- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Aqueous solution containing different concentration (0.5, 0.6 and 1.0%) (w/v) of Polyvinyl pyrrolodon-Iodine (PVP-I) complex, a well-known antiseptic; is prepared and the stability
and homogeneity of these solution is assessed as per the ICH Guidelines and International Harmonized Protocol respectively. The solutions were found to be sufficiently homogeneous and stable
for a year at 25 °C (60%RH). Measurement uncertainty of the prepared PVP-I solutions were estimated by identifying possible sources of uncertainty using Ishikawa diagram and preparing
uncertainty budget based on scope of calibration laboratory. The stable and homogenized PVP-I solution is to be used in a clinical trial for the application on oro and nasopharynx against
novel SARS-CoV-2 Virus. SIMILAR CONTENT BEING VIEWED BY OTHERS _IN VITRO_ VIRUCIDAL ACTIVITY OF POVIDONE IODINE GARGLE AND MOUTHWASH AGAINST SARS-COV-2: IMPLICATIONS FOR DENTAL PRACTICE
Article 10 December 2020 MOUTHRINSES AND SARS-COV-2 VIRAL LOAD IN SALIVA: A LIVING SYSTEMATIC REVIEW Article 24 May 2022 VALIDATED HPLC METHOD FOR SIMULTANEOUS DETERMINATION OF AZELASTINE
HYDROCHLORIDE FLUTICASONE PROPIONATE AND OXYMETAZOLINE IN NASAL MUCOSA AND NASOPHARYNGEAL SWABS FROM REAL HUMAN SAMPLES Article Open access 04 February 2025 INTRODUCTION In the late 2019 the
unexpected inception of COVID-19 commenced due to novel coronavirus, SARS-CoV-2; and has been declared as pandemic by WHO because of its nature and severity. Though vaccination program has
already been implemented in many countries, it will take a long time to vaccinate this huge world population. But concern still remains whether this global vaccination program declines the
exposure or affected rate1. Hence precautionary measures like social distancing, lock down, personal hygiene, testing, contact tracing, and universal masking are still in effect worldwide.
But issues like material of construction, uses and disposal practice of face masks have raised questions on sustainable environmental point of view2,3. Therefore an additional preventive
measure alongside with mask, sanitization and social distancing should be taken until all of the population is being vaccinated or any other sustainable solution appears. So far it has been
known that the nasal cavity, oropharynx and nasopharynx are the primary target cell of novel corona virus SARS-CoV-24,5,6 where the host cell becomes infected due to interaction between
viral spike protein (SP) and host cell receptor Angiotensin Converting Enzyme 2 (ACE2) or Basigen/EMMPRIN (CD147)7. Viral Access and contamination of the mentioned pathway can be prevented
by wearing mask but the infection incidence, if happens, despite wearing mask or not, can be reduced upon applying an effective antiseptic agent that is able in preventing viral colonization
or load. Povidone-iodine, a stable chemical complex of polyvinylpyrrolidone (PVP), is an established antiseptic with a broad spectrum used extensively from 0.5 to 10% in mouth wash, hand
wash, skin preparation, ophthalmic surgery and oral surgery8,9,10,11. The antiseptic action of PVP-I starts when free iodine dissociates from the polymer complex that rapidly penetrates the
microbes and oxidizes the nucleic acid and its protein structures12. Several reports have shown that, not only active in vitro against SARS-COV (epidemic of 2002–03) & MERS-COV (epidemic
of 2012–13) virus, but also against SARS-CoV-2 virus13,14,15,16. Stability of a pharmaceutical product is defined as the ability of a particular formulation in a specific
container/enclosure system to remain within its physical, chemical, microbiological, toxicological, protective and informational specifications17. In other words, it is the extent of a
product to remain within its specification from packaging to use. Stability testing assesses the effect of environmental factors on the quality of the drug substance or a formulated product
that is utilized to estimate its shelf life; appropriate storage conditions and suggests labeling instructions. Furthermore, the data produced during the stability testing is a crucial
requirement for regulatory approval of any drug or formulation18. On the other hand, homogeneity is defined as the degree of uniformity distributed throughout a quantity of a material or
solution. Drug product homogeneity defines the sameness of quality attribute(s) across an entire batch. From product quality and regulatory perspective, homogeneity within a batch and
consistency between manufactured batches are keys to safeguard public health against numerous sources of variability19. Furthermore, quality control (QC) testing performed during release and
stability studies demands homogeneity assessment of product batches for the justification of sample size used19. Uncertainty is usually defined as the parameter associated with the
measurement which illustrates reasonably attributed value dispersions of the measurand20. Every measurement has a degree of uncertainty irrespective of precision and accuracy. This is caused
by two factors, the measuring instrument limitation (systematic error) and the personnel`s skill performing the measurements (random error). Estimation of uncertainties associated with
results helps experts to observe their findings more precisely. It is important to compare results between different laboratories or from the same laboratory at different times, with
confidence. This can be attained by ensuring the same ‘reference points’ used by all laboratories through establishing a chain of calibrations leading to primary national or international
standards, ideally the Systeme Internationale (SI) units of measurement. This unbroken chain of comparisons leading to a known reference value provides ‘traceability’ to a common reference
point, ensuring that different experimenters are using the same measurement units. Traceability is therefore closely connected to uncertainty. Traceability provides the means of placing all
related measurements on a consistent measurement scale, whereas uncertainty characterizes the ‘strength’ of the links in the chain and the agreement to be expected between laboratories
performing similar measurements21. Hence, the purpose of this study is to assess the homogeneity and stability of prepared different concentration of PVP-I solution which will be used in a
clinical trial for the application in on oro-nasopharings against novel SARS-CoV-2 Virus. This study alongside successful completion of clinical trial will provide an advantage to the
pharmaceutical industries regarding the shelf life of the product and regulatory approval as well. Uncertainty estimation will ensure traceability up to SI unit from metrological aspect.
MATERIALS & METHODS CHEMICALS All chemicals and reagents were USP or ACS grade and were used as received basis. PVP-I (purity as available iodine: 11.2%), acetic acid (purity: 99.7%),
starch (pH of 2% solution: 6.0–7.5), sodium thiosulfate (purity: 99.5%) was obtained from Sigma-Aldrich (Germany) and glycerin (purity: 99.5%) was supplied by Merck (Germany). Sterile water
was used in preparation of different concentration of PVP-I solution. Deionized water (pH 5.65; conductivity < 2.0 μS/cm), produced from own plant of BRiCM was used in all other purpose
unless mentioned otherwise. EQUIPMENT All pH values were measured by Thermo Scientific, USA pH meter (Model: Orion Star A215), weighing were performed using Mettler Toledo, Switzerland
electrical Laboratory balance (Model: AL204) and absorbance were measured by Shimadzu, Japan UV–Visible Spectrophotometer (Model: UV-1800). Sterile water were produced in Perlong, China
vertical pressure steam sterilizer (Model: PTS-B50L) at 120 °C and 15lbs for 20 min. PREPARATION OF DIFFERENT CONCENTRATION OF PVP-I SOLUTION 0.5% (w/v), 0.6% (w/v) and 1.0% (w/v) PVP-I
solution were prepared by sterile water in a dark place. Formulation of different concentration of PVP-I solution is shown in Table 1. Prepared solutions were then bottled in 20 mL PTFE
amber colored oro-nasal spray bottle for stability & homogeneity assessment and for further application on oro-nasopharings against novel SARS-CoV-2 Virus. DETERMINATION OF AVAILABLE
IODINE Available iodine in the prepared solution were determined according the official analytical method of the USP. Briefly, 1 mL sample solution added to 150 mL deionized water and
stirred for 1 h in dark. 0.1 mL dilute acetic acid added and titrated with 0.1 M sodium thiosulfate using starch solution as indicator. 1 mL of 0.1 M sodium thiosulfate is equivalent to
12.69 mg of available iodine. MEASUREMENT OF ABSORBANCE AT 368 NM Prepared PVP-I solution were first diluted 10times by deionized water and then measured at 368 nm against deionized water as
reference. HOMOGENEITY ASSESSMENT Homogeneity of different concentration of PVP-I solution were assessed at different storage conditions i.e. 25 °C (60%RH), 30 °C (65%RH) and 40 °C (75%RH)
on the following day of formulation. A set of 10 oro-nosal spray bottle from each formulation were randomly selected. Each sample was analyzed in duplicate. Statistical analysis like Cochran
Test on pH, available iodine and absorbance of the PVP-I solutions was executed for homogeneity assessment22. STABILITY ASSESSMENT Stability of prepared PVP-I solutions were assessed at 25
°C (60%RH), 30 °C (65%RH) and 40 °C (75%RH) for the period of 12 months23,24. Same set of oro-nosal spray bottle that were used for homogeneity assessment were also used for this purpose.
pH, available iodine and absorbance of the PVP-I solutions were measured at 2 months interval. The mean values of results were used for statistical analysis to establish a linear regression
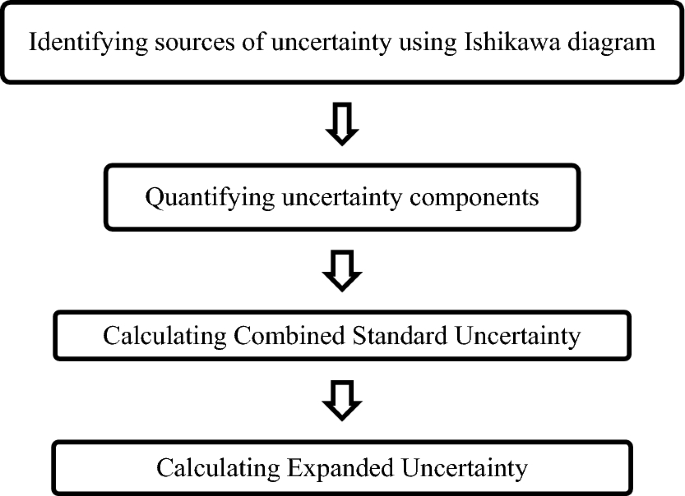
and statistical significance were investigated using one way ANOVA22,25. MEASUREMENT UNCERTAINTY ESTIMATION Following flow chart will be helpful in estimating measurement uncertainty.
Ishikawa diagram is constructed for the parameter pH, available iodine and absorbance to find out possible sources of uncertainty. Uncertainty from balance, volumetric flask, measuring
cylinder, burette, pH meter, homogeneity, stability and repeatability were identified. The value of uncertainty arising from balance, volumetric flask, measuring cylinder, burette and pH
meter were obtained from respective calibration certificate. Standard deviation of repeatability and stability were directly considered as standard uncertainty. The estimative of standard
uncertainty due to homogeneity (_u__hom_) was calculated by Eq. (1)26 $$u_{hom} = \sqrt {\frac{{MS_{within} }}{n}} \cdot \sqrt[4]{{\frac{2}{{f_{within} }}}}$$ (1) where _MS__within_
represents the mean square within groups of one way ANNOVA, _f__within_ represents degree of freedom of _MS__within_ one way ANNOVA and _n_ represents number of replicates. Once all the
uncertainty components are quantified, the combined standard uncertainty (_u__c_) is calculated using Eq. (2)21 $$u_{c} = \sqrt {\sum \left( {\frac{{u_{xi} }}{xi}} \right)^{2} }$$ (2) where
_u__xi__/xi_ represents uncertainties of each componenet expressed as relative standard deviation. Finally the expanded uncertainty (_U_) is calculated using Eq. (3) $$U = k \, * \, u_{c}$$
(3) where _k_ = 2, the coverage factor at 95% confidence interval. RESULTS & DISCUSSIONS HOMOGENEITY OF DIFFERENT CONCENTRATION OF PVP-I SOLUTION The homogeneity of formulated PVP-I
solutions can be understood by performing Cochran test. Cochran`s test statistic is calculated and compared with the appropriate critical value. If the _C__Cochran_ < _C__Critical_; then
the solution is considered to be homogeneous21. The critical values of 7–20 pairs at 95% confidence level are mentioned in Table 2. Cochran’s test statistic is calculated by following
equation; $${C}_{Cochran}= \frac{{D}_{max}^{2}}{\sum_{m}{D}_{i}^{2}}$$ where \({D}_{i}^{2}\) = squared difference of each pair, \({D}_{max}^{2}\) = maximum squared difference,
\({C}_{Cochran}\)= Cochran test statistic. The critical value for 10 pair is found to be 0.602 from Table 2. Since all of calculated _C__Cochran_ < _C__Critical_; it can be said that all
the prepared PVP-I solution (0.5%, 0.6% and 1.0%) is homogeneous at all of the studied storage conditions (Table 3). An example for calculating the Cochran’s test statistic of pH of 0.6%
PVP-I solution at storage conditions 25 °C (60%RH) is illustrated below in Table 4. STABILITY OF DIFFERENT CONCENTRATION OF PVP-I SOLUTION Stability of 0.5, 0.6 and 1.0% PVP-I solution were
assessed at 25 °C (60%RH), 30 °C (65%RH) and 40 °C (75%RH) based on the pH, Available Iodine % and absorbance at 368 nm of 10 times diluted solution. Regression line for each of the
parameter of each prepared solution at above mentioned storage condition was constructed for the period of 12 months with 2 months interval (Figs. 1, 2, and 3). Regression line is generally
constructed for graphical observation of the investigated data collected over a period of time which helps to find out any sort of instability quickly at a glance. This approach may often
mislead in assessing the stability of a product. Hence, a more reliable approach like one way ANOVA has been introduced where statistical significant difference is observed between initial
and final data. For any product to be considered as stable, there should be no significant differences between starting and ending time. Hence, the prepared PVP-I solution that has _p_ value
> 0.05 for all of the studied parameter (pH, available iodine and absorbance) should be considered stable. The _p_ value for each parameter at the studied storage condition is shown in
Table 5. From Fig. 1, it can be seen that the pH of all prepared solution at every storage condition steadily decreases except for 0.5% PVP-I solution at 25 °C and 0.6% PVP-I solution at 25
°C and 30 °C. As for available iodine and absorbance at 368 nm of 10 times dilution, it can be seen from Figs. 2 and 3 that, the available iodine of 0.5% PVP-I solution at 25 °C and 30 °C
and 0.6% PVP-I solution at 25 °C remains unchanged; the absorbance of 0.5% PVP-I solution at 25 °C, 30 °C and 40 °C and 0.6% PVP-I solution at 25 °C and 30 °C changes negligibly. These
findings from the figures are also supported by the _p_ value shown in Table 5. In light of Table 5 and Figs. 1, 2, and 3 it can be said that 0.5% and 0.6% PVP-I solution is stable for 12
months if stored at 25 °C (60%RH). As seen from Table 5 and Figs. 1, 2 and 3, 1.0% PVP-I solution is not stable at any of the storage condition. A strong negative correlation was found
(Table 6) between available iodine and absorbance at all the storage condition for 1.0% PVP-I solution which might be the cause of instability of the said solution. MEASUREMENT UNCERTAINTY
ESTIMATION OF 0.5% AND 0.6% PVP-I SOLUTION Since 0.5% and 0.6% PVP-I solution at 25 °C were found to be stable from stability assessment, uncertainty of only these solutions at stable
condition were estimated. In order to estimate the uncertainty, an uncertainty budget is needed to be prepared first. Based on source of uncertainty and scope of calibration laboratory the
uncertainty budget is prepared. The Ishikawa diagram/fish bone diagram also known as cause and effect diagram is designed in Figs. 4, 5, and 6 to identify the uncertainty associated in
measuring pH, available iodine and absorbance of 0.5% and 0.6% PVP-I solution27. The uncertainty budget with the expanded uncertainty calculated using Eqs. (1), (2), and (3) for the said
parameters of 0.5% and 0.6% PVP-I solution is tabulated in Tables 7, 8, and 9 and contribution of sources of uncertainty in estimating measurement uncertainty is shown in Figs. 7, 8, and 9.
It has been seen that repeatability contributed the most in estimating measurement uncertainty for pH and absorbance and stability the second most contributor for both of these cases. This
might be because of the standard deviation which was considered as the uncertainty for repeatability and stability. Whereas for the case available iodine, uncertainty from the burette
calibration contributed the most. APPLICATION OF PVP-I SOLUTION ON ORO-NASOPHARINGS AGAINST NOVEL SARS-COV-2 VIRUS A clinical trial on human has already begun in three hospitals of
Bangladesh where these formulated PVP-I solutions (0.5–0.6%) were being applied on oropharings and nasopharings of RT-PCR confirmed COVID-19 patients to assess the viricidal effect of PVP-I
against SARS-CoV-2. Two puffs in nose and three puffs in mouth (each puff contains about 80 µL solution) were applied as shown in Fig. 10. Ethical approval was taken from respective
authority and a written consent was also taken from the patient who was under the trial. The clearance of SARS-CoV-2 was tested after single time application of formulated PVP-I solution and
compared with the corresponding controls. The results were found to be promising so far. More than 80% were found to be negative after single application of formulated PVP-I solution.
Detailed results with statistics will be published as soon as the trial is complete. CONCLUSION This study demonstrated that the prepared 0.5% and 0.6% PVP-I formulation is stable and
sufficiently homogeneous at 25 °C (60%RH). Both of the solutions were found to be stable during the studied period of a year. Further stability study will be carried out to investigate
whether these solutions will be stable for another year. Measurement uncertainty of these two formulations were estimated and found to be as follows, pH: 4.86 ± 0.05, Available Iodine: 0.06
± 0.01% & absorbance (at 368 nm): 1.032 ± 0.12 for 0.5% PVP-I solution; and pH: 4.82 ± 0.04, Available Iodine: 0.08 ± 0.01% & absorbance (at 368 nm): 1.946 ± 0.22 for 0.5% PVP-I
solution. The clinical trial that is undergoing using these two formulations after single application on oro-nasopharings against novel SARS-CoV-2 Virus showed promising result so far. The
formulated solutions may require some modification or development if significant number of discomfort or irritation is reported by the patients during the clinical trial. In that case
another study will be proposed to investigate the effect of modification or development on the original formulation. REFERENCES * Briz-Redón, Á. & Serrano-Aroca, Á. On the association
between COVID-19 vaccination levels and incidence and lethality rates at a regional scale in Spain. _Stoch. Environ. Res. Risk Assess._ 5, 1–8. https://doi.org/10.1007/s00477-021-02166-y
(2022). Article Google Scholar * Tuñón-Molina, A. _et al._ Protective face masks: current status and future trends. _ACS Appl. Mater. Interfaces._ 13(48), 56725–56751.
https://doi.org/10.1021/acsami.1c12227 (2021). Article CAS PubMed Google Scholar * Babaahmadi, V., Amid, H., Naeimirad, M. & Ramakrishna, S. Biodegradable and multifunctional
surgical face masks: A brief review on demands during COVID-19 pandemic, recent developments, and future perspectives. _Sci. Total Environ._ 798, 149233.
https://doi.org/10.1016/j.scitotenv.2021.149233 (2021). Article ADS CAS PubMed PubMed Central Google Scholar * To, K. K. _et al._ Consistent detection of 2019 novel coronavirus in
saliva. _Clin. Infect. Dis._ 71, 841–843 (2020). Article CAS PubMed Google Scholar * Zou, L. _et al._ SARS-CoV-2 viral load in upper respiratory specimens of infected patients. _N. Engl.
J. Med._ 382(12), 1177–1179 (2020). Article PubMed PubMed Central Google Scholar * Khurshid, Z., Asiri, F. Y. I. & Al Wadaani, H. Human saliva: Noninvasive fluid for detecting novel
coronavirus. _Int. J. Environ. Res. Public Health_ 17(7), 2225 (2020). Article CAS PubMed PubMed Central Google Scholar * Sarma, P. _et al._ Therapeutic options for the treatment of
2019-novel coronavirus: An evidence-based approach. _Indian J. Pharmacol._ 52(1), 1 (2020). Article PubMed PubMed Central Google Scholar * Urias, D. S., Varghese, M., Simunich, T.,
Morrissey, S. & Dumire, R. Preoperative decolonization to reduce infections in urgent lower extremity repairs. _Eur. J. Trauma Emerg. Surg._ 44(5), 787–793 (2018). Article PubMed
Google Scholar * Koerner, J. C., George, M. J., Meyer, D. R., Rosco, M. G. & Habib, M. M. Povidone-iodine concentration and dosing in cataract surgery. _Surv. Ophthalmol._ 63(6),
862–868 (2018). Article PubMed Google Scholar * Silas, M. R., Schroeder, R. M., Thomson, R. B. & Myers, W. G. Optimizing the antisepsis protocol: Effectiveness of 3 povidone-iodine
1.0% applications versus a single application of povidone-iodine 5.0%. _J. Cataract Refract. Surg._ 43(3), 400–404 (2017). Article PubMed Google Scholar * Domingo, M. A., Farrales, M. S.,
Loya, R. M., Pura, M. A. & Uy, H. The effect of 1% povidone iodine as a pre-procedural mouthrinse in 20 patients with varying degrees of oral hygiene. _J. Philipp. Dent. Assoc._ 48(2),
31–38 (1996). CAS PubMed Google Scholar * da Silveira, T. D., de Figueiredo, M. A. Z., Cherubini, K., de Oliveira, S. D. & Salum, F. G. The topical effect of chlorhexidine and
povidone-iodine in the repair of oral wounds. _Stomatologija_ 21(2), 35–41 (2019). Google Scholar * Eggers, M., Eickmann, M. & Zorn, J. Rapid and effective virucidal activity of
povidone-iodine products against Middle East respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus Ankara (MVA). _Infect. Dis. Therapy_ 4(4), 491–501 (2015). Article
Google Scholar * Lange, C. _et al._ Expression of the COVID-19 receptor ACE2 in the human conjunctiva. _J. Med. Virol._ 92(10), 2081–2086 (2020). Article CAS PubMed PubMed Central
Google Scholar * Hassandarvish, P. _et al._ In vitro virucidal activity of povidone iodine gargle and mouthwash against SARS-CoV-2: Implications for dental practice. _Br. Dental J._
https://doi.org/10.1038/s41415-020-2402-0 (2020). Article Google Scholar * Pelletier, J. S. _et al._ Efficacy of povidone-iodine nasal and oral antiseptic preparations against severe acute
respiratory syndrome coronavirus 2 (SARS-CoV-2). _Ear Nose Throat J._ https://doi.org/10.1177/0145561320957237 (2020). Article PubMed Google Scholar * Kommanaboyina, B. & Rhodes, C.
T. Trends in stability testing, with emphasis on stability during distribution and storage. _Drug Dev. Ind. Pharm._ 25, 857–867 (1999). Article CAS PubMed Google Scholar * Bajaj, S.,
Singla, D. & Sakhuja, N. Stability testing of pharmaceutical products. _J. Appl. Pharm. Sci._ 02(03), 129–138 (2012). Google Scholar * Doymaz, F., Ye, F. & Burdick, R. Product
homogeneity assessment during validation of biopharmaceutical drug product manufacturing process. In _Quality by Design for Biopharmaceutical Drug Product Development AAPS Advances in the
Pharmaceutical Sciences Series_ Vol. 18 (eds Jameel, F. _et al._) (Springer, 2015). Google Scholar * Guide to The Expression of Uncertainty in Measurement. ISO, Geneva. (ISBN 92–67–10188–9)
(Reprinted 1995: Reissued as ISO Guide 98–3 (2008), also available from http://www.bipm.org as JCGM 100:2008) (1993). * Ellison, S. L. R. & Williams, A. _Quantifying Uncertainty in
Analytical Measurement_ 3rd edn. (EURACHEM/CITAC Guide CG 4, 2012). Google Scholar * Thompson, M., Ellison, S. L. & Wood, R. The international harmonized protocol for the proficiency
testing of analytical chemistry laboratories- IUPAC technical report. _Pure. Appl. Chem_ 78, 145–196 (2006). Article CAS Google Scholar * ICH Q1A(R2). _Stability Testing Guidelines:
Stability Testing of New Drug Substances and Products_. ICH Steering Committee (2003). * WHO. _Stability Studies in a Global Environment_. Geneva meeting working document QAS/05.146 with
comments (2004). * Thompson, M. Instability and heterogeneity: A new approach needed. _Accredit. Qual. Assur._ 13, 581–584 (2008). Article CAS Google Scholar * ISO Guide 35. _Reference
Materials-General and Statistical Principles for Certification_. International Organization for Standardization (ISO), Geneva (2006). * ISO 9004–4:1993. _Total Quality Management. Part 2_.
In _Guidelines for quality improvement_. ISO, Geneva (1993). Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Bangladesh Reference Institute for Chemical Measurements
(BRiCM), Dr-Qudrat-I-Khuda Road, Dhanmondi, Dhaka, 1205, Bangladesh Md. Moniruzzaman, Mamudul Hasan Razu, Sad Al Rezwan Rahman, Nayan Kumer Kundu, Sabiha Kamal & Mala Khan Authors * Md.
Moniruzzaman View author publications You can also search for this author inPubMed Google Scholar * Mamudul Hasan Razu View author publications You can also search for this author inPubMed
Google Scholar * Sad Al Rezwan Rahman View author publications You can also search for this author inPubMed Google Scholar * Nayan Kumer Kundu View author publications You can also search
for this author inPubMed Google Scholar * Sabiha Kamal View author publications You can also search for this author inPubMed Google Scholar * Mala Khan View author publications You can also
search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Md. Moniruzzaman. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with
regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's
Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Moniruzzaman, M., Razu, M.H., Rahman, S.A. _et al._ Stability, homogeneity and
measurement uncertainty estimation of PVP-I solutions for the application on oro and nasopharynx against SARS-CoV-2. _Sci Rep_ 14, 7268 (2024). https://doi.org/10.1038/s41598-024-52346-3
Download citation * Received: 21 August 2021 * Accepted: 09 March 2022 * Published: 27 March 2024 * DOI: https://doi.org/10.1038/s41598-024-52346-3 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative










