Laboratory (MRITL), for use
as a positive control. Slides were deparaffinised in EZ Prep Volume Adjust (Ventana Co., Arizona, USA) according to manufacturer’s instructions and washed in TRIS-based Reaction Buffer (pH
7.6). Antigen retrieval was achieved using heat and TRIS–EDTA-boric acid (pH 8.4, Ventana Co.) for 60 min. Ultraviolet inhibitor blocking solution was applied for 4 min before a further
30-min incubation at room temperature with rabbit polyclonal anti-human C4d antibody (Cell Marque, Sigma Aldrich, Missouri, USA, 1:75 dilution). Slides were then incubated in horseradish
peroxidase-linked secondary antibody for 8 min before an 8-min incubation in diaminobenzidine chromogen. To amplify positive staining, a copper enhancer was applied for 4 min, before a
12-min counterstain in Haematoxylin II and 4 min in bluing reagent. Finally, slides were dehydrated by sequential washes in solutions of 70% (2 × 3 min), 95% (2 × 3 min) and 100% industrial
methylated spirit (ThermoFisher) (3 × 3 min) before 1 × 2 min and 2 × 10 min immersions in Histoclear (Thermo Fisher). After staining, placental slides were mounted with DPX and coverslips
and left to dry. Immunohistochemical staining for HLA-Class I and II in placental tissue was undertaken at the University of Manchester using a Leica BOND RX Research Stainer. Slides were
cut and prepared as previously described, dewaxed and heated in citrate buffer for 30 min in Leica BOND standard solutions for antigen retrieval. Endogenous peroxidase was quenched using 3%
(v/v) hydrogen peroxide (VWR International, Pennsylvania, USA) for 10 min at room temperature. Following 2 × 5 min washes in distilled water, blocking of non-specific antibody binding was
conducted using 10% (v/v) normal goat serum (Sigma-Aldrich) in 0.1% TBS-Tween (Biotium, UK) for 30 min at room temperature. Sections were then incubated for one hour at 37 °C with mouse
monoclonal anti-human HLA Class I (Abcam, 5 μg/ml) or Class II (Santa Cruz Biotechnology, Texas, USA 5 μg/ml) diluted in blocking solution. After a 5 min wash in distilled water, 2 × 5 min
washes in Leica wash solution and another 5 min in distilled water, sections were incubated with biotinylated polyclonal goat anti-mouse secondary antibody (AAT Bioquest, California, USA 3.3
µg/ml) for 30 min at room temperature followed by washes in distilled water and wash solution as previously described. Avidin-peroxidase (Sigma-Aldrich, 5 µg/ml in TBS) was applied for 30
min at room temperature to amplify the secondary antibody signal before 2 × 5 min washes in wash solution and 1 × 5 min wash in distilled water. Diaminobenzidine (DAB) chromogen (BD
Biosciences, New Jersey, USA) was reconstituted according to manufacturer’s instructions and dropped onto each section for secondary antibody detection. Following an 8-min incubation and
5-min wash in distilled water, placental sections were finally incubated (2 s) in haematoxylin as a nuclear counterstain. Slides were then left in warm water for 5 min and then transferred
into cold water. Dehydration of sections was conducted by sequential incubation in solutions of 70% (2 × 3 min), 95% (2 × 3 min) and 100% IMS (3 × 3 min) before 1 × 2 min and 2 × 10 min
incubations in Histoclear (National Diagnostics, Georgia, USA). DPX mounting medium was dropped onto each section before coverslip application and storage. Negative sections utilised to
ensure antibody specificity were treated identically except for omission of primary antibody which was replaced with non-immune mouse IgG (Sigma-Aldrich) at the same working concentration.
Spleen tissue was used as a positive control for both HLA-Class I and HLA-Class II antibodies. C4d and HLA Class I and II slides were scanned at the University of Manchester Bioimaging
Facility using brightfield microscopy and a 3D Histech Panoramic 250 Flash Slide Scanner. Grading of C4d deposition and HLA expression was carried out by two separate reviewers blinded to
sample identity. Scanned slides were opened using QuPath Version 0.3.0 RRID:SCR_018257 computerised image analysis software22 and a 1mm2 grid superimposed upon the scanned slide image. A
random number generator was then used to select eight regions for blinded semi-quantitative analysis of staining intensity. Intervillous C4d and HLA positivity was graded on a scale of 0 to
3; 0 = 0% to 5% of villi affected; 1 = 5% to 25%; 2 = 25% to 75%; and 3 ≥ 75% as previously described17. Only positive staining along the apical surface of villi which was in contact with
maternal blood was considered in the analysis. C4d and HLA scores from the centre and edge placental regions were averaged to provide a measure for each placenta, before both separate
reviewer’s scores were averaged to give an overall score. ANTI-HLA ANTIBODY SCREENING OF MATERNAL PLASMA For detection of class I and II anti-HLA antibodies, maternal EDTA plasma samples
were processed by MRITL. A mixed Luminex assay was used to initially determine any presence of anti-HLA antibodies, consisting of LABScreen Mixed Beads (One Lambda Inc, Thermo Fisher) coated
in several HLA antigens and each possessing a unique combination of fluorochromes. Maternal plasma was incubated with the mixed beads at 22 °C for 15 min before washing in LABScreen wash
buffer according to manufacturer’s protocol. R-phycoerythrin (PE)-conjugated goat anti-human IgG and PE-conjugated donkey anti-human IgM secondary antibodies were then added for 5 min at 22
°C to allow detection of bound antibody. After a final wash in wash buffer, beads were resuspended in buffer before transfer to a 96-well PCR plate for Luminex LabScan3D analysis using a
dual laser system to identify the presence of antibody in patient plasma binding to beads, giving a measurement of mean fluorescence intensity (MFI). Data were inputted into HLA Fusion One
Lambda Software and checked with established criteria from the MRITL for detection of false positives or negatives. Samples testing positive were then retested in the same manner but with
specific LABScreen beads possessing only a single antigen for identification of anti-HLA antibody specificity. Antibodies with an MFI value of 2000 or more were considered positive in
accordance with MRITL criteria. MATERNAL AND FETAL HLA GENOTYPING In the case of a positive anti-HLA antibody screen, maternal and fetal DNA (or maternal DNA alone where fetal tissue was not
available) were extracted for determination of HLA genotype from thawed EDTA buffy coat and snap frozen umbilical cord samples, respectively. Samples were processed using the DNeasy Blood
& Tissue Kit (Qiagen, Germany) according to kit instructions. Extracted DNA samples were analysed at the MRITL to determine HLA genotype via the Luminex LABType™ Sequence Specific
Oligonucleotide (SSO) HLA Typing protocol to fluorescently tag and identify HLA alleles. Results were reviewed by a State Registered Clinical Scientist (LBF) to confirm HLA genotype and
establish any false positive bead reactions. The number of fetal-specific anti-HLA antibodies (FSAs) and their MFI was then noted in positive cases where fetal DNA was available. PERCENTAGE
CALCULATED REACTION FREQUENCY Using maternal anti-HLA antibody specificities and HLA genotype, percentage calculated Reaction Frequency (%cRF) was established for each participant testing
positive via input of the results of Luminex screen into the NHS Blood and Transplant Kidney %cRF tool23. %cRF reflects the percentage of donors anticipated to have an unsuitable HLA profile
in the context of renal transplantation and is based on the HLA type from 10,000 donors, whose HLA type reflects those of the UK general population. Organ recipients with no HLA
incompatible donors are assigned a %cRF of 0% and are unsensitised with 100% of donors being suitable, whereas those with a score of > 80% are highly sensitised and prove more challenging
to find a suitable donor, as > 80% of donors are considered unsuitable. PREDICTION OF CROSSMATCH RESULTS In participants with FSAs, results of T and B cell flow cytometry crossmatch
(FXCM) and complement-dependent cytotoxicity (CDC) crossmatch were predicted using a formula developed by the MRITL24. HLA specificities and respective FSA MFI values were inputted to give
an estimation of positive or negative result. Where results were in between the in-house cut-off values for a positive or negative result, these were described as equivocal, and could not be
reliably predicted. STATISTICAL ANALYSIS All statistical analysis and creation of graphs was undertaken using GraphPad Prism v9 (GraphPad Software, USA). Normality was determined by the
Shapiro–Wilk test. Continuous demographic data were analysed using ordinary one-way ANOVA with Dunn’s multiple comparisons test or Kruskal–Wallis test for normally distributed and
non-normally distributed data, respectively. Interobserver agreement of C4d and HLA staining was determined by calculation of the weighted Kappa between reviewer scores and grading was
analysed via Kruskal–Wallis test with Dunn’s multiple comparisons where this was significant. Categorical demographic data and proportions of antibody-positive participants were analysed
using Fisher’s exact test. For %cRF values the Mann–Whitney test was run to determine statistical differences. Statistical significance was set at _P_ < 0.05 for each test. To relate
HLA-Class I and II expression with CHI severity, scores were compared to CD68+ counts previously carried out on placentas from index cases of CHI18 RESULTS PARTICIPANT DEMOGRAPHIC
CHARACTERISTICS Seventeen index cases of CHI had available placental tissue as FFPE embedded blocks, with eight healthy controls provided by the Department of Paediatric Histopathology. A
further nineteen healthy controls and fifteen women in subsequent pregnancies after CHI were recruited to the study. For two index cases, demographic information and pregnancy outcomes could
not be obtained as records were unavailable or participants received their care at another centre. Maternal demographic and pregnancy outcome data corresponding to one subsequent pregnancy
was limited as the participant chose to give birth at another hospital. Despite this, these pregnancies were included in the study due to availability of placental tissue and the rarity of
CHI. An overview of recruitment for the study is provided in Supplementary Fig. 1. Demographic characteristics of remaining study participants and pregnancy outcomes are shown in Table 1.
There were no significant differences in maternal age, BMI, ethnicity or pregnancies conceived via oocyte donor. Gravidity and parity were significantly higher in index pregnancies compared
to controls_._ In women returning for care in a subsequent pregnancy following diagnosis, parity was significantly lower compared to index cases of CHI. Women returning for care in
subsequent pregnancies had a higher incidence of autoimmune disease compared to controls (_P_ = 0.012). Adverse outcome was more common in index pregnancies with CHI compared to control (_P_
< 0.0001) and subsequent pregnancies (_P_ < 0.0001). Rates of Caesarean section were higher in control pregnancies compared to index cases of CHI (_P_ = 0.0003), and gestation at
delivery was decreased in index (_P_ = 0.0031) and subsequent pregnancies (_P_ = 0.016). Individualised birthweight centile was significantly decreased in index cases of CHI compared to
control (_P_ < 0.0001) and subsequent pregnancies (_P_ = 0.016_)_. There was a higher proportion of male fetuses in subsequent pregnancies compared to controls (_P_ = 0.02_)._ By
definition, all index cases had a diagnosis of CHI listed on their placental histopathology report. Placental tissue was sent for histopathological examination in ten subsequent pregnancies.
There was no histopathological diagnosis of recurrent CHI noted in any subsequent pregnancies. Eleven of fifteen (73.3%) subsequent pregnancies were medicated with aspirin, low molecular
weight heparin, hydroxychloroquine and prednisolone. The remaining four (26.7%) subsequent pregnancies were treated with aspirin, low-molecular-weight heparin and hydroxychloroquine only.
C4D DEPOSITION IN INDEX CASES OF CHI FFPE tissue was available for analysis in pregnancies from twenty-five healthy controls, seventeen index cases of CHI and eleven subsequent pregnancies.
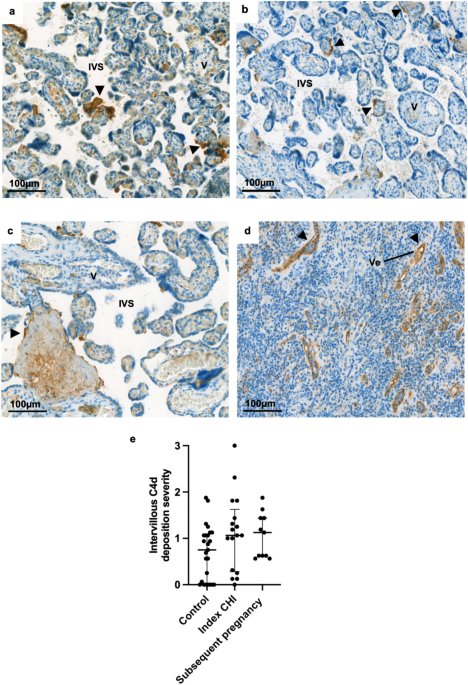
C4d was apparent in healthy control placentas (Fig. 1a), those from index cases of CHI (Fig. 1b) and subsequent pregnancies (Fig. 1c). C4d localised along the apical surface of the
syncytiotrophoblast, appearing similar to the vessels of a rejected kidney (Fig. 1d). Weighted Kappa for interobserver agreement was calculated at 0.43, indicating moderate agreement.
Amongst all three groups, the degree of C4d deposition varied widely between cases, there was no significant difference between the levels of C4d deposition in index CHI (median 1.06 [IQR
0.3–1.6]) and subsequent pregnancies (1.13 [IQR 0.6–1.4]) compared to controls (0.75 [IQR 0–1.1]) (Fig. 1e). The extent of C4d deposition was compared to the degree of macrophage
infiltration previously quantified in these cases, which showed no relationship (r = -0.001, _P_ = 0.99). EXPRESSION OF HLA-CLASS I AND II AT THE MATERNAL–FETAL INTERFACE IN CHI HLA-Class I
expression was low within placentas of healthy controls, index cases of CHI and subsequent pregnancies, with only a small degree of HLA-Class I expression on the surface of certain villi in
areas where the syncytiotrophoblast layer appeared denuded (Fig. 2a-c). In index cases of CHI, the majority of positive HLA-Class I staining was observed in infiltrating maternal
macrophages, however there were occasional focal areas of expression around what appeared to be necrotic villi and areas of fibrin deposition (Fig. 2b). HLA-Class II was rarely observed at
the maternal–fetal interface in healthy control pregnancies and was instead mainly found in Hofbauer cells within fetal villi (Fig. 2d). In contrast, index cases of CHI demonstrated linear
positive staining for HLA-Class II on the apical surface of certain areas of syncytiotrophoblast (Fig. 2e). This staining was often in proximity to areas of macrophage infiltration. Most
placentas of subsequent pregnancies closely resembled healthy controls, with only sparse areas of HLA-Class II in contact with the intervillous space (Fig. 2f). The weighted Kappa for
grading of HLA expression along the apical surface of the syncytiotrophoblast was 0.36, indicating fair agreement. No significant difference was found in average HLA-Class I expression
between healthy control pregnancies (median 0.38 [IQR 0.2–0.6]), index cases of CHI (0.63 [IQR 0.3–0.9]) and subsequent pregnancies (0.38 [IQR 0.2–0.6]) (Fig. 3a). Average HLA-Class II
expression was significantly increased in index cases of CHI (0.44 [IQR 0.1–0.7]) compared to healthy controls (0.06 [IQR 0–0.2]) (_P_ = 0.0002), but not subsequent pregnancies (0.13 [IQR
0–0.3]) (Fig. 3b). Syncytiotrophoblast HLA-Class II expression in index cases of CHI demonstrated a positive relationship both with the extent of intervillous CD68+ macrophage infiltration
(r = 0.67, _P_ = 0.004) (Fig. 3c) and C4d deposition (r = 0.48, _P_ = _0.05_) (Fig. 3d). MATERNAL ANTI-HLA ANTIBODY SCREENING Seventeen controls and fourteen participants with previous CHI
had plasma available for analysis by Luminex for the presence of anti-HLA antibodies. There was no statistically significant difference in the proportion of healthy control participants
tested positive for anti-HLA antibodies (Class I and II) compared to those with a history of CHI (8/17 (47.1%) vs 5/14 (35.7%), respectively, Fig. 4a). There was also no significant
difference in the median level of sensitisation between antibody positive healthy controls and women with a diagnosis of CHI (99.0 [interquartile range (IQR) 36.3–100] vs 86.0 [IQR
37.0–99.5], respectively) (Fig. 4b). Five of eight (62.5%) anti-HLA antibody positive controls were classed as highly sensitised with a %cRF > 80%, compared to three out of five (60.0%)
participants with previous CHI. Amongst anti-HLA positive participants, a similar proportion of those with a history of CHI displayed Class I positivity compared to controls (4/5 (80.0%) vs
6/8 (75.0%), respectively) (Fig. 4c). Four of five (80.0%) participants with CHI tested positive for Class II anti-HLA antibodies compared to seven of eight controls (87.5%) (Fig. 4d). These
variations in proportions were not statistically significant. Six antibody positive control participants and three participants with previous CHI had matched placental tissue available for
analysis. Amongst these who tested positive for anti-HLA antibodies, %cRF was not related to the severity of intervillous C4d deposition or HLA-Class II expression (Fig. 4e and f,
respectively). SCREENING FOR FETAL-SPECIFIC ANTIBODIES Umbilical cord tissue matched to maternal plasma was available for HLA genotype analysis in all eight antibody-positive healthy
controls and three women with a diagnosis of CHI in a previous pregnancy. Fetal DNA was unavailable in the remainder of pregnancies from women with previous CHI as two participants had
delivered at another centre, and one placenta had not been sent for research. FSAs were evident in five of eight (62.5%) anti-HLA antibody positive control pregnancies compared to all three
cases of previous CHI with available fetal DNA (100%) (Fig. 5). There was no statistically significant difference in the number of FSAs in healthy controls compared to cases of previous CHI
(2 [IQR 0–3.8] vs 5 [1.0–6.0], respectively) (Fig. 5a). Furthermore, in participants diagnosed with CHI there was no difference in individual MFI value compared to those found in control
pregnancies (23,884 [IQR 4723–28628] vs 17,881 [IQR 8345–26500], respectively) (Fig. 5b). Two of three participants with previous CHI exhibited high total MFI of FSAs, though overall there
was no statistically significant difference compared to healthy controls (median 108,810 [IQR 3519- 111520] versus 62,132 [IQR 41067-65662], respectively) (Fig. 5d). FSA specificities are
listed in Supplementary Table 1. Using the MFI values of FSAs, the results of FXCM and CDC crossmatch were predicted (Table 2). In one case of previous CHI (33.3%), the B cell FXCM result
was equivocal. There were no significant differences in predicted T or B cell positivity for either FXCM or CDC crossmatch in healthy controls or women with a history of CHI. DISCUSSION
Currently, the pathophysiology of CHI is incompletely understood, though pathological similarities shared with rejected allografts, its high recurrence rate, and reported association with
maternal autoimmune disease have resulted in the hypothesis that it is a disorder of maternal anti-fetal rejection10,11,12,13,17,20. Individual case reports on CHI have also described the
presence of elevated maternal anti-HLA antibody levels and increased HLA expression at the maternal–fetal interface15,16. Despite this evidence, extensive transplant-style crossmatching has
yet to be applied to cases of CHI in a more robust case–control study design. Here, virtual crossmatching was used to determine whether CHI resembles organ rejection with respect to C4d
deposition and the presence of antibodies directed towards fetal HLA. Placental HLA expression was also investigated to determine whether this is altered in a larger cohort of CHI cases. C4d
deposition has been described in several reports of placentas with CHI15,17,25, reminiscent of the appearance of a rejected kidney biopsy26,27. Critically, these studies did not compare C4d
deposition in index cases of CHI to healthy controls. Here, we found no differences in placental C4d staining between CHI and healthy pregnancies, in part due to the wide variation in the
amounts of staining seen in each group. In common with our study, earlier studies of placentas from women with systemic lupus erythematosus (SLE) and antiphospholipid syndrome reported that
C4d deposition in healthy controls varies from no detectable staining to a small amount around the apical surface of the syncytiotrophoblast, similar to the staining pattern observed
here28,29. However, the C4d staining in healthy controls appears elevated compared to previous investigations; it should be noted that the criteria used to grade C4d deposition varies
between studies, limiting comparability. Here we found moderate interobserver agreement (κ = 0.43), indicating a degree of subjectivity, which is consistent with wider issues reported in the
diagnosis and grading of inflammation in CHI. Specifically, Reus et al.16 stated a similar Kappa value of 0.54 for interobserver variability in grading of CHI lesions. Therefore, less
subjective methods to grade inflammatory features in CHI need to be developed, for example using computerised quantification or proteomic analyses. In addition, other pathological features
e.g., elevated fibrin deposition are apparent in some, but not all placentas with CHI, so C4d likewise might also be exaggerated in specific cases. This is supported by our finding that
there was no relationship between CHI severity and C4d deposition. Terry et al.30 also stipulated that CHI is “a unique inflammatory process that arises in a permissive environment after
exposure to an appropriate trigger”. If this is the case, it may be plausible that complement activation in CHI acts in combination with other aberrant inflammatory processes to cause
pathology, whereas in healthy pregnancy its activity is limited, e.g. by complement regulatory factors richly expressed on the placenta31. Further characterisation of immunoregulatory
molecules in CHI in future may help to confirm or refute this hypothesis. The proportion of participants with anti-HLA antibodies did not differ significantly between controls and women with
previous CHI, at 47.1% versus 40.0%, respectively. Additionally, there were no differences in the proportion of positive predicted crossmatch results, %cRF value, the number of FSAs or
their MFI. With regard to anti-HLA antibodies, pregnancy is considered to be both the most common and strongest sensitising event, and anti-HLA positivity is reported in 29–49% of parous
women32,33,34,35. The mechanism of HLA sensitisation in healthy pregnancy appears to be as a result of extracellular vesicles released from the placenta into the maternal circulation and
interaction with fetal cells at delivery providing a source of fetal antigen36,37,38. Previous studies of a total of six women with CHI, reported maternal anti-HLA antibody positivity to be
as high as 75%, though only one investigated a control group, consisting of seven participants15,16. In contrast, evidence from this more robust study instead suggests that anti-HLA antibody
sensitisation in women with previous pregnancies complicated by CHI is comparable to parous healthy controls. Nevertheless, despite this lack of difference in HLA antibody profiles between
healthy pregnancies and those with a history of CHI, index cases within our cohort did exhibit elevated HLA-Class II at the maternal–fetal interface. It is well recognised that in healthy
pregnancy the semi-allogeneic human placenta is tolerated by the maternal immune system, largely thanks to downregulation of HLA by the villous trophoblast2. With this in mind, it is
reasonable to hypothesise that an altered HLA profile in placentas with CHI may mean that anti-HLA antibodies are more relevant in these pregnancies, even if the level of sensitisation is
similar to healthy controls. As HLA-Class II expression in placentas from index cases of CHI within our cohort showed a positive relationship with the extent of C4d deposition and maternal
CD68+ macrophage infiltration, it is tempting to speculate that binding of anti-HLA antibodies to the trophoblast may occur in severe cases of CHI, particularly as HLA-Class II expression
was observed in proximity to areas of intervillous macrophage infiltration. Further investigation is required to determine how these inflammatory features may be related, for example it
would be worthwhile to determine whether C4d deposition is localised to areas where HLA is upregulated on the trophoblast, suggesting evidence of antibody binding at the maternal–fetal
interface. Our finding of HLA-Class II on the syncytiotrophoblast surface aligns with an observation made in Benachi et al.’s15 study of two placentas with CHI, although importantly no
significant difference in overall HLA-Class I expression was found here after semi-quantitative grading in a larger cohort. It should be noted that within our study, interobserver agreement
was only fair, again emphasising the need for less subjective measurement of inflammatory features in CHI. In a similar study into villitis of unknown etiology, a related inflammatory
placental condition, Enninga et al. analysed gene expression via microarray and found altered expression of Class-II mRNA and upregulation of pathways associated with graft rejection. Use of
similar methods in CHI may therefore provide better insight into its pathophysiology and reduce subjectivity associated with manual grading. As well as anti-HLA antibodies, CHI has been
associated with maternal autoimmune disease and fetal and neonatal alloimmune thrombocytopenia (FNAIT)11,12,39. However, the link between autoantibodies and development of CHI remains
unclear and reported rates of autoimmune disease in women with CHI vary widely, from 12 to 58%11,12,18. In studies of women with antiphospholipid syndrome, use of hydroxychloroquine was
associated with reduced titres of autoantibodies which bind to trophoblast in vitro and initiate release of pro-inflammatory cytokines40,41. In subsequent pregnancies following a diagnosis
of CHI, hydroxychloroquine and other immunomodulatory medications e.g. prednisolone are often prescribed with an aim to reduce the maternal inflammatory response42. It is therefore
conceivable that maternal antibodies may have been present in index pregnancies affected by CHI but without accompanying C4d deposition, similar to a phenomenon termed as C4d-negative
rejection, recently acknowledged in antibody-mediated rejection wherein complement is not detected in graft biopsies27. Applying the same experimental method in index CHI pregnancies is
limited by the fact that maternal plasma taken during pregnancy from these cases would not be available as diagnosis is made after delivery. An alternative method would be to directly apply
anti-human IgG or IgM to stored placental tissue from these cases to highlight any bound anti-placental antibody, similar to that used in the diagnosis of lupus via skin biopsies, known as
the lupus band test43. Obtaining plasma from women with antiphospholipid syndrome would also likely serve as a suitable positive control to ensure antibodies are detected via the assay. As
CHI has been observed in cases of FNAIT and autoimmune disease, it is also plausible that it may occur as a result of a variety of antibodies which are capable of binding to antigens
expressed on the placenta, rather than HLA alone. This may help to explain why some, but not all participants with CHI tested positive for anti-HLA antibodies within this study. As a
diagnosis of CHI is only made after an affected pregnancy, the plasma analysed in this study was limited to subsequent pregnancies, all of which were receiving a form of thromboprophylactic
and immunomodulatory treatment that may have prevented CHI recurrence18. Following solid organ transplantation, prednisolone is used routinely as maintenance immunosuppression to prolong
graft survival. It is therefore possible that any anti-HLA antibodies which may have been present in previous pregnancies were reduced as a result of medication. This limitation extends
beyond this study and into research on CHI as a whole, as plasma is rarely collected at the time of index pregnancies and women are increasingly beginning to take immunosuppressive
medications even before conception, making screening for maternal antibodies in this period and pregnancy before treatment difficult. Furthermore, ethical issues surrounding randomised
control trials using placebo or less effective medications in women with multiple previous poor pregnancy outcomes means that obtaining plasma from untreated pregnant women is unlikely. To
address the confounding factor of medication, it may be worthwhile in future to obtain ethical permission to obtain blood for anti-HLA antibodies outside of pregnancy, before
immunomodulatory treatment has commenced. Preliminary data from two women with CHI suggested that fetal-specific anti-HLA antibodies are stable for up to 17 months, further justifying the
use of sampling following the first affected pregnancy15. The previously referenced study identifying CHI in 40.7% of placentas from women with FNAIT tested for antibodies in plasma at four
separate timepoints from 16–20 weeks’ gestation39. Antibody-positive women were defined as those with a positive screen in at least one of four samples, as antibodies may be transient. In
contrast, plasma from only one time point during gestation was available for analysis here, which could have negatively affected Luminex results. Thus, further investigations using maternal
plasma sampled as soon as possible after the index case and longitudinal studies may give more accurate insight into the role of anti-HLA antibodies and how they fluctuate throughout
gestation. To date, this study represents the largest attempt to apply crossmatching techniques in samples from women with a previous diagnosis of CHI. In collaboration with an accredited
Transplantation Laboratory, extensive anti-HLA antibody screening was undertaken to a high standard as used in the clinical setting to determine donor-recipient suitability pre-transplant.
In the majority of cases, matched placental tissue and fetal DNA was also available, which allowed any fetal-directed antibodies to be identified and for the prediction of crossmatch
results. Though no differences were found, this may be a result of immunomodulatory therapy taken by participants, and sample size was limited for these variables as only three and five
cases of CHI and controls had antibodies toward their fetus, respectively. As CHI is a rare disease, increasing involvement of affected women into research studies and facilitating
collaboration between centres specialising in its treatment will be essential to provide a larger sample size to more thoroughly investigate the specific relevance of antibodies which may be
directed towards fetal HLA. As CHI has been hypothesised as an inappropriate immune response toward fetal HLA inherited from the father, both previous studies to investigate anti-HLA
antibodies had paternal DNA available for genotyping15,16. In this study, analysis was restricted to fetal DNA due to the fact that collection of paternal plasma samples was not covered by
ethical agreements. This meant that only anti-HLA antibodies directed toward the fetus of the current pregnancy could be identified, as opposed to other specificities which may have been
present in past pregnancies with CHI. Thus, future studies investigating the immune aetiology of CHI should include collection and analysis of paternal DNA. Though CHI has been likened to
maternal rejection of the placenta, evidence of increased C4d deposition and increased maternal anti-fetal antibodies could not be confirmed. Despite this, we have described for the first
time in a relatively large cohort of CHI cases that HLA-Class II expression is upregulated at the maternal–fetal interface. This leads us to hypothesise that anti-HLA antibodies may hold
more relevance in the pathophysiology of CHI compared to healthy pregnancies where trophoblast HLA expression is restricted. What remains to be answered is whether increased HLA expression
in CHI is causative, or a secondary response to placental inflammation. Due to the heterogeneity in the degree of HLA-Class II upregulation within our cohort, we argue that the latter is
more likely, however the initial inflammatory trigger in CHI is yet to be identified. In investigating the role of HLA in CHI, treatment using immunosuppressive medication in subsequent
pregnancies and subjectivity in the grading of inflammation are likely confounding factors. Further study using transcriptomic analysis of inflammatory pathways and detection of antibodies
after an initial diagnosis of CHI is therefore required, both to identify its cause and potential therapeutic targets. DATA AVAILABILITY The datasets generated during and/or analysed during
the current study are available from the corresponding author on reasonable request. REFERENCES * Rodger JC, Drake BL. The Enigma of the Fetal Graft. Am Sci. 1987;75: 51–57. Available:
http://www.jstor.org/stable/27854450 * Apps, R. _et al._ Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads
to characterize allotype specificities of anti-HLA antibodies. _Immunology_ 127, 26–39. https://doi.org/10.1111/j.1365-2567.2008.03019.x (2009). Article CAS PubMed PubMed Central Google
Scholar * Collins, M. K., Tay, C.-S. & Erlebacher, A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. _J.
Clin. Investig._ 119, 2062–2073. https://doi.org/10.1172/jci38714 (2009). Article CAS PubMed PubMed Central Google Scholar * Co, E. C. _et al._ Maternal decidual macrophages inhibit NK
cell killing of invasive cytotrophoblasts during human pregnancy. _Biol. Reprod._ 88, 155. https://doi.org/10.1095/biolreprod.112.099465 (2013). Article PubMed PubMed Central Google
Scholar * Somerset, D. A., Zheng, Y., Kilby, M. D., Sansom, D. M. & Drayson, M. T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory
T-cell subset. _Immunology_ 112, 38–43. https://doi.org/10.1111/j.1365-2567.2004.01869.x (2004). Article CAS PubMed PubMed Central Google Scholar * Nadeau-Vallée, M. _et al._ Sterile
inflammation and pregnancy complications: A review. _Reproduction_ 152, R277–R292. https://doi.org/10.1530/rep-16-0453 (2016). Article CAS PubMed Google Scholar * Kim, E. N. _et al._
Clinicopathological characteristics of miscarriages featuring placental massive Perivillous fibrin deposition. _Placenta_ https://doi.org/10.1016/j.placenta.2019.07.006 (2019). Article
PubMed PubMed Central Google Scholar * Kim, C. J., Romero, R., Chaemsaithong, P. & Kim, J. S. Chronic inflammation of the placenta: Definition, classification, pathogenesis, and
clinical significance. _Am. J. Obstet. Gynecol._ 213, 53–69. https://doi.org/10.1016/j.ajog.2015.08.041 (2015). Article Google Scholar * Labarrere, C. & Mullen, E. Fibrinoid and
trophoblastic necrosis with massive chronic intervillositis: An extreme variant of villitis of unknown etiology. _Am. J. Reprod. Immunol._ 15, 85–91.
https://doi.org/10.1111/j.1600-0897.1987.tb00162.x (1987). Article CAS Google Scholar * Bos, M. _et al._ Towards standardized criteria for diagnosing chronic intervillositis of unknown
etiology: A systematic review. _Placenta_ 61, 80–88. https://doi.org/10.1016/j.placenta.2017.11.012 (2018). Article CAS PubMed Google Scholar * Revaux, A. _et al._ Antiphospholipid
syndrome and other autoimmune diseases associated with chronic intervillositis. _Arch. Gynecol. Obstet._ 291, 1229–1236. https://doi.org/10.1007/s00404-014-3536-6 (2015). Article CAS
PubMed Google Scholar * Mekinian, A. _et al._ Chronic histiocytic intervillositis: Outcome, associated diseases and treatment in a multicenter prospective study. _Autoimmunity_ 48, 40–45.
https://doi.org/10.3109/08916934.2014.939267 (2015). Article CAS PubMed Google Scholar * Contro, E., deSouza, R. & Bhide, A. Chronic intervillositis of the placenta: A systematic
review. _Placenta_ 31, 1106–1110. https://doi.org/10.1016/j.placenta.2010.10.005 (2010). Article CAS PubMed Google Scholar * Parant, O., Capdet, J., Kessler, S., Aziza, J. & Berrebi,
A. Chronic intervillositis of unknown etiology (CIUE): Relation between placental lesions and perinatal outcome. _Eur. J. Obstet. Gynecol. Reprod. Biol._ 143, 9–13.
https://doi.org/10.1016/j.ejogrb.2008.06.012 (2009). Article PubMed Google Scholar * Benachi, A. _et al._ Chronic histiocytic intervillositis: Manifestation of placental
alloantibody-mediated rejection. _AJOG_ https://doi.org/10.1016/j.ajog.2021.06.051 (2021). Article Google Scholar * Reus, A. D. _et al._ An immunological basis for chronic histiocytic
intervillositis in recurrent fetal loss. _Am. J. Reprod. Immunol._ 70, 230–237. https://doi.org/10.1111/aji.12125 (2013). Article CAS PubMed Google Scholar * Bendon, R. W. _et al._
Significance of C4d Immunostaining in placental chronic intervillositis. _Pediatr. Dev. Pathol._ 18, 362–368. https://doi.org/10.2350/14-12-1582-OA.1 (2015). Article PubMed Google Scholar
* Brady, C. _et al._ Immunomodulatory therapy reduces the severity of placental lesions in chronic histiocytic intervillositis. _Front. Med._ https://doi.org/10.3389/fmed.2021.753220
(2021). Article Google Scholar * Abdulghani, S., Moretti, F., Gruslin, A. & Grynspan, D. Recurrent massive perivillous fibrin deposition and chronic intervillositis treated with
heparin and intravenous immunoglobulin: A case report. _JOGC_ 39, 676–681. https://doi.org/10.1016/j.jogc.2017.03.089 (2017). Article PubMed Google Scholar * Brady, C. A. _et al._ Chronic
histiocytic intervillositis: A breakdown in immune tolerance comparable to allograft rejection?. _Am. J. Reprod. Immunol._ 85, e13373. https://doi.org/10.1111/aji.13373 (2021). Article CAS
PubMed Google Scholar * Gardosi J, Williams A, Hugh O FA. Customised Centile Calculator, GROW Version 2.1.6.1. In: Gestation Network [Internet]. 2020. Available: www.gestation.net *
Bankhead, P. _et al._ QuPath: Open source software for digital pathology image analysis. _Sci. Rep._ 7, 1–7. https://doi.org/10.1038/s41598-017-17204-5 (2017). Article CAS Google Scholar
* NHS Blood and Transplant. Kidney Calculated Reaction Frequency Tool. https://www.odt.nhs.uk/transplantation/tools-policies-and-guidance/calculators/ * Flynn, P. A., Fernando, S.,
Worthington, J. E. & Poulton, K. V. Predicting flow cytometry crossmatch results from single-antigen bead testing. _Int. J. Immunogenet._ 51, 93–99. https://doi.org/10.1111/iji.1265825
(2024). Article CAS PubMed Google Scholar * Sato, Y. _et al._ CD39 downregulation in chronic intervillositis of unknown etiology. _Virchows Arch._ 475, 357–364.
https://doi.org/10.1007/s00428-019-02598-6 (2019). Article CAS PubMed Google Scholar * Roufosse, C. _et al._ A 2018 reference guide to the banff classification of renal allograft
pathology. _Transplantation_ 102, 1795–1814. https://doi.org/10.1097/tp.0000000000002366 (2018). Article PubMed PubMed Central Google Scholar * Loupy, A. _et al._ The Banff 2019 kidney
meeting report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. _Am. J. Transplant._ https://doi.org/10.1111/ajt.15898 (2020). Article PubMed
PubMed Central Google Scholar * Matrai, C. E., Rand, J. H. & Baergen, R. N. Absence of distinct immunohistochemical distribution of annexin A5, C3b, C4d, and C5b–9 in placentas from
patients with antiphospholipid antibodies, preeclampsia, and systemic lupus erythematosus. _Pediatr. Dev. Pathol._ 22, 431–439. https://doi.org/10.1177/1093526619836025 (2019). Article
PubMed Google Scholar * Minamiguchi, S. _et al._ Complement split product C4d deposition in placenta in systemic lupus erythematosus and pregnancy-induced hypertension. _Pathol. Int._ 63,
150–157. https://doi.org/10.1111/pin.12041 (2013). Article CAS PubMed Google Scholar * Terry, J. & Bedaiwy, M. A. Placental interferon signaling is involved in chronic
intervillositis of unknown etiology. _Placenta_ 124, 5–11. https://doi.org/10.1016/j.placenta.2022.05.006 (2022). Article CAS PubMed Google Scholar * Holmes, C. H. _et al._ Complement
regulatory proteins at the feto-maternal interface during human placental development: Distribution of CD59 by comparison with membrane cofactor protein (CD46) and decay accelerating factor
(CD55). _Eur. J. Immunol._ 22, 1579–1585. https://doi.org/10.1002/eji.1830220635 (1992). Article CAS PubMed Google Scholar * Triulzi, D. J. _et al._ The effect of previous pregnancy and
transfusion on HLA alloimmunization in blood donors: Implications for a transfusion-related acute lung injury risk reduction strategy. _Transfusion (Paris)_ 49, 1825–1835.
https://doi.org/10.1111/j.1537-2995.2009.02206.x (2009). Article Google Scholar * De Clippel, D. _et al._ Screening for HLA antibodies in plateletpheresis donors with a history of
transfusion or pregnancy. _Transfusion (Paris)_ 54, 3036–3042. https://doi.org/10.1111/trf.12727 (2014). Article CAS Google Scholar * Vilches, M. & Nieto, A. Analysis of
pregnancy-induced anti-HLA antibodies using luminex platform. _Transplant. Proc._ 47, 2608–2610. https://doi.org/10.1016/j.transproceed.2015.09.032 (2015). Article CAS PubMed Google
Scholar * Akgul, S. U. _et al._ Association between HLA antibodies and different sensitization events in renal transplant candidates. _Transplant. Proc._ 49, 425–429.
https://doi.org/10.1016/j.transproceed.2017.02.004 (2017). Article CAS PubMed Google Scholar * Bianchi, D. W. _et al._ Detection of fetal cells with 47, XY,+21 karyotype in maternal
peripheral blood. _Hum. Genet._ 90, 368–370. https://doi.org/10.1007/bf00220460 (1992). Article CAS PubMed Google Scholar * Sabapatha, A., Gercel-taylor, C. & Taylor, D. D. Specific
isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. _Am. J. Reprod. Immunol._ 56, 345–355.
https://doi.org/10.1111/j.1600-0897.2006.00435.x (2006). Article CAS PubMed Google Scholar * Hönger, G. _et al._ Frequency and determinants of pregnancy-induced child-specific
sensitization. _Am. J. Transplant._ 13, 746–753. https://doi.org/10.1111/ajt.12048 (2013). Article CAS PubMed Google Scholar * Nedberg, N. H. _et al._ Platelet alloimmunization is
associated with low grade chronic histiocytic intervillositis—A new link to a rare placental lesion?. _Placenta_ 112, 89–96. https://doi.org/10.1016/j.placenta.2021.07.291 (2021). Article
CAS PubMed Google Scholar * Nuri, E. _et al._ Long-term use of hydroxychloroquine reduces antiphospholipid antibodies levels in patients with primary antiphospholipid syndrome. _Immunol.
Res._ 65, 17–24. https://doi.org/10.1007/s12026-016-8812-z (2017). Article CAS PubMed Google Scholar * Mulla, M. J. _et al._ Antiphospholipid antibodies induce a pro-inflammatory
response in first trimester trophoblast via the TLR4/MyD88 pathway. _Am. J. Reprod. Immunol._ 62, 96–111. https://doi.org/10.1111/j.1600-0897.2009.00717.x (2009). Article CAS PubMed
PubMed Central Google Scholar * Moar, L. _et al._ Chronic histiocytic intervillositis (CHI): Current treatments and perinatal outcomes, a systematic review and a meta-analysis. _Front
Endocrinol (Lausanne)_ https://doi.org/10.3389/fendo.2022.945543 (2022). Article PubMed Google Scholar * Reich, A., Marcinow, K. & Bialynicki-Birula, R. The lupus band test in
systemic lupus erythematosus patients. _Ther. Clin. Risk Manag._ 7, 27. https://doi.org/10.2147/TCRM.S10145 (2011). Article PubMed PubMed Central Google Scholar Download references
ACKNOWLEDGEMENTS This study was funded by Tommy’s Baby Charity. The authors would like to acknowledge the significant input that Dr Ian Crocker had into the conception and undertaking of the
research project reported here. Sadly, Dr Crocker died in August 2022, prior to the submission of this manuscript. AUTHOR INFORMATION Author notes * Ian P. Crocker is deceased. AUTHORS AND
AFFILIATIONS * Tommy’s Maternal and Fetal Health Research Centre, St Mary’s Hospital, The University of Manchester, Manchester, UK Chloe A. Brady, Chloe Moss, Zhiyong Zou, Ian P. Crocker
& Alexander E. P. Heazell * Transplantation Laboratory, Manchester Royal Infirmary, Manchester University NHS Foundation Trust, Manchester, UK Laura B. Ford * Saint Mary’s Hospital,
Manchester University NHS Foundation Trust, Manchester, UK Alexander E. P. Heazell Authors * Chloe A. Brady View author publications You can also search for this author inPubMed Google
Scholar * Laura B. Ford View author publications You can also search for this author inPubMed Google Scholar * Chloe Moss View author publications You can also search for this author
inPubMed Google Scholar * Zhiyong Zou View author publications You can also search for this author inPubMed Google Scholar * Ian P. Crocker View author publications You can also search for
this author inPubMed Google Scholar * Alexander E. P. Heazell View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS C.A.B, I.P.C and A.E.P.H
contributed toward study design and planning. C.A.B performed experiments and data collection, analysis and interpretation and writing of the manuscript. L.B.F assisted with experiments,
data analysis and interpretation and the preparation of the manuscript. Z.Z and C.M assisted with image analysis and made equal contribution towards the manuscript. All authors aside from
I.P.C had input in the revision of the manuscript. CORRESPONDING AUTHOR Correspondence to Chloe A. Brady. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY FIGURE 1. SUPPLEMENTARY TABLE 1. SUPPLEMENTARY LEGENDS. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative
Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Brady, C.A., Ford, L.B., Moss, C. _et al._ Virtual crossmatching reveals
upregulation of placental HLA-Class II in chronic histiocytic intervillositis. _Sci Rep_ 14, 18714 (2024). https://doi.org/10.1038/s41598-024-69315-5 Download citation * Received: 09 June
2023 * Accepted: 02 August 2024 * Published: 12 August 2024 * DOI: https://doi.org/10.1038/s41598-024-69315-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative







