- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Brain organoids, which are three-dimensional cell culture models, have the ability to mimic certain structural and functional aspects of the human brain. However, creating these
organoids can be a complicated and difficult process due to various technological hurdles. This study presents a method for effectively generating cerebral organoids from human induced
pluripotent stem cells (hiPSCs) using electromagnetic gold nanoparticles (AuNPs). By exposing mature cerebral organoids to magnetized AuNPs, we were able to cultivate them in less than 3
weeks. The initial differentiation and neural induction of the neurosphere occurred within the first week, followed by maturation, including regional patterning and the formation of complex
networks, during the subsequent 2 weeks under the influence of magnetized AuNPs. Furthermore, we observed a significant enhancement in neurogenic maturation in the brain organoids, as
evidenced by increased histone acetylation in the presence of electromagnetic AuNPs. Consequently, electromagnetic AuNPs offer a promising in vitro system for efficiently generating more
advanced human brain organoids that closely resemble the complexity of the human brain. SIMILAR CONTENT BEING VIEWED BY OTHERS MICROFLUIDIC DEVICE WITH BRAIN EXTRACELLULAR MATRIX PROMOTES
STRUCTURAL AND FUNCTIONAL MATURATION OF HUMAN BRAIN ORGANOIDS Article Open access 05 August 2021 SPATIO-TEMPORAL DYNAMICS ENHANCE CELLULAR DIVERSITY, NEURONAL FUNCTION AND FURTHER MATURATION
OF HUMAN CEREBRAL ORGANOIDS Article Open access 14 February 2023 NOVEL MODEL OF CORTICAL–MENINGEAL ORGANOID CO-CULTURE SYSTEM IMPROVES HUMAN CORTICAL BRAIN ORGANOID CYTOARCHITECTURE Article
Open access 14 May 2023 INTRODUCTION Brain organoids offer a remarkable opportunity to closely mimic the complexity of the human brain and study its development, model diseases, and screen
potential drugs1,2. These organoids are typically derived from pluripotent stem cells that are guided to differentiate into various types of brain cells, including neurons and glial cells.
Through self-organization, these cells form intricate networks that resemble the developing human brain3,4. Consequently, brain organoids have emerged as a valuable tool for investigating
brain development, disease mechanisms, and drug responses. For instance, researchers have developed cerebral and midbrain organoids to overcome the challenges associated with limited access
to and complexity of human brain tissues5,6,7. Furthermore, the application of neurological disease organoid models, such as those for Alzheimer's, Parkinson's, Autism, and
Huntington's disease, has significantly contributed to our understanding of these conditions within a three-dimensional environment that closely resembles the human
brain8,9,10,11,12,13,14,15. Thus, brain organoids hold immense promise for comprehending brain development, diseases, and serve as a valuable platform for disease modeling, drug screening,
and translational medicine. However, generating mature brain organoids is a time-consuming process involving multiple steps, including three-dimensional differentiation and maturation. The
efficiency of producing fully developed brain organoids is significantly low, with many organoids failing to reach complete maturation or failing to develop altogether. Recent pioneering
efforts have aimed to address these limitations and enhance the efficiency and reproducibility of brain organoid generation16,17,18. Notably, the utilization of the air–liquid interface
system in the organoid culture has demonstrated improvements in neuronal survival and axon outgrowth within cerebral organoids19. Additionally, the development of brain organoid-on-a-chip
technology has provided perfusable microfluidic platforms with multicellular architectures, facilitating the organization and neural differentiation of mature brain organoids20. Despite
these significant advancements, generating brain organoids that faithfully replicate the characteristics of the human brain remains an ongoing challenge. The time-intensive nature of the
process and the need for maturation present major obstacles to the broader application of brain organoids in research and clinical settings. Previously, our studies have demonstrated the
remarkable potential of extremely low-frequency electromagnetic fields (EL-EMF) or magnetized gold nanoparticles in neuronal fate conversion and direct neuronal reprogramming, significantly
enhancing the efficiency of these processes21,22. In these studies, we observed a robust activation of the histone acetyltransferase, Brd2, resulting in the acetylation of histones H4K12 and
H3K27 at the promoter regions of neuronal-specific genes. This activation ultimately improved the efficiency of neuronal reprogramming21. Moreover, another study involving electromagnetized
AuNP stimulation in the hippocampus of EMF-exposed mice demonstrated increased histone acetylation, leading to the activation of adult neural stem cells and enhanced neurogenesis in an aged
mouse model22. These findings highlight the potential of magnetized AuNPs as a promising strategy for improving neuronal cell fate determination and its implications in regenerative
medicine. In this study, we present a highly efficient approach for generating mature 3D brain organoids by employing magnetized AuNPs. Our findings reveal a remarkable maturation of brain
organoids within a mere 3-week period following treatment with magnetized AuNPs. Notably, these organoids closely resemble brain organoids cultured for 12 weeks using conventional methods.
Our results demonstrate that magnetized AuNPs significantly enhance the efficiency and reproducibility of brain organoid generation, primarily through the upregulation of histone acetylation
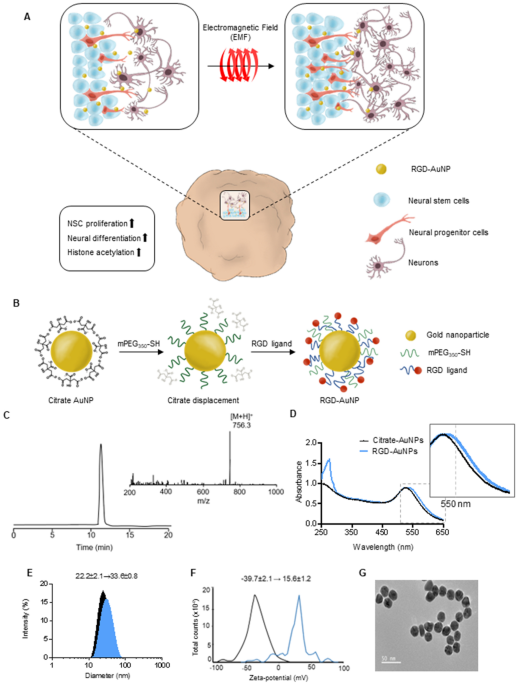
(Fig. 1A). Consequently, our study highlights the exceptional advantages of electromagnetic stimulation using AuNPs exposed to EL-EMF, as it offers scalability, reproducibility, and
superior efficiency in generating organoids that closely emulate the intricate structure and function of the human brain. RESULTS AND DISCUSSION PREPARATION OF MAGNETIZED AUNPS FOR INDUCING
BRAIN ORGANOIDS Cerebral organoids, representing a complex 3D cell culture model that partially recapitulates the characteristics of the human cerebral brain, hold immense potential for
various applications18. To improve the generation efficiency of cerebral organoids using electromagnetized AuNPs, we employed a two-step preparation process to create magnetizable RGD
(arginine-glycine-aspartic acid)-conjugated AuNPs (Fig. 1B). We chose to coat the AuNPs with RGD, which is a well-known binding motif found in fibronectins. This is driven by the fact that
AuNPs have a limited surface area, necessitating surface modification to achieve a high density of tightly binding ligands, such as RGD23. This modification is crucial to enable efficient
and specific coating for neuronal applications24,25,26. Following the reports from previous studies, we modified the AuNPs by incorporating thiol-containing ligands, mercapto
(methoxypolyethylene glycol and mPEF350-SH), and RGD peptides. This modification aimed to induce magnetic polarization and enhance cell adhesion to the AuNP substrates (Fig. 1C)27. To assess
the properties of RGD-conjugated AuNPs, we employed UV–Vis spectroscopy in conjunction with dynamic light scattering and ζ-potential measurements. The absorption spectrum of the
RGD-conjugated AuNPs exhibited a shift towards 532 nm compared to the citrate-conjugated AuNPs (Fig. 1D). Additionally, the hydrodynamic radii of the AuNPs increased from 22.2 ± 2.1 nm to
33.6 ± 0.8 nm, and the ζ-potential shifted from − 39.7 ± 2.1 mV to 15.6 ± 1.2 mV upon the introduction of RGD-containing peptides (Fig. 1E,F). Field emission scanning electron microscopy
(FE-SEM) analysis confirmed the well-dispersed distribution of the RGD-AuNPs (Fig. 1G). To investigate the potential of magnetized AuNPs in promoting the generation of cerebral organoids, we
developed a modified protocol based on the self-organizing principles of human induced pluripotent stem cell (iPSC) cultures from previous studies1,13. Initially, a single iPSC-derived
embryonic body was mixed with RDG-conjugated AuNPs at a concentration of 8 × 1012 nanoparticles/mL in Matrigel, forming a three-dimensional structure (Fig. 2A). Based on previous research
indicating the neurogenic potential of EL-EMF exposures with a frequency of 60 Hz and an intensity of 1 mT, the 3D aggregated cultures were treated with epidermal growth factor (EGF) and
fibroblast growth factor-8 (FGF8) under the same EMF condition for 5 days to promote the patterning of neuroectodermal fate. Subsequently, to induce the maturation of the cerebral organoids,
the AuNP-treated organoids were exposed to the same EMF condition for additional 2 weeks in the presence of brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor
(GDNF), and ascorbic acid (Fig. 2A). Remarkably, our observations revealed a significant increase in PAX6 + NESTIN + neural stem cells within the electromagnetized AuNP-treated group,
indicating a significant enhancement of neurogenesis in the cerebral organoids after 2 weeks of exposure to EMF-exposed AuNPs (Fig. 2B–G). However, we did observe a slight increase in size
compared to the EMF or AuNPs-only control group (Fig. S1A). Considering a previous study by Fayol et al. that reported EMF exposure-induced cell condensation and volume reduction during
chondrogenic differentiation of stem cells28, we further confirmed cell proliferation within the electromagnetized AuNP brain organoids. Significantly increased Ki67-positive cells in the
magnetized AuNP brain organoids suggest that EMF stimulation contributes to the augmented size of the AuNP organoids primarily through the activation of neuronal proliferation (Fig. S1B, C).
Moreover, a cell viability assay was conducted to evaluate the cytotoxicity of AuNPs in the cerebral organoids, revealing no significant differences between the control group and the
magnetized AuNPs-treated organoids (Fig. S1D). PROMOTION OF CEREBRAL ORGANOID MATURATION BY ELECTROMAGNETIZED AUNPS To assess the impact of electromagnetized AuNPs on the functional
maturation of cerebral organoids, we proceeded to examine the expression of marker genes associated with the maturation process. Initially, we observed a significant increase in the
population of DCX+, TUJ1+, and MAP2+ neuronal cells in the brain organoids exposed to EMF for 2 weeks (Fig. 3A,B). Moreover, EMF-exposed AuNP organoids exhibited a substantial rise in
VGLUT1+ mature glutamatergic neurons and CTIP2+ cortical-layer neurons (Fig. 3A,B, Fig. S1E). Consistently, RT-PCR analysis of markers for dorsal pallial (CTIP2 and SATB2), cholinergic
(ChAT), and GABAergic (GAD67) neurons demonstrated significant increases in the brain organoids subjected to EMF, indicating the effective maturation of the organoids facilitated by
magnetized AuNPs (Fig. 3C,D, Fig. S1F). Furthermore, mature neuronal markers including NEFL, MAPT, Synapsin1, and MAP2 exhibited significant upregulation after 3 weeks of EMF treatment in
the cerebral organoids (Fig. 3E). Notably, the expression levels of these mature marker genes in brain organoids treated with magnetized AuNPs for 2 weeks closely resembled those in the
control brain organoids cultured for 8 weeks using conventional methods (Fig. 3E). Moreover, the expression levels of these mature marker genes were significantly increased in brain
organoids treated with magnetized AuNPs for 7 weeks compared to the control brain organoids cultured for 8 weeks using conventional methods (Fig. 3E). To confirm the functional maturation of
the brain organoids, we employed the human synapsin 1 (hSYN1) gene promoter to drive neuronal synapse-specific expression of a red fluorescent protein (RFP) in functional neurons29.
Consistently, we observed a significant increase in the number of RFP-positive cells in the EMF-exposed AuNP brain organoids (Fig. 3F,G), indicating that electromagnetized AuNPs facilitate
efficient functional maturation in 3D cerebral organoids. MECHANISM OF MAGNETIZED AUNPS-MEDIATED BRAIN ORGANOGENESIS To elucidate the mechanism underlying magnetized AuNPs-mediated brain
organogenesis, we investigated the potential activation of specific histone modifiers during the development of cerebral organoids. Our analysis revealed a significant upregulation of the
lysine acetyltransferase 2B (KAT2B) gene, known for its preferential acetylation of histone H3, in the magnetized AuNP-treated organoids exposed to electromagnetic fields (EMF) (Fig. 4A).
However, no noticeable differences were observed in the expression of other epigenetic modifiers (Fig. 4A). In line with these findings, we observed a marked increase in the population of
MAP2+ and KAT2B+ cells in the AuNP-treated organoids under EMF exposure conditions (Fig. 4B,C). Furthermore, semiquantitative Western blotting demonstrated higher levels of KMT2B in the
EMF-exposed AuNP organoids compared to the control organoids at days 7 and 14 (Fig. 4D). To further investigate the functional implications, we performed chromatin immunoprecipitation
(ChIP)-qPCR assays to assess the binding of KAT2B to the promoter regions of neural-specific genes. Interestingly, we found a significant enrichment of KAT2B occupancy at the promoter
regions of SLC2A1 and MT3 in the EMF-exposed AuNP organoids (Fig. 4E,F). Collectively, these findings suggest that electromagnetic stimulation facilitated by AuNPs promotes the
three-dimensional differentiation of cerebral organoids by modulating the activity of KMT2B and its impact on the accessibility of neural-specific genes. These results imply that histone
acetylation may mediate the process of EMF-stimulated AuNP-induced brain organogenesis. H3K9 ACETYLATION PLAYS A CRUCIAL ROLE IN ELECTROMAGNETIZED AUNPS-INDUCED BRAIN ORGANOGENESIS KAT2B is
an acetyltransferase enzyme responsible for H3K9 acetylation (H3K9ac) during neural lineage differentiation30,31. To investigate the involvement of H3K9ac in brain organoid development
induced by electromagnetized AuNPs, we examined the levels of H3K9ac in cerebral organoids. Remarkably, we found that electromagnetized AuNPs significantly increased the number of
H3K9ac-expressing cells in the brain organoids (Fig. 5A,B). Additionally, the relative intensity of H3K9ac-expressing cells was significantly higher in AuNP-treated organoids exposed to EMF
(Fig. 5C). This increase in H3K9ac persisted at days 7 and 14 (Fig. S2A), and even at 21 days after exposure to the EMF/AuNP conditions (Fig. 5D,E). Notably, the relative intensities of
H3K27me3 and H3K4me3, which are established as key epigenetic markers for repressive and active regulation of neurogenic genes, respectively, did not show significant differences after
exposure to electromagnetized AuNPs (Fig. 5E). These findings suggest that EMF stimulation in conjunction with AuNPs contributed specifically to increased histone acetylation in the 3D brain
organoids. Furthermore, we examined the expression of oncogenes, DNA damage marker, and inflammatory marker such as Brca1, Parp1, and Cox2 in magnetized 3D brain organoids32,33. However, we
did not observe significant differences in the relative expression of these genes following exposure to electromagnetized AuNPs (Fig. S2B). To further investigate the functional role of
KAT2B in electromagnetized AuNP-treated brain organoids under EMF stimulation, we employed a shRNA lentivirus to knockdown KAT2B expression. Western blot analysis confirmed the differential
expression of KAT2B upon knockdown (Fig. S3A,B). Notably, the observed increase in H3K9ac levels under electromagnetized AuNP conditions induced by EMF was significantly diminished in the 3D
organoids following KAT2B knockdown (Fig. S3C). Moreover, we observed a significant reduction in the population of NESTIN- and KI67-positive cells in the electromagnetized AuNP organoids
upon KAT2B knockdown (Fig. 5F–H). Consistently, the numbers of NESTIN+ and SOX2+ cells were significantly decreased in the electromagnetized AuNP organoids treated with KAT2B shRNA (Fig.
5I,K). Similarly, inhibition of KAT2B led to a significant decrease in the number of MAP2− and TUJ1-positive neurons in the electromagnetized AuNP organoids (Fig. 5J,L). These results
suggest that electromagnetized AuNPs functionally activate the development of 3D organoids through the involvement of KAT2B, which plays a critical role in the regulation of neural-specific
loci under EMF exposure conditions. CONCLUSIONS Brain organoids offer a valuable opportunity to model the unique characteristics of brain development and diseases. However, existing
protocols for generating brain organoids have limitations, including slow maturation and inconsistent results. In this study, we present a significant advancement by demonstrating that
EL-EMF mediated magnetized AuNPs greatly enhance the efficiency of neuronal differentiation and maturation in cerebral organoids compared to conventional methods. Extremely low-frequency
electromagnetic fields (EL-EMF) have distinct advantages over static fields or other magnetic treatments when it comes to influencing cellular responses. Firstly, EL-EMF induces dynamic
changes in the electromagnetic environment, providing a versatile and nuanced modulation of cellular responses compared to static fields. Secondly, EL-EMF can penetrate tissues more
effectively, allowing for deeper interaction with cells and tissues, making it suitable for studying complex cellular systems and three-dimensional tissue constructs34. Additionally, EL-EMF
interacts with cellular components and biomolecules, triggering intracellular signaling pathways and molecular events that lead to diverse cellular responses35. In contrast, static fields or
other magnetic treatments may lack the specificity and effectiveness in modulating these complex cellular processes36,37. These unique characteristics of EL-EMF make it a crucial tool for
investigating and manipulating cellular responses in various biological contexts. Furthermore, we have discovered the involvement of a histone modifier, KAT2B, which responds to the
stimulation of AuNPs under EMF exposure. This finding suggests that effective generation of mature brain-like structures is achieved through epigenetic histone acetylation. However, the
precise mechanisms by which electromagnetic fields (EMF) influence neuronal migration and proliferation are not fully understood. Nonetheless, it is clear that EMF-induced KAT2B-mediated
histone acetylation directly influences neuronal proliferation and migration. Our experimental results consistently demonstrate the binding of KAT2B to the promoter regions of
neural-specific genes during EMF-induced 3D brain organogenesis. These genes play a crucial role in promoting neural development, resulting in an enlargement of the organoid diameter and an
upregulation of neural stem cell gene expression in response to EMF treatment. Taken together, our findings provide a powerful platform to investigate the mechanisms underlying brain
development and diseases, as well as to validate robust tools for developing medicines. MATERIALS AND METHODS AUNP SYNTHESIS AND PREPARATION OF RGD-AUNPS Following the previous methods, the
CYGRGDS peptide was synthesized according to the standard 9-fluorenylmethoxycarbonyl-based solid phase peptide synthesis protocol, on a Rink amide AM resin (0.1 mmol/g). In addition, 20 nm
AuNPs were also prepared following a standard protocol38,39. Briefly, an aqueous solution of 0.3 mM HAuCl4·3H2O was brought to a boil with vigorous stirring, and a 10 mM sodium citrate
solution was added. After boiling for 7 min, the heating resource was removed, and the colloid was stirred for another 15 min. The AuNP solution was characterized by UV–Vis spectroscopy and
an absorbance maximum at 520 nm were obtained. The size and surface charge of the AuNPs was measured using dynamic light scattering. Arginine-glycine-aspartate (RGD)-conjugated AuNPs were
obtained from citrate-coated AuNPs through a modified place exchange reaction with thiol ligands40. Briefly, mPEG350-SH solution (10 mM, 0.1 mL) was added to the citrate-coated AuNP solution
(28.3 μg/mL, 2 mL) and gently mixed. After incubating at 4 °C for 24 h, the supernatant was removed by centrifugation and the RGD peptide (0.5 mM, 1 mL) was mixed with the PEG-coated AuNPs.
After 24 h treatment, the modified AuNPs were washed with ddH2O and resuspended in ddH2O. Arginine-glycine-aspartate (RGD)-conjugated AuNPs were characterized by UV–Vis spectroscopy,
dynamic light scattering, and ζ-potential measurement. LENTIVIRUS GENERATION AND VIRAL INFECTION _KAT2B_ shRNA (Target sequence: GCAGATACCAAACAAGTTTAT) was purchased from Applied Biological
Materials Inc. Lentivirus production was prepared as described previously41. Briefly, the lentivirus was produced in HEK293T cells grown in Dulbecco’s Modified Eagle Medium containing 10%
fetal bovine serum and 1% penicillin/streptomycin. Afterward, the cells were transfected with the lentivirus construct, _KAT2B_ shRNA, and packaging plasmid, psPAX2, and pMD2.G vectors using
calcium phosphate co-precipitation. One day after transfection, the medium was changed, and the viruses were harvested 48–72 h later. After approximately 24 h EMF exposure, the embedded
AuNP brain organoids with Matrigel were infected with the lentivirus, _KAT2B_ shRNA, twice in 2 days. GENERATION OF ELECTROMAGNETIZED AUNP-HUMAN CEREBRAL ORGANOIDS Human control fibroblasts
(GM23967, Male, 52YR) were purchased from the Coriell Cell Repository and cultured in human cell culture medium containing Dulbecco’s Modified Eagle Medium, 10% fetal bovine serum, 1%
nonessential amino acids (Gibco), 0.1% β-mercaptoethanol (Gibco), and 1% penicillin/streptomycin (Gibco). To generate hiPSCs from fibroblasts, the cells were infected with a lentivirus
construct harboring Oct4, Sox2, C-myc, and Klf4, twice over 2 days. The hiPSC lines were cultured in StemMACS iPS-Brew XF (Miltenyi Biotec, 130-104-368), containing StemMACS Supplement
(Miltenyi Biotec) on Matrigel Matrix (Corning, #354234) coated plates at 37 °C and 5% CO2. The hiPSCs were singularized using Accutase (Stemcell Technologies, #07920) to induce their
dissociation into single cells, while the EBs were maintained in a human embryonic stem cell growth medium without a ROCK inhibitor for 3 days. A single embryoid body (EB) measuring 450–500
µm in diameter was transferred to pre-warmed 6-well tissue culture plates containing 5 µL of Human ESC medium supplemented with 1 µL of RGD-AuNPs nanoparticles (at a concentration of 8 ×
1012 nanoparticles/mL). To prepared electromagnetized AuNP-human cerebral organoids, the EB-AuNPs mixture was embedded in a 10 µL Matrigel matrix and allowed to polymerize for 10 min at room
temperature. The EBs conjugated with the RGD-AuNPs were maintained in an incubator at 37 °C and 5% CO2. Human embryonic stem cell growth medium was replaced with neural stem cell medium
(Advanced DMEM/F12 and neurobasal medium, 1 × N2, 1 × B27, 5% Albumax-I, 2 mM GlutaMAX, 0.1 mM beta-mercaptoethanol, 3 µM CHIR99021, 0.5 µM A83-01, and 10 ng/mL LIF) containing fibroblast
growth factor-2 (20 ng/mL) and epidermal growth factor (20 ng/mL) for 5 days. To promote organoid patterning, aggregated cells were cultured in a neural stem cell medium containing 200 nM
ascorbic acid and 20 ng/mL brain-derived neurotrophic factor for 1 week. To promote organoid maturation, organoids were cultured in cerebral organoid differentiation medium (Advanced
DMEM/F12 and neurobasal medium, 1 × N2, 1 × B27, 5% Albumax-I, 2 mM GlutaMAX, 0.1 mM beta-mercaptoethanol) containing 200 nM ascorbic acid, 20 ng/mL brain-derived neurotrophic factor, and 20
ng/mL glial cell-derived neurotrophic factor from day 12 to day 21. The culture medium was changed every three days, and the organoids were maintained on an orbital shaker (PSU-10i, Biosan)
in an incubator at 37 °C and 5% CO2. FLOW CYTOMETRY The organoids were dissociated using papain for 15 min, with pipetting every 5 min to facilitate cell dissociation. Dissociated organoids
were washed with 1 × DPBS (Gibco, 14190-144), then, filtered using a cell strainer (SPL, 352340) to remove cell debris. The remaining cells were resuspended in 4% paraformaldehyde in
phosphate-buffered saline and incubated for 10 min. The fixed cells were washed twice with 1% bovine serum albumin in phosphate-buffered saline and resuspended in fluorescence-activated cell
sorting buffer. Flow cytometry was performed using Accuri equipment (Becton–Dickinson), and data analysis was conducted by FlowJo vX software (TreeStar). IMMUNOFLUORESCENT STAINING ANALYSIS
Organoids were fixed in 4% paraformaldehyde for 1 h after being washed with 1 × phosphate-buffered saline and then incubated in a 30% sucrose solution overnight. Following the previously
published protocol13, organoid sections were immunostained using the following primary antibodies: PAX6 (Invitrogen, 42-6600), NESTIN (Invitrogen, PA5-47378), KI67 (Invitrogen, 14-5698-82),
SOX2 (Millipore, AB5603), TUJ1 (Sigma, T2200), MAP2 (Cell Signaling, 4542 s), DCX (Cell signaling, 4604), VGLUT1 (Invitrogen, 48-2400), TBR1 (Abcam, ab31940), NEUN (Millipore, MAP377 KAT2B
(Abcam, ab12188), and H3K9ac (Abcam, ab4441). Appropriate secondary antibodies (Invitrogen) were used for amplifying the signal. Next, nuclei were labeled with 6-diamidino-2-phenylindole
(DAPI, Invitrogen). Sections were mounted in Fluoromount-G mounting medium and images were captured using a confocal laser scanning microscope (ZEISS, LSM800). The number of Nesin positive
cells were counted in three, non-overlapping visual field at a magnification of × 20. The percentages of immunopositive cells were calculated per 100 cells, visualized with DAPI staining42.
WESTERN BLOT ANALYSIS Organoid samples for all conditions were lysed in lysis buffer (1% NP-40, 0.5% DOC, 0.1% SDS, 150 mmol/L NaCl in 50 mmol/L Tris, pH 8.0, Sigma-Aldrich; and 1 ×
proteinase inhibitor mixture, Roche) after being dissociated with a homogenizer and washed with 1 × phosphate-buffered saline. After adding 5 × SDS loading buffer and boiling at 99 °C for 5
min, the extracted proteins were electrophoresed on a 12% sodium dodecyl sulfate–polyacrylamide gel and transferred to nitrocellulose membranes (GE Healthcare Bio-Sciences). The membranes
were probed with the following primary antibodies: anti-KAT2B (1:500, Abcam, ab12188), anti-H3K9ac (1:1000, Abcam, ab4441), anti-H3K27me3 (1:1000, Millipore, 07-449), anti-H3K4me3 (1:500,
Abcam, Ab8580), anti-histone H3 (1:1000, AbFrontier, LF-MA02330), and beta-actin (1:1000, AbFrontier, LF-PA0207). QUANTITATIVE REAL-TIME POLYMERASE CHAIN REACTION ANALYSIS A previously
published protocol was used to isolate total RNA from organoid samples43. To synthesize complementary DNA from isolated RNA, the AccuPower RT-PCR PreMix (Bioneer) was used according to the
manufacturer’s protocols. Quantitative RT-PCR was performed using Platinum SYBR green qPCR SuperMix (Invitrogen) in a Rotor-Gene Q real-time PCR cycler (QIAGEN). Expression of all target
genes was normalized against the expression of glyceraldehyde-3-phosphate dehydrogenase, GAPDH, in each organoid sample. STATISTICAL ANALYSIS All data are presented as the mean ± SEM of each
independent experiment. Values of ‘n’ indicate the number of independent experiments performed or the number of individual experiments. Statistical analyses were performed with GraphPad
Prism. The p-values were calculated by Student’s t-test, one-way ANOVA, and two-way ANOVA tests followed by Tukey–Kramer multiple comparisons test. All of the statistical details for each
experiment have been documented in the individual figure legends. DATA AVAILABILITY The authors have no data to share since all data are shown in the submitted manuscript. REFERENCES *
Lancaster, M. A. _et al._ Cerebral organoids model human brain development and microcephaly. _Nature_ 501, 373–379. https://doi.org/10.1038/nature12517 (2013). Article ADS CAS PubMed
Google Scholar * Kanton, S. _et al._ Organoid single-cell genomic atlas uncovers human-specific features of brain development. _Nature_ 574, 418–422.
https://doi.org/10.1038/s41586-019-1654-9 (2019). Article ADS CAS PubMed Google Scholar * Cakir, B. _et al._ Engineering of human brain organoids with a functional vascular-like system.
_Nat. Methods_ 16, 1169–1175. https://doi.org/10.1038/s41592-019-0586-5 (2019). Article CAS PubMed PubMed Central Google Scholar * Trujillo, C. A. _et al._ Complex oscillatory waves
emerging from cortical organoids model early human brain network development. _Cell Stem Cell_ 25, 558-569.e557. https://doi.org/10.1016/j.stem.2019.08.002 (2019). Article CAS PubMed
PubMed Central Google Scholar * Jo, J. _et al._ Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. _Cell Stem
Cell_ 19, 248–257. https://doi.org/10.1016/j.stem.2016.07.005 (2016). Article CAS PubMed PubMed Central Google Scholar * Muguruma, K., Nishiyama, A., Kawakami, H., Hashimoto, K. &
Sasai, Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. _Cell Rep._ 10, 537–550. https://doi.org/10.1016/j.celrep.2014.12.051 (2015).
Article CAS PubMed Google Scholar * Xiang, Y. _et al._ hESC-derived thalamic organoids form reciprocal projections when fused with cortical organoids. _Cell Stem Cell_ 24, 487-497.e487.
https://doi.org/10.1016/j.stem.2018.12.015 (2019). Article CAS PubMed PubMed Central Google Scholar * Liu, C. _et al._ Mitochondrial HSF1 triggers mitochondrial dysfunction and
neurodegeneration in Huntington’s disease. _EMBO Mol. Med._ 14, e15851. https://doi.org/10.15252/emmm.202215851 (2022). Article CAS PubMed PubMed Central Google Scholar * Conforti, P.
_et al._ Faulty neuronal determination and cell polarization are reverted by modulating HD early phenotypes. _Proc. Natl. Acad. Sci. USA_ 115, E762-e771.
https://doi.org/10.1073/pnas.1715865115 (2018). Article CAS PubMed PubMed Central Google Scholar * Villa, C. E. _et al._ CHD8 haploinsufficiency links autism to transient alterations in
excitatory and inhibitory trajectories. _Cell Rep._ 39, 110615. https://doi.org/10.1016/j.celrep.2022.110615 (2022). Article CAS PubMed Google Scholar * Paulsen, B. _et al._ Autism
genes converge on asynchronous development of shared neuron classes. _Nature_ 602, 268–273. https://doi.org/10.1038/s41586-021-04358-6 (2022). Article ADS CAS PubMed PubMed Central
Google Scholar * Wulansari, N. _et al._ Neurodevelopmental defects and neurodegenerative phenotypes in human brain organoids carrying Parkinson’s disease-linked DNAJC6 mutations. _Sci.
Adv._ 7, 1540. https://doi.org/10.1126/sciadv.abb1540 (2021). Article ADS CAS Google Scholar * Kim, H. _et al._ Modeling G2019S-LRRK2 sporadic Parkinson’s disease in 3D midbrain
organoids. _Stem Cell Rep._ 12, 518–531. https://doi.org/10.1016/j.stemcr.2019.01.020 (2019). Article CAS Google Scholar * Zhao, J. _et al._ APOE4 exacerbates synapse loss and
neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids. _Nat. Commun._ 11, 5540. https://doi.org/10.1038/s41467-020-19264-0 (2020). Article ADS CAS PubMed
PubMed Central Google Scholar * Choi, S. H. _et al._ A three-dimensional human neural cell culture model of Alzheimer’s disease. _Nature_ 515, 274–278. https://doi.org/10.1038/nature13800
(2014). Article ADS CAS PubMed PubMed Central Google Scholar * Lancaster, M. A. _et al._ Guided self-organization and cortical plate formation in human brain organoids. _Nat.
Biotechnol._ 35, 659–666. https://doi.org/10.1038/nbt.3906 (2017). Article CAS PubMed PubMed Central Google Scholar * Qian, X. _et al._ Generation of human brain region-specific
organoids using a miniaturized spinning bioreactor. _Nat. Protoc._ 13, 565–580. https://doi.org/10.1038/nprot.2017.152 (2018). Article CAS PubMed PubMed Central Google Scholar *
Velasco, S. _et al._ Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. _Nature_ 570, 523–527. https://doi.org/10.1038/s41586-019-1289-x (2019).
Article ADS CAS PubMed PubMed Central Google Scholar * Giandomenico, S. L. _et al._ Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output.
_Nat. Neurosci._ 22, 669–679. https://doi.org/10.1038/s41593-019-0350-2 (2019). Article CAS PubMed PubMed Central Google Scholar * Wang, Y., Wang, L., Guo, Y., Zhu, Y. & Qin, J.
Engineering stem cell-derived 3D brain organoids in a perfusable organ-on-a-chip system. _RSC Adv._ 8, 1677–1685. https://doi.org/10.1039/c7ra11714k (2018). Article ADS CAS PubMed PubMed
Central Google Scholar * Yoo, J. _et al._ Electromagnetized gold nanoparticles mediate direct lineage reprogramming into induced dopamine neurons in vivo for Parkinson’s disease therapy.
_Nat. Nanotechnol._ 12, 1006–1014. https://doi.org/10.1038/nnano.2017.133 (2017). Article ADS CAS PubMed Google Scholar * Chang, Y. _et al._ Electromagnetized gold nanoparticles improve
neurogenesis and cognition in the aged brain. _Biomaterials_ 278, 121157. https://doi.org/10.1016/j.biomaterials.2021.121157 (2021). Article CAS PubMed Google Scholar * Takahashi, S.
_et al._ The RGD motif in fibronectin is essential for development but dispensable for fibril assembly. _J. Cell Biol._ 178, 167–178. https://doi.org/10.1083/jcb.200703021 (2007). Article
CAS PubMed PubMed Central Google Scholar * Revkova, V. A. _et al._ Spidroin silk fibers with bioactive motifs of extracellular proteins for neural tissue engineering. _ACS Omega_ 6,
15264–15273. https://doi.org/10.1021/acsomega.1c01576 (2021). Article CAS PubMed PubMed Central Google Scholar * Ananthanarayanan, B., Little, L., Schaffer, D. V., Healy, K. E. &
Tirrell, M. Neural stem cell adhesion and proliferation on phospholipid bilayers functionalized with RGD peptides. _Biomaterials_ 31, 8706–8715.
https://doi.org/10.1016/j.biomaterials.2010.07.104 (2010). Article CAS PubMed PubMed Central Google Scholar * Naghdi, P. _et al._ Survival, proliferation and differentiation enhancement
of neural stem cells cultured in three-dimensional polyethylene glycol-RGD hydrogel with tenascin. _J. Tissue Eng. Regen. Med._ 10, 199–208. https://doi.org/10.1002/term.1958 (2016).
Article CAS PubMed Google Scholar * García-Álvarez, R., Hadjidemetriou, M., Sánchez-Iglesias, A., Liz-Marzán, L. M. & Kostarelos, K. In vivo formation of protein corona on gold
nanoparticles. The effect of their size and shape. _Nanoscale_ 10, 1256–1264. https://doi.org/10.1039/c7nr08322j (2018). Article CAS PubMed Google Scholar * Fayol, D. _et al._ Use of
magnetic forces to promote stem cell aggregation during differentiation, and cartilage tissue modeling. _Adv. Mater._ 25, 2611–2616. https://doi.org/10.1002/adma.201300342 (2013). Article
CAS PubMed Google Scholar * Kim, H. _et al._ Modelling APOE ɛ3/4 allele-associated sporadic Alzheimer’s disease in an induced neuron. _Brain_ 140, 2193–2209.
https://doi.org/10.1093/brain/awx144 (2017). Article PubMed Google Scholar * Du, Y. _et al._ Nucleosome eviction along with H3K9ac deposition enhances Sox2 binding during human
neuroectodermal commitment. _Cell Death Differ._ 24, 1121–1131. https://doi.org/10.1038/cdd.2017.62 (2017). Article CAS PubMed PubMed Central Google Scholar * Qiao, Y., Wang, R., Yang,
X., Tang, K. & Jing, N. Dual roles of histone H3 lysine 9 acetylation in human embryonic stem cell pluripotency and neural differentiation. _J. Biol. Chem._ 290, 2508–2520.
https://doi.org/10.1074/jbc.M114.603761 (2015). Article CAS PubMed Google Scholar * Yang, F. _et al._ Epigenetic modifications of interleukin-6 in synovial fibroblasts from
osteoarthritis patients. _Sci. Rep._ 7, 43592. https://doi.org/10.1038/srep43592 (2017). Article ADS PubMed PubMed Central Google Scholar * Kaeser, M. D. & Iggo, R. D.
Promoter-specific p53-dependent histone acetylation following DNA damage. _Oncogene_ 23, 4007–4013. https://doi.org/10.1038/sj.onc.1207536 (2004). Article CAS PubMed Google Scholar *
Foletti, A., Lisi, A., Ledda, M., de Carlo, F. & Grimaldi, S. Cellular ELF signals as a possible tool in informative medicine. _Electromagn. Biol. Med._ 28, 71–79.
https://doi.org/10.1080/15368370802708801 (2009). Article PubMed Google Scholar * He, Y. L. _et al._ Exposure to extremely low-frequency electromagnetic fields modulates Na+ currents in
rat cerebellar granule cells through increase of AA/PGE2 and EP receptor-mediated cAMP/PKA pathway. _PLoS ONE_ 8, e54376. https://doi.org/10.1371/journal.pone.0054376 (2013). Article ADS
CAS PubMed PubMed Central Google Scholar * Fassina, L. _et al._ Electromagnetic enhancement of a culture of human SAOS-2 osteoblasts seeded onto titanium fiber-mesh scaffolds. _J.
Biomed. Mater. Res. A_ 87, 750–759. https://doi.org/10.1002/jbm.a.31827 (2008). Article CAS PubMed Google Scholar * Luo, F. _et al._ Effects of pulsed electromagnetic field frequencies
on the osteogenic differentiation of human mesenchymal stem cells. _Orthopedics_ 35, e526-531. https://doi.org/10.3928/01477447-20120327-11 (2012). Article PubMed Google Scholar *
Turkevich, J., Stevenson, P. C. & Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. _Discuss. Faraday Soc._ 11, 55–75 (1951). Article Google
Scholar * Luby, A. O., Breitner, E. K. & Comfort, K. K. Preliminary protein corona formation stabilizes gold nanoparticles and improves deposition efficiency. _Appl. Nanosci._ 6,
827–836. https://doi.org/10.1007/s13204-015-0501-z (2016). Article ADS CAS Google Scholar * Lee, E. _et al._ Molecular origin of AuNPs-induced cytotoxicity and mechanistic study. _Sci.
Rep._ 9, 2494. https://doi.org/10.1038/s41598-019-39579-3 (2019). Article ADS CAS PubMed PubMed Central Google Scholar * Kim, H., Kim, S., Cho, B., Shin, J. & Kim, J. APOE
ε4-dependent effects on the early amyloid pathology in induced neurons of patients with Alzheimer’s disease. _Transl. Neurodegener._ 11, 45. https://doi.org/10.1186/s40035-022-00319-9
(2022). Article CAS PubMed PubMed Central Google Scholar * Suzuki, S., Namiki, J., Shibata, S., Mastuzaki, Y. & Okano, H. The neural stem/progenitor cell marker nestin is expressed
in proliferative endothelial cells, but not in mature vasculature. _J. Histochem. Cytochem._ 58, 721–730. https://doi.org/10.1369/jhc.2010.955609 (2010). Article CAS PubMed PubMed Central
Google Scholar * Kim, H. _et al._ Dormant state of quiescent neural stem cells links Shank3 mutation to autism development. _Mol. Psychiatry_ 27, 2751–2765.
https://doi.org/10.1038/s41380-022-01563-1 (2022). Article CAS PubMed Google Scholar Download references FUNDING This work was supported by Korean Fund for Regenerative Medicine funded
by Ministry of Science and ICT, and Ministry of Health and Welfare (2021M3E5E5096464, Republic of Korea) and Basic Science Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (NRF-2022R1A6A1A03053343 and NRF-2021R1I1A1A01052628). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Laboratory of Stem Cells & Gene Editing,
Department of Chemistry, Dongguk University, Pildong-Ro 1-Gil 30, Jung-Gu, Seoul, 04620, Republic of Korea Hongwon Kim, Yoo-Jung Lee & Jongpil Kim * Department of Chemistry and Chemical
Biology, Rutgers, The State University of New Jersey, Piscataway, NJ, 08854, USA Hongwon Kim * Laboratory of Protein Engineering, Department of Biomedical Engineering, Dongguk University,
Seoul, 04620, Republic of Korea Youngeun Kwon Authors * Hongwon Kim View author publications You can also search for this author inPubMed Google Scholar * Yoo-Jung Lee View author
publications You can also search for this author inPubMed Google Scholar * Youngeun Kwon View author publications You can also search for this author inPubMed Google Scholar * Jongpil Kim
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS H.K. and J.K. contributed the ideas and designed the experiments. H.K. and Y.J.L. performed
the experiments and analyzed the data. All authors contributed to the writing of the manuscript. CORRESPONDING AUTHOR Correspondence to Jongpil Kim. ETHICS DECLARATIONS COMPETING INTERESTS
The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's
Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kim, H., Lee, YJ., Kwon, Y. _et al._ Efficient generation of brain organoids using
magnetized gold nanoparticles. _Sci Rep_ 13, 21240 (2023). https://doi.org/10.1038/s41598-023-48655-8 Download citation * Received: 03 April 2023 * Accepted: 29 November 2023 * Published: 01
December 2023 * DOI: https://doi.org/10.1038/s41598-023-48655-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative