- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Biliary tract cancers are rare cancers with poor prognosis due to a lack of therapeutic options, especially after the failure of first-line systemic treatment. Targeted treatments
for this clinical situation are promising and have entered clinical practice. We aimed to describe the overall survival of matched targeted treatment after first-line treatment in patients
with biliary tract cancers in an Austrian real-world multicenter cohort. We performed a multicenter retrospective chart review of patients with biliary tract cancer between September 2015
and January 2022. Data, including comprehensive molecular characteristics—next generation sequencing (NGS) and immunohistochemistry (IHC), clinical history, surgical procedures, ablative
treatments, patient history, and systemic chemotherapy, were extracted from the records of the participating institutions. Targeted treatment was matched according to the ESMO scale for the
clinical actionability of molecular targets (ESCAT). We identified 159 patients with the available molecular characteristics. A total of 79 patients underwent second-line treatment. Of
these, 36 patients received matched targeted treatment beyond the first-line and were compared with 43 patients treated with cytotoxic chemotherapy in terms of efficacy outcomes. For Tier
I/II alterations, we observed a progression free survival ratio (PFStargeted/PFSpre-chemotherapy) of 1.86, p = 0.059. The overall survival for patients receiving at least two lines of
systemic treatment significantly favored the targeted approach, with an overall survival of 22.3 months (95% CI 14.7–29.3) vs. 17.5 months (95% CI 1.7–19.8; p = 0.048). Our results
underscore the value of targeted treatment approaches based on extended molecular characterization of biliary tract cancer to improve clinical outcomes. SIMILAR CONTENT BEING VIEWED BY
OTHERS PRECISION ONCOLOGY FOR INTRAHEPATIC CHOLANGIOCARCINOMA IN CLINICAL PRACTICE Article Open access 19 August 2022 CURRENT AND NOVEL THERAPEUTIC OPPORTUNITIES FOR SYSTEMIC THERAPY IN
BILIARY CANCER Article 22 July 2020 COMPREHENSIVE CLINICOPATHOLOGICAL AND GENOMIC PROFILING OF GALLBLADDER CANCER REVEALS ACTIONABLE TARGETS IN HALF OF PATIENTS Article Open access 05
November 2022 INTRODUCTION Biliary tract cancers (BTC) comprise a heterogeneous group of rare tumors with a poor prognosis. The incidence in Western countries is rising, especially for the
intrahepatic subtype, which may be linked to metabolic-associated conditions such as obesity, type-2 diabetes, or non-alcoholic liver disease1,2. Overall survival (OS) is still poor, with a
5-year OS rate of < 20% across all subtypes1,3,4. Recently, a new first-line standard incorporating checkpoint inhibitors (CPI) in a first-line metastatic setting has emerged5. Treatment
after the first-line predominantly comprises cytotoxic chemotherapy. However, the proof of its effectiveness is rather weak, depending on the retrospective analyses and two prospective
randomized trials6,7,8. The advent of sophisticated molecular techniques has paved the way for precision medicine in BTCs. It is estimated that up to 50% of BTCs are potential candidates for
molecular-informed therapy9,10. Several smaller phase I/II trials, basket trials, and case series show the potential of targeted treatment regimens in second-line and beyond11,12,13,14.
Currently, testing for fibroblast growth factor receptor 2 (_FGFR2)_ or neurotrophic tropomyosin kinase receptors (_NTRK)_ fusion, isocitrate dehydrogenase 1 _IDH1_ or B-rapidly accelerated
fibrosarcoma kinase (_BRAF)_ hotspot mutations, and human epidermal growth factor receptor 2 (_HER2/neu)_ amplification or microsatellite status is strongly recommended by the new BTC ESMO
guidelines4. Tackling other pathogenic variants found in next-generation sequencing (NGS) reports seems of less value. Whether the strategy of comprehensive molecular profiling—NGS,
immunohistochemistry (IHC)—of all BTCs, at least in the second-line setting, is efficient in terms of outcome parameters in real-world conditions remains unclear. Although retrospective
series suggest a clinical benefit of molecular profiling, these analyses are hampered by the local availability of targeted treatments, advances in molecular techniques that expand potential
molecular vulnerabilities, and ethnic differences in the molecular profile of BTC11,13. Therefore, the aim of this study was to assess the efficacy of matched targeted treatment in
second-line and beyond therapy in patients with BTC in an Austrian real-world multicenter cohort. MATERIALS AND METHODS PATIENTS In this trial, we conducted a retrospective chart review of
patients diagnosed with BTC at five cancer centers in Austria between September 2015 and January 2022. Patients were included in the study if they had a histologically proven diagnosis of
BTC and molecular profiling of tumor tissue. Decisions for individual molecular-matched therapeutic options were based on current scientific and clinical experience, clinical trial
availability, and approved agents at the time. Data collected from the patients’ electronic records included demographics, clinical history, surgery, local therapeutic approaches,
chemotherapy, targeted therapies, response to targeted treatment, and survival status as of September 2022. Stages were defined according to the TNM 8th edition. STATEMENT OF ETHICS Written
informed consent for molecular analysis was obtained from all patients during the routine clinical workflow, and the study protocol was approved by the local committees on human research
(Ethics Committee Land Oberoesterreich, 1100/2023 and Ethics Committee of the City of Vienna, EK-1099/2021), ensuring that it conformed to the ethical guidelines of the 1975 Declaration of
Helsinki. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. MOLECULAR PROFILING AND CLASSIFICATION OF MUTATIONS
Genetic variants were considered for statistical analysis according to criteria as stated by Cao et al.15. We tested two variant classification systems in precision oncology that are
typically used in molecular tumor boards. ESCAT, as a framework for the actionability of molecular targets16, and the National Center for Tumor Diseases (NCT) Heidelberg variant classifier
were used to rank the clinical evidence for the matched targeted therapies17,18,19. Sample collection and NGS molecular profiling were performed using tumor tissues obtained from core tumor
biopsies or surgical samples. DNA was analyzed using customized NGS panels: the TruSight Tumor 170—Illumina hybrid capture platform (170 genes for mutations) amplifications or fusions) or
the FoundationOne CDx hybrid-capture NGS service platform (324 genes for both mutations and fusions) Foundation Medicine. Inc). Additionally, data on defective mismatch repair
(dMMR)/microsatellite instability (MSI) status, PD-1 or PDL-1 and _HER2neu_ or _EGFR_ IHC were collected when reported. STUDY END POINTS Our primary objective was to assess the overall
survival (OS) of patients with ESCAT I-IV or NCT m1-m4 alterations matched to targeted drugs compared with those treated with chemotherapy. OS was defined as the time from the starting date
of first-line systemic chemotherapy to the date of death; patients without documented death on the cut-off date were censored on the date the patient was last known to be alive. The
secondary objectives were to assess (i) the time from progression or failure of first-line treatment to death or last follow-up (OS@ 2nd line) and (ii) determine the overall response rate
(ORR). Time to failure of strategy (TFS) was defined as the time from the start of the first-line chemotherapy to the start of the second-line treatment (matched targeted treatment or
non-targeted therapy equivalent to chemotherapy). PFS was defined as the time from the start of chemotherapy or matched targeted treatment to the date of the first documentation of disease
progression or death from any cause, whichever occurred. The activity of targeted treatment was further described using the PFS ratio, defined as PFS achieved on matched targeted treatment
divided by PFS or TFS observed on previous therapy. We used the modified PFS (mPFS) approach proposed by Mock et al.20. An mPFS ratio of ≥ 1.3 was considered beneficial based on the
considerations of Bailey et al. and Von Hoff et al. using each patient as his or her individual control, which is a study endpoint for precision medicine studies as recommended by health
agencies such as the European Medicines Agency21,22. Survival data derived from statistical analysis is expressed as median values. STATISTICS Descriptive statistics were used to summarize
the baseline data of all patients with the molecular test results. Logistic regression was used to estimate the odds ratio for receiving targeted treatment according to the ESCAT or NCT
classification of alterations. Survival analysis was conducted for patients who received at least first-line palliative chemotherapy, and further analysis of the benefit of targeted
therapies was restricted only to patients who received second-line treatment. Time-to-event endpoints were estimated using the Kaplan–Meier method and log-rank test for statistical
comparison. Cox proportional hazards models were used to obtain HRs with 95% confidence intervals (CIs). Multivariate Cox models were used to calculate HR with 95% CIs after adjusting for
potential confounders (age, ECOG status, sex, primary tumor location, primary tumor resection, stage at diagnosis, and local ablative therapy). The mPFS ratios were calculated according to
Mock et al., and paired comparisons were performed using the paired Wilcoxon test20. The P-values were two-sided. All statistical analyses were performed using Stata software package (Stata.
version 17.0; StataCorp and GraphPad Prism 9.0 (GraphPad Software. Inc. San Diego. CA). CONSENT TO PARTICIPATE Written informed consent for molecular analysis was obtained from each patient
during the routine clinical workflow. RESULTS PATIENT CHARACTERISTICS In our analysis, we included patients with biliary tract cancer and available comprehensive molecular characterization
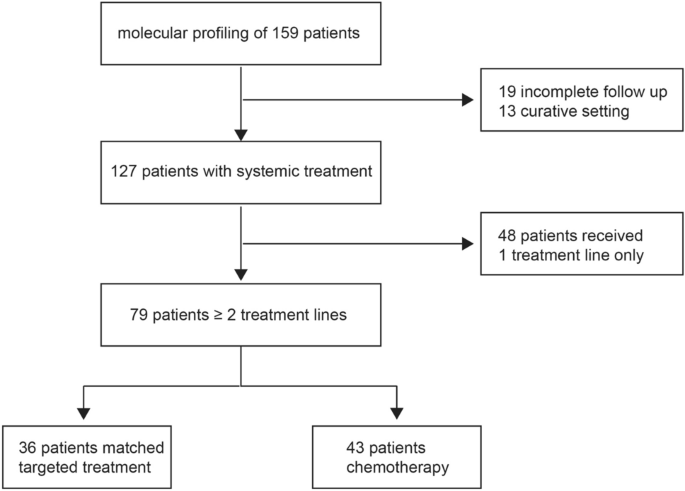
data from five Austrian cancer centers. The data cut-off date was September 2022 (Fig. 1). In total, the molecular profile of 159 patients with biliary tract cancer was available. For
further analysis, 19 patients were excluded due to incomplete clinical follow-up data, and 13 patients were excluded because they were still in the curative setting. Therefore, 127 patients
received at least one line of systemic treatment. The description of the molecular landscape of extrahepatic (eCC) and gallbladder cancer (GBC) vs. intrahepatic biliary tract cancer (iCC)
was based on this cohort (Fig. 2A,B). The three most prevalent altered genes, _TP53_ (32.6% for eCC vs. 22.2% for iCC; p = 0.21), _KRAS_ (19.6% for eCC vs.17.3% for iCC; p = 0.81), and
_CDKN2A_ (15.2% for eCC vs. 18.5% for iCC; p = 0.81), were evenly distributed. Whereas in iCC alterations in genes dedicated as ESCAT I/II (_FGFR2_ fusion, _BRAF-V600E_ mut., _Her2neu_
amplification, _IDH1_ mut., MSI and _NTRK_ Fusion) according to the latest ESMO guidelines for molecular testing in biliary tract cancer were more frequent than in eCC (27.5% vs. 10.5%; p =
0.0093) (Fig. 2A,B; Supplementary Table). The treatment patterns across lines of therapy are depicted in Fig. 3. 62.2% of patients with advanced unresectable or metastatic biliary tract
cancer proceeded to 2nd line treatment and 32.2% received at least 3rd line therapy. Reasons not proceeding to 2nd line were in most cases deterioration of ECOG performance status (N = 22;
17.3%) or death while on first line treatment (N = 17; 13.4%). After failure of first line strategy, 36 patients who received a matched targeted treatment beyond the first line were compared
in terms of efficacy outcomes with 43 patients treated with cytotoxic chemotherapy (Fig. 1). Patients who received targeted treatment were significantly younger than those who received
chemotherapy alone; 61.9 vs. 67.6 years (p = 0.012). However, this age difference became insignificant when patients not suitable for second-line therapy were excluded (61.9 vs 64.6 years; p
> 0.05). The description of the group differences between matched targeted treatment (TT) and non-targeted therapy (NTT) versus non-targeted therapy, including only patients with
second-line treatment (NTT2), is depicted in Table 1. The male patient rate was consistent between the groups (TT 69.4%; NTT 56%; NTT2 65.1%; p > 0.05). The ECOG performance status was
also balanced between the groups. We observed a higher proportion of iCC in the group of patients who were eligible for matched targeted treatment than in the chemotherapy group (TT 83.3%
vs. NTT 56% vs. NTT2 55.8; p = 0.0041; p = 0.026). No significant difference in tumor stage distribution was detected between the two groups. Patients with targeted treatment were more often
prone to primary tumor resection 41.7% vs. 33% vs. 34.9%; p = 0.412; p = 0.643) and ablative therapy (27.8% vs. 15.4% vs. 18.6%; p = 0.133; p = 0.422) during the course of the disease
(Table 1). Most patients received a platinum-based combination as the first-line systemic treatment (80.6% vs. 84.6% vs. 90.7%; p = 0.602; p = 0.214). Second-line chemotherapy consisted
mostly of irinotecan or platinum-based chemotherapy. In addition, in the targeted group, 47.2% of patients were already suitable for the matched targeted approach. In total, patients in the
matched targeted treatment group received a median of one treatment line more (three vs. two lines; p = 0.023) than the chemotherapy group suitable for at least second-line therapy (Table
1). ACTIVITY OF TARGETED TREATMENT In the matched targeted treatment group, the most prevalent target genes were _FGFR_ (36.1%), _BRAF_ (11.1%), _HER2neu_ (11.1%) and MSI (11.1%). Tier I/II
alterations were found most frequently in our cohort (23 patients, 63.9%). Among these, _FGFR2_-Fusion (30.4%), _BRAF V600E_ mutation (13%), _Her2neu_ amplification (17.4%), high PDL-1
expression (17.4%), and high MSI (17.4%) were reported. Tiers III/IV consisted of alterations in _FGFR2/3_ (38.5%), _BRCA1/2_ (23.1%), _PIK3CA_ (15.4%), _EGFR_ (15.4%), or _BRAF non V600E_
(7.7%) (Fig. 2A,B; Supplementary Table). In most patients (N = 17 or 47.2%), matched targeted treatment was initiated as second-line therapy, and 36.1% received it as 3rd line (Table 1). The
overall response (ORR) rate was 39%, with two complete responses (CR) in patients harboring MSI and 12 patients with partial remissions (PR). 6 patients (16,7%) achieved stable disease
(SD). The disease control rate (DCR) defined as CR + PR + SD was 55.6% (Fig. 4B). To further assess the clinical activity of this targeted approach, we compared the PFS of the targeted
approach with that of the previous treatment. For Tier I/II alterations, we observed a modified PFS ratio (PFStargeted/PFSpre-chemotherapy) of 1.86 that was borderline significant (p =
0.059) (Fig. 4C). We further showed that FGFR2 fusion and MSI patients had the greatest benefit, with an mPFS ratio of 2.66 (p = 0.0186) (Fig. 4A). The mPFS ratio for ESCAT III/IV
alterations was 0.77 (p = 0.946) with only 4 patients having a mPFS ratio greater than 1 (Fig. 4D). EFFICACY OF TARGETED TREATMENT Most of the patients received platinum-based first-line
therapy. The time to failure of the first-line strategy was not different between the two groups and was 4.76 months (95% CI 2.926–9.436) for the targeted group vs. 6.51 months (95% CI
4.997–8.449) in the chemotherapy group (HR 1.26; 95% CI 0.777–2.038; p = 0.35; Fig. 5C). OS in the overall cohort was 22.32 months (95% CI 14.663- 29.195) in the targeted group (N = 36) vs.
11.74 (95% CI 8.153–16.636) in the chemotherapy group (N = 91). HR of 0.45 (95% CI 0.275–0.722; p = 0.001; Fig. 5A). Further comparison of patients who received at least two lines of therapy
showed a significant OS benefit of almost 5 months for the targeted treatment group (22.32 months; 95% CI 14.663–29.195; vs. 17.49 months; 95% CI 11.74–19.79). This resulted in HR 0.58 (95%
CI 0.335–0.994; p = 0.048) (Fig. 5B). After adjusting for factors such as sex, ECOG status, primary tumor resection, localization and stage in a multivariate analysis, the OS difference
remained significant (p = 0.018) with a HR of 0.45 (95% CI 0.234–0.871; Fig. 6). After the start of second-line treatment, OS was 12.36 months (95% CI 7.792–19.167) in the matched targeted
treatment group and 8.48 months (95% CI 4.405–10.422) in the chemotherapy group (HR 0.54; 95% CI 0.309–0.926; p = 0.025; Fig. 5D). Statistical significance remained in the multivariate
analysis (HR 0.44; 95% CI 0.229–0.854; p = 0.015). EFFICACY OF TARGETED TREATMENT ACCORDING TO VARIANT CLASSIFICATION SYSTEMS IN PRECISION ONCOLOGY Subdividing the targeted group according
to the level of evidence for each group showed an OS benefit after first-line failure for ESCAT I/II compared to chemotherapy patients 12.43 months 95% CI 6.148–NR) vs. 8.48 months (95% CI
4.405–10.422 HR of 0.44; 95% CI 0.219–0.866; p = 0.018) (Fig. 7). Comparable results were obtained from NCT m1A/B classified variants; OS was 14.96 months 95% CI 6.15–NR) (Supplementary
Figure). For ESCAT III/IV alteration, OS was only numerically different with 12.36 months 95% CI 7.364–19.167; HR 0.78 95% CI 0.396–1.555; p = 0.47 compared to the chemotherapy group (Fig.
7). Among ESCAT I/II alterations, _FGFR2_ fusion-positive tumors treated with Pemigatinib and MSI tumors treated with checkpoint inhibitors showed the greatest benefit (N = 11; OS NR; 95% CI
9.863-NR) (Fig. 7). DISCUSSION The main focus of the trial was to demonstrate the efficacy of precision medicine in BTC in routine clinical practice, as the current evidence is
predominantly derived from Asian cohorts, patients from phase 1/2 clinical trials, or distinct anatomical subtypes13,14,23,24. Recommendations for targeted therapies as proposed, for
example, in molecular tumor boards are often hampered by missing availability of the matched treatment due to the lack of cost coverage by insurance companies, clinical deterioration during
NGS turnaround times, or low evidence levels influencing the treating physician or patients25. In our cohort, 28% of the patients in the metastatic setting with complete molecular profiling
were candidates for precision oncology. This percentage is slightly lower than the assumed 40–50% of potential exploitable targets in BTC3. In our setting, this could simply be due to the
lack of available matched treatment for _IDH1/2_, _KRAS G12C_ or _MET_ amplification, as well as some missed opportunities in _FGFR2_ altered or MSI high patients. The overall molecular
makeup of our cohort reflected the results described in the literature. The reason for the lower frequency of _IDH1/2_ mutations in our patients remains elusive. OS BENEFIT OF OVERALL
STRATEGY Our trial showed that the strategy of advanced molecular characterization in BTC almost doubled the OS compared with sequential chemotherapy. After excluding patients in the
chemotherapy cohort who were not able to receive second-line treatment (due to rapid tumor progression, worsening of the ECOG performance status, decline of further cytostatic treatment, or
missing target for second-line targeted treatment), we compared a more homogenous group of patients receiving at least two lines of systemic treatment. An OS benefit of 5 months was
observed. As the time to failure of the first-line strategy was not different between the groups, we could clearly delineate that the OS advantage depends on the implementation of molecular
informed treatments beyond the first-line. This provided a OS benefit of over 4 months when second-line treatment was initiated. The OS time beyond failure of first-line strategy of 8.5
months was similar to results reported from prospective ABC trials or retrospective real-world cohorts6,7,26. Therefore, the underperformance of our control group can largely be excluded.
EFFICACY ACCORDING TO ESCAT CLASSIFICATION André et al. showed that the clinical benefit of matched targeted treatment for breast cancer seems to be confined to ESCAT I/II tiers27. The
results from Verdaguer et al. suggest that a benefit is derived for a cohort of BTC from higher-ranked ESCAT recommendations14. In a subgroup analysis of our data, we can show the dominance
of ESCAT I and II tiers, a result that is maintained when applying a different variant classification system (the NCT variant classifier). The choice of a Tier I/II-matched therapy as a
modified mPFS to correct for false-positive or false-negative results, as shown by Mock et al., met the threshold of 1.3, which is commonly used in precision oncology to determine the
benefit of matched targeted treatment20. These improved PFS ratios were reflected in an OS advantage. For the subgroup of _FGFR2_ Fusion or MSI patients, the mPFS ratio was even more
pronounced, suggesting that OS improvement might predominantly be based on _FGFR2_ Fusion and MSI patients, resulting in even more granularity in ESCTA I/II tiers. The clinical benefits of
Tiers III and IV remain controversial. Our results still advocate discussing III/IV in molecular tumor boards and applying matched therapy may be beyond second-line or searching for
available clinical trials. SINGLE TARGETS The results for MSI or _FGFR2_ Fusion are in line with smaller prospective molecular-selected trials showing an OS advantage in MSI patients or
_FGFR2_ Fusion patients in contrast to other class I or II targets28,29,30. We cannot exclude the possibility that _FGFR2_ Alterations have an additional prognostic impact, in addition to
their predictive role for _FGFR2_ inhibitors31,32. PDL1 blockade, in addition to MSI based on PDL1 expression as a stratification marker, is a class II recommendation based on smaller phase
II trials33. On an individual basis, we observed responses lasting up to 8 months. These results reinforce the implementation of checkpoint inhibition in CCA, with or without chemotherapy.
In contrast, we did not find targeting _Her2neu_ with Trastuzumab +/− Pertuzumab despite promising phase II data34. All four patients showed tumor progression. One reason might be that
different co-mutations affect the clinical outcome parameters. However, according to the data from ASCO 2022 and reports from other tumor types, targeting _HER2neu_ with
Trastuzumab-deruxtecan would probably yield better results35. Unfortunately, blocking of _IDH1_ was not available in Europe. Access to _IDH1_ inhibitors, such as Ivosidenib, will further
expand the therapeutic options and may further improve the clinical benefit for patients with BTC. LIMITATIONS A drawback of our trial was that MSI and TMB testing was not performed
routinely in every patient; thus, patients who may have been candidates for checkpoint inhibitors were potentially missed. Furthermore, the extent of the NGS panel was not consistent in our
patients. This reflects the diagnostic advances in molecular techniques over time and the different availability of NGS panel diagnostics in various centers. Further limitations are due to
the retrospective nature of our analysis and, presumably, tumor spatial and temporal heterogeneity. Ct-DNA isolated from blood or bile samples could overcome these limitations. SUMMARY Our
results underscore the value of comprehensive molecular testing for BTC and reinforce the statement of the ESMO guidelines that every patient with BTC should undergo molecular
characterization. We clearly showed that, in contrast to unmatched cytotoxic chemotherapy, the application of molecular matched targeted treatment leads to an OS benefit in a real-world
setting. Further research must focus on the resistance mechanisms of the targeted agents and how to overcome these limitations. DATA AVAILABILITY The datasets used and/or analysed during the
current study are available from the corresponding author on reasonable request. REFERENCES * Izquierdo-Sanchez, L. _et al._ Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic
and therapeutic insights from the ENSCCA Registry. _J. Hepatol._ 76, 1109–1121 (2022). Article PubMed CAS Google Scholar * Clements, O., Eliahoo, J., Kim, J. U., Taylor-Robinson, S. D.
& Khan, S. A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. _J. Hepatol._ 72, 95–103 (2020). Article PubMed Google Scholar
* Valle, J. W., Kelley, R. K., Nervi, B., Oh, D.-Y. & Zhu, A. X. Biliary tract cancer. _Lancet_ 397, 428–444 (2021). Article PubMed CAS Google Scholar * Vogel, A. _et al._ Biliary
tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. _Ann. Oncol._ https://doi.org/10.1016/j.annonc.2022.10.506 (2022). Article PubMed Google Scholar *
Oh, D.-Y. _et al._ Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. _Nejm Évid_ 1, (2022). * Moik, F. _et al._ Benefit of second-line systemic chemotherapy for
advanced biliary tract cancer: A propensity score analysis. _Sci. Rep._ 9, 5548 (2019). Article ADS PubMed PubMed Central Google Scholar * Lamarca, A. _et al._ Second-line FOLFOX
chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. _Lancet Oncol._ 22, 690–701 (2021). Article
PubMed PubMed Central CAS Google Scholar * Yoo, C. _et al._ Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer
after progression on gemcitabine plus cisplatin (NIFTY): A multicentre, open-label, randomised, phase 2b study. _Lancet Oncol._ 22, 1560–1572 (2021). Article PubMed CAS Google Scholar *
Jan, Y.-H. _et al._ Comprehensive assessment of actionable genomic alterations in primary colorectal carcinoma using targeted next-generation sequencing. _Br. J. Cancer_ 127, 1304–1311
(2022). Article PubMed PubMed Central CAS Google Scholar * Taghizadeh, H., Müllauer, L., Mader, R. & Prager, G. W. Applied precision cancer medicine in metastatic biliary tract
cancer. _Hepatol. Int._ 14, 288–295 (2020). Article PubMed CAS Google Scholar * Verlingue, L. _et al._ Precision medicine for patients with advanced biliary tract cancers: An effective
strategy within the prospective MOSCATO-01 trial. _Eur. J. Cancer_ 87, 122–130 (2017). Article PubMed Google Scholar * Subbiah, V. _et al._ Dabrafenib plus trametinib in patients with
BRAF V600E-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. _Lancet Oncol._ 21, 1234–1243 (2020). Article PubMed CAS Google Scholar *
Tomczak, A. _et al._ Precision oncology for intrahepatic cholangiocarcinoma in clinical practice. _Br. J. Cancer_ 127, 1701–1708 (2022). Article PubMed PubMed Central CAS Google Scholar
* Verdaguer, H. _et al._ ESMO Scale for Clinical Actionability of Molecular Targets driving targeted treatment in patients with cholangiocarcinoma. _Clin. Cancer Res._ 28, 1662–1671
(2022). Article PubMed CAS Google Scholar * Cao, J. _et al._ Intrahepatic cholangiocarcinoma: Genomic heterogeneity between eastern and western patients. _Jco Precis. Oncol._ 4,
PO.18.00414 (2020). PubMed PubMed Central Google Scholar * Mateo, J. _et al._ A framework to rank genomic alterations as targets for cancer precision medicine: The ESMO scale for clinical
actionability of molecular targets (ESCAT). _Ann. Oncol._ 29, 1895–1902 (2018). Article PubMed PubMed Central CAS Google Scholar * Horak, P. _et al._ Precision oncology based on omics
data: The NCT Heidelberg experience. _Int. J. Cancer_ 141, 877–886 (2017). Article PubMed CAS Google Scholar * Horak, P. _et al._ Standards for the classification of pathogenicity of
somatic variants in cancer (oncogenicity): Joint recommendations of Clinical Genome Resource (ClinGen), Cancer Genomics Consortium (CGC), and Variant Interpretation for Cancer Consortium
(VICC). _Genet. Med._ 24, 986–998 (2022). Article PubMed PubMed Central CAS Google Scholar * Leichsenring, J. _et al._ Variant classification in precision oncology. _Int. J. Cancer_
145, 2996–3010 (2019). Article PubMed CAS Google Scholar * Mock, A. _et al._ Community-driven development of a modified progression-free survival ratio for precision oncology. _Esmo
Open_ 4, e000583 (2019). Article PubMed PubMed Central Google Scholar * Bailey, C. H. _et al._ Progression-free survival decreases with each subsequent therapy in patients presenting for
phase I clinical trials. _J. Cancer_ 3, 7–13 (2011). Article PubMed PubMed Central Google Scholar * Hoff, D. D. V. _et al._ Pilot study using molecular profiling of patients’ tumors to
find potential targets and select treatments for their refractory cancers. _J. Clin. Oncol._ 28, 4877–4883 (2010). Article Google Scholar * Takada, K. _et al._ Effect of comprehensive
cancer genomic profiling on therapeutic strategies and clinical outcomes in patients with advanced biliary tract cancer: A prospective multicenter study. _Front. Oncol._ 12, 988527 (2022).
Article PubMed PubMed Central CAS Google Scholar * Gray, S. _et al._ Targeted therapies for perihilar cholangiocarcinoma. _Cancers_ 14, 1789 (2022). Article PubMed PubMed Central CAS
Google Scholar * Heinrich, K. _et al._ Lessons learned: The first consecutive 1000 patients of the CCCMunichLMU Molecular Tumor Board. _J. Cancer Res. Clin._
https://doi.org/10.1007/s00432-022-04165-0 (2022). Article Google Scholar * Lamarca, A., Hubner, R. A., Ryder, W. D. & Valle, J. W. Second-line chemotherapy in advanced biliary cancer:
A systematic review. _Ann. Oncol._ 25, 2328–2338 (2014). Article PubMed CAS Google Scholar * Andre, F. _et al._ Genomics to select treatment for patients with metastatic breast cancer.
_Nature_ 610, 343–348 (2022). Article ADS PubMed CAS Google Scholar * Marabelle, A. _et al._ Efficacy of pembrolizumab in patients with noncolorectal high microsatellite
instability/mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 Study. _J Clin Oncol_ 38, 1–10 (2020). Article PubMed CAS Google Scholar * Maio, M. _et al._
Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: Updated analysis from the phase II KEYNOTE-158 study. _Ann. Oncol._ 33, 929–938 (2022). Article PubMed
CAS Google Scholar * Abou-Alfa, G. K. _et al._ Pemigatinib for previously treated locally advanced/metastatic cholangiocarcinoma (CCA): Update of FIGHT-202. _J. Clin. Oncol._ 39,
4086–4086 (2021). Article Google Scholar * Pavicevic, S. _et al._ Prognostic and predictive molecular markers in cholangiocarcinoma. _Cancers_ 14, 1026 (2022). Article PubMed PubMed
Central CAS Google Scholar * Rizzato, M. _et al._ Prognostic impact of FGFR2/3 alterations in patients with biliary tract cancers receiving systemic chemotherapy: The BITCOIN study. _Eur.
J. Cancer Oxf. Engl._ 1990(166), 165–175 (2021). Google Scholar * Kim, R. D. _et al._ A phase 2 multi-institutional study of nivolumab for patients with advanced refractory biliary tract
cancer. _JAMA Oncol._ 6, 888–894 (2020). Article PubMed Google Scholar * Lee, C. _et al._ A phase II trial of trastuzumab plus modified-FOLFOX for gemcitabine/cisplatin refractory
HER2-positive biliary tract cancer (BTC): Multi-institutional study of the Korean Cancer Study Group (KCSG-HB19–14). _J. Clin. Oncol._ 39, 4161 (2021). Article Google Scholar * Ohba, A.
_et al._ Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): An investigator-initiated multicenter phase 2
study (HERB trial). _J. Clin. Oncol._ 40, 4006–4006 (2022). Article Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Internal Medicine I for
Hematology with Stem Cell Transplantation, Hemostaseology, and Medical Oncology, Ordensklinikum Linz, Seilerstaette 4, 4010, Linz, Austria Bernhard Doleschal & Andreas Petzer *
Department of Internal Medicine, University Hospital St. Pölten, St. Pölten, Austria Hossein Taghizadeh * Laboratory for Molecular Genetic Diagnostics, Ordensklinikum Linz, Linz, Austria
Gerald Webersinke * Department of Oncology and Hematology, Kepler University Clinic Linz, Linz, Austria Gudrun Piringer & Clemens A. Schmitt * Medical Faculty, Johannes Kepler University
Linz, Linz, Austria Gudrun Piringer, Patrick Kirchweger, Clemens A. Schmitt & Holger Rumpold * Department of Internal Medicine, State Hospital Pyhrn Eisenwurzen, Steyr, Austria Georg
Schreil * Department of Internal Medicine, State Hospital Rohrbach, Rohrbach, Austria Jörn Decker & Karl J. Aichberger * Department of General and Visceral Surgery, Ordensklinikum Linz,
Linz, Austria Patrick Kirchweger * Gastrointestinal Cancer Center, Ordensklinikum Linz, Linz, Austria Patrick Kirchweger & Holger Rumpold * Department of Internal Medicine IV, Hospital
Wels-Grieskirchen, Wels, Austria Josef Thaler * Division of Oncology, Department of Medicine I, Medical University Vienna, Vienna, Austria Gerald W. Prager Authors * Bernhard Doleschal View
author publications You can also search for this author inPubMed Google Scholar * Hossein Taghizadeh View author publications You can also search for this author inPubMed Google Scholar *
Gerald Webersinke View author publications You can also search for this author inPubMed Google Scholar * Gudrun Piringer View author publications You can also search for this author inPubMed
Google Scholar * Georg Schreil View author publications You can also search for this author inPubMed Google Scholar * Jörn Decker View author publications You can also search for this
author inPubMed Google Scholar * Karl J. Aichberger View author publications You can also search for this author inPubMed Google Scholar * Patrick Kirchweger View author publications You can
also search for this author inPubMed Google Scholar * Josef Thaler View author publications You can also search for this author inPubMed Google Scholar * Andreas Petzer View author
publications You can also search for this author inPubMed Google Scholar * Clemens A. Schmitt View author publications You can also search for this author inPubMed Google Scholar * Gerald W.
Prager View author publications You can also search for this author inPubMed Google Scholar * Holger Rumpold View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS B.D., H.T., G.W., G.P., G.S., J.D, K.J.A., P.K., J.T. provided the data. B.D. and H.R. drafted the manuscript. H.R. and B.D. did the statistical analysis. H.R.
conceptualization and supervising. B.D., H.T., G.W.P., C.A.S. and H.R. contributed to the interpretation of the data. B.D., H.T., G.P., A.P., J.T., C.A.S., K.J.A., G.W.P. and H.R. revised
the manuscript. The final version was approved by all authors. All authors give consent for publication. CORRESPONDING AUTHOR Correspondence to Bernhard Doleschal. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published
maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY LEGENDS. SUPPLEMENTARY FIGURE 1. SUPPLEMENTARY TABLE 1. RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in
this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's
Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Doleschal, B., Taghizadeh, H., Webersinke, G.
_et al._ Real world evidence reveals improved survival outcomes in biliary tract cancer through molecular matched targeted treatment. _Sci Rep_ 13, 15421 (2023).
https://doi.org/10.1038/s41598-023-42083-4 Download citation * Received: 18 July 2023 * Accepted: 05 September 2023 * Published: 18 September 2023 * DOI:
https://doi.org/10.1038/s41598-023-42083-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative






