- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Heavy metal ions can be introduced into the water through several point and non-point sources including leather industry, coal mining, agriculture activity and domestic waste.
Regrettably, these toxic heavy metals may pose a threat to both humans and animals, particularly when they infiltrate water and soil. Heavy metal poisoning can lead to many health
complications, such as liver and renal dysfunction, dermatological difficulties, and potentially even malignancies. To mitigate the risk of heavy metal ion exposure to humans and animals, it
is imperative to extract them from places that have been polluted. Several conventional methods such as ion exchange, reverse osmosis, ultrafiltration, membrane filtration and chemical
precipitation have been used for the removal of heavy metal ions. However, these methods have high operation costs and generate secondary pollutants during water treatment. Biosorption is an
alternative approach to eliminating heavy metals from water that involves employing eco-friendly and cost-effective biomass. This review is focused on the heavy metal ions contamination in
the water, biosorption methods for heavy metal removal and mathematical modeling to explain the behaviour of heavy metal adsorption. This review can be helpful to the researchers to design
wastewater treatment plants for sustainable wastewater treatment. SIMILAR CONTENT BEING VIEWED BY OTHERS REMOVAL OF HEAVY METAL IONS FROM WASTEWATER: A COMPREHENSIVE AND CRITICAL REVIEW
Article Open access 08 July 2021 THE EFFICIENCY OF REMOVING HEAVY METAL IONS FROM INDUSTRIAL ELECTROPOLISHING WASTEWATER USING NATURAL MATERIALS Article Open access 22 October 2022
EXPERIMENTAL MODELLING STUDIES ON THE REMOVAL OF DYES AND HEAVY METAL IONS USING ZNFE2O4 NANOPARTICLES Article Open access 09 April 2022 INTRODUCTION The issue of heavy metal pollution is
increasingly pervasive on a global scale. Heavy metals are naturally occurring elements that are present in the earth's crust. However, excessive amounts of heavy metals can pose a
significant risk1. Some compounds, such as heavy metals, are resistant to decomposition and can accumulate in people and animals when they enter the food chain2. Metals can enter the
environment through natural means or human actions including waste disposal, industrial manufacturing, and mining3. Mining poses a significant danger by potentially displacing and spreading
heavy metals to surrounding regions during flooding or windstorms4. It is important to acknowledge and address environmental hazards to safeguard the well-being of both humans and the
natural world5. Heavy metal-induced water pollution can have detrimental impacts on human health6,7. These metals can enter our systems via polluted water and food8. They can bind with
organic groups, resulting in the formation of detrimental chemicals that can induce damaging effects on our cells9. Multiple techniques exist for extracting these metals from polluted water;
however, they are accompanied by drawbacks such as the production of additional pollutants or exorbitant expenses10,11,12,13. Hence, it is crucial to devise appropriate biological
techniques for the remediation of heavy metals14. Biosorption is an efficient and eco-friendly technology created to remove heavy metal ions from polluted water, offering both
cost-effectiveness and environmental benefits15. Biosorption methods can replace conventional methods and can be considered as suitable alternative to existing physiochemical methods due to
the eco-friendly and cost-effective nature of biosorption techniques. The biosorption method relies on the utilization of various types of raw materials derived from agro-waste, plant
residue, and algal and microbial biomass16. Biosorption is a metabolically independent method that does not require the participation of living organisms, making it a more straightforward
and user-friendly technology17. A diverse range of materials, such as rice and wheat husks, activated carbon, agricultural waste, bananas and citrus peels, and green-synthesized
nanoparticles, can be effectively used for biosorption18,19,20. It is important to emphasise that these materials have a distinct surface character that greatly enhances their capacity to
absorb the heavy metal ions found in the water21,22. This review is focused on the heavy metals contamination sources including point and non-point sources of heavy metal ions contamination
in the water. This review also provides detailed information on the biosorption method for heavy metal removal. In addition, the behaviour of biosorption is also described by mathematical
models including isotherms, thermodynamics and kinetics. WATER QUALITY ASSESSMENT WATER QUALITY CRITERIA A thorough examination of a wide range of variables that are well-known and
recognized as key indicators to accurately characterize the overall quality of water23. The World Health Organization recommends the maximum allowable limits for water physicochemical
parameters, as shown in Table 1. This comprehensive understanding and assessment of the various variables are based on the findings and conclusions drawn from a meticulous study conducted by
several researchers24. To ensure the utmost safety and well-being of users who rely on water, whether it is for consumption, recreational activities, or any other specific purpose, several
essential water quality criteria are implemented and enforced. These criteria are meticulously designed to regulate and control the maximum allowable concentration level of specific
substances within a given medium, be it water, sediment, or biota. The primary objective behind these criteria is to prevent and eliminate any potential risks or harmful effects that may
arise from exposure to excessive levels of such substances. It is important to note that these water quality criteria are particularly crucial and significant when the medium, such as water,
sediment, or biota, is continuously utilized or relied upon for a specific purpose. This emphasis on continuous usage further highlights the necessity and importance of establishing and
adhering to these criteria to ensure long-term safety and sustainability. It is imperative to acknowledge and recognize the multifaceted nature and complexity of these physicochemical
parameters, as they collectively play a pivotal role in determining and shaping the overall quality and characteristics of water. Their interconnected and interdependent nature necessitates
a thorough understanding of each parameter's influence and impact on water quality. Moreover, the presence and concentration of heavy metals in water are of particular concern and
importance due to their potential toxicity and detrimental effects on both human health and the environment25. The establishment and enforcement of water quality criteria, alongside regular
monitoring and assessment of heavy metals concentration, are crucial in safeguarding and preserving the integrity and safety of water resources26. The water quality criteria are vital
components of ensuring the safety, sustainability, and overall well-being of users who rely on water for various purposes. These criteria are meticulously formulated based on a detailed
understanding and examination of numerous variables that accurately characterize water quality. The comprehensive assessment of physicochemical parameters, including dissolved oxygen levels,
pH, temperature, conductivity, BOD, COD, TDS, minerals, and heavy metals concentration, is essential in maintaining and protecting the quality and integrity of water resources27. Following
the comprehensive and exhaustive evaluation and analysis of the ambient water quality concerning the implementation and execution of appropriate and effective measures and actions to
adequately and proficiently manage and control pollution for all types of discharges, including those occurring in the upstream sections of the water bodies28. It is significant to
acknowledge and recognize that this particular mechanism and approach also serves and functions as a means and tool to facilitate and support the growth, development, and establishment of
various industries, thereby emphasizing and emphasizing the crucial and pivotal significance and role it plays in the overall and comprehensive framework and structure of environmental
management. It is imperative and essential to explicitly state and specify that under no circumstances and situations are industries permitted or authorized to release or discharge any form
or type of waste or effluent materials into the river sections28,29. WATER QUALITY ASSESSMENT AND MANAGEMENT There is a problem with water quality around the world. The preservation of
public health, food security, biodiversity, and additional ecosystem services are progressively endangered by the intensifying and escalating pollution of fresh water in both developed and
developing nations30,31,32,33. A noteworthy association exists between pollution and economic advancement, with population growth, agricultural expansion, industrial expansion, and energy
production all contributing to the discharge of untreated or uncontrolled wastewater into surface and groundwater bodies. Despite recent preliminary evaluations of water quality worldwide,
the extent of the predicament remains uncertain34. Water quality needs to be protected and improved effectively and efficiently with better information about the issues involved. Government
and private agencies are working on water quality assessment and management35. * 1. The development and implementation of a comprehensive water resources plan, policy formulation,
coordination, and guidance. * 2. Irrigation, flood control, and multi-purpose projects need to be closely monitored, supervised, inspected, cleaned, and monitored for their effectiveness. *
3. Groundwater development is the process of developing groundwater resources, establishing utilizable resources, and formulating policies for their exploitation, along with the supervision
of state-level groundwater development activities and the support that is provided to them. * 4. The development of a comprehensive perspective regarding the water resources of a nation and
the assessment of the water balance across various basins and sub-basins are key considerations in the evaluation of inter-basin transfer feasibility. The primary initiatives that are
currently being undertaken involve a comprehensive investigation into the management of groundwater, both at macro and micro levels. These measures play a crucial role in ensuring the
sustainable management of groundwater resources. It is of paramount importance to prioritize these initiatives to guarantee the long-term viability of groundwater resources36. Furthermore,
the Board, in collaboration with concerned state government agencies, conducts periodic evaluations of replenishable groundwater resources in the country. This collaborative approach ensures
a comprehensive and informed understanding of the current state of groundwater resources37. The Central Pollution Control Board (CPCB) of India and the Environmental Protection Agency (USA)
are authoritative bodies, that exercise their oversight over the numerous state boards by setting emission standards and establishing ambient standards38. These bodies play a crucial role
in mitigating the adverse effects of pollution by conducting nationwide surveys to evaluate the existing state of pollution. To achieve this goal, the Environmental Protection Agency has
implemented two comprehensive monitoring programs for inland water quality. Through these programs, a network of 480 measurement stations such as tanneries, chemical plants, textile mills
and distilleries has been established across the primary river basins in the country39. These measurement stations serve as crucial points of data collection and analysis, enabling a
comprehensive understanding of water quality40,41,42,43. Moreover, it is essential to recognize the significance of the field of International Environmental Law (IEL) in safeguarding our
planet's environment, which is a shared resource. At AIDA, it is necessary to actively engage with this field daily, utilizing its principles and frameworks to support individuals and
communities in their efforts to protect the environment. Preserving the environment is closely intertwined with the protection of foundational human rights. Therefore, our work in the field
of IEL not only seeks to safeguard the environment but also aims to uphold and promote these fundamental rights that are inextricably linked to the environment. Through our commitment to the
principles and practices of IEL, to strive to contribute to a sustainable and equitable future for all44. OCCURRENCE OF HEAVY METALS IN THE ENVIRONMENT For each 10% increase in the usage of
pesticides, this phenomenon can be observed. Many investigations have been carried out on the subject of wastewater and its impact on human health. A study examining the influence of
irrigation water quality on human health discovered higher rates of illness in the villages that employed wastewater for irrigation in comparison to the control village45. A study conducted
by Bartone46 observed that water pollution serves as both a cause and an effect in the connections between agriculture and human health46. The contamination of heavy metals in water is also
influenced by natural factors such as volcanic activity, metal corrosion, metal evaporation from soil and water, soil erosion, and geological weathering47. In comparison to the global
average level, the concentration of trace elements (> 0.05 mg/L hexavalent chromium and > 0.01 mg/L arsenic) in water quality on the Child Loess Plateau is found to be higher. The
quality of river water, when poor, is associated with high levels of sodium and salinity hazards. In the case of surface water bodies, a wide range of pollution sources, including both point
and non-point sources, have a significant impact on water quality48. POINT SOURCE Point sources of toxic heavy metal ions contamination in water are defined as particular types of pollution
that cause high amounts of heavy metal ions contamination in water. It is important to release contaminants from the sources and directly inject them into the nearest water bodies or
environmental sources49. Industrial units situated on the banks of the rivers serve as major heavy metal contamination sources in the water. The point sources of heavy metals contamination
are described into two main categories49. INDUSTRIAL SOURCES Industrial wastewater plays a major role in the heavy metals contamination in the water. Industrial wastewater contains several
hazardous chemicals including heavy metal ions that are directly or indirectly released into the environment. These heavy metal ions accumulate in the food chain and affect human beings
including terrestrial and aquatic animals50. Based on a survey by the central pollution control board, 260 million litres of industrial wastewater daily released into the Ganga River in
India51. According to a report released by the Ministry of Ecology and Environment of China, the nation released a total of 25.02 billion tons of industrial wastewater in the year 2019,
which is equivalent to approximately 68.5 billion litres per day52. As stated in a report published by the World Bank, industries operating in Bangladesh discharge an approximate amount of
1.5 million cubic meters (equivalent to 1.5 billion litres) of untreated or partially treated wastewater into rivers and other water bodies daily53. There are several industries which cause
heavy metal contamination in water paper, sugar, textiles, steel, battery, leather, chemicals, pharmaceuticals, metal works, and food industries discharge their wastewater into the
environment54,55. DOMESTIC SOURCES OF POLLUTION The domestic source of water contamination is the second major part of a point source. Domestic sources also depend on the collection of waste
and their dumping56,57. Domestic pollution can be reduced if wastewater is properly treated before discharging into the environment58. The main components of domestic sources are microbes
and organic matter. Domestic waste also contains a large amount of metals and salt including chlorides, detergents, oils and grease. The Yamuna River in India is highly polluted by domestic
sources, about 85% of the other sources of contamination59. In China, the sources of water contamination from within the country comprise industrial emissions, untresated domestic sewage,
and agricultural overflow. Based on current information, industrial wastewater is a significant contributor to water pollution, as more than 60% of China's underground water and a third
of its surface water are classified as unsuitable for human use due to contamination60. In Bangladesh, various factors like insufficient sewage treatment, industrial wastage, and
agricultural runoff contribute to water pollution. Studies suggest that a significant percentage of surface water in Bangladesh, around 85%, is contaminated. This contamination mainly stems
from domestic and industrial sources, resulting in severe health problems for millions of people who depend on polluted water sources53. NONPOINT OR DIFFUSED SOURCE OF POLLUTION The
contributions originating from sources that are spread out and not concentrated in one specific location are deemed to be of lesser significance when compared to the contributions from
sources that are concentrated in one specific location. This is primarily because these diffused sources lack specificity in terms of their characteristics and attributes, and also due to
their sheer abundance in number61. When pollutants, such as harmful substances or contaminants, are discharged and flow into a body of water, they are categorized as nonpoint sources. These
nonpoint sources can arise from various activities or areas, without a specific source or location to attribute them to. For instance, runoff from a field has the potential to carry
fertilizers and pesticides into a nearby stream, thereby contributing to the pollution of the water. The fertilizers and pesticides used in agriculture contain several types of metal
compounds62. These metals cause several types of contamination in the water. The occurrence of monsoon, which is a period characterized by heavy rainfall, plays a significant role in the
process of leaching, drainage, and surface water runoff63,64. These processes serve as mechanisms or pathways through which pollution is transported from the catchment area of a river to the
river itself. It is important to note that the pollution being transported in this manner is predominantly diffused in nature, meaning that it is made up of various components that are not
concentrated in one specific location. These components include but are not limited to topsoil, organic matter, plant residues, nutrients, toxicants, and microorganisms. Thus, the diffused
pollution being transported in this manner encompasses a diverse range of substances and materials62,64. AGRICULTURAL SOURCES OF POLLUTION The pollution of rivers caused by agricultural
activities is linked to a variety of crucial elements, specifically, the remnants left behind from agricultural practices, the utilization of fertilizers and pesticides, the rearing of
livestock, and the excessive accumulation of salts that arise as a consequence of the implementation of irrigation water65. The waste generated from agricultural activities within the
watershed of the river undergoes a natural process of decomposition, ultimately culminating in the contamination of the river66. The agricultural residues are also part of the food chain
specially utilized by bacteria and fungi. Their microorganisms break down agricultural waste and these degraded waste materials are responsible for water contamination67. LANDFILL AND
DUMPING OF TOXIC WASTE The dumping and landfill of hazardous materials are done carefully and follow the guidelines of CPCB, India and other environmental protection agencies. The scope of
Municipal Solid Waste (MSW) is wide-ranging, encompassing not only household waste but also healthcare and industrial refuse68. However, it is concerning that there is a lack of adequate
categorization for these different types of waste, resulting in their indiscriminate deposition into a single landfill. This indiscriminate disposal practice has significant consequences for
the environment and public health, as it leads to severe pollution of both the immediate and surrounding areas69. The landfill, being the primary location for the disposal of solid waste,
plays a central role in these detrimental effects. The repercussions of such disposal methods are far-reaching, with environmental pollution and the spread of diseases being particularly
severe outcomes70. A specific concern in the context of open dumping sites is the transportation of leachate, which serves as a prominent source of heavy metals in various environmental
compartments such as surface and groundwater, soil, and vegetation71. The heavy metals that are of particular concern in this regard include Cd, Cr, Cu, Pb, Ni, and Zn, as they pose
significant issues due to their presence and potential for harm72. Furthermore, the impurity of wastewater has emerged as a direct cause of the contamination of food crops, further
exacerbating the overall issue at hand73. OTHER SOURCES OF WATER POLLUTION There exist additional origins of water contamination, including but not limited to the excessive utilization of
water for bathing and clothes washing, the practice of cattle wading, and the act of open defecation74. Bathing and cloth washing in the river are among the most prevalent activities that
are closely associated with water pollution. In various towns and villages located along rivers, it is customary to lead cattle to the river for drinking and bathing75. The impact of
cattle-related activities on water quality cannot be underestimated. This is evident through the direct release of urine, dung, and both organic and inorganic matter that gets washed off
from the cattle. These activities not only contaminate the water but also have a significant influence on its overall quality. Moreover, when cattle wade through rivers, they disturb the
sediments present at the riverbed, further exacerbating the situation by introducing additional pollutants into the water76. It is important to note that these issues are not limited to
rural areas alone. Even in urban areas, especially in slum clusters where proper sanitation facilities are lacking, open defecation is rampant. This leads to a surplus of waste being dumped
into open spaces. Consequently, a considerable portion of the population resorts to using either the catchment area or the river itself as a means of waste disposal. This further contributes
to the introduction of organic pollution and pathogens into the river water, exacerbating the already compromised quality77,78,79,80. BIOSORPTION OF HEAVY METAL IONS There exist various
methodologies by which wastewater may be cleansed of hazardous compounds, including but not limited to heavy metals. One such technique, referred to as biosorption, involves the utilization
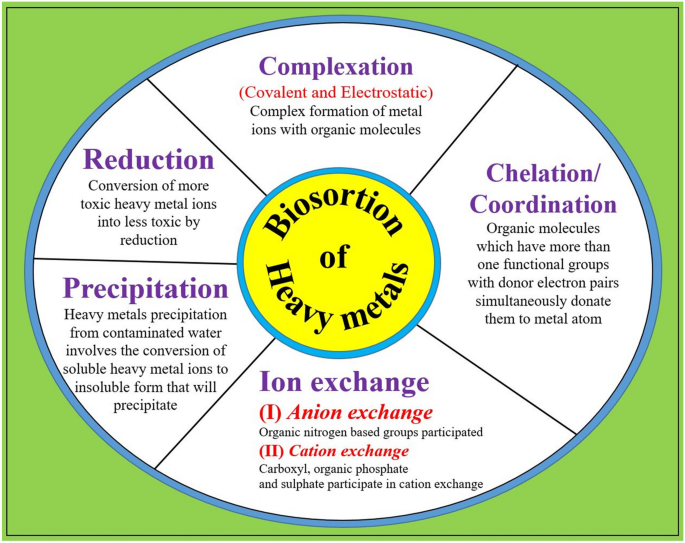
of expired microbial biomass for the express purpose of extracting these aforementioned metals81. This particular approach is further elucidated and visually demonstrated within a schematic
representation, as denoted by Fig. 1. It is widely believed that dead plant material can be used to remove heavy metals from polluted water. This process, called biosorption, happens when
the metal ions stick to the surface of the dead plant material82. Interestingly, living plants can also do this. In living plants, the metal ions can stick to the surface of the cells or get
absorbed through the plant's metabolic processes. This is an important process to help clean up polluted water83. In the process of removing harmful heavy metal ions from water, a
natural and cost-effective solution is to use biosorbents83. These are materials that contain certain functional groups, such as amino, amide, imidazole, sulfonate, and carboxyl groups, that
can bond with heavy metal ions to remove them from water84. The effectiveness of biosorbents depends on the variety and concentration of functional groups present on their surface, as well
as their surface shape85. The rough and porous surface of biosorbents is better at binding heavy metal ions and removing them from water86. Scientists use various methods such as FT-IR, SEM,
EDX, NMR, and XRD to analyze the surface shape and functional groups of biosorbents and ensure their quality87. The process of biosorption can be influenced by numerous factors, including
the utilization of specific microorganisms, the existence of various metal ions (including those that contend with the target metal), temperature, and pH88,89. If the pH level decreases, the
competition among metal ions that possess a positive charge can intensify89. Conversely, if the pH level rises, a greater number of surface binding sites become accessible. The mechanism of
biosorption for hexavalent chromium ions (Cr (VI)) is rather intricate90. These ions possess the ability to adhere to groups on the surface that are positively charged and subsequently
undergo a transformation into trivalent chromium ions (Cr (III)) via diverse pathways. Ordinarily, this process transpires in three distinct stages91. The initial step in the biosorption
process involves the attachment of positively charged surface functional groups to negatively charged Cr (VI) ions. The subsequent stage of the biosorption process is the reduction process.
The conversion of Cr (VI) to Cr (III) is facilitated by electron donor groups17,92,93. Heavy metals biosorption capacity of different adsorbents are mentioned in the Table 2, 3, 4 and 5.
MODELLING APPROACHES FOR HEAVY METALS BIOSORPTION ISOTHERM MODELS Isotherms, which are indispensable tools in the realm of adsorption investigations, are primarily concerned with the
meticulous and intricate analysis of the multifaceted and convoluted correlation existing between the adsorption capacity of a given substance and the residual concentration of heavy metal
ions that are inherently present therein, all while ensuring that the temperature remains constant. In the vast field of adsorption, an abundance of isotherm models has been introduced and
extensively employed, encompassing, yet not limited to, the renowned Freundlich, Langmuir, Temkin, Halsey, Harkin-Jura (H-J), D-R, Redlich-Peterson, and Jovanovic isotherm models, all of
which possess their own distinct merits and drawbacks when it comes to the accurate prediction of adsorption behaviour113. LANGMUIR ISOTHERM The fundamental principle that forms the basis
for the Langmuir isotherm is founded on the concept of monolayer adsorption, which takes place exclusively on a homogenous adsorbent. This phenomenon is achieved by disregarding any
potential surface interaction that may occur between two molecules that have been absorbed into the adsorbent material114. The mathematical expression is shown in Eq. (1).
$$\frac{{C}_{e}}{{q}_{e}} = \frac{1}{{Q}^{0}b} + \frac{{C}_{e}}{{Q}^{0}}$$ (1) Recent investigations have employed the Langmuir isotherm to explore adsorption phenomena in diverse domains,
including environmental remediation115,116. FREUNDLICH ISOTHERM In stark contrast, the model known as the Freundlich adsorption isotherm delves into the intricate realm of multilayer
adsorption occurring on the surface of an adsorbent that is characterized by its heterogeneity. This particular model serves the purpose of elucidating the underlying mechanisms involved in
the process of adsorption, which is fundamentally centred around the deposition of numerous layers of molecules onto the surface of said adsorbent. This elaborate process is achieved through
a meticulous examination of the heterogeneity displayed by the surface of the adsorbent, as well as a thorough analysis of the intricate interactions that transpire between the absorbing
molecules and the material constituting the adsorbent117. The linear form of Freundlich isotherm is given in Eq. (2). $${\mathrm{log }\, q}_{e} =\mathrm\, { log }kf + \frac{1}{n}\mathrm{log
}\, {C}_{e}$$ (2) The investigation of heavy metal adsorption processes, especially in environmental remediation, has been the focus of recent studies that have utilized the Freundlich
isotherm115,118. TEMKIN ISOTHERM The Temkin isotherm model offers a prognostication of equivalent binding energies for the adsorption on surfaces, thereby enabling a more comprehensive
comprehension of the process. It has been noted that the heat associated with adsorption rises linearly alongside the number of binding sites within a given layer. This implies that the
adsorption process is predominantly influenced by the even dissemination of binding energies, albeit only until a certain threshold, for all molecules present within said layer119. The
Temkin isotherm is shown in Eq. (3). $$q_{e} = \frac{RT}{{b_{T} }}{\text{ln\, A}}_{{\text{T}}} + \frac{RT}{{b_{T} }}{\text{ln C}}_{{\text{e}}}$$ (3) Recent investigations have recently
employed the Temkin isotherm, a widely utilized mathematical model, to comprehensively examine the intricate mechanisms underlying heavy metal adsorption processes, with a specific focus on
their application in the realm of environmental remediation, as explicated by the works of Nguyen et al.115 and Raji et al.120. DUBININ–RADUSHKEVICH (D–R) ISOTHERM The D–R isotherm model
posits that the adsorption process of heavy metal ions is deeply contingent upon the intricate and nuanced characteristics intrinsic to the structure of the adsorbent material121. The linear
form of D–R isotherm is shown in Eq. (4). $${\text{ln}}\, q_{e} = {\text{ ln Q}}_{{\text{D - R}}} - \, \beta \, \varepsilon^{2}$$ (4) where, QD–R (mol/g) and ꞵ (mol2 kJ−2) are the D–R
constants, calculated from the intercept and slope of the plot between ln qe and ɛ2. Here, ɛ is Polanyi potential and is calculated from Eq. (5). $${\upvarepsilon }^{2}=RT\mathrm{ ln}\,
\left(1+\frac{1}{{C}_{e}}\right)$$ (5) where R is the universal gas constant (8.341 J mol−1 K−1) and T is the temperature (K). The relationship between the free energy of adsorption and the
D–R isotherm constant is established. The free energy signifies the amount of energy required for the adsorption of one mole of adsorbate. It is possible to determine this value by utilizing
Eq. (6). $$E=\frac{1}{\sqrt{-2\beta }}$$ (6) where E (kJ mol−1) is the free energy which denotes whether the adsorption system is physical or chemical. Recent research has employed the D–R
isotherm, referred to as the Dubinin-Radushkevich isotherm, as a valuable tool in investigating and exploring the mechanisms and processes of heavy metal adsorption, especially in the
context of environmental remediation efforts115,122. HALSEY ISOTHERM On the contrary, the Halsey isotherm model delineates the phenomenon of multilayer adsorption occurring at a
significantly greater spatial separation from the surface of the adsorbent123. Equation (7) exhibits the Halsey isotherm.
$${q}_{e}=\frac{1}{{n}_{H}}{I}_{n}{K}_{H}-\frac{1}{{n}_{H}}{\text{ln}}\, {C}_{qe}$$ (7) Recent studies have applied the Halsey isotherm to investigate heavy metal adsorption processes,
especially in environmental remediation124. HARKIN–JURA (H–J) ISOTHERM The Harkin–Jura (H–J) isotherm model talks about how adsorbents (materials used to remove pollutants from liquids or
gases) can have multiple layers of pollutants sticking to their surface125. H–J isotherm is shown in Eq. (8). $$\frac{1}{{q}_{e}^{2}}= \frac{B}{A}- \left(\frac{1}{A}\right)log\, {C}_{e}$$
(8) where B and A are the model constants. B and A can be calculated from the slope and intercept of the plot between \(\frac{1}{{q}_{e}^{2}}\) versus \(log{ C}_{e}\). Liosis et al.126 and
Czikkely et al.127 described H–R isotherm modeling in their study for heavy metal adsorption processes, especially in environmental remediation. THERMODYNAMICS To put it simply, we can study
how certain materials interact with each other under different temperatures. If we see a positive change in one property called enthalpy (∆H°), it means that the process needs more heat to
happen and we can make it happen faster by increasing the temperature. On the other hand, if we see a negative change in another property called Gibbs free energy (∆G°), it means that the
process can happen on its own and will happen faster if we increase the temperature128. Thermodynamic parameters were calculated by using Eqs. (9), (10) and (11). $$\Delta {\text{G}}^\circ
\, = \, - {\text{RT ln}}\, k_{c}$$ (9) $$kc = \frac{{C}_{Ae}}{{C}_{e}}$$ (10) $$\mathrm{ln }kc = \frac{\mathrm{\Delta S}^{\_\!\!\!\!\circ} }{R}-\frac{\mathrm{\Delta H}^{\_\!\!\!\!\circ}
}{RT}$$ (11) where, _C__ae_ (mg L−1) is the equilibrium concentration, Ce (mg L−1) denotes equilibrium metal ion concentration in the bulk solution, T is the reaction temperature (K) and R
is the universal constant (8.314 J mol−1 K−1). The value of ΔSº and ΔHº were determined using the intercept and slope of the plot between ln _k__c_ versus 1/_T_129. KINETICS The
comprehension of how metal ions adhere to the exterior of an adsorbent is of utmost significance in the endeavour to formulate efficient wastewater treatment systems130. The influence
exerted on this process by the attributes of the adsorbent can be assessed with the aid of various models131. Presently, our investigation involves the experimentation with diverse models to
prognosticate the speed with which heavy metal ions will attach themselves to the surface of a distinct material capable of eliminating them from wastewater. PSEUDO-FIRST ORDER KINETICS The
pseudo-first-order model refers to how certain substances attach to surfaces. It's a way to understand how quickly this attachment happens, and it's often used in scientific
research. The model is described by a mathematical equation, which helps researchers study these processes in more detail (Eq. 12).
$$log\left({q}_{e}-{q}_{t}\right)={\text{log}}\left({q}_{e}\right)-\frac{{k}_{s}}{2.303}t$$ (12) where ks is the equilibrium rate constant and calculated from the slope
\(log({q}_{e}-{q}_{t}\)) vs time (t). The _q__t_ and _q__e_ are the adsorption capacities (mg/g) at time _t_ and equilibrium, respectively132. PSEUDO-SECOND ORDER KINETICS The process
involves the absorption of a material onto a surface. It is believed that the rate at which this happens is limited by the ability of the material to stick to the surface. This process
includes a type of absorption that involves a chemical reaction133. The mathematical expression of the pseudo-second-order model is shown in Eq. (13). $$\frac{t}{{q}_{t}}=
\frac{1}{{k\mathrm{^{\prime}}}_{2}{q}_{e}}+\frac{1}{{q}_{e}}\mathrm{t }$$ (13) $$h= {k^{\prime}}_{2}{q}_{e}^{2}$$ (14) Here, \({k{\prime}}_{2}\) and h are constants that can be calculated
from the plot between _t/q__t_ vs _t_. Recent studies have successfully applied this model to study heavy metal adsorption processes120,133. SIGNIFICANCE OF BIOSORPTION METHODS FOR HEAVY
METAL REDUCTION Biosorption, particularly the utilization of natural or modified biomaterials, presents a promising environmentally friendly technique for the reduction of heavy metals. It
presents several benefits, such as the utilization of low-cost adsorbents, achieving high efficiency, and requiring minimal chemical resources. The pseudo-second-order kinetics model,
commonly employed in the explanation of biosorption, grants valuable insights into the underlying mechanisms of the process. The comprehension of these mechanisms is of utmost importance to
optimize biosorption processes and develop effective treatment systems. In summary, biosorption makes a significant contribution to the present understanding of environmentally friendly
approaches to heavy metal reduction, providing sustainable solutions for the remediation of the environment. CONCLUSION AND FUTURE PROSPECTS Water contamination caused by heavy metals is a
significant problem that affects both humans and animals. Heavy metal ions can cause severe health problems such as liver and kidney damage, skin disorders, cognitive impairment and even
cancer. To prevent the harmful effects of these toxic metals, it is important to find an eco-friendly and cost-effective method to remove heavy metal ions contamination from wastewater.
Biosorption is an eco-friendly method based on the biomass derived from plant, algal, and agricultural waste and microbes. This method is environmentally friendly and does not require much
investment. This review provides basic to advanced knowledge to the research about heavy metal contamination and their eco-friendly removal process. DATA AVAILABILITY All data generated or
analysed during this study are included in this published article. REFERENCES * Rahman, Z. & Singh, V. P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd),
chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. _Environ. Monit. Assess._ 191, 419 (2019). Article PubMed Google Scholar * Jarup, L. Hazards of
heavy metal contamination. _Br. Med. Bull._ 68, 167–182 (2003). Article PubMed Google Scholar * Martin, Y. E. & Johnson, E. A. Biogeosciences survey: Studying interactions of the
biosphere with the lithosphere, hydrosphere and atmosphere. _Prog. Phys. Geogr._ 36, 833–852 (2012). Article Google Scholar * Kinuthia, G. K. _et al._ Levels of heavy metals in wastewater
and soil samples from open drainage channels in Nairobi, Kenya: Community health implication. _Sci. Rep._ 10, 8434. https://doi.org/10.1038/s41598-020-65359-5 (2020). Article ADS PubMed
PubMed Central CAS Google Scholar * Singh, R., Gautam, N., Mishra, A. & Gupta, R. Heavy metals and living systems: An overview. _Indian J. Pharmacol._ 43, 246–253 (2011). Article
PubMed PubMed Central CAS Google Scholar * Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K. & Sutton, D. J. Heavy metals toxicity and the environment. _EXS_ 101, 133–164 (2012).
PubMed PubMed Central Google Scholar * Wu, X. _et al._ A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. _Environ. Sci. Pollut. Res. Int._
23, 8244–8259 (2016). Article PubMed CAS Google Scholar * Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B. & Beeregowda, K. N. Toxicity, mechanism and health effects of some
heavy metals. _Interdiscip. Toxicol._ 7, 60–72 (2014). Article PubMed PubMed Central Google Scholar * Jan, A. T. _et al._ Heavy metals and human health: Mechanistic insight into
toxicity and counter defense system of antioxidants. _Int. J. Mol. Sci._ 16, 29592–29630 (2015). Article PubMed PubMed Central CAS Google Scholar * Briffa, J., Sinagra, E. &
Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. _Heliyon_ 6, e04691. https://doi.org/10.1016/j.heliyon.2020.e04691 (2020). Article PubMed
PubMed Central CAS Google Scholar * Khulbe, K. C. & Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. _Appl. Water Sci._ 8, 19.
https://doi.org/10.1007/s13201-018-0661-6 (2018). Article ADS CAS Google Scholar * Qasem, N. A. A., Mohammed, R. H. & Lawal, D. U. Removal of heavy metal ions from wastewater: A
comprehensive and critical review. _npj Clean Water_ 4, 36. https://doi.org/10.1038/s41545-021-00127-0 (2021). Article CAS Google Scholar * Kurniawan, T. A. & Chan, G. Y. S.
Physico-chemical treatment techniques for wastewater. _Chem. Eng. J._ 118, 83–98 (2006). Article CAS Google Scholar * Anirudhan, T. S. & Sreekumari, S. S. Adsorptive removal of heavy
metal ions from industrial effluents using activated carbon derived from waste coconut buttons. _J. Environ. Sci._ 23, 1989–1998 (2011). Article CAS Google Scholar * Wołowiec, M.,
Komorowska-Kaufman, M., Pruss, A., Rzepa, G. & Bajda, T. Removal of heavy metals and metalloids from water using drinking water treatment residuals as adsorbents: A review. _Minerals_ 9,
487. https://doi.org/10.3390/min9080487 (2019). Article ADS CAS Google Scholar * Singh, V. _et al._ Simultaneous removal of ternary heavy metal ions by a newly isolated _Microbacterium
paraoxydans_ strain VSVM IIT(BHU) from coal washery effluent. _BioMetals_ https://doi.org/10.1007/s10534-022-00476-4 (2022). Article PubMed Google Scholar * Jobby, R., Jha, P., Yadav, A.
K. & Desai, N. Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: A comprehensive review. _Chemosphere_ 207, 255–266 (2018). Article ADS PubMed CAS Google Scholar *
Bhatti, H. N. _et al._ Efficient removal of dyes using carboxymethyl cellulose/alginate/polyvinyl alcohol/rice husk composite: Adsorption/desorption, kinetics and recycling studies. _Int. J.
Biol. Macromol._ 150, 861–870. https://doi.org/10.1016/j.ijbiomac.2020.02.093 (2020). Article PubMed CAS Google Scholar * Akpomie, K. G. & Conradie, J. Banana peel as a biosorbent
for the decontamination of water pollutants: A review. _Environ. Chem. Lett._ 18(4), 1085–1112. https://doi.org/10.1007/s10311-020-00995-x (2020). Article CAS Google Scholar * Ratnasari,
A. _et al._ Mass transfer mechanisms of water pollutions adsorption mediated by different natural adsorbents. _Environ. Qual. Manag._ 32(1), 95–104. https://doi.org/10.1002/tqem.21849
(2022). Article Google Scholar * Gadd, G. M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. _J. Chem. Technol.
Biotechnol._ 84, 13–28 (2007). Article Google Scholar * Singh, V. _et al._ Hexavalent-chromium-induced oxidative stress and the protective role of antioxidants against cellular toxicity.
_Antioxidants_ 12, 2375. https://doi.org/10.3390/antiox11122375 (2022). Article CAS Google Scholar * Okwitanti, Y. _et al._ Investigation of rainwater quality at different rooftop types:
A case study at the large islamic boarding school in Madura. _Desalin. Water Treat._ 256, 217–220. https://doi.org/10.5004/dwt.2022.28352 (2022). Article CAS Google Scholar * Hasan, I.
Water quality assessment: A case study of the Jhenai River in Bangladesh. _RA J. Appl. Res._ https://doi.org/10.31142/rajar/v4i7.08 (2018). Article Google Scholar * Loh, Z. Z. _et al._
Comparative assessments on wastewater treatment technologies for potential of wastewater recycling. _Desalin Water Treat._ 261, 151–158. https://doi.org/10.5004/dwt.2022.28527 (2022).
Article CAS Google Scholar * Ratnasari, A. _et al._ Bioremediation of micropollutants using living and non-living algae—Current perspectives and challenges. _Environ. Pollut._ 292,
118474. https://doi.org/10.1016/j.envpol.2021.118474 (2022). Article PubMed CAS Google Scholar * Modelling of water quality-based emission limits for industrial discharges in rivers.
_Water Sci. Technol._ _39_(4). https://doi.org/10.1016/s0273-1223(99)00077-3 (1999). * Evaluating measures to control the impact of agricultural phosphorus on water quality. _Water Sci.
Technol._ 39(12). https://doi.org/10.1016/s0273-1223(99)00330-3 (1999). * Jahan, S. & Strezov, V. Water quality assessment of Australian ports using water quality evaluation indices.
_PLOS ONE_ 12(12), e0189284. https://doi.org/10.1371/journal.pone.0189284 (2017). Article PubMed PubMed Central CAS Google Scholar * Drinking Water Quality Monitoring & Surveillance
Framework. https://jaljeevanmission.gov.in/sites/default/files/guideline/WQMS-Framework.pdf. Accessed 27 July 2023 (2023). * du Plessis, A. Persistent degradation: Global water quality
challenges and required actions. _One Earth_ 5(2), 129–131. https://doi.org/10.1016/j.oneear.2022.01.005 (2022). Article ADS Google Scholar * Chehade, E. UN Environment’s Freshwater
Strategy 2017–2021: Tackling global water quality challenges. _Desalin. Water Treat._ 176, 429–429. https://doi.org/10.5004/dwt.2020.25554 (2020). Article Google Scholar * Central Ground
Water Board, Ministry of Water Resources, RD &GR Government of India. https://cgwb.gov.in/aboutcgwb.html. * Owan, V. J. Practicum report carried out in Government Primary School
Obufa-Esuk, 205 Goldie Street, Calabar, Cross River State. _SSRN Electron. J._ https://doi.org/10.2139/ssrn.3221786 (2018). Article Google Scholar * CPCB | Central Pollution Control Board.
https://cpcb.nic.in/Introduction/. Accessed 27 July 2023 (2023). * Water Quality Monitoring (WQM). System for River Ganga Overview. https://cpcb.nic.in/ngrba/WQM_overvew.php. Accessed 27
July 2023 (2023). * Borozan, A. B. _et al_. Soil pollution with heavy metals and bioremediation methods. _AgroLife Sci. J._ 10(1). https://doi.org/10.17930/AGL202115 (2021). * Global
Environment Monitoring System for Freshwater. _CEO Water Mandate_. https://ceowatermandate.org/resources/global-environment-monitoring-system-for-freshwater-2019/ (2019). * Bhardwaj, R.M.
Water quality monitoring in India achievements and constraints. In _IWG-Env, International Work Session on Water Statistics, Vienna, June 20–22_ (2005). * Nivetha, C. & Sangeetha, S. P.
A literature survey on water quality of Indian water bodies. _Mater. Today Proc._ 33, 412–414. https://doi.org/10.1016/j.matpr.2020.04.552 (2020). Article CAS Google Scholar *
International Environmental Law.
https://www.americanbar.org/groups/public_education/publications/insights-on-law-and-society/volume-19/insights-vol--19---issue-1/international-environmental-law/. Accessed 27 July 2023
(2023). * Xiao, J., Wang, L., Deng, L. & Jin, Z. Characteristics, sources, water quality and health risk assessment of trace elements in river water and well water in the Chinese Loess
Plateau. _Sci. Total Environ._ 650, 2004–2012. https://doi.org/10.1016/j.scitotenv.2018.09.322 (2019). Article ADS PubMed CAS Google Scholar * Blanco, A. & Roper, W. E. Remote
sensing techniques to detect surface water quality constituents in coastal and inland water bodies from point or non point pollution sources. _Proc. Water Environ. Feder._ 2007(17),
2039–2067. https://doi.org/10.2175/193864707788115915 (2007). Article Google Scholar * Lancaster, M. Green chemistry: An introductory text. In _RSC Paperbacks Series_.
https://doi.org/10.1039/9781847551009 (2007). * Srinivasan, J. T. & Reddy, V. R. Impact of irrigation water quality on human health: A case study in India. _Ecol. Econ._ 68(11),
2800–2807. https://doi.org/10.1016/j.ecolecon.2009.04.019 (2009). Article Google Scholar * Bartone, C. R. & Arlosoroff, S. Irrigation reuse of pond effluents in developing countries.
_Water Sci. Technol._ 19(12), 289–297. https://doi.org/10.2166/wst.1987.0159 (1987). Article Google Scholar * Zacchaeus, O. O. _et al._ Effects of industrialization on groundwater quality
in Shagamu and Ota industrial areas of Ogun State, Nigeria. _Heliyon_ 6(7), e04353. https://doi.org/10.1016/j.heliyon.2020.e04353 (2020). Article PubMed PubMed Central Google Scholar *
Dutta, V., Dubey, D. & Kumar, S. Cleaning the River Ganga: Impact of lockdown on water quality and future implications on river rejuvenation strategies. _Sci. Total Environ._ 743,
140756. https://doi.org/10.1016/j.scitotenv.2020.140756 (2020). Article PubMed PubMed Central CAS Google Scholar * Wu, S. _et al._ Treatment of industrial effluents in constructed
wetlands: Challenges, operational strategies and overall performance. _Environ. Pollut._ 201, 107–120. https://doi.org/10.1016/j.envpol.2015.03.006 (2015). Article PubMed CAS Google
Scholar * Annual Report. Central Pollution Control Board, India. https://yamunariverproject.wp.tulane.edu/wp-content/uploads/sites/507/2021/01/cpcb_2009-water-quality-status.pdf. Accessed
27 July 2023 (2023). * Kowalik-Klimczak, A. & Stanislawek, E. Reclamation of water from dairy wastewater using polymeric nanofiltration membranes. _Desalin. Water Treat._ 128, 364–371.
https://doi.org/10.5004/dwt.2018.22981 (2018). Article CAS Google Scholar * Guo, X. _et al._ Industrial water pollution discharge taxes in China: A multi-sector dynamic analysis. _Water_
10(12), 1742. https://doi.org/10.3390/w10121742 (2018). Article CAS Google Scholar * Hasan, Md. K., Shahriar, A. & Jim, K. U. Water pollution in Bangladesh and its impact on public
health. _Heliyon_ 5(8), e02145. https://doi.org/10.1016/j.heliyon.2019.e02145 (2019). Article PubMed PubMed Central Google Scholar * Ratnasari, A., Syafiuddin, A., Kueh, A. B. H.,
Suhartono, S. & Hadibarata, T. Opportunities and challenges for sustainable bioremediation of natural and synthetic estrogens as emerging water contaminants using bacteria, fungi, and
algae. _Water Air Soil Pollut._ https://doi.org/10.1007/s11270-021-05183-3 (2021). Article Google Scholar * Wang, Z. _et al._ Water level decline in a reservoir: Implications for water
quality variation and pollution source identification. _Int. J. Environ. Res. Public Health_ 17(7), 2400. https://doi.org/10.3390/ijerph17072400 (2020). Article PubMed PubMed Central CAS
Google Scholar * Koul, B., Yadav, D., Singh, S., Kumar, M. & Song, M. Insights into the domestic wastewater treatment (DWWT) regimes: A review. _Water_ 14, 3542.
https://doi.org/10.3390/w14213542 (2022). Article CAS Google Scholar * Alvarez, S., Asci, S. & Vorotnikova, E. Valuing the potential benefits of water quality improvements in
watersheds affected by non-point source pollution. _Water_ 8(4), 112. https://doi.org/10.3390/w8040112 (2016). Article Google Scholar * Ecotechnological methods for managing non-point
source pollution in watersheds, lakes and reservoirs. _Water Sci. Technol._ 33(4–5). https://doi.org/10.1016/0273-1223(96)00216-8 (1996). * Anjum, S. & Rana, S. Impact of environmental
pollutants on agriculture and food system. _Adv. Microb. Tech. Agric. Environ. Health Manag._ 2023, 133–151. https://doi.org/10.1016/b978-0-323-91643-1.00005-3 (2023). Article Google
Scholar * Yu, X., Geng, Y., Heck, P. & Xue, B. A review of China’s rural water management. _Sustainability_ 7(5), 5773–5792. https://doi.org/10.3390/su7055773 (2015). Article Google
Scholar * Arvanitoyannis, I. S. & Varzakas, T. H. Vegetable waste management: Treatment methods and potential uses of treated waste. _Waste Manag. Food Indus._ 2008, 703–761.
https://doi.org/10.1016/b978-012373654-3.50014-6 (2008). Article Google Scholar * Alengebawy, A., Abdelkhalek, S. T., Qureshi, S. R. & Wang, M.-Q. Heavy metals and pesticides toxicity
in agricultural soil and plants: Ecological risks and human health implications. _Toxics_ 9(3), 42. https://doi.org/10.3390/toxics9030042 (2021). Article PubMed PubMed Central CAS Google
Scholar * Kapoor, D. & Singh, M. P. Heavy metal contamination in water and its possible sources. _Heavy Met. Environ._ 2021, 179–189. https://doi.org/10.1016/b978-0-12-821656-9.00010-9
(2021). Article Google Scholar * Goyal, V. C., Singh, O., Singh, R., Chhoden, K. & Malyan, S. K. Appraisal of heavy metal pollution in the water resources of western Uttar Pradesh.
_India Assoc. Risks Environ. Adv._ 8, 100230. https://doi.org/10.1016/j.envadv.2022.100230 (2022). Article CAS Google Scholar * Ratnasari, A. _et al._ Prospective biodegradation of
organic and nitrogenous pollutants from palm oil mill effluent by acidophilic bacteria and archaea. _Bioresour. Technol. Rep._ 15, 100809. https://doi.org/10.1016/j.biteb.2021.100809 (2021).
Article CAS Google Scholar * Vongdala, N., Tran, H.-D., Xuan, T., Teschke, R. & Khanh, T. Heavy metal accumulation in water, soil, and plants of municipal solid waste landfill in
Vientiane, Laos. _Int. J. Environ. Res. Public Health_ 16(1), 22. https://doi.org/10.3390/ijerph16010022 (2018). Article PubMed PubMed Central CAS Google Scholar * Bakis, R. &
Tuncan, A. An investigation of heavy metal and migration through groundwater from the landfill area of Eskisehir in Turkey. _Environ. Monit. Assess._ 176(1–4), 87–98.
https://doi.org/10.1007/s10661-010-1568-3 (2010). Article PubMed Google Scholar * Giusti, L. A review of waste management practices and their impact on human health. _Waste Manag._ 29(8),
2227–2239. https://doi.org/10.1016/j.wasman.2009.03.028 (2009). Article PubMed CAS Google Scholar * Kanmani, S. & Gandhimathi, R. Assessment of heavy metal contamination in soil due
to leachate migration from an open dumping site. _Appl. Water Sci._ 3(1), 193–205. https://doi.org/10.1007/s13201-012-0072-z (2012). Article ADS CAS Google Scholar * Sridhara Chary, N.,
Kamala, C. T. & Samuel Suman Raj, D. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. _Ecotoxicol. Environ. Saf._ 69(3),
513–524. https://doi.org/10.1016/j.ecoenv.2007.04.013 (2008). Article PubMed CAS Google Scholar * Chen, L. _et al._ Heavy metals in food crops, soil, and water in the lihe river
watershed of the Taihu Region and their potential health risks when ingested. _Sci. Total Environ._ 615, 141–149. https://doi.org/10.1016/j.scitotenv.2017.09.230 (2018). Article ADS PubMed
CAS Google Scholar * Ratnasari, A., Syafiuddin, A., Mehmood, M. A. & Boopathy, R. A review of the vermicomposting process of organic and inorganic waste in soils: Additives effects,
bioconversion process, and recommendations. _Bioresour. Technol. Rep._ 21, 101332. https://doi.org/10.1016/j.biteb.2023.101332 (2023). Article CAS Google Scholar * OthienoOdwori, E. &
WanambachaWakhungu, J. Assessment of physico-chemical and bacteriological quality of drinking water sources in Kakamega County, Kenya. _Asian J. Environ. Ecol._ 2023, 45–63.
https://doi.org/10.9734/ajee/2023/v20i1432 (2023). Article Google Scholar * Potgieter, N., Karambwe, S., Mudau, L. S., Barnard, T. & Traore, A. Human enteric pathogens in eight rivers
used as rural household drinking water sources in the northern region of South Africa. _Int. J. Environ. Res. Public Health_ 17(6), 2079. https://doi.org/10.3390/ijerph17062079 (2020).
Article PubMed PubMed Central CAS Google Scholar * Traoré, A. _et al._ The impact of human activities on microbial quality of rivers in the Vhembe District, South Africa. _Int. J.
Environ. Res. Public Health_ 13(8), 817. https://doi.org/10.3390/ijerph13080817 (2016). Article PubMed PubMed Central CAS Google Scholar * Kongprajug, A. _et al._ Human and animal
microbial source tracking in a tropical river with multiple land use activities. _Int. J. Hyg. Environ. Health_ 222(4), 645–654. https://doi.org/10.1016/j.ijheh.2019.01.005 (2019). Article
PubMed CAS Google Scholar * Zafar, S., Aqil, F. & Ahmad, I. Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agricultural soil.
_Bioresour. Technol_ 98, 2557–2561 (2007). Article PubMed CAS Google Scholar * Singh, V. & Mishra, V. Microbial removal of Cr (VI) by a new bacterial strain isolated from the site
contaminated with coal mine effluents. _J. Environ. Chem. Eng._ 9, 106279. https://doi.org/10.1016/j.jece.2021.106279 (2021). Article CAS Google Scholar * Wuana, R. A. & Okieimen, F.
E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. _Int. Scholar. Res. Not._ 2011, 402647.
https://doi.org/10.5402/2011/402647 (2011). Article Google Scholar * Quinn, M. J. & Sherlock, J. C. The correspondence between UK ‘action levels’ for lead in blood and in water. _Food
Addit. Contam._ 7, 387–424 (1990). Article PubMed CAS Google Scholar * Khulbe, K. C. & Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. _Appl.
Water Sci._ https://doi.org/10.1007/s13201-018-0661-6 (2018). Article Google Scholar * Wang, L. K., Wang, M.-H.S., Hung, Y.-T., Shammas, N. K. & Chen, J. P. _Handbook of Advanced
Industrial and Hazardous Wastes Management_ (CRC Press, 2017). Book Google Scholar * Peng, S.-H. _et al._ Biosorption of copper, zinc, cadmium and chromium ions from aqueous solution by
natural foxtail millet shell. _Ecotoxicol. Environ. Saf._ 165, 61–69. https://doi.org/10.1016/j.ecoenv.2018.08.084 (2018). Article PubMed CAS Google Scholar * Wang, J. & Chen, C.
Biosorption of heavy metals by _Saccharomyces cerevisiae_: A review. _Biotechnol. Adv._ 24(5), 427–451. https://doi.org/10.1016/j.biotechadv.2006.03.001 (2006). Article PubMed CAS Google
Scholar * Verma, N. & Sharma, R. Bioremediation of toxic heavy metals: A patent review. _Recent Patents Biotechnol._ https://doi.org/10.2174/1872208311666170111111631 (2017). Article
Google Scholar * Ojuederie, O. & Babalola, O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: A review. _Int. J. Environ. Res. Public Health_ 14(12),
1504. https://doi.org/10.3390/ijerph14121504 (2017). Article PubMed PubMed Central CAS Google Scholar * Zhang, W. _et al._ Enhanced heavy metal removal from an aqueous environment using
an eco-friendly and sustainable adsorbent. _Sci. Rep_ https://doi.org/10.1038/s41598-020-73570-7 (2020). Article PubMed PubMed Central Google Scholar * Chojnacka, K. Biosorption and
bioaccumulation—The prospects for practical applications. _Environ. Int._ 36(3), 299–307. https://doi.org/10.1016/j.envint.2009.12.001 (2010). Article PubMed CAS Google Scholar *
Duwiejuah, A. B., Abubakari, A. H., Quainoo, A. K. & Amadu, Y. Review of biochar properties and remediation of metal pollution of water and soil. _J. Health Pollut._
https://doi.org/10.5696/2156-9614-10.27.200902 (2020). Article PubMed PubMed Central Google Scholar * Dias, M. A., Rosa, C. A., Linardi, V. R., Conte, R. A. & De Castro, H. F.
Application of factorial design to study of heavy metals biosorption by waste biomass from beverage distillery. _Appl. Biochem. Biotechnol._ 91–93(1–9), 413–422.
https://doi.org/10.1385/abab:91-93:1-9:413 (2001). Article PubMed Google Scholar * Netzahuatl-Muñoz, A. R., Aranda-García, E. & Cristiani-Urbina, E. Chromium recovery from
chromium-loaded cupressus lusitanica bark in two-stage desorption processes. _Plants_ 12(18), 3222. https://doi.org/10.3390/plants12183222 (2023). Article PubMed PubMed Central CAS
Google Scholar * Park, D., Yun, Y.-S. & Park, J. M. Studies on hexavalent chromium biosorption by chemically-treated biomass of _Ecklonia_ Sp.. _Chemosphere_ 60(10), 1356–1364.
https://doi.org/10.1016/j.chemosphere.2005.02.020 (2005). Article ADS PubMed CAS Google Scholar * Park, D., Yun, Y.-S., Ahn, C. K. & Park, J. M. Kinetics of the reduction of
hexavalent chromium with the brown seaweed Ecklonia biomass. _Chemosphere_ 66(5), 939–946. https://doi.org/10.1016/j.chemosphere.2006.05.068 (2007). Article ADS PubMed CAS Google Scholar
* Deng, L., Zhang, Y., Qin, J., Wang, X. & Zhu, X. Biosorption of Cr(VI) from aqueous solutions by nonliving green algae _Cladophora albida_. _Miner. Eng._ 22(4), 372–377.
https://doi.org/10.1016/j.mineng.2008.10.006 (2009). Article CAS Google Scholar * Moghal, A. A. B. _et al._ Heavy metal immobilization studies and enhancement in geotechnical properties
of cohesive soils by EICP technique. _Appl. Sci._ 10(21), 7568. https://doi.org/10.3390/app10217568 (2020). Article CAS Google Scholar * Khanpour-Alikelayeh, E., Partovinia, A., Talebi,
A. & Kermanian, H. Enhanced biodegradation of light crude oil by immobilized _Bacillus licheniformis_ in fabricated alginate beads through electrospray technique. _Environ. Monit.
Assess._ https://doi.org/10.1007/s10661-021-09104-z (2021). Article PubMed Google Scholar * Pal, D. & Maiti, S. K. An approach to counter sediment toxicity by immobilization of heavy
metals using waste fish scale derived biosorbent. _Ecotoxicol. Environ. Saf._ 187, 109833. https://doi.org/10.1016/j.ecoenv.2019.109833 (2020). Article PubMed CAS Google Scholar *
Dadrasnia, A., Chuan Wei, K., Shahsavari, N., Azirun, M. & Ismail, S. Biosorption potential of _Bacillus salmalaya_ strain 139SI for removal of Cr(VI) from aqueous solution. _Int. J.
Environ. Res. Public Health_ 12(12), 15321–15338. https://doi.org/10.3390/ijerph121214985 (2015). Article PubMed PubMed Central CAS Google Scholar * Fernández-López, J. A., Angosto, J.
M. & Avilés, M. D. Biosorption of hexavalent chromium from aqueous medium with OpuntiaBiomass. _Sci. World J._ 2014, 1–8. https://doi.org/10.1155/2014/670249 (2014). Article CAS Google
Scholar * Nandhagopal, K., Munuswamy, E. & Krishnan, V. Biosorption of chromium vi by ubiquitous dictyota biomas. _Int. J. Pharm. Biol. Sci._ 8, 27–131 (2018). Google Scholar * Hiew,
B. Y. Z., Lee, L. Y., Lee, X. J., Thangalazhy-Gopakumar, S. & Gan, S. Utilisation of environmentally friendly Okara-based biosorbent for cadmium(II) removal. _Environ. Sci. Pollut. Res._
28(30), 40608–40622. https://doi.org/10.1007/s11356-020-09594-3 (2020). Article CAS Google Scholar * Garg, U., Kaur, M. P., Jawa, G. K., Sud, D. & Garg, V. K. Removal of cadmium (II)
from aqueous solutions by adsorption on agricultural waste biomass. _J. Hazard. Mater._ 154(1–3), 1149–1157. https://doi.org/10.1016/j.jhazmat.2007.11.040 (2008). Article PubMed CAS
Google Scholar * Liu, L. & Fan, S. Removal of cadmium in aqueous solution using wheat straw biochar: Effect of minerals and mechanism. _Environ. Sci. Pollut. Res._ 25(9), 8688–8700.
https://doi.org/10.1007/s11356-017-1189-2 (2018). Article MathSciNet CAS Google Scholar * Hou, Y. _et al._ Biosorption of cadmium and manganese using free cells of _Klebsiella_ sp.
isolated from waste water. _PLOS ONE_ 10(10), e0140962. https://doi.org/10.1371/journal.pone.0140962 (2015). Article PubMed PubMed Central CAS Google Scholar * Abdel-Aty, A. M., Ammar,
N. S., Abdel Ghafar, H. H. & Ali, R. K. Biosorption of cadmium and lead from aqueous solution by fresh water alga _Anabaena sphaerica_ biomass. _J. Adv. Res._ 4(4), 367–374
https://doi.org/10.1016/j.jare.2012.07.004 (2014). * Yu, X., Zhao, J., Liu, X., Sun, L., Tian, J. & Wu, N. Cadmium pollution impact on the bacterial community structure of arable soil
and the isolation of the cadmium resistant bacteria. _Front. Microbiol_ https://doi.org/10.3389/fmicb.2021.698834 (2021). * ul Haq, A., Saeed, M., Anjum, S., Bokhari, T. H., Usman, M. &
Tubbsum, S. Evaluation of sorption mechanism of Pb (II) and Ni (II) onto pea (_Pisum sativum_) peels. _J. Oleo Sci._ 66(7), 735–743 https://doi.org/10.5650/jos.ess17020 (2017). *
Nagashanmugam, K. B. & Srinivasan, K. Evaluation of carbons derived from gingelly oil cake for the removal of lead(II) from aqueous solutions. _J. Environ. Sci. Eng._ 52, 349–360 (2010).
PubMed CAS Google Scholar * Rafatullah, M., Sulaiman, O., Hashim, R. & Ahmad, A. Adsorption of copper (II), chromium (III), nickel (II) and lead (II) ions from aqueous solutions by
Meranti sawdust. _J. Hazard. Mater._ 170(2–3), 969–977. https://doi.org/10.1016/j.jhazmat.2009.05.066 (2009). Article PubMed CAS Google Scholar * Yuvaraja, G., Krishnaiah, N., Subbaiah,
M. V. & Krishnaiah, A. Biosorption of Pb(II) from aqueous solution by _Solanum melongena_ leaf powder as a low-cost biosorbent prepared from agricultural waste. _Colloids Surf. B
Biointerfaces_ 114, 75–81. https://doi.org/10.1016/j.colsurfb.2013.09.039 (2014). Article PubMed CAS Google Scholar * Sarada, B., Prasad, M. K., Kumar, K. K. & Murthy, C. Potential
use of leaf biomass, _Araucaria heterophylla_ for removal of Pb+2. _Int. J. Phytoremediat._ 15(8), 756–773. https://doi.org/10.1080/15226514.2012.735289 (2013). Article CAS Google Scholar
* Costa, W. D. _et al._ Removal of copper(II) ions and lead(II) from aqueous solutions using seeds of _Azadirachta indica_ A. Juss as bioadsorvent. _Environ. Res._ 183, 109213.
https://doi.org/10.1016/j.envres.2020.109213 (2020). Article PubMed CAS Google Scholar * Ayawei, N., Ebelegi, A. N. & Wankasi, D. Modelling and interpretation of adsorption
isotherms. _J. Chem._ 2017, 1–11. https://doi.org/10.1155/2017/3039817 (2017). Article CAS Google Scholar * Kalam, S., Abu-Khamsin, S. A., Kamal, M. S. & Patil, S. Surfactant
adsorption isotherms: A review. _ACS Omega_ 6(48), 32342–32348. https://doi.org/10.1021/acsomega.1c04661 (2021). Article PubMed PubMed Central CAS Google Scholar * Nguyen, T. T. _et
al._ Application of Langmuir and Freundlich isotherms for adsorption of heavy metals onto natural adsorbents: A review. _Environ. Technol. Innov._ 25, 102052 (2022). Google Scholar * Zhao,
Y. _et al._ Adsorption of acetone and ethanol over metal–organic framework MIL-101(Cr): Equilibrium, kinetic, and thermodynamic studies. _Chem. Eng. J._ 416, 129100 (2021). Google Scholar *
Edet, U. A. & Ifelebuegu, A. O. Kinetics, isotherms, and thermodynamic modeling of the adsorption of phosphates from model wastewater using recycled brick waste. _Processes_ 8(6), 665.
https://doi.org/10.3390/pr8060665 (2020). Article CAS Google Scholar * Wang, L. _et al._ Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater:
A review. _Sci. Total Environ._ 668, 1298–1309. https://doi.org/10.1016/j.scitotenv.2019.03.011 (2019). Article ADS PubMed CAS Google Scholar * Dada, A.O. Langmuir, Freundlich, Temkin
and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. _IOSR J. Appl. Chem._ 3(1), 38–45 https://doi.org/10.9790/5736-0313845
(2012). * Raji, Z., Karim, A., Karam, A. & Khalloufi, S. Adsorption of heavy metals: Mechanisms, kinetics, and applications of various adsorbents in wastewater remediation—A review.
_Waste_ 1(3), 775–805. https://doi.org/10.3390/waste1030046 (2023). Article Google Scholar * Murphy, O. P., Vashishtha, M., Palanisamy, P. & Kumar, K. V. A review on the adsorption
isotherms and design calculations for the optimization of adsorbent mass and contact time. _ACS Omega_ 8(20), 17407–17430. https://doi.org/10.1021/acsomega.2c08155 (2023). Article PubMed
PubMed Central CAS Google Scholar * Hu, Q. & Zhang, Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. _J. Mol. Liq._ 277,
646–648. https://doi.org/10.1016/j.molliq.2019.01.005 (2019). Article CAS Google Scholar * Batool, F., Akbar, J., Iqbal, S., Noreen, S. & Bukhari, S. N. A. Study of isothermal,
kinetic, and thermodynamic parameters for adsorption of cadmium: An overview of linear and nonlinear approach and error analysis. _Bioinorgan. Chem. Appl._ 2018, 1–11.
https://doi.org/10.1155/2018/3463724 (2018). Article CAS Google Scholar * Akpomie, K. G., Conradie, J., Adegoke, K. A., Oyedotun, K. O., Ighalo, J.O., Amaku, J. F., Olisah, C., Adeola, A.
O. & Iwuozor, K. O. Adsorption mechanism and modeling of radionuclides and heavy metals onto ZnO nanoparticles: A review. _Appl. Water Sci._ 13(1)
https://doi.org/10.1007/s13201-022-01827-9 (2022). * Torrik, E., Soleimani, M. & Ravanchi, M. T. Application of kinetic models for heavy metal adsorption in the single and multicomponent
adsorption system. _Int. J. Environ. Res._ 13(5), 813–828. https://doi.org/10.1007/s41742-019-00219-3 (2019). Article CAS Google Scholar * Liosis, C., Papadopoulou, A., Karvelas, E.,
Karakasidis, T. E. & Sarris, I. E. Heavy metal adsorption using magnetic nanoparticles for water purification: A critical review. _Materials_ 14(24), 7500.
https://doi.org/10.3390/ma14247500 (2021). Article ADS PubMed PubMed Central CAS Google Scholar * Czikkely, M., Neubauer, E., Fekete, I., Ymeri, P. & Fogarassy, C. Review of heavy
metal adsorption processes by several organic matters from wastewaters. _Water_ 10(10), 1377. https://doi.org/10.3390/w10101377 (2018). Article CAS Google Scholar * Igberase, E., Osifo,
P. & Ofomaja, A. The adsorption of Pb, Zn, Cu, Ni, and Cd by modified ligand in a single component aqueous solution: Equilibrium, kinetic, thermodynamic, and desorption studies. _Int. J.
Anal. Chem._ 2017, 1–15. https://doi.org/10.1155/2017/6150209 (2017). Article CAS Google Scholar * Olawale, S. A. _et al._ Thermodynamics and mechanism of the adsorption of heavy metal
ions on keratin biomasses for wastewater detoxification. _Adsorp. Sci. Technol._ 2022, 1–13. https://doi.org/10.1155/2022/7384924 (2022). Article CAS Google Scholar * Ratnasari, A.
Modified polymer membranes for the removal of pharmaceutical active compounds in wastewater and its mechanism—A review. _Bioengineered_ 14(1) https://doi.org/10.1080/21655979.2023.2252234
(2023). * Singh, M., Rayaz, M. & Arti, R. Isotherm and kinetic studies for sorption of Cr(VI) onto prosopis cineraria leaf powder: A comparison of linear and non‐linear regression
analysis. _Environ. Prog. Sustain. Energy_ https://doi.org/10.1002/ep.14259 (2023). * Bakar, S. A. _et al._ Kinetics and isotherms of heavy metals removal from laundry greywater by chitosan
ceramic beads. _Environ. Adv._ 13, 100391. https://doi.org/10.1016/j.envadv.2023.100391 (2023). Article CAS Google Scholar * Robati, D. Pseudo-second-order kinetic equations for modeling
adsorption systems for removal of lead ions using multi-walled carbon nanotube. _J. Nanostruct. Chem._ 3(1) https://doi.org/10.1186/2193-8865-3-55 (2013). Download references
ACKNOWLEDGEMENTS It is our sincere gratitude to the ICMR-RMRIMS, Patna for providing the necessary facilities for this study. Dr. Veer Singh thanks the Department of Health Research (DHR)
for granting the Young Scientist Award (File No.R.12014/37/2022-HR). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biochemistry, ICMR-Rajendra Memorial Research Institute of
Medical Sciences, Patna, 800007, India Veer Singh, Ghufran Ahmed, Sonali Vedika, Pinki Kumar & Ashish Kumar * Department of Microbiology, ICMR-Rajendra Memorial Research Institute of
Medical Sciences, Patna, 800007, India Sanjay K. Chaturvedi * Centre of Experimental Medicine and Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi, 221005, India
Sachchida Nand Rai * Faculty of Biotechnology, University of Agricultural Sciences and Veterinary Medicine of Bucharest, 011464, Bucharest, Romania Emanuel Vamanu Authors * Veer Singh View
author publications You can also search for this author inPubMed Google Scholar * Ghufran Ahmed View author publications You can also search for this author inPubMed Google Scholar * Sonali
Vedika View author publications You can also search for this author inPubMed Google Scholar * Pinki Kumar View author publications You can also search for this author inPubMed Google Scholar
* Sanjay K. Chaturvedi View author publications You can also search for this author inPubMed Google Scholar * Sachchida Nand Rai View author publications You can also search for this author
inPubMed Google Scholar * Emanuel Vamanu View author publications You can also search for this author inPubMed Google Scholar * Ashish Kumar View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS Veer Singh: Performed experiments, Writing original draft preparation including figures and Conceptualization. Ghufran Ahmed, Sonali
Vedika, Pinki Kumar, Sachchida Nand Rai, Sanjay K Chaturvedi: Reviewing and Editing. Ashish Kumar and Emanuel Vamanu: Supervision, Writing- Reviewing and Editing. CORRESPONDING AUTHORS
Correspondence to Emanuel Vamanu or Ashish Kumar. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative
Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the
original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in
the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your
intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence,
visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Singh, V., Ahmed, G., Vedika, S. _et al._ Toxic heavy metal ions
contamination in water and their sustainable reduction by eco-friendly methods: isotherms, thermodynamics and kinetics study. _Sci Rep_ 14, 7595 (2024).
https://doi.org/10.1038/s41598-024-58061-3 Download citation * Received: 26 January 2024 * Accepted: 25 March 2024 * Published: 31 March 2024 * DOI:
https://doi.org/10.1038/s41598-024-58061-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Heavy metals * Water contamination * Biosorption *
Eco-friendly biosorbent