- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT There are many surgical techniques (packing, Pringle maneuver, etc.) and hemostatic agents to manage hepatic bleeding in trauma surgery. This study compares the effectiveness of two
different types of hemostatic agents, one is an active flowable hemostat and the other is a passive hemostat made of modified absorbable polymers [MAP]. Both surgical technique and
hemostatic agents can be used together as a means of controlling bleeding. We have hypothesized that a single hemostatic agent might be as effective as a unique hemostatic surgical
technique. Twenty swine were prospectively randomized to receive either active Flowable (Floseal) or passive MAP powder (PerClot) hemostatic agents. We used a novel severe liver injury model
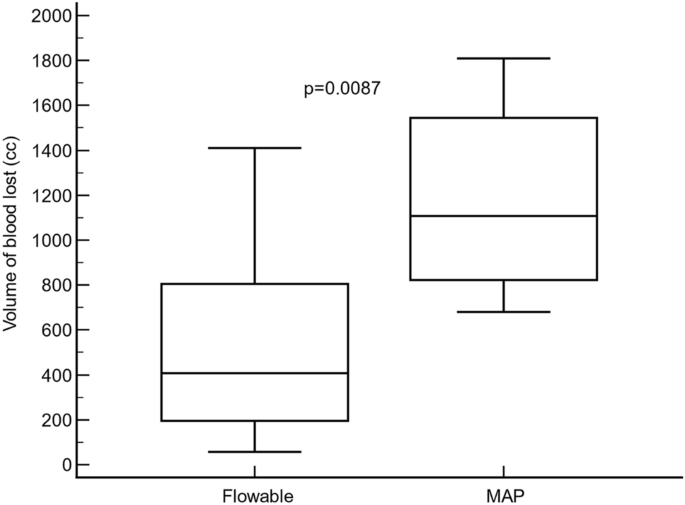
that caused exsanguinating hemorrhage. The main outcome measure was total blood loss volume. The total volume of blood loss, from hepatic injury to minute 120, was significantly lower in
the Flowable group (407.5 cm3; IqR: 195.0–805.0 cm3) compared to MAP group (1107.5 cm3; IqR: 822.5 to 1544.5 cm3) (Hodges–Lehmann median difference: − 645.0 cm3; 95% CI: − 1144.0 to − 280.0
cm3; p = 0.0087). The rate of blood loss was significantly lower in the flowable group compared with the MAP group as measured from time of injury to minutes 3, 9, 12, and 120 (except for 6
min). The mean arterial pressure gradually recovered in the flowable group by 24 h, whereas in the MAP group, the mean arterial pressure was consistently stayed below baseline values.
Kaplan–Meier survival analysis indicated similar rates of death between study groups (Logrank test p = 0.3395). Both the flowable and the MAP hemostatic agents were able to effectively
control surgical bleeding in a novel severe liver injury model, however, the flowable gelatin–thrombin agent provided quicker and better bleed control. SIMILAR CONTENT BEING VIEWED BY OTHERS
AN INJECTABLE, EXPANDABLE POLYACRYLAMIDE CRYOGEL DECREASES BLOOD LOSS AND IMPROVES SURVIVAL IN A PORCINE MODEL OF JUNCTIONAL HEMORRHAGE Article Open access 08 February 2025 EFFICACY AND
SAFETY EVALUATION OF A NOVEL HEMOSTATIC GELATIN MATRIX FOR INTRAOPERATIVE HEMOSTASIS: A PROSPECTIVE, RANDOMIZED CONTROLLED TRIAL Article Open access 09 November 2024 A COMPARATIVE ANALYSIS
OF COLLAGEN BASED DRESSINGS AND POLYSACCHARIDE MICROSPHERES FOR HEMOSTASIS MANAGEMENT IN HEPATIC STAB WOUNDS Article Open access 30 January 2025 INTRODUCTION Bleeding is a prevalent
complication of liver surgery that negatively affects clinical outcomes. Surgical bleeding of the liver can lead to a significant increase in morbidity and mortality rates, longer surgical
procedures, longer hospital stays, and increased costs1,2,3. Therefore, reducing surgical bleeding may positively impact clinical and economic aspects1,4. Different strategies have been used
to reduce surgical bleeding, specifically, hemostatic products have been used and classified in different ways based on their mechanism of action. One of the most widely used
classifications for hemostatic products is based on whether the product provides a physical structure around which platelets can aggregate to form a clot (passive) or whether the product
delivers its mechanism of action on the clotting cascade in a biologically active manner (active)5. Among these, topical hemostatic agents have been used to improve surgical hemostasis1,6.
Currently, various hemostatic agents are available as adjunctive measures to control surgical bleeding. Some active agents, including fibrinogen and thrombin, actively participate at the end
of the coagulation cascade to form fibrin clots7,8,9. These agents can be effectively used in patients with spontaneous or drug-induced coagulation disorders and are effective for a wide
range of bleeding rates (including pulsatile arterial bleeding)5,9. Others, such as porcine gelatin10, oxidized cellulose11, and plant-derived polysaccharide spheres12 are known as passive
hemostatic agents. They activate and aggregate platelets and form a matrix at the site of bleeding, allowing clotting to occur9. Their efficacy depends on the patient’s own fibrin production
to achieve hemostasis, so they are only appropriate for patients who have an intact coagulation system9,10,11,12. In previous studies, we investigated the ease of use and strength of a
novel modified absorbable polymer (MAP) powder (PerClot) compared with a surgical technique13. The purpose of the current study was to compare this novel MAP powder hemostatic agent with a
well-known gelatin–thrombin flowable product, as a single hemostatic treatment, without any other surgical technique, in the same severe experimental liver hemorrhage model. MATERIALS AND
METHODS STUDY DESIGN This prospective, randomized, and experimental study was conducted on 20 female swine (Large White) in the Surgical Research Unit of the Hospital Central de la Defensa
“Gómez Ulla” (Madrid, Spain). The study protocol (Register number: ES280790000187) was approved by the Ethics Committee, Hospital Central de la Defensa Education Committee, and Council of
the Environment of the Community of Madrid. The study was conducted in accordance with the Spanish and European legislation regarding animal experimentation, and all methods were reported in
accordance with the ARRIVE guidelines. At the end of the study (24 h after the procedure), the animals were euthanized with an anesthetic overdose in accordance with the current
legislation. STUDY ANIMALS Twenty healthy female swine (Sus corfa, Large White), with an average weight of 35.5 kg (33.5–40.0 kg), underwent a quarantine period, and a veterinary examination
to rule out the presence of any underlying disease prior to study start. STUDY GROUPS Animals were randomly assigned to the Flowable group receiving treatment with a gelatin–thrombin
flowable hemostatic agent (Floseal, Baxter Healthcare Corporation Hayward, CA, USA) utilizing two 5 mL syringes, for a total of 10 mL or the MAP group receiving a MAP powder hemostatic agent
(PerClot, Polysaccharide Hemostatic System, Baxter Healthcare Corporation Deerfield, IL, USA) receiving two packages of 5 g each, with a total of 10 g. PROCEDURES ANESTHETIC PROCEDURE AND
MONITORING Anesthesia (atropine sulfate, azaperone, midazolam, ketamine 2%, sevoflurane, fentanyl, and phenylephrine) and fluids were administered via a venous line. A tube ranging from 6.5
to 7.5 mm connected to a ventilator (rate 12–15 breaths/min), was used to intubate the animals. The animals were monitored using electrocardiography, pulse oximetry, vaginal temperature
probe, and capnography. In addition, a femoral arterial probe was used for the invasive monitoring of blood pressure and heart rate. Balanced saline solution was administered prior to the
surgical procedure in order to assure both groups were equally hydrated. SURGICAL PROCEDURE After performing an extended right subcostal laparotomy, the middle (segment IV) and left
(segments II and III) suprahepatic veins were located using echo-Doppler (Logic V2; General Electric, Chicago, IL, USA), where two incisions, 2 cm long and 5 cm deep, were made on the liver
parenchyma using a No. 20 scalpel blade. Post-mortem, the investigators verified that both veins had been completely sectioned. After injury, hemostatic agents were applied according to the
manufacturer’s instructions14,15. Two 5 mL syringes, with a total of 10 mL of gelatin–thrombin flowable hemostatic agent, were applied. For MAP, two packages of 5 g each for a total of 10 g
were applied directly, applying pressure to the injury site for 3 min. No further product or compression was applied, and hemostasis was evaluated at 6, 9, 12, 60, and 120 min. In this
method, hemostatic agents were prepared prior to hepatic injury, to ensure the time required to apply the hemostatic compound was consistent for both groups. Ringer Lactate Solution (RLS)
was administered to restore hydration and fluid balance. To equalize the volume of administered RLS to the measured blood lost by the liver injury, RLS fluid was given at a rate of 1000
ml/h, starting after hepatic injury to minute 12. This was followed by a 500 ml/h infusion from minute 13 to minute 60. For the time period between minute 61 and minute 120, the rate of RLS
administration was calculated in order to complete the reposition volume. By minute 120, the animals received an equal volume of RLS in accordance with how much blood was lost. The blood was
removed using a surgical aspirator (Flexivac®) and gauze packing pads. The volume of blood loss was calculated using the following formula: “v = [(b1 − a1) + (b2 − a2)]/1.04”, where “b1”
was the weight of the tank of the surgical aspirator loaded with blood, “a1” was the dry weight of the tank (without blood), “b2” was the weight of the surgical pads soaked in blood,“a2” was
the dry weight of the surgical pads (without blood) and 1.04 was the numerical constant representing the blood density of the swine model16. OUTCOMES PRIMARY OUTCOME The primary endpoint
was measured as the total volume of blood lost in the time period after hepatic injury to minute 120. SECONDARY OUTCOMES Secondary outcome measures included: the proportion of animals in
which hemostasis was achieved at minute 3, 6, 9, 12, 60, and 120, heart rate (beats per minute, bpm), mean arterial pressure, and survival rate at 120 min. The amount of time required to
apply the hemostatic product was recorded and used as an indicator to measure the difficulty in applying the hemostatic agent. It was assumed that a longer application time correlated with a
greater degree of difficulty. STATISTICAL ANALYSIS A standard statistical analysis was performed using MedCalc® Statistical Software version 20.218 (MedCalc Software Ltd, Ostend, Belgium;
https://www.medcalc.org; 2023). Descriptive statistics such as number (percentage) and median (interquartile range, IqR) were used as appropriate. Intragroup comparisons of blood loss and
hemodynamic parameters were performed using the Friedman’s two-way analysis test. The Mann–Whitney _U_ test was used to compare the different parameters between the flowable and MAP groups.
Survival rates were plotted for the study groups using Kaplan–Meier analysis and compared using a Log-rank test. Linear regression analysis was used to assess the relationship between the
time of application (independent variable) and the volume of blood lost at 120 min (dependent variable). Categorical variables were compared using the chi-square test and Fisher’s exact
test, as needed. A p-value of less than 0.05 was considered significant. Animals that died during the study were entered as 0 to denote a censored observation. ETHICAL APPROVAL The study
protocol (Register number: ES280790000187) was approved by the Ethics Committee, Hospital Central de la Defensa Education Committee, and Council of the Environment of the Community of
Madrid. The study was conducted in accordance with the Spanish and European legislation regarding animal experimentation, and all methods were reported in accordance with the ARRIVE
guidelines. RESULTS PREOPERATIVE VALUES Twenty-four female swine were enrolled in the study, but four of the flowable group animals did not pass the quarantine. Eight (40.0%) pigs in the
flowable group and twelve (60.0%) pigs in the MAP group were included in the study. In the overall study sample, the median (IqR) body weight was 35.5 kg (range: 33.5–40.0 kg), with no
significant differences between groups (Hodges–Lehmann median difference: 3.3 kg; 95% confidence interval: − 2.5 to 9.5 kg; p < 0.2022). The baseline clinical characteristics of the
animals are summarized in Table 1. Briefly, the baseline variables were comparable between groups, with the exception of the volume of balanced saline solution administered, which was
significantly greater in the MAP group (Hodges–Lehmann median difference: 167.5 cm3; 95% CI: 89.0–306.7 cm3; p < 0.0009) (Table 1). PRIMARY ENDPOINT The total volume of blood loss, from
hepatic injury to minute 120, was significantly lower in the flowable group (407.5 cm3; IqR: 195.0–805.0 cm3) compared to the MAP group (1107.5 cm3; IqR: 822.5–1544.5 cm3) (Hodges–Lehmann
median difference: − 645.0 cm3; 95% CI: − 1144.0 to − 280.0 cm3; p < 0.0087) (Fig. 1). SECONDARY OUTCOMES In the flowable group, the median (IqR) blood volume lost was 240.0 cm3
(120.0–415.0 cm3), from hepatic injury to minute 3; 70.0 cm3 (27.5–117.5 cm3) from minute 3 to minute 6; 27.5 cm3 (10.0–75.0 cm3) from minute 6 to minute 9; 20.0 cm3 (5.0–60.0 cm3) from
minute 9 to minute 12; 22.5 cm3 (5.0–100.0 cm3) from minute 12 to minute 60; and 10.0 cm3 (1.3–10.0 cm3) from minute 60 to minute 120 (p < 0.0001, Friedman rank sum test). In the MAP
group, the median (IqR) blood volume lost was 570.0 cm3 (480.0–660.0 cm3) from injury to minute 3; 115.0 cm3 (77.5–207.5 cm3) from minute 3 to minute 6; 107.5 cm3 (77.5–175.0 cm3) from
minute 6 to minute 9; 85.0 cm3 (52.5–232.5 cm3) from minute 9 to minute 12; 50.0 cm3 (35.0–215.0 cm3) from minute 12 to minute 60; and 410.0 cm3 (35.0–53.8 cm3) from minute 60 to minute 120
(p < 0.0001, Friedman rank sum test). With the exception of the minute 6 measurement (Hodges–Lehmann median difference: − 57.0 cm3; 95% CI: − 155.0 to 10.0 cm3, p < 0.1136), the volume
of blood lost was significantly lower in the flowable group compared with the MAP group from injury to minute 3 (Hodges–Lehmann median difference: − 327.5 cm3; 95% CI: − 490.0 to − 90.0
cm3, p < 0.0097); from minute 6 to minute 9 (Hodges–Lehmann median difference: − 80.0 cm3; 95% CI: − 140.0 to − 40.0 cm3, p < 0.0017); from minute 9 to minute 12 (Hodges–Lehmann median
difference: − 60.0 cm3; 95% CI: − 200.0 to − 15.0 cm3, p < 0.0205); from minute 12 to minute 60 (Hodges–Lehmann median difference: − 35.0 cm3; 95% CI: − 175.0 to − 5.0 cm3, p <
0.0304); and from minute 60 to minute 120 (Hodges–Lehmann median difference: − 35.0 cm3; 95% CI: − 50.0 to − 10.0 cm3, p < 0.0176) (Fig. 2). In the flowable group, the mean arterial
pressure was significantly higher at minutes 3, 6, 9, 12, and 60 compared to the MAP group (Fig. 3A). The mean arterial pressure gradually recovered in the flowable group by 24 h, whereas in
the MAP group, the mean arterial pressure was consistently below baseline values (Fig. 3A). The heart rate remained stable throughout the study in both the flowable (Friedman test, p <
0.9983) and MAP groups (Friedman test, p < 0.7979), with no significant differences between the study groups at any of the time points measured (Fig. 3B). Kaplan–Meier survival analysis
indicated similar rates of death between the study groups (hazard ratio: 0.41; 95% CI: 0.07–2.52); Logrank test p < 0.3395) (Fig. 4). The application time was similar in the flowable
(median: 28.0 s; IqR: 21.0–33.0 s) and in the MAP (median: 32.0 s; IqR: 29.0–37.5 s) groups (Hodges–Lehmann median difference: − 4.5 s; 95% confidence interval: − 12.0 to 3.0 s; p <
0.1747). Linear regression analysis did not show a significant correlation between the time needed for application and the volume of blood lost from injury to minute 120 in either the
flowable group (R2 = 0.001, p = 0.9376, regression line slope − 1.6 cm3/s; where 95% CI ranged from − 49.7 to 46.5 cm3/s) nor in the MAP group (R2 = 0.230, p = 0.1145, regression line slope
29.1 cm3/s; where 95% CI ranged from − 8.4 to 66.6 cm3/s). Overall, there were no significant differences in the slopes between the two groups (mean difference, − 30.7; standard error, 25.6;
p < 0.2481) (Fig. 5). Similarly, there was not a correlation between the time needed for application and the volume of blood lost from injury to minute 12 in either the flowable group
(R2 = 0.00, p < 0.9205, regression line slope − 0.0 cm3/s; where 95% CI ranged from − 0.03 to 0.03 cm3/s) nor in the MAP group (R2 = 0.26, p < 0.0900, regression line slope 0.01 cm3/s;
where 95% CI ranged from − 0.00 to 0.02 cm3/s). Overall, there were no significant differences in the slopes between the two groups (mean difference: − 0.01, standard error, 0.01; p <
0.3322). DISCUSSION ACTIVE OR PASSIVE HEMOSTATIC AGENT? Hemostasis may be defined as a multilayered cellular and molecular response that stops hemorrhage at the site of tissue injury. The
capacity to improve hemostasis during surgical procedures is a key factor in preventing blood loss, reducing perioperative morbidity and surgery times, and improving surgical outcomes17. A
variety of these hemostatic agents are available, and have been categorizing based on their mechanism of action. In general, hemostatic agents can be divided into active and passive
hemostatic agents, depending on how they interact within a patient’s coagulation cascade, and both groups have utility in different procedures as adjunctive therapies for controlling
surgical bleeding when conventional methods are inefficient or impractical5. Active hemostatic agents provide their mechanism of action at the end of the clotting cascade in a biological
manner bypassing the initial steps and facilitating formation of the fibrin clot even when other aspects of the coagulation cascade may be dysfunctional. Whereas, passive hemostatic agents
are most effective for minimal bleeding scenarios and rely on a patient having an intact coagulation cascade so they can activate platelets by providing a structure where platelets can
aggregate and activate while relying on the patient’s intact coagulation cascade and ability to produce clotting factors to support clot formation5. Various novel hemostatic agents have been
developed for use in open and laparoscopic procedures9,18,19. In the active hemostatic group, we chose a flowable gelatin–thrombin-based product because it is a highly efficacious topical
hemostatic agent18,20. Floseal is a well-known flowable agent composed of cross-linked hydrolyzed bovine gelatin (500–600 µm particles) and human thrombin (500 IU/mL)14. In the current
study, the evaluated passive hemostatic agent was PerClot. We chose this product because it has improved chemical characteristics over previous MAP and it has also shown to improve survival
and blood loss when compared to a surgical technique (perihepatic packing) in swine13. PerClot is composed of modified absorbable polymer (MAP) granules that have a molecular structure that
rapidly absorbs water, forming a gelled adhesive matrix15. It is a passive hemostatic agent that provides a mechanical barrier against further bleeding and results in the accumulation of
platelets, red blood cells, and coagulation proteins (thrombin, fibrinogen, etc.) while providing a physical structure around which platelets can aggregate and promote the normal
physiological clotting cascade5,21, and it has been designed as an adjunct hemostatic to control bleeding during surgical procedures or following traumatic injuries17. WHICH HEMOSTATIC AGENT
WORKS BETTER ALONE? To the best of our knowledge, this is the first study to compare PerClot, a passive hemostatic agent, with Floseal, an active hemostatic agent, in a novel and severe
experimental liver injury model, without any other surgical maneuver. Other authors used hemostasis under greatly reduced flow conditions as the products were applied during a perihepatic
packing or Pringle maneuver22. In one study, a hemostatic agent was used in addition to a surgical technique, making it very difficult to decipher whether the reduction of blood loss was due
to the hemostatic agent, or the surgical technique, or a combination of both23. Furthermore, if hemostasis is used as a single treatment, complications associated with other surgical
techniques, such as increases in abdominal pressure23,24 or intra-abdominal abscess25 may be avoided. SIMILARITIES Both products performed sufficiently in this study of severe hemorrhage, as
we did not find significant differences between the groups in terms of survival rates, heart rate, arterial pressure, or application time of the hemostatic agent (Figs. 3, 4). In addition,
both hemostatic agents effectively controlled bleeding by 12 min. In this study, the application time was used as a surrogate to measure ease of usability of the products. We did not find
any significant differences in the speed of application between groups. Moreover, we did not find a correlation between the time taken to apply the hemostatic agent and the volume of blood
lost at minutes 12 and 120, in either group (Fig. 5). DIFFERENCES The primary endpoint of this study was the overall difference in blood loss, as it was hypothesized that the volume of blood
loss could be a good parameter for analyzing hemostatic strength. We observed that Floseal had a stronger hemostatic effect compared with PerClot, due to the fact that the flowable group
resulted in less blood loss (407.5 cm3) compared with the powder group (1107.5 cm3) (p < 0.0087). (Fig. 1). Moreover, Floseal was faster than PerClot in achieving hemostasis, and its
effect was more stable in the first few minutes. A hemostatic effect was observed as early as minute 3, giving Floseal a significant advantage over PerClot (Fig. 2). Later, at minute 6, both
products seemed to equalize in their effect, and afterwards, at minutes 9 and 12, a significant difference was seen between the two groups (although the volume decreased). The speed and
stability of coagulation could be related to the thrombin component in Floseal. The greater the thrombin concentration of the hemostatic agent, the faster the hemostatic effect was achieved.
Regarding blood loss, this study observed that most of the animals achieved hemostasis by minute 12, with continued bleeding in a few animals. In fact, five animals died (4 in the MAP group
and one in the flowable group). Despite this difference, it might be said that both hemostatic agents statistically controlled bleeding by 12 min. To the best of our knowledge, this is the
first study to compare the rate of blood loss and survival after treatment with PerClot and Floseal in a severe swine liver injury model. The results are comparable with another study that
used Floseal versus another MAP (Arista)26. Specifically, our results are consistent with this heparinized porcine hepatic abrasion model of capsular tears, in that Floseal provided greater
control of bleeding, and the hemostatic success of the flowable agent was 17.5%, 40%, and 57.5% greater than that of the MAP agent (Arista) at 2, 5, and 10 min after application,
respectively. Also, there is an important difference between both products related on the implementation application. The applicator of The PerClot may become blocked if the applicator
becomes occluded (blocked) by blood. Therefore, it can should be administered as close as possible to the source of bleeding by scattering the product at a certain distance from the bleeding
point so that the product is properly distributed13,15. In contrast, we introduced the cannula of Floseal into the bloody injury, and there was no risk of rendering the system useless
occluding the applicator tip. Although there was a probability of embolism if the product was introduced into a major vessel, we did not observe any thrombosis or distant embolism in this
study. COULD IT BE APPLIED IN CLINICAL PRACTICE? Floseal has been used in various clinical scenarios, such as cardiac, thoracic, and nephrectomy surgeries8,14,27. In a prospective,
randomized clinical trial of 309 patients with cardiac, vascular, or spinal surgery, the authors reported the control of bleeding at 3 min (85% vs. 48%, p < 0.001) compared to a
thrombin-soaked gel foam, with a mean bleed control of 2.8 min. Moreover, in the cardiac surgery cohort, the control of bleeding was 77% vs. 0% at minute 3 (p < 0.0037) and 92% vs. 40% (p
< 0.0057) at minute 10 in “severe bleeding”28. On the other hand, PerClot had fewer reportings compared to Floseal, but also observed a decreased time of hemostasis29,30,31. Our
preclinical observations agree with the results of this clinical trial; both are efficient surgical hemostatic products, but Floseal is quicker and more stable. CHARACTERISTICS OF LIVER
INJURY The novel wound model implemented in this study was unique from others used previously, in that it was incisive, severe, and reproducible32,33,34,35,36,37. Additionally, it produces a
standard wound that can be accurately compared across studies13. LIMITATIONS Both PerClot and Floseal, are a sampling of the large variety of active and passive hemostatic agents currently
available. Therefore, to further compare the clinical outcomes of active and passive hemostatic agents, new prospectives and randomized trials are needed. Of note, this study used a novel,
severe experimental liver injury model that caused massive hemorrhage. Therefore, caution must be exercised when applying these findings in clinical practice. CONCLUSIONS According to the
results of this study, both Floseal and PerClot hemostatic agents effectively controlled surgical bleeding in this severe liver injury model, although the Floseal provided quicker and better
bleeding control. DATA AVAILABILITY Data and material are available on request for ethical or legal reasons by contacting: [email protected]. REFERENCES * Corral, M., Ferko,
N., Hollmann, S., Broder, M. S. & Chang, E. Health and economic outcomes associated with uncontrolled surgical bleeding: A retrospective analysis of the Premier Perspectives Database.
_ClinicoEconomics Outcomes Res. CEOR_ 7, 409–421 (2015). Google Scholar * Ghadimi, K., Levy, J. H. & Welsby, I. J. Perioperative management of the bleeding patient. _Br. J. Anesth._
117(suppl 3), iii18–iii30 (2016). Article CAS Google Scholar * Stokes, M. E. _et al._ Impact of bleeding-related complications and/or blood product transfusions on hospital costs in
inpatient surgical patients. _BMC Health Serv. Res._ 11, 135 (2011). Article PubMed PubMed Central Google Scholar * Shander, A. Financial and clinical outcomes associated with surgical
bleeding complications. _Surgery_ 142(4 Suppl), S20–S25 (2007). Article PubMed Google Scholar * Iannitti, D. A., Kim, C., Ito, D. & Epstein, J. Impact of an active hemostatic product
treatment approach on bleeding-related complications and hospital costs among inpatient surgeries in the United States. _J. Med. Econ._ 24(1), 514–523 (2021). Article PubMed Google Scholar
* Wright, J. D. _et al._ Patterns of use of hemostatic agents in patients undergoing major surgery. _J. Surg. Res._ 186(1), 458–466 (2014). Article PubMed Google Scholar * Chapman, W.
C. _et al._ A phase 3, randomized, double-blind comparative study of the efficacy and safety of topical recombinant human thrombin and bovine thrombin in surgical hemostasis. _J. Am. Coll.
Surg._ 205(2), 256–265 (2007). Article PubMed Google Scholar * Echave, M., Oyagüez, I. & Casado, M. A. Use of Floseal, a human gelatine-thrombin matrix sealant, in surgery: A
systematic review. _BMC Surg._ 14, 111 (2014). Article PubMed PubMed Central Google Scholar * Chiara, O. _et al._ A systematic review on the use of topical hemostats in trauma and
emergency surgery. _BMC Surg._ 18(1), 1–20 (2018). Article Google Scholar * Ragusa, R. _et al._ Use of gelatin powder added to rifamycin versus bone wax in sternal wound hemostasis after
cardiac surgery. _Interact. Cardiovasc. Thorac. Surg._ 6(1), 52–55 (2007). Article PubMed Google Scholar * Lewis, K. M. _et al._ Comparison of regenerated and non-regenerated oxidized
cellulose hemostatic agents. _Eur. Surg. ACA Acta Chir. Austriaca_ 45(4), 213–220 (2013). Article CAS Google Scholar * Tscholl, V. _et al._ Prospective randomized study evaluating the
effects of PerClot (Polysaccharide Hemostatic System) application in patients with high bleeding risk undergoing cardiac rhythm device implantation. _Int. J. Cardiol._ 248, 84–91 (2017).
Article PubMed Google Scholar * José, S. D. V. F., Luis, D. N., Juan, G. M., Antonio, D. P. & Lidia, S. R. Utility of microporous polysaccharide hemospheres in severe hepatic trauma:
Experimental study of hemostatic strength and ease of use. _Injury_ 54(2), 339–344 (2023). Article PubMed Google Scholar * Floseal Hemostatic Matrix, 10 mL. Instructions for use. Baxter
Healthcare Corporation. Updated July 4, 2014. https://www.fffenterprises.com/assets/downloads/pi-Surgical%20Sealant_Floseal_Baxter.pdf. Accessed Aug 14, 2023. * PerClot Polysaccharide
Hemostatic System. Instruction for use. Starch Medical Incorporated. https://www.cryolife.com/wp-content/uploads/2019/08/PerClot-IFU-Rev.K-201901-.pdf. Accessed Aug 14, 2023. * Madrid, V. A.
_Aprovechamiento de los subproductos carnicos_ 1st edn, 35–43 (Editorial Mundi-Prensa, 1999). Google Scholar * Wu, B. _et al._ Perioperative outcomes and hospital costs associated with
flowable gelatin hemostatic matrix for lumbar surgeries in real world hospital setting. _J. Med. Econ._ 22(9), 917–923 (2019). Article PubMed Google Scholar * Gabay, M. & Boucher, B.
A. An essential primer for understanding the role of topical hemostats, surgical sealants, and adhesives for maintaining hemostasis. _Pharmacotherapy_ 33(9), 935–955 (2013). Article PubMed
Google Scholar * Tompeck, A. J. _et al._ A comprehensive review of topical hemostatic agents: The good, the bad, and the novel. _J. Trauma Acute Care Surg._ 88(1), e1–e21 (2020). Article
CAS PubMed Google Scholar * Lewis, K. M. _et al._ Comparison of two gelatin and thrombin combination hemostats in a porcine liver abrasion model. _J. Investig. Surg._ 26(3), 141–148
(2013). Article Google Scholar * Sheppard, O. O. & Foje, N. A. Topical coagulant agents. _Surg. Clin. North Am._ 102(1), 65–83 (2022). Article PubMed Google Scholar * Pusateri, A.
E. _et al._ Making sense of the preclinical literature on advanced hemostatic products. _J. Trauma Inj. Infect. Crit. Care_ 60(3), 674–682 (2006). Article Google Scholar * Meldrum, D. R.
_et al._ Barney Resident Research Award. Cardiopulmonary hazards of perihepatic packing for major liver injuries. _Am. J. Surg._ 170(6), 532–537 (1995). Article Google Scholar * Meldrum,
D. R. _et al._ Prospective characterization and selective management of the abdominal compartment syndrome. _Am. J. Surg._ 174(6), 663–667 (1997). Article Google Scholar * Nicol, A. J.,
Hommes, M., Primrose, R., Navsaria, P. H. & Krige, J. E. J. Packing for control of hemorrhage in major liver trauma. _World J. Surg._ 31(3), 569–574 (2007). Article CAS PubMed Google
Scholar * Lewis, K. M., Atlee, H., Mannone, A., Lin, L. & Goppelt, A. Efficacy of hemostatic matrix and microporous polysaccharide hemospheres. _J. Surg. Res._ 193(2), 825–830 (2015).
Article CAS PubMed Google Scholar * Ghimire, S. _et al._ Polymeric materials for hemostatic wound healing. _Pharmaceutics_ 13(12), 2127 (2021). Article CAS PubMed PubMed Central
Google Scholar * Oz, M. C., Rondinone, J. F. & Shargill, N. S. Floseal matrix: New generation topical hemostatic sealant. _J. Cardiac Surg._ 18(6), 486–493 (2003). Article Google
Scholar * Beyer, B. _et al._ 1023 Impact of a polysaccharide hemostat on bleeding complications and pelvic lymphocele rates after radical prostatectomy: Initial results of a prospective
randomized trial. _Eur. Urol. Suppl._ 12(1), e1023 (2013). Article Google Scholar * Bruckner, B. A. _et al._ Microporous polysaccharide hemosphere absorbable hemostat use in cardiothoracic
surgical procedures. _J. Cardiothorac. Surg._ 9(1), 1–7 (2014). Article Google Scholar * Jacobs, D. G., & Chrisas, A. B. (2020). Surgical techniques for managing hepatic
injury—UpToDate. Updated February 24, 2022. https://www.uptodate.com/contents/surgical-techniques-for-managing-hepatic-injury. Accessed Aug 14, 2023. * Jewelewicz, D. D., Cohn, S. M.,
Crookes, B. A. & Proctor, K. G. Modified rapid deployment hemostat bandage reduces blood loss and mortality in coagulopathic pigs with severe liver injury. _J. Trauma_ 55(2), 275–281
(2003). Article PubMed Google Scholar * Cohn, S. M., Cross, J. H., Ivy, M. E., Feinstein, A. J. & Samotowka, M. A. Fibrin glue terminates massive bleeding after complex hepatic
injury. _J. Trauma_ 45(4), 666–672 (1998). Article CAS PubMed Google Scholar * Holcomb, J. B. _et al._ Effect of dry fibrin sealant dressings versus gauze packing on blood loss in grade
V liver injuries in resuscitated swine. _J. Trauma_ 46(1), 49–57 (1999). Article CAS PubMed Google Scholar * Duggan, M. J. _et al._ Development of a lethal, closed-abdomen grade v
hepato-portal injury model in non-coagulopathic swine. _J. Surg. Res._ 182(1), 101–107 (2013). Article PubMed Google Scholar * Fonouni, H. _et al._ Hemostatic efficiency of modern topical
sealants: Comparative evaluation after liver resection and splenic laceration in a swine model. _J. Biomed. Mater. Res. Part B Appl. Biomater._ 106(3), 1307–1316 (2018). Article CAS
Google Scholar * Slezak, P., Keibl, C., Redl, H., Labahn, D. & Gulle, H. An efficacy comparison of two hemostatic agents in a porcine liver bleeding model: Gelatin/thrombin flowable
matrix versus collagen/thrombin powder. _J. Investig. Surg._ 33(9), 828–838 (2020). Article Google Scholar Download references ACKNOWLEDGEMENTS The commander and crew of the warship Santa
María for supporting the writing of the manuscript on board the Indic Ocean. J Barroso for help with literature research. Medical writing and editorial assistant services provided by Ciencia
y Deporte S.L. Support for this assistance was funded by Baxter. FUNDING MedCor contributed to this study by supplying the products and partial financing for animal husbandry costs (eight
pigs), and performed the first translation of this article. The Central Hospital of Defense “Gómez Ulla” contributed to the study by financing 16 pigs and all the surgical materials. All
members of the study belonged to the Spanish Ministry of Defense. Independent support for this assistance was funded by Baxter. Baxter was neither involved in the preparation of the
recommendations nor did the company influence scientific consensus.Open access fees have been supported by a grant (PID 2021-123045OB-100) from the public agency (Ministry of Science and
Technology of Spain) AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Central Hospital of Defense, General and Digestive Unit, Spanish Ministry of Defense, Glorieta del Ejército, 1, 28047,
Madrid, Spain Francisco José Sánchez del Valle, Luis De Nicolás & Guillermo Fernández * Central Hospital of Defense, Unit of Surgical Research, Spanish Ministry of Defense, Madrid, Spain
Pedro Fernández * University of Alcalá de Henares, Madrid, Spain Esther Gómez * Complutense University, Madrid, Spain Inmaculada Aranaz Corral Authors * Francisco José Sánchez del Valle
View author publications You can also search for this author inPubMed Google Scholar * Luis De Nicolás View author publications You can also search for this author inPubMed Google Scholar *
Guillermo Fernández View author publications You can also search for this author inPubMed Google Scholar * Pedro Fernández View author publications You can also search for this author
inPubMed Google Scholar * Esther Gómez View author publications You can also search for this author inPubMed Google Scholar * Inmaculada Aranaz Corral View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS S.F. was responsible for the study conception and design. S.F., L.N., G.F., and P.F. were responsible for the surgical
procedures, and S.F. and E.G. were responsible for drafting the article. All authors agreed to act as guarantors of this work. CORRESPONDING AUTHOR Correspondence to Francisco José Sánchez
del Valle. ETHICS DECLARATIONS COMPETING INTERESTS The author is a research member of Transbiomat (research group of the “Universidad Complutense de Madrid”) The authors declare no competing
interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The
images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is
not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission
directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Sánchez
del Valle, F.J., De Nicolás, L., Fernández, G. _et al._ Comparison of a gelatin thrombin versus a modified absorbable polymer as a unique treatment for severe hepatic hemorrhage in swine.
_Sci Rep_ 13, 20854 (2023). https://doi.org/10.1038/s41598-023-41983-9 Download citation * Received: 28 December 2022 * Accepted: 04 September 2023 * Published: 27 November 2023 * DOI:
https://doi.org/10.1038/s41598-023-41983-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative








