- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Gastric cancer is one of the most common malignancies worldwide, and the third leading cause of cancer-related death. The identification of novel biomarkers and therapeutic targets
is critical to improve the prognosis. A total of 380 patients with primary gastric cancer from the TCGA database were analyzed. The receiver operating characteristic curves were plotted. We
further evaluated the independent prognostic ability of NPC2 expression for overall survival (OS) and relapse-free survival (RFS) through the Kaplan–Meier curve and Cox analysis. The NPC2
expression was significantly higher (_P_ < 0.001) in gastric cancer. High NPC2 expression was significantly (_P_ < 0.0001) associated with poor OS and poor RFS. The age, stage,
radiation therapy, residual tumor, and NPC2 expression showed independent prognostic value for OS. The gender and NPC2 expression showed independent prognostic value for RFS. The higher NPC2
expression was observed in gastric cancer, compared with adjacent normal tissue (_P_ < 0.001), confirmed by the IHC staining. The CCK-8 assay showed that NPC2 knockdown inhibits cell
proliferation while NPC2 overexpression promotes cell proliferation (_P_ < 0.05). NPC2 expression may serve as a promising prognostic biomarker for patients with gastric cancer. SIMILAR
CONTENT BEING VIEWED BY OTHERS THE TUMOR BIOLOGICAL SIGNIFICANCE OF RNF43 AND LRP1B IN GASTRIC CANCER IS COMPLEX AND CONTEXT-DEPENDENT Article Open access 23 February 2023 HIF1A PROTEIN
EXPRESSION IS CORRELATED WITH CLINICAL FEATURES IN GASTRIC CANCER: AN UPDATED SYSTEMATIC REVIEW AND META-ANALYSIS Article Open access 14 June 2024 RE-EVALUATION OF THE RELATIONSHIP BETWEEN
PRPC EXPRESSION AND PATIENT PROGNOSIS IN PRIMARY ESOPHAGEAL SQUAMOUS CELL CARCINOMA AND PRIMARY HEPATOCELLULAR CARCINOMA Article Open access 28 December 2024 INTRODUCTION Gastric cancer,
originated from gastric mucosal epithelium, is one of the most common malignancies worldwide threatening human health1. Gastric cancer is the third leading cause of cancer-related death with
more than 1 million new cases each year2. Due to the limited understanding of molecular mechanisms and poor prognosis, the mortality rate of gastric cancer is still high3. Gastric cancer
has an insidious onset and atypical early symptoms are often not appreciated by patients4. The majority of patients are found to have advanced gastric cancer, and their 5-year survival rates
tend to be less than 10%5. Therefore, identification of novel biomarkers and therapeutic targets is critical to improve the diagnosis, treatment and prognosis of gastric cancer6, 7. NPC2
(NPC intracellular cholesterol transporter 2) is an intracellular cholesterol transporting protein that affects the cholesterol homeostasis regulatory system8, 9. In recent years, increasing
evidence has revealed the important role of NPC2 in the development and progression of various human cancers, including gastric cancer10. There is a very strong association between NPC2
expression and tumors, with a signature of overexpression in breast, colon, as well as lung cancers11. However, the clinical significance and prognostic value of NPC2 expression in gastric
cancer remain largely unknown. The Cancer Genome Atlas (TCGA) is a comprehensive and integrated database that provides a wealth of genomic, transcriptomic, and clinical information on
multiple types of cancer, including gastric cancer12, 13. In this study, we aimed to assess the correlation between NPC2 expression in gastric cancer and clinicopathologic characteristics
through analyzing data from the TCGA database. The receiver operating characteristic (ROC) curves were plotted to analyze the diagnostic value of NPC2. We further evaluated the independent
prognostic ability of NPC2 expression for overall survival (OS) and relapse-free survival (RFS) through the Kaplan–Meier curve and Cox analysis. The ability of NPC2 to predict OS and RFS was
reflected by nomogram. Finally, the NPC2 expression was validated in the samples collected from patients and gastric cancer cell. The findings could provide new insights into the clinical
significance of NPC2 expression in gastric cancer and its potential as a diagnostic and prognostic biomarker. METHODS DATA COLLECTION Data was obtained from TCGA database
(https://www.cancergenome.nih.gov). The raw data was preprocessed to remove outliers and normalize the expression levels of NPC2. A total of 380 patients with primary gastric cancer were
involved (Supplementary Table 1). The clinicopathologic characteristics and expression levels of NPC2 in gastric cancer were analyzed by Fisher’s exact test and chi-square test, and
non-parametric rank sum test, respectively14. The study was approved by the Ethics Committee of the First Hospital of Shanxi Medical University, and all methods were performed in accordance
with the relevant guidelines and regulations. The 30 patient samples were obtained with appropriate informed consent and processed with approval. DIAGNOSTIC VALUE ANALYSIS The diagnostic
value of NPC2 expression was analyzed by plotting the ROC curve and calculating the area under the curve (AUC). The AUC was used to determine the accuracy of NPC2 in diagnosing gastric
cancer. SURVIVAL ANALYSIS The Kaplan–Meier curve was used to evaluate the prognostic value of NPC2 expression for OS and RFS15. The log-rank test was used to compare the survival curves
between different subgroups. UNIVARIATE AND MULTIVARIATE COX ANALYSIS Univariate Cox analysis was performed to assess the univariate association between NPC2 expression and survival.
Multivariate Cox analysis was performed to evaluate the independent prognostic ability of NPC2 expression after adjusting for other potential confounding factors. NOMOGRAM ANALYSIS The
nomogram was used to reflect the ability of NPC2 to predict OS and RFS. The concordance index (C-index) was calculated to assess the predictive accuracy of the nomogram16. GSEA ANALYSIS The
online GSEA analysis was performed after searching the TCGA for evaluation of NPC2 expression and enriched pathways17. REAL-TIME QUANTITATIVE PCR The RT-qPCR was performed using the kit
(Invitrogen, Thermo Fisher, USA) according to the instruction. 2−ΔΔCt method was used for quantification. The NPC2 primers (forward primer: 5′-TGT GGC TCA GTG GCT TAG G-3′; reverse primer:
5′-CCA GGA AGG GAT TTC ACA CA-3′) and β-actin primers (forward primer: 5′-ACC CCA AAG CCA ACA GA-3′; reverse primer: 5′-CCA GAG TCC ATC ACA ATA CC-3′) were used. IMMUNOHISTOCHEMISTRY The
immunohistochemistry (IHC) staining was performed as reported18. Anti-NPC2 primary antibody (MA5-29450, Thermo Fisher, USA) and corresponding rabbit secondary antibody were used. CELL
CULTURE AND TRANSFECTION The cells were purchased from American Tissue Culture Collection and cultured in the necessary environment as reported19. There were four groups including si-NPC2
transfected group, NPC2 overexpressed group, si-NC transfected group as negative control, and un-transfected group as control. The validation of transfection efficiency was performed using
qRT-PCR and western blot. WESTERN BLOT To confirm NPC2 knockdown, Western blot analysis was performed as previously reported20. Primary antibody NPC2 (sc-30346, Santa Cruz Biotechnology,
USA) and goat secondary antibody (sc-2354, Santa Cruz Biotechnology, USA) were used. CELL PROLIFERATION ASSAY The cell proliferation assay was performed by CCK-8 in AGS and SGC-7901 cell as
reported20. After plasmid transfection, the cells were cultured for 24 h. Then, the CCK-8 reagent (10 μL) was added and kept for 20 min. The absorbance at 490 nm was measured. STATISTICAL
ANALYSIS All statistical analyses were performed using the R software (version 3.5.2). The Wilcoxon rank sum and the Kruskal–Wallis test was used for comparing two and multiple groups,
respectively. A two-sided P value of < 0.05 was considered statistically significant. RESULTS CHARACTERISTICS OF PATIENTS WITH GASTRIC CANCER The characteristics of patients with gastric
cancer were evaluated in both groups according to high and low NPC2 expression (Supplementary Table 2). The histologic grade (_P_ = 0.048), T classification (_P_ = 0.0081), and vital status
(_P_ = 0.018) were significantly different in two groups. At the same time, there were no statistical differences in age (_P_ = 0.8347), gender (_P_ = 0.4134), histological type (_P_ =
0.0594), stage (_P_ = 0.2087), N classification (_P_ = 0.198), M classification (_P_ = 0.7266), radiation therapy (_P_ = 0.0581), and residual tumor (_P_ = 0.62). NPC2 EXPRESSION IN GASTRIC
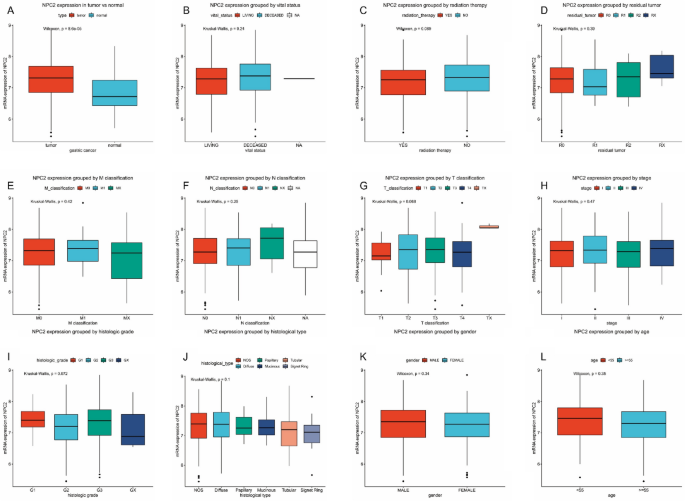
CANCER Compared with normal gastric tissue, the NPC2 expression was significantly higher (_P_ < 0.001) in gastric cancer (Fig. 1A). Besides, the subgroup analysis was performed (Fig.
1B–L), including vital status, radiation therapy, residual tumor, TNM classification, stage, histological type, histologic grade, gender, and age. However, no statistical differences were
found. DIAGNOSTIC VALUE OF NPC2 EXPRESSION The ROC curve analysis showed that NPC2 expression had a high diagnostic value for gastric cancer with AUC of 0.696 (Supplementary Figure 1A).
Moreover, the AUC for stage I-IV was 0.660 (Supplementary Figure 1B), 0.721 (Supplementary Figure 1C), 0.678 (Supplementary Figure 1D), and 0.716 (Supplementary Figure 1E). HIGH NPC2
EXPRESSION IS ASSOCIATED WITH POOR OS AND RFS The Kaplan–Meier curves showed high NPC2 expression was significantly (_P_ < 0.0001) associated with poor OS (Fig. 2A) and poor RFS (Fig.
2B). OS GROUPED NPC2 EXPRESSION The OS grouped NPC2 expression was significantly different in stage I/II, stage III/IV, stage G1/G2, stage G3/G4, stage I, stage II, stage III, stage G2,
stage G3, T3, T4, N0, M0, R0, male, and older patients (Fig. 3A–P). Others were not significant (Supplementary Figure 2). By univariate and multivariate analysis (Supplementary Figure 3A–B),
the age [hazard ratio (HR): 1.825, 95% confidence interval (CI): 1.122–2.967, P = 0.015], stage (HR: 1.320, 95% CI: 1.049–1.661, _P_ = 0.018), radiation therapy (HR: 0.471, 95% CI:
0.318–0.699, _P_ < 0.001), residual tumor (HR: 1.695, 95% CI: 1.290–2.227, _P_ < 0.001), and NPC2 expression (HR: 1.664, 95% CI: 1.190–2.327, _P_ = 0.003) showed independent prognostic
value for OS. RFS GROUPED NPC2 EXPRESSION The RFS grouped NPC2 expression was significantly different in stage I/II, stage III/IV, stage G3/G4, stage II, stage III, stage G3, T3, T4, N0,
M0, R0, R1/R2, male, female, and older patients (Fig. 4A–O). Others were not significant (Supplementary Figure 4). By univariate and multivariate analysis (Supplementary Figure 5A–B), the
gender (HR: 1.960, 95% CI: 1.132–3.394, _P_ = 0.016) and NPC2 expression (HR: 2.097, 95% CI: 1.289–3.412, _P_ = 0.003) showed independent prognostic value for RFS. PREDICTIVE VALUE OF NPC2
EXPRESSION IN OS AND RFS The nomogram was used to further evaluate the ability of NPC2 to predict OS (Fig. 5A,B) and RFS (Fig. 6A,B). The results showed that NPC2 expression was a
significant predictor of OS and RFS, with high predictive accuracy (Figs. 5C–H, 6C–H). Specifically, high NPC2 expression could predict shorter OS and RFS. HIGH NPC2 EXPRESSION-ENRICHED
PATHWAYS The GSEA analysis (Supplementary Table 3) showed that high NPC2 expression was significant associated (_P_ < 0.05) with asthma (Fig. 7A), cytokine-cytokine receptor interaction
(Fig. 7B), drug metabolism other enzymes (Fig. 7C), natural killer cell mediated cytotoxicity (Fig. 7D), primary bile acid biosynthesis (Fig. 7E), and systemic lupus erythematosus (Fig. 7F).
HIGH NPC2 EXPRESSION IN TISSUE The higher NPC2 expression was observed in gastric cancer, compared with adjacent normal tissue (_P_ < 0.001, Fig. 8A). The IHC staining further confirmed
the high NPC2 expression in gastric cancer (Fig. 8B). Moreover, the prognostic significance of NPC2 expression was validated by RT-PCR with another cohort consisting of 58 living patients
and 32 deceased patients (Supplementary Figure 6). Higher NPC2 expression was associated with poor survival (_P_ < 0.05). HIGH NPC2 EXPRESSION IN CELL Besides, the NPC2 expression was
evaluated in normal cells including HaCaT and RGM-1 (Human gastric epithelial cell), and gastric cancer cells including MGC-803 (Human low differentiated gastric cancer), HGC-27 (Human
undifferentiated gastric cancer), SGC-7901 (Human gastric cancer with lymph node metastasis), AGS (Untreated human gastric adenocarcinoma cells). The NPC2 expression was higher in gastric
cancer than normal cells (_P_ < 0.05, Fig. 8C). Particularly, the NPC2 expression was highest in AGS, which was used for subsequent cell proliferation assay. NPC2 KNOCKDOWN INHIBITS CELL
PROLIFERATION First, the knockdown and overexpression efficiency were examined (Supplementary Figure 7 and Fig. 8D). The si-NPC2 treated AGS cell showed significant decreased NPC2 expression
(_P_ < 0.05), and O-NPC2 treated AGS cell showed significant increased NPC2 expression (_P_ < 0.01). Then, the CCK-8 assay showed that NPC2 knockdown inhibits AGS cell proliferation
while NPC2 overexpression promotes cell proliferation (_P_ < 0.05, Fig. 8E). The involvements of NPC2 in proliferation was validated in SGC-7901 cell as well (Supplementary Figure 8).
DISCUSSION Clinical stage at diagnosis directly determines the prognosis of patients with gastric cancer. Patients with localized, early-stage gastric cancer usually have a high 5-year
overall survival rate (OS > 60%), whereas patients with local and distant metastases of gastric cancer have a significantly decreased 5-year OS of 30% and 5%, respectively21.
Unfortunately, due to the occult nature and atypical nature of early clinical symptoms of gastric cancer, more than 60% of patients present with regional or distant metastasis at
diagnosis22. Surgical resection is the best treatment option for patients with early-stage gastric cancer, and chemotherapy is the most important treatment for patients with unresectable or
advanced metastasis. However, gastric cancer patients often respond poorly or even do not respond to chemotherapy due to their inherent or acquired resistance, becoming the most common cause
of treatment failure. Therefore, the low rate of early diagnosis and chemoresistance are the main reasons for the poor prognosis of gastric cancer. In the present study, we assessed the
correlation between NPC2 expression and clinicopathologic characteristics in gastric cancer patients using data from The Cancer Genome Atlas (TCGA) database. Our findings indicated that NPC2
expression is significantly associated with certain clinicopathological features and can serve as a promising prognostic biomarker for gastric cancer patients. Abnormal expression of NPC2
is closely associated with the progression of multiple malignant tumors. NPC2 expression is increased in patients with lung adenocarcinoma and their pleural effusions, but it is not clear
whether NPC2 plays a role in lung adenocarcinoma23. The presence of NPC2 protein in pleural effusions of lung adenocarcinoma suggests that it may be used as a potential diagnostic marker for
lung cancer. NPC2 overexpression correlates with the HER-2 subtype, suggesting that NPC2 upregulation may be a favorable prognostic predictor in breast cancer24. Notably, reduced expression
of NPC2 was particularly observed in liver tumor tissues compared with normal counterpart tissues25. NPC2 is associated with lipid metabolism and the innate immune system, and it influences
the formation and progression of various types of cancer cells, playing critical regulatory functions. Wei et al. found a relatively higher expression of NPC2 in glioblastoma and NPC2
overexpression inhibited cell proliferation26. Adachi et al. found silkworm NPC2 protein inhibits the growth of FM3A murine breast cancer cells27. Wang et al. reported that NPC2
overexpression decreased PDGF-BB-induced cell proliferation by inhibiting p38, JNK, and AKT phosphorylation28. Yet, the mechanism underlying the involvements of NPC2 in proliferation has
reached no consensus. The TCGA database involving huge data of gene related expression profile is keeping updated, and the number of patients may vary slightly at different time points.
Further studies with larger sample sizes and longer follow-up periods are needed to validate our results and to determine the optimal cutoff value for NPC2 expression in gastric cancer
patients. Additionally, it is essential to evaluate the feasibility and accuracy of NPC2 expression as a diagnostic and therapeutic target in gastric cancer. CONCLUSION In conclusion, our
findings provide evidence that NPC2 expression may serve as a promising prognostic biomarker for patients with gastric cancer. This information could have important implications for the
early diagnosis, therapeutic strategies and personalized management of gastric cancer. The findings of this study can improve the therapy of GC patients by providing a novel therapeutic
target and basis for further drug development. DATA AVAILABILITY The data were obtained from public database. The experiment data are available at reasonable request. REFERENCES * Smyth, E.
C., Nilsson, M., Grabsch, H. I., van Grieken, N. C. & Lordick, F. Gastric cancer. _Lancet (London, England)_ 396, 635–648. https://doi.org/10.1016/s0140-6736(20)31288-5 (2020). Article
CAS PubMed Google Scholar * Ajani, J. A. _et al._ Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. _J. Natl. Compr. Cancer Netw. JNCCN_ 20, 167–192.
https://doi.org/10.6004/jnccn.2022.0008 (2022). Article CAS PubMed Google Scholar * Joshi, S. S. & Badgwell, B. D. Current treatment and recent progress in gastric cancer. _CA Cancer
J. Clin._ 71, 264–279. https://doi.org/10.3322/caac.21657 (2021). Article PubMed Central Google Scholar * Zhao, R. R. _et al._ Role of annexin A family in tumorigenesis and
chemoresistance of gastric cancer. _Neoplasma_ 69, 251–263. https://doi.org/10.4149/neo_2021_210629N872 (2022). Article CAS PubMed Google Scholar * Li, G. Z., Doherty, G. M. & Wang,
J. Surgical management of gastric cancer: A review. _JAMA Surg._ 157, 446–454. https://doi.org/10.1001/jamasurg.2022.0182 (2022). Article PubMed Google Scholar * Matsuoka, T. &
Yashiro, M. Biomarkers of gastric cancer: Current topics and future perspective. _World J. Gastroenterol._ 24, 2818–2832. https://doi.org/10.3748/wjg.v24.i26.2818 (2018). Article CAS
PubMed PubMed Central Google Scholar * de Mello, R. A. & Amaral, G. A. Current and potential biomarkers in gastric cancer: A critical review of the literature. _Future Oncol._ 17,
3383–3396. https://doi.org/10.2217/fon-2021-0084 (2021). Article CAS PubMed Google Scholar * Qian, H. _et al._ Structural basis of low-pH-dependent lysosomal cholesterol egress by NPC1
and NPC2. _Cell_ 182, 98-111.e118. https://doi.org/10.1016/j.cell.2020.05.020 (2020). Article CAS PubMed Google Scholar * Cologna, S. M. & Rosenhouse-Dantsker, A. Insights into the
molecular mechanisms of cholesterol binding to the NPC1 and NPC2 proteins. _Adv. Exp. Med. Biol._ 1135, 139–160. https://doi.org/10.1007/978-3-030-14265-0_8 (2019). Article CAS PubMed
Google Scholar * Xu, Y., Zhang, Q., Tan, L., Xie, X. & Zhao, Y. The characteristics and biological significance of NPC2: Mutation and disease. _Mutat. Res. Rev. Mutat. Res._ 782,
108284. https://doi.org/10.1016/j.mrrev.2019.108284 (2019). Article CAS PubMed Google Scholar * Sugawara, M. _et al._ A novel role for Niemann–Pick disease type 2C protein in papillae
formation. _PLoS ONE_ 6, e15777. https://doi.org/10.1371/journal.pone.0015777 (2011). Article ADS CAS PubMed Central Google Scholar * Wang, Z., Jensen, M. A. & Zenklusen, J. C. A
practical guide to the cancer genome atlas (TCGA). _Methods Mol. Biol. (Clifton, N.J.)_ 1418, 111–141. https://doi.org/10.1007/978-1-4939-3578-9_6 (2016). Article Google Scholar *
Nshizirungu, J. P. & Bennis, S. Reproduction of the Cancer Genome Atlas (TCGA) and Asian Cancer Research Group (ACRG) gastric cancer molecular classifications and their association with
clinicopathological characteristics and overall survival in Moroccan patients. _Des. Mark._ https://doi.org/10.1155/2021/9980410 (2021). Article Google Scholar * Cai, H. & Jiao, Y. Low
CYP24A1 mRNA expression and its role in prognosis of breast cancer. _Sci. Rep._ 9, 13714. https://doi.org/10.1038/s41598-019-50214-z (2019). Article ADS CAS PubMed PubMed Central
Google Scholar * Zhang, D. _et al._ Scoring system for tumor-infiltrating lymphocytes and its prognostic value for gastric cancer. _Front. Immunol._ 10, 71.
https://doi.org/10.3389/fimmu.2019.00071 (2019). Article CAS PubMed PubMed Central Google Scholar * Dong, D. _et al._ Deep learning radiomic nomogram can predict the number of lymph
node metastasis in locally advanced gastric cancer: an international multicenter study. _Ann. Oncol._ 31, 912–920. https://doi.org/10.1016/j.annonc.2020.04.003 (2020). Article CAS PubMed
Google Scholar * Chi, Y., Wang, H., Wang, F. & Ding, M. PHTF2 regulates lipids metabolism in gastric cancer. _Aging_ 12, 6600–6610. https://doi.org/10.18632/aging.102995 (2020). Article
CAS PubMed PubMed Central Google Scholar * Xie, J. W. _et al._ m(6)A modification-mediated BATF2 acts as a tumor suppressor in gastric cancer through inhibition of ERK signaling. _Mol.
Cancer_ 19, 114. https://doi.org/10.1186/s12943-020-01223-4 (2020). Article CAS PubMed PubMed Central Google Scholar * Tian, S. _et al._ SERPINH1 regulates EMT and gastric cancer
metastasis via the Wnt/β-catenin signaling pathway. _Aging_ 12, 3574–3593. https://doi.org/10.18632/aging.102831 (2020). Article CAS PubMed PubMed Central Google Scholar * Cai, H. _et
al._ Ataxia telangiectasia mutated inhibitor-loaded copper sulfide nanoparticles for low-temperature photothermal therapy of hepatocellular carcinoma. _Acta Biomater._ 127, 276–286.
https://doi.org/10.1016/j.actbio.2021.03.051 (2021). Article CAS PubMed Google Scholar * Norwood, D. A., Montalvan, E. E., Dominguez, R. L. & Morgan, D. R. Gastric cancer: Emerging
trends in prevention, diagnosis, and treatment. _Gastroenterol. Clin. N. Am._ 51, 501–518. https://doi.org/10.1016/j.gtc.2022.05.001 (2022). Article Google Scholar * Thrift, A. P. &
El-Serag, H. B. Burden of gastric cancer. _Clin. Gastroenterol. Hepatol._ 18, 534–542. https://doi.org/10.1016/j.cgh.2019.07.045 (2020). Article PubMed Google Scholar * Pernemalm, M. _et
al._ Use of narrow-range peptide IEF to improve detection of lung adenocarcinoma markers in plasma and pleural effusion. _Proteomics_ 9, 3414–3424. https://doi.org/10.1002/pmic.200800814
(2009). Article CAS Google Scholar * Liao, Y. J. _et al._ Characterization of Niemann–Pick Type C2 protein expression in multiple cancers using a novel NPC2 monoclonal antibody. _PLoS
ONE_ 8, e77586. https://doi.org/10.1371/journal.pone.0077586 (2013). Article ADS CAS PubMed PubMed Central Google Scholar * Liao, Y. J. _et al._ Niemann-Pick type C2 protein regulates
liver cancer progression via modulating ERK1/2 pathway: Clinicopathological correlations and therapeutical implications. _Int. J. Cancer_ 137, 1341–1351. https://doi.org/10.1002/ijc.29507
(2015). Article CAS PubMed Google Scholar * Wei, D. _et al._ NPC2 as a prognostic biomarker for glioblastoma based on integrated bioinformatics analysis and cytological experiments.
_Front. Genet._ 12, 611442. https://doi.org/10.3389/fgene.2021.611442 (2021). Article CAS PubMed PubMed Central Google Scholar * Adachi, T., Matsumoto, Y., Inagaki, Y. & Sekimizu,
K. Niemann-Pick disease type C2 protein induces autophagy and inhibits growth in FM3A breast cancer cells. _Drug Discov. Ther._ 9, 282–288. https://doi.org/10.5582/ddt.2015.01014 (2015).
Article CAS PubMed Google Scholar * Wang, Y. H. & Twu, Y. C. Niemann–Pick type C2 protein regulates free cholesterol accumulation and influences hepatic stellate cell proliferation
and mitochondrial respiration function. _Int. J. Mol. Sci._ https://doi.org/10.3390/ijms19061678 (2018). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS
Thank for the TCGA database for providing data. FUNDING This study was funded by the First Hospital of Shanxi Medical University (No. SYYYRC-2022002). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Gastroenterology, First Hospital of Shanxi Medical University, Taiyuan, 030000, China Xing Chen * Faculty of Graduate Studies, Shanxi Medical University,
Taiyuan, 030000, China Yunzhuang Yao, Jinnan Ren, Junhui Lu, Yue Sui & Jingwen Gong * Heping Hospital Affiliated to Changzhi Medical College, Changzhi, China Yunzhuang Yao Authors *
Yunzhuang Yao View author publications You can also search for this author inPubMed Google Scholar * Jinnan Ren View author publications You can also search for this author inPubMed Google
Scholar * Junhui Lu View author publications You can also search for this author inPubMed Google Scholar * Yue Sui View author publications You can also search for this author inPubMed
Google Scholar * Jingwen Gong View author publications You can also search for this author inPubMed Google Scholar * Xing Chen View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS X.C., and Y.Y. designed this study and had full access to all of data in the study; J.R. extracted the data; J.L. analyzed and interpreted the data;
S.Y., and J.G. wrote the paper. All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Xing Chen. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits
use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless
indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory
regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yao, Y., Ren, J., Lu, J. _et al._ Prognostic significance of high NPC2 expression
in gastric cancer. _Sci Rep_ 13, 20710 (2023). https://doi.org/10.1038/s41598-023-47882-3 Download citation * Received: 19 April 2023 * Accepted: 20 November 2023 * Published: 24 November
2023 * DOI: https://doi.org/10.1038/s41598-023-47882-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative