- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT At present, the separation technology of fluorite and calcite is still immature, and the research in this paper can promote the improvement of the separation technology of fluorite
and calcite. The selective inhibition mechanism of tannin and humate sodium on calcite was studied by means of actual ore flotation test, single mineral flotation test, Zeta potential
measurement and FT-IR spectroscopy. The results show that the mixture of tannin and sodium humate inhibitor has a good inhibitory effect on carbonate under weak alkaline condition. The
reaction products of sodium humate, tannin and calcium ions in solution interact with organic compounds adsorbed on the surface of calcite, forming multilayer adsorption on the surface of
calcite, making calcite more hydrophilic. Based on density functional theory, Materials Studio (MS) was used to calculate the relevant adsorption energy, and the result was as follows: (a)
compared with fluorite, tannin and humate sodium molecules are more easily adsorbed on the surface of calcite. (b) Compared with calcite alone adsorption of tannin molecules or sodium humate
molecules, the adsorption state will be more stable, and the effect of tannin and sodium humate synergistic inhibition of calcite is better than the effect of inhibition alone. Therefore,
using tannin and sodium humate as a combination inhibitor can effectively separate fluorite and calcite, which will promote the development and utilization of fluorite ore in industry.
SIMILAR CONTENT BEING VIEWED BY OTHERS FLOTATION SEPARATION OF PYRITE AND CARBONATE MINERALS FROM A CARLIN-TYPE GOLD DEPOSIT IN GUIZHOU BY A NEW COMBINED COLLECTOR AND ITS ADSORPTION
MECHANISM Article Open access 11 November 2024 ENHANCING FLOTATION SEPARATION OF CHALCOPYRITE AND MAGNESIUM SILICATE MINERALS BY SURFACE SYNERGISM BETWEEN PAAS AND GA Article Open access 18
March 2021 ADSORPTION AND SAFE IMMOBILIZATION OF SR IONS IN MODIFIED ZEOLITE MATRICES Article Open access 04 November 2023 INTRODUCTION The world is rich in total fluorspar resources, but is
facing shortage of high quality mineral resources, such as China1. Carbonized fluorite is one of the main types of fluorspar, which is abundant, and the main gangue of this ore is calcite.
Flotation is the most efficient method to recover this type of fluorite. Fluorite and calcite contain Ca2+. During flotation, the physical and chemical properties of the two minerals are
very similar due to the interaction of dissolved minerals, dissolved ions and mineral surfaces. Both fluorite and calcite contain Ca2+, and collectors and inhibitors are more likely to
interact with Ca2+ on the mineral surface, so collectors and inhibitors have similar effects on the surface of the two minerals, making it difficult to separate calcite from fluorite2,3. And
the degree of difficulty in separation is closely related to the ratio of fluorite content to calcite mineral content. The smaller the ratio, the more difficult the separation of minerals.
Generally, such fluorite deposits with ratios less than 4 to 5 are classified as refractory fluorite deposits4,5. The flotation separation of fluorite and calcite has been increasingly
studied. However, its flotation separation technology is not mature, especially for this kind of difficult ore with two very similar physical and chemical properties of minerals6,7. There
have been some reports8,9,10,11 on the use of single or mixed acidic water glass, water glass, baked gum, sodium hexametaphosphate, caustic soda, citric acid and sodium lignosulfonate as
calcite depressants in the flotation of fluorite ores at appropriate pH. It was shown that 2-phosphonobutane -1, 2, 4-tricarboxylic acid can selectively inhibit calcite at pH 9.0 under the
condition of sodium oleate as a trapping agent, which also provides a new option for calcite depressants12.The study13 showed that using oleic acid as a trapping agent, the flotation
performance of calcite in weak acid media was slightly worse than that of fluorite. If the appropriate inhibitor is combined with the collector, the grade and recovery of fluorite can be
improved. In fluorite ore, appropriate inhibitors can selectively interact with the surface of calcite but not with the surface of fluorite, making the surface of calcite more hydrophilic,
collectors can interact with the surface of fluorite but not with the surface of calcite, making the surface of fluorite more hydrophobic, so appropriate inhibitors and collectors are used
in combination. It can effectively separate fluorite and calcite and improve the grade and recovery rate of fluorite. The flotation separation of carbonated fluorite ores will be a
long-standing problem in fluorite flotation. Therefore, it is very important to carry out flotation separation studies of refractory carbonate fluorite ores for the development and
utilization of such resources. In this study, fluorite flotation tests were performed by using a mixture of tannin and sodium humate as a depressant of calcite. On this basis, the selective
inhibition mechanism of tannin and sodium humate on calcite was studied by means of Zeta potential measurement and FT-IR spectroscopy. MATERIALS AND METHODS MATERIALS The pure mineral
samples of fluorite and calcite were obtained from Guangzhou Mingfa Mineral Specimen Manufacturing Co., LTD. The samples of − 0.075 mm particle size and − 0.075 mm + 0.038 mm particle size
were prepared by hand sorting, crushing, grinding and screening for pure mineral flotation test. − 0.038 mm sample size for infrared spectroscopy and other tests. The multi-element chemical
analysis of pure mineral samples of fluorite and calcite was carried out. The results are shown in Table 1 below. It can be seen from the elemental analysis of Table 1 that the purity of
fluorite and calcite is above 98%, and the purity of fluorite and calcite selected in the test meets the requirements of pure mineral test. The actual fluorite ore was taken from Qingrong
County, Guizhou Province, China. It was crushed and dry ground to − 2.0 mm. The results of chemical multi-element analysis of the samples are shown in Table 2. The results in Table 2 show
that the CaF2 content in the ore is very low. The main components of gangue are CaCO3 and SiO2, whose content is as high as 47.59%, and the content of CaF2 is close. The ratio of CaF2 to
CaCO3 is 2.64, which shows that the ore is insoluble fluorite ore. The study of the mineral composition and the characteristics of the mineral assemblage showed that the main mineral in the
sample was fluorite. The gangue minerals are mainly quartz and calcite, followed by pyrite, gypsum, barite, clay minerals, etc. Sodium humate and tannin were used as flotation inhibitors.
Sodium humate was composed of C9H8Na2O4 and the grade was analytically pure. It was purchased from Shandong Greenwater Chemical Co., LTD. The tannin composition is C76H52O46, the grade is
analytically pure, and it was purchased from Nanhai Jiangshun Chemical Products Factory in Foshan City. FLOTATION TEST The actual flotation test of fluorite ore was carried out at room
temperature. The laboratory-prepared oleic acid (at a concentration of 1 mol/L) is heated and dissolved in water before being added to the flotation operation. Acidified water glass is
configured according to the volume ratio of water glass 4: concentrated sulfuric acid 1: water 40. Other agents are chemical pure products. The actual ore flotation test uses XFDIV1.0 single
cell flotation machine. After the flotation is completed, the floating products and the bottom products of the flotation cell are dried, weighed, calculated the yield and carried out
chemical analysis to obtain the grade and recovery of fluorite. Flotation exploration and rough conditions tests were performed using a mixture of sodium phosphate, sodium fluosilicate,
tannin, sodium humate, tannin and sodium humate. For ease of expression, the mixtures of tannin and sodium humate are referred to as Df01 and Df02, respectively. Based on these tests, the
effect of different types of depressants on flocculentation was tested at the dosage of 100 g/ton of depressant, 1000 g/ton of sodium carbonate, 300 g/ton of water glass, 300 g/ton of oleic
acid and a grinding fineness of − 0.074 mm 75%. SINGLE MINERAL FLOTATION TEST Single mineral flotation tests were carried out at room temperature using an XFGII tank flotation machine with a
speed of 2010 r/min. Take 4 g of sample into the flotation cell for each test, add 40 mL of deionized water and stir for 1 min. Adjust the pH with hydrochloric acid or NaOH solution for 2
min. The concentration of HCl or NaOH used for pH regulation is 1 mol/L. Add the depressant sodium oleate at 2-min intervals. Manually scrape off the foam for 5 min. The product is dried and
weighed. Calculation of mineral recovery by weight of product. ZETA POTENTIAL MEASUREMENTS The Zetasizer 2000 Zeta potential analyzer was used to determine the potential. Grind the sample
with an agate mortar to – 5 μm. Place 50 mg of sample at a time in a small beaker, add deionized water, adjust the pH with HCl or NaOH, stir for 2 min, add the depressant and stir for 10
min. The potential was measured and repeated three times and the average value was taken. FT-IR SPECTROSCOPY The NEXU-670 infrared spectrometer is used for infrared spectral analysis. Before
the IR spectroscopy test, tannin and sodium humate solutions were prepared with deionized water and placed in 250 mL beakers, respectively, and the tannic acid solution was adjusted to
neutral with 2% NaOH. The sample was ground to – 5 μm, added to a certain amount of the prepared solution, stirred for 10 min, let sit for 20 min, and the supernatant was separated. Then add
deionized water and stir, let it stand for a certain time, and separate out the supernatant. Repeat the washing three times. Filtered through a conical funnel and washed three times with
deionized water. The filtered solid was placed in a surface dish and dried naturally. MOLECULAR SIMULATION Molecular simulation mainly involves constructing models at the atomic level of
minerals with the help of computers, and then simulating the structure and motion of mineral molecules to further obtain various physical or chemical properties of the system under study.
Compared to traditional theoretical and experimental studies, molecular simulations have the advantages of low cost, high safety, and the ability to present properties at the microscopic
level. MaterialsStudio (MS) is a materials simulation software developed by BIOVIA. It includes various theoretical methods such as quantum mechanics, molecular mechanics and dynamics and
Monte Carlo, and integrates as many as 23 functional modules such as CASTEP, DMol3, Forcite, and Sorption to enable cross-scale studies from microscopic electronic structure resolution to
macroscopic performance prediction14. The adsorption energy simulations obtained from MS simulations help to understand the adsorption mechanism of tannin and sodium humate molecules in
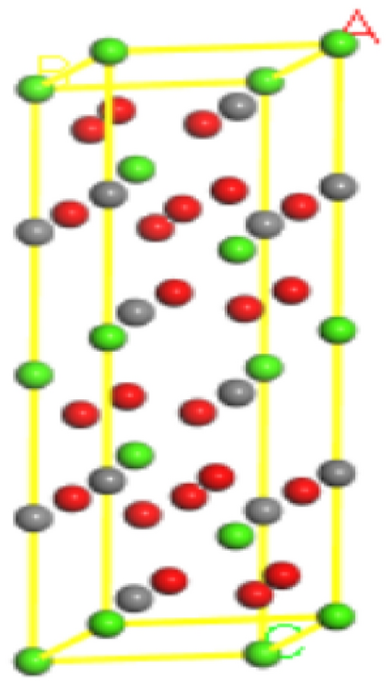
calcite and fluorite, and provide theoretical support for efficient flotation separation of fluorite. CALCULATION OF ADSORPTION ENERGY The crystal modeling of calcite was referred to the
calcite crystal model in the Crystal Structure Database of the American Mineralogist, whose cell optimized prism lengths are a = 7.061 nm, b = 4.990 nm, and c = 4.990 nm, and the inter-prism
angles are α = 90°, β = 60°, and γ = 120°, respectively, and the corresponding cell structures are shown in Fig. 1. After calculations in the Morphology module of Materials Studio (MS)
software, the most stable (1 − 1 2) surface of calcite was selected as the adsorption surface, and the tannin molecules were adsorbed after the steps of faceting, supercell and the
establishment of vacuum layer, and the related energies were calculated. The optimized conformation of tannin and the optimized conformation of sodium humate are shown in Fig. 2, and the
surface model of calcite (1 − 1 2) after structural optimization is shown in Fig. 3. The same calculation method and calculation steps were used to calculate the adsorption energy of
fluorite for tannin adsorption with sodium humate. The crystal modeling of fluorite was referred to the fluorite crystal model in the American Mineralogist Crystal Structure Database, and
its cell was optimized with prism lengths of a = b = c = 5.407 nm and inter-prism angles of α = β = γ = 90, respectively, and the corresponding cell structure is shown in Fig. 4. After the
calculation of Morphology module of Materials Studio (MS) software, the most stable (1 1 1) surface of fluorite was selected as the adsorption surface. The crystal model of fluorite with the
optimized surface model of fluorite (1 1 1) is shown in Fig. 4. The positive or negative adsorption energy can visually represent the stability of the system after adsorption of foreign
impurities. If the adsorption energy is less than zero, it means that the system releases energy when adsorbing foreign impurities and the system is stable after adsorption; if the
adsorption energy is greater than zero, it means that the system absorbs energy when adsorbing foreign impurities and the system is unstable after adsorption15. When it is negative and the
absolute value is larger, the easier the adsorption process is and the correspondingly more stable the system after adsorption16. The adsorption energy is calculated by subtracting the
energy of each part before adsorption from the energy of the system after adsorption. The expression of adsorption energy is as follows: $$ {\text{E}}_{{{\text{ads}}}} = {\text{
E}}_{{{\text{calcite }} + {\text{ tannin}}}} - {\text{E}}_{{{\text{calcite}}}} - {\text{E}}_{{{\text{tannin}}}} , $$ (1) $$ {\text{E}}_{{{\text{ads}}}} = {\text{ E}}_{{{\text{calcite }} +
{\text{ sodium humate}}}} - {\text{ E}}_{{{\text{calcite}}}} - {\text{ E}}_{{{\text{humate}}}} , $$ (2) $$ {\text{E}}_{{{\text{ads}}}} = {\text{ E}}_{{{\text{fluorite }} + {\text{ tannin}}}}
- {\text{E}}_{{{\text{fluorite}}}} - {\text{E}}_{{{\text{tannin}}}} , $$ (3) $$ {\text{E}}_{{{\text{ads}}}} = {\text{ E}}_{{{\text{fluorspar }} + {\text{ sodium humate}}}} - {\text{
E}}_{{{\text{fluorspar}}}} - {\text{ E}}_{{\text{sodium humate}}} , $$ (4) $$ {\text{E}}_{{{\text{ads}}}} = {\text{ E}}_{{{\text{calcite }} + {\text{ sodium humate }} + {\text{ tannin}}}} -
{\text{ E}}_{{{\text{calcite}}}} - {\text{ E}}_{{{\text{tannin}}}} - {\text{ E}}_{{\text{sodium humate}}} , $$ (5) In the formula: Eads is the adsorption energy; Ecalcite + tannin is the
total energy of calcite (1 − 1 2) after surface adsorption of tannin; Ecalcite + sodium humate is the total energy of calcite (1 − 1 2) after surface adsorption of sodium humate; Efluorite +
tannin is the total energy of fluorite (1 1 1) after surface adsorption of tannin; Efluorite + sodium humate is the total energy of fluorite (1 1 1) after surface adsorption of sodium
humate. Ecalcite + sodium humate + tannin is the total energy of calcite (1 − 1 2) after surface adsorption of sodium humate and tannin; Ecalcite is the energy of calcite crystals; Efluorite
is the energy of fluorite crystals; Etannin is the energy of tannin molecules; Esodium humate is the energy of sodium humate molecules. Table 4 shows the adsorption energies of calcite (1 −
1 2) surface and fluorite (1 1 1) surface after adsorption of tannin and sodium humate molecules, respectively, and Table 5 shows the adsorption energies of calcite (1 − 1 2) surface after
adsorption of tannin and sodium humate molecules, respectively, and the adsorption energies of calcite (1 − 1 2) surface after adsorption of both tannin and sodium humate. The optimized
molecular model of calcite (1 − 1 2) surface adsorbed tannin, the optimized molecular model of calcite (1 − 1 2) surface adsorbed sodium humate, the optimized molecular model of fluorite (1
− 1 2) surface adsorbed tannin, the optimized molecular model of fluorite (1 − 1 2) surface adsorbed sodium humate, and the optimized molecular model of calcite (1 − 1 2) surface adsorbed
tannin and sodium humate are shown in Fig. 5. RESULTS FLOTATION TEST * 1. The effect of different types of depressants on flotation. The test flow and flotation agent scheme are shown in
Fig. 6. Some of the test results are shown in Table 3. The results in Table 3 show that the recovery of CaF2 was 91.01% with a grade of 79.82% when a mixed depressant Df01 (1:5 mixture of
tannin and sodium humate) was used for the crude float. The flotation result was the best. When sodium phosphate was used as a depressant, the flotation result was the worst, followed by
sodium fluorosilicate. When tannin or sodium humate was added, the CaF2 grade of fluorite crude concentrate was improved. The recovery of CaF2 was lower when tannin was added and higher when
sodium humate was added, which indicated that the depressant of tannin was stronger than other depressants and the selectivity of sodium humate was better than other depressants. The
depressant effect of the mixture of tannin and sodium humate was the best. the test results of Df01 dosage on the flotation effect are shown in Fig. 7. As can be seen from Fig. 7, the
concentrate grade increases significantly with the increase of Df01 dosage, but it also has a greater impact on the concentrate recovery. Especially when the dosage exceeds 100 g/t, the
recovery rate is greatly reduced. * 2. Closed circuit flotation test. Based on rough condition tests and process tests, closed-circuit tests were conducted. The closed circuit flotation flow
chart and flotation reagent scheme are shown in Fig. 6. The results are shown in Table 4. The results show that the final fluorite concentrate CaF2, CaCO3 and SiO2 content indexes meet the
three grade standards for the chemical requirements of fluorite concentrate. It can be seen that good indicators can be obtained with this process. SINGLE MINERAL FLOTATION TEST The effect
of tannin and sodium humate on the flotation of fluorite and calcite minerals was investigated. From Fig. 8, it can be seen that tannin has a depressant effect on the flotation of fluorite.
This effect increases with increasing pH and with increasing tannin concentration. Due to tannins, the recovery of fluorspar decreased from nearly 80% to less than 60% when the pH was 5.
When the pH is 11, the recovery of fluorspar drops to less than 20%. As can be seen from Fig. 9, the recovery of calcite was less than 20% at around pH 7 under the depressant of tannins. The
recovery increased with increasing pH, reaching the highest value near pH 9. The recovery of calcite decreases linearly when the pH is greater than 9, approaching 0 at around pH 11. As can
be seen in Fig. 10, sodium humate has little effect on the flotation of fluorite. The flotation recovery of fluorite did not vary much with the concentration of sodium humate. It can be seen
from Fig. 11 that sodium humate has a strong depressant effect on the flotation of calcite. In the pH range of 6–10, the recovery of calcite decreased from more than 40% to less than 20%
with the addition of sodium humate. The recovery decreased with the increase of sodium humate concentration. The recovery of calcite was 0 when the tannin concentration was 1 g/L and the pH
value was around 6.5. The above experimental results show that tannins can inhibit both minerals under alkaline conditions with pH values greater than 9. In the pH range of 6–8, tannins
inhibited calcite more than fluorite. Sodium humate has a strong depressant effect on calcite and little depressant effect on fluorite flotation. Therefore, sodium humate has a good
selective depressant effect on calcite. The relationship between the amount of tannin, the amount of sodium humate and the adsorption of fluorite and calcite was investigated at pH 8, and
the results are shown in Fig. 12. The results were that the adsorption capacity of calcite for tannin and sodium humate was greater than that of fluorite, and the adsorption amount increased
with the increase of reagent dosage. However, when the amount of tannins exceeded 0.15 mg/g, the adsorption of tannins on minerals increased very little. At this time, the adsorption amount
of calcite for tannin was about 0.055 mg/g, and that of fluorite was about 0.033 m/g. When the amount of sodium humate exceeded 0.15 mg/g, the adsorption increase rate of sodium humate was
low. At this time, the adsorption amount of calcite to sodium humate was about 0.045 mg/g, and that of fluorite was about 0.022 mg/g. ZETA POTENTIAL MEASUREMENT The effect of tannin and
sodium humate adsorption on the calcite surface charge was investigated by measuring the potential. The results in Fig. 13 show that the zeta potential on the calcite surface is negative
with the addition of sodium humate and tannin, and the negative value increases with the increase of pH. The surface of calcite is negatively charged when the pH value is greater than 11.
After the addition of sodium humate and tannins, the adsorption of sodium humate and tannins significantly reduced the surface potential of the minerals. This is because before the addition
of tannin and sodium humate, the surface of calcite is covered with metal cations such as Ca2+, making the surface of calcite positively charged, after the addition of tannin and sodium
humate, tannin and sodium humate interact with metal cations such as Ca2+ on the surface of calcite, reducing the number of cations on the surface of calcite, making the surface of calcite
negatively charged. Therefore, the adsorption of sodium humate and tannins on calcite surface is chemisorption. FT-IR SPECTROSCOPY Figure 14 shows the IR spectra of calcite, calcite and
tannin adsorbed to tannins. The IR spectra of calcite and tannin at 2505/cm, 875/cm, 710/cm, show a strong peak which is the vibrational peak of –COO17. The IR spectra of calcite adsorbed on
tannins showed a new weak absorption peak at 1200/cm and 1036/cm compared to the IR spectra of calcite. The absorption peak at 1200/cm is the -O-vibration peak formed by the adsorption of
calcite by tannins, while 1036/cm is the C–H vibration peak formed by the adsorption of calcite by tannins. Figure 15 shows the IR spectra of calcite, calcite and sodium humate with adsorbed
sodium humate. The IR spectra of sodium humate are at 1026/cm, 1373/cm, 1577/cm, and 3423/cm with C–O–C, C–N, C=C, and –OH stretching vibrational peaks, respectively18,19. Therefore,
aromatic compounds such as –OH, –NH2, COOH and C–O–C were found in sodium humate. The IR spectra of calcite adsorbed by sodium humate compared to the IR spectra of calcite showed strong
absorption peaks due to calcite. However, there is a new weak absorption peak at 1028/cm, which may be an asymmetric peak of the ether group (C–O–C) in sodium humate. MOLECULAR SIMULATION As
shown in Table 5, calcite adsorbed tannin and sodium humate with larger adsorption energy, and the adsorbed tannin and sodium humate were not easily detached, and the adsorption state was
more stable; on the contrary, fluorite adsorbed tannin and sodium humate with smaller adsorption energy, and the adsorbed tannin and sodium humate were easily detached, and the adsorption
state was not stable. Calcite is more easily adsorbed on the calcite surface under the same conditions as fluorite, both tannin and sodium humate, making calcite more hydrophilic. It is
easier to selectively separate calcite and fluorite in the flotation process. It can be seen from Table 6 that the absolute values of adsorption energy of calcite adsorbing tannin and sodium
humate at the same time are higher than those of calcite adsorbing tannin alone and calcite adsorbing sodium humate alone, and the state of calcite adsorbing tannin and sodium humate
existing at the same time is relatively more stable, so the effect of tannin and sodium humate synergistically inhibiting calcite is better than the effect of tannin and sodium humate
inhibiting calcite alone. DISCUSSION Sodium humate is mainly composed of C, O and a small amount of H, N and S. It is an amorphous macromolecular compound. Sodium humate has a large number
of functional groups such as carboxyl, phenolic hydroxyl, alcohol hydroxyl, hydroxyquinone, amino, and sulfonic acid groups20,21. Ellagic acids are molecules with large amorphous substances,
and tannins have a large number of polar groups in their molecular structure, mainly –O and –COOH22. Among them, the molecular structure of tannins is shown in Fig. 16. From previous
studies, it was shown that calcite has a greater adsorption capacity for tannin and sodium humate than fluorite at pH = 8. This is the reason why sodium humate and tannin can be used as
depressants for fluorite flotation of calcite. Similarly, the above-mentioned study showed that sodium humate and tannins were chemisorbed on the calcite surface. The PZCs of calcite and
fluorite were 9.7 and 11, respectively. When pH = 8, both minerals are positively charged because the anions in both minerals are preferentially dissolved. Mineral surfaces have exposed Ca
protons with which polar groups such as carboxyl and hydroxyl groups in the structure of sodium humates and tannins bind, complex or chelate, thus adsorbing to the mineral surface. Other
polar groups not adsorbed with the mineral interact with water molecules, which makes calcite more hydrophilic and prevents the mineral surface from interacting with oleic acid. The model of
tannin adsorption on calcite is shown in Fig. 1722. Since calcite dissolves faster than fluorite and has more Ca protons on its surface than fluorite surface, the adsorption capacity of
calcite surface for sodium humate and tannin is greater than that of fluorite. Also, the calcium ions dissolved from calcite are much larger than fluorite, and these ions are also chelated
with sodium humate and tannic acid to form organic salts. The chemical reaction between tannins and calcium ions is shown in Fig. 1823. These organic salts adsorb on the organic compounds on
the surface of calcite, forming multiple layers of adsorption on the surface of calcite. The presence of a large number of polar functional groups in these adsorption products further makes
the surface of calcite minerals hydrophilic and hinders the adsorption of the trapping agent on calcite, thus inhibiting the flotation of calcite. The multilayer adsorption model of calcite
surface is shown in Fig. 19. CONCLUSION * 1. Under weak alkaline conditions, tannin and sodium humate compounds have the best depressant effect on calcite. The optimal flotation conditions
for the refractory fluorite ore in Guizhou were determined through experiments. The fineness of grinding is − 0.074 mm, accounting for 75%, the amount of sodium carbonate is 1000 g/t, the
amount of water glass is 300 g/t, the amount of Df01 is 100 g/t, and the amount of oleic acid is 300 g/t. Through the flotation process of primary roughing, primary sweeping and four-stage
selection of fluorite ore, a qualified fluorite concentrate with CaF2 grade of 95.52% and recovery of 91.20% was obtained, meeting the demand for chemical tertiary fluorite concentrate. * 2.
Sodium humate has a strong inhibiting ability to calcite, while it has a weak inhibiting effect on fluorite flotation. Sodium humate has a good selective depressant effect on calcite.
Tannin has a strong depressant effect on both minerals under alkaline conditions at pH values greater than 9. In the pH range of 6–8, it is stronger than fluorite for calcite. * 3. Sodium
humate and tannins are chemisorbed on the surface of calcite. The adsorption capacity of tannin and sodium humate on calcite was greater than that of fluorite at pH 8. Adsorption of the
reagents resulted in a negative surface dynamic potential for calcite and fluorite, and the negative value increased with increasing pH. After the adsorption of tannins on calcite, new
chemical bonds with –O- and C–H appear on the calcite surface. After calcite adsorption, a new absorption peak also appeared on the calcite surface. * 4. Polar groups in the molecules of
sodium humate and tannins, such as carboxyl and hydroxyl groups, bind, complex or chelate with calcium ions on the surface of calcite. At the same time, sodium humate and tannins react with
calcium ions in solution to produce organic calcium salts. These calcium salts interact with organic compounds adsorbed on the calcite surface, forming multiple layers of adsorption on the
calcite surface, thus making the calcite more hydrophilic and hindering the adsorption of the trapping agent. * 5. Based on density flooding theory, Materials Studio (MS) software was used
to calculate the adsorption energy associated between these molecules of calcite, fluorite, tannin, and sodium humate, and the results were obtained as follows: (a) Tannin molecules and
sodium humate molecules are more easily adsorbed on the surface of calcite compared to fluorite. (b) Compared to calcite adsorption of tannin molecules or sodium humate molecules alone,
simultaneous adsorption of both states would be more stable, and tannin and sodium humate synergistically inhibited calcite better than alone. DATA AVAILABILITY All data generated or
analyzed during this study are included in this published article. REFERENCES * Li, L. _et al._ Fluorite ore beneficiation technology progress in China. _J. Miner. Resourc. Protect. Utiliz._
6, 46–53. https://doi.org/10.13779/j.carol_carroll_nki (2015). Article CAS Google Scholar * Song, Q. _et al._ Guizhou a calcite type fluorite ore flotation experiment study. _J. Chem.
Miner. Process._ 46(6), 10–21. https://doi.org/10.16283/j.carolcarrollnkihgkwyjg (2017). Article Google Scholar * Yin, W. _et al._ Beneficiation test of Pingquan calcite fluorite ore.
_Min. Metall._ 01, 1–4 (2008). Google Scholar * Kienko, L. A. _et al._ Lower temperature flotation of carbonate-fluorite ores. _J. Miner. Sci._ 46, 317–323.
https://doi.org/10.1007/s10913-010-0039-0 (2010). Article Google Scholar * Wenbo, Z., Josue, M., Roberto, T., Hector, V. & Shaoxian, S. Flotation of fluorite from ores by using
acidized water glass as depressant. _Miner. Eng._ 45, 142–145 (2013). Article Google Scholar * Zhang, W. _et al._ Study on flotation process of a carbonate fluorite ore. _Nonferr. Metals
(Miner. Process. Sect.)_ 04, 48–52 (2014). Google Scholar * Huang, L., Ge, Y. & Xiong, X. Flotation test of high calcium magnesium fluorite. _Nonferr. Metals_ 36(04), 32–34 (2013). CAS
Google Scholar * Xiaobo, Z. _et al._ Effect of a CA depressant on flotation separation of celestite from fluorite and calcite using SDS as a collector. _Miner. Eng._ 111, 201–208.
https://doi.org/10.1016/j.mineng (2017). Article Google Scholar * Zhang, Y. _et al._ Flotation separation of scheelite from fluorite using sodium polyacrylate as inhibitor. _Minerals_ 7,
102. https://doi.org/10.3390/min7060102 (2017). Article CAS ADS Google Scholar * Gao, Z., Gao, Y., Zhu, Y., Hu, Y. & Sun, W. Selective flotation of calcite from fluorite: A novel
reagent schedule. _Minerals_ 6, 114. https://doi.org/10.3390/min6040114 (2016). Article CAS ADS Google Scholar * Ren, Z. _et al._ Selective separation of fluorite, barite and calcite
with valonea extract and sodium fluosilicate as depressants. _Minerals_ 7, 24. https://doi.org/10.3390/min7020024 (2017). Article CAS ADS Google Scholar * Zhiyong, G. _et al._ Selective
flotation of scheelite from calcite using a novel reagent scheme. _Mineral Process. Extract. Metall. Rev._ 43(2), 137–149. https://doi.org/10.1080/08827508.2020.1825956 (2022). Article CAS
Google Scholar * Chen, C. Effect of inorganic anions on flotation behavior of three typical calcium-containing salts and its mechanism. _Central South Univ._ 2011, 742 (2011). ADS Google
Scholar * Wang, X. _et al._ Application of materials studio to gas adsorption in metal-organic frame materials. _Modern Chem. Ind._ 9(11), 34–43.
https://doi.org/10.16606/j.carol_carroll_nki (2021). Article Google Scholar * Fei, S. _et al._ Effect of N/Rh co-doped rutile TiO_2 surface on optical gas sensing characteristics of CO
gas. _Chin. J. Lasers_ 46(11), 214–221 (2019). Google Scholar * Hao, Y. _et al._ Molecular simulation of activated hydroxyl group adsorbed by methanol on Zn-modified Sr/γ-Al_2O_3 surface.
_Trans. Chin. Soc. Agric. Eng._ 38(09), 253–260 (2012). Google Scholar * Lan, P. Extraction of condensed tannin from grape residue and preparation of tannyl adhesive. _Nanjing Forestry
Univ._ 2013, 859 (2013). Google Scholar * Tong, Y., Zhang, X. & Su, X. Infrared spectroscopy and thermogravimetric analysis study of humic acid. _J. Modified Urea-formaldehyde Resin
Adhesive China_ 9, 32–36. https://doi.org/10.13416/j.carolcarrolla (2010). Article Google Scholar * Li, H. J. Study on extraction and characterization of humic acid and fulvic acid.
_Huazhong Univ. Sci. Technol._ 2012, 14 (2012). Google Scholar * McDonnell, R. _et al._ Characterization of humic substances in heathland and forested peat soils of the Wicklow mountains.
_Biol. Env. Proc. R. Irish Acad._ 101(3), 187–197 (2001). Google Scholar * Lin, S. _et al._ Oxidative depression of arsenopyrite by using calcium hypochlorite and sodium humate. _Minerals_
8, 463. https://doi.org/10.3390/min8100463 (2018). Article CAS ADS Google Scholar * Zhu, Y. S. & Zhu, J. G. _Chemical Principle of Flotation Reagent_ (Central South University of
Technology Press, 1987). Google Scholar * Cao, M. L. Simulation study on the interaction mechanism between tannins and calcium-containing minerals. _Nonferrous Metals Miner. Process. Sect._
6, 33–35 (1996). Google Scholar Download references ACKNOWLEDGEMENTS This study did not receive any specific funding from funding agencies in the public, commercial or non-profit sectors.
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * College of Mining, Guizhou University, Guiyang, 220025, China Zhi-xiong Zhu, Guang-Hua Nie, Yun Tang, Ying Jiang, Biyang Tuo & Jiaxin Li *
National & Local Joint Laboratory of Engineering for Effective Utilization of 3 Regional Mineral Resources from Karst Areas, Guiyang, 220025, China Zhi-xiong Zhu, Guang-Hua Nie, Yun
Tang, Ying Jiang, Biyang Tuo & Jiaxin Li * Guizhou Key Laboratory of Comprehensive Utilization of Nonmetallic Mineral Resources, Guiyang, 220025, China Zhi-xiong Zhu, Guang-Hua Nie, Yun
Tang, Ying Jiang, Biyang Tuo & Jiaxin Li * College of Mining, Guizhou University, Guiyang, 550025, China Guang-Hua Nie Authors * Zhi-xiong Zhu View author publications You can also
search for this author inPubMed Google Scholar * Guang-Hua Nie View author publications You can also search for this author inPubMed Google Scholar * Yun Tang View author publications You
can also search for this author inPubMed Google Scholar * Ying Jiang View author publications You can also search for this author inPubMed Google Scholar * Biyang Tuo View author
publications You can also search for this author inPubMed Google Scholar * Jiaxin Li View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Z.Z.
prepared the main manuscript text, G.N. directed the writing of the manuscript, T.Y., Y.J., B.T., J.L. assisted experiment. All the authors reviewed the manuscript. CORRESPONDING AUTHOR
Correspondence to Guang-Hua Nie. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains
neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the
article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your
intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence,
visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhu, Zx., Nie, GH., Tang, Y. _et al._ Study on synergistic inhibition and
mechanism of flotation separation of fluorite and calcite by tannin and sodium humate. _Sci Rep_ 14, 381 (2024). https://doi.org/10.1038/s41598-023-50233-x Download citation * Received: 07
July 2023 * Accepted: 17 December 2023 * Published: 03 January 2024 * DOI: https://doi.org/10.1038/s41598-023-50233-x SHARE THIS ARTICLE Anyone you share the following link with will be able
to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative









