- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT In this study, we conducted direct synthesis of a dual metal–organic framework (Ni/Co-Hemin MOF) on phosphorous-doped reduced graphene oxide (PrGO) to serve as an active material in
high-performance asymmetrical supercapacitors. The nanocomposite was utilized as an active material in supercapacitors, exhibiting a noteworthy specific capacitance of 963 C g−1 at 1.0 A
g−1, along with a high rate capability of 68.3% upon increasing the current density by 20 times, and superior cycling stability. Our comprehensive characterization and control experiments
indicated that the improved performance can be attributed to the combined effect of the dual MOF and the presence of phosphorous, influencing the battery-type supercapacitor behavior of GO.
Additionally, we fabricated an asymmetric hybrid supercapacitor (AHSC) using Ni/Co-Hemin/PrGO/Nickel foam (NF) and activated carbon (AC)/NF. This AHSC demonstrated a specific capacitance of
281 C g−1 at 1.0 A g−1, an operating voltage of 1.80 V, an impressive energy density of 70.3 Wh kg−1 at a high power density of 0.9 kW kg−1. Notably, three AHSC devices connected in series
successfully powered a clock for approximately 42 min. These findings highlight the potential application of Hemin-based MOFs in advanced supercapacitor systems. SIMILAR CONTENT BEING VIEWED
BY OTHERS RAPID COLD PLASMA SYNTHESIS OF COBALT METAL–ORGANIC FRAMEWORK/REDUCED GRAPHENE OXIDE NANOCOMPOSITES FOR USE AS SUPERCAPACITOR ELECTRODES Article Open access 13 September 2023
SUPERCAPACITOR PERFORMANCE OF POROUS NICKEL COBALTITE NANOSHEETS Article Open access 03 November 2020 FABRICATION OF 3D BINDER-FREE GRAPHENE NIO ELECTRODE FOR HIGHLY STABLE SUPERCAPATTERY
Article Open access 08 July 2020 INTRODUCTION Energy is considered the most critical scientific subject in the twenty-first century1,2. To survive the earth, renewable energy is essential to
reducing greenhouse gas emissions and air pollution3. Hence, new energy generation technologies like solar4, wind5, and fuel cells6 require devices to store energy. Li-ion batteries and
supercapacitors are the two main electrical energy storage systems. They developed over the years for portable devices as well as smart grid deployments7. Supercapacitors can store a large
amount of charge compared to conventional capacitors, deliver energy quickly, have fast charging ability, have a long lifetime, offer superior low-temperature performance, are eco-friendly,
and have low costs. Moreover, unlike batteries, they do not explode even if it is overcharged8,9,10,11. On the other hand, Ragone plot12 illustrates supercapacitors' importance in their
high specific power density. Besides, the capacitance in supercapacitors is influenced by equivalent series resistance and electrode and electrolyte materials and affects the operating
voltage13,14. Therefore, for the best performance of a supercapacitor, it needs to have high capacitance, high operating voltage, and low resistance15. From these, electrode material, among
all parameters, plays an essential role in developing the supercapacitor performance15. In other words, Hybrid supercapacitor devices are crucial for the advancement of electrochemical
energy storage systems that can offer high energy storage capacity at a low operational cost16. One of these electrode materials that appears effective is MOFs, porous hybrid materials
consisting of metal ions or metal clusters coordinated to organic linkers17,18,19. Structures of this type offer the following advantages: high internal surface area, high porosity,
structural and chemical tunability, and good stability. Besides, MOFs can be controlled in terms of porosity due to pore uniformity and atomic-level structure, dimension, geometry,
functionality, and flexibility in network topology20,21. But, most pristine MOFs are poor conductors22,23. To overcome this deficiency, one of the most common strategies is to combine MOFs
with carbon materials (reduced graphene oxide and carbon nanotubes) or conductive polymers (polypyrrole and polyaniline)24. Additionally, the incorporation of reduced graphene oxide (rGO) in
composites can serve as an effective means of impeding the aggregation and restacking of graphene, both during the fabrication process and in practical usage25. The exceptional mechanical
and chemical durability exhibited by rGO makes it an excellent scaffold material for the active component, which can effectively mitigate structural degradation and thereby enhance the
cyclic stability of the system26. This research synthesized dual MOFs/ PrGO as an active material. In other words, carbon-based materials such as GO have been extensively studied and
utilized in industrial production for capacitors and batteries. The advantages of GO for use in supercapacitors are abundance, non-toxicity, facile manufacturing, low cost, high chemical
stability, good electronic and mechanical properties, high specific surface area, and a vast working temperature range27,28,29. However, the performance of GO in supercapacitors is limited
due to nanosheet stacking and agglomeration. This problem exists because of the van der Waals forces and _π–π_ interactions, therefore, it dramatically decreases the specific capacitance30.
To put it more simply, macroscopic properties are affected by the electrode material microstructure31. Some researchers are used different methods, such as synthesis GO with porous 3D
architectures in the form of hydrogels, organic gels, and foams to provide a larger surface area and better electron transport channels30,32. The other method is modifying GO, including
chemical doping with different heteroatoms (F, B, S, N, and P), which provide capacitive behavior and reduce the aggregation of GO sheets33,34. Among these heteroatoms, phosphorus great much
attention for doping GO because the electronegativity of P (2.19) is lower than C (2.55), and the atomic radius size of P (195 pm) is more than C (170 pm), which causes polarization and
distortion in the graphene lattice followed by the specific capacitance, surface area, and electrochemical performance were improved35,36. In other words, when phosphorus is doped in GO, P
electrons contribute to the graphene _π_-system to increase charge carriers30. This study uses MOFs with hemin ligands for supercapacitor applications for the first time. Hemin, an
iron-containing porphyrin with chlorine, is widely used to synthesize electrocatalysts for fabricating electrochemical sensors37,38 and energy production, such as hydrogen and oxygen
evolution reactions39,40. Besides, porphyrin-base structures such as Hemin have several advantages for utilizing active materials in supercapacitors. These structures have the following
benefits: non-toxicity, low cost, and highly exposed active site in the active substance due to its highly conjugated structure (M–N4)41,42. Therefore, we tried synthesizing dual Ni/Co-Hemin
MOF (NCH) and compositing with PrGO through the one-pot co-synthesis method to increase their cycle life, specific surface area, and pseudo-capacitance behavior. This method causes the
uniform distribution of Ni-MOF and Co-MOF particles, and PrGO acts as a conductive bridge. Afterward, various spectroscopic and microscopic methods studied the structure of the obtained
nanocomposite. The electrochemical behavior of this nanocomposite was first investigated in a three-electrode system for supercapacitor applications. The results of this study revealed that
NCH/PrGO nanocomposite is a promising active material as an AHSC with wide operating voltage and acceptable value of energy density versus several related materials (Fig. S1). RESULTS AND
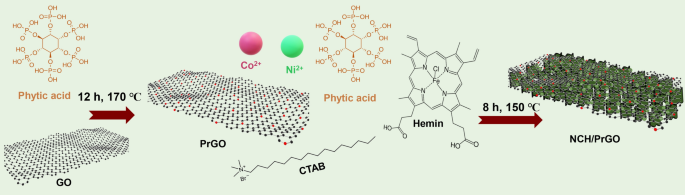
DISCUSSION CHARACTERIZATION First, GO was doped with P using a facile hydrothermal method that used phytic acid as a phosphorous source to obtain PrGO hydrogel. Afterward, the novel NCH MOF
was grown on graphene sheets. Moreover, phytic acid and CTAB are used as nucleating and kinetic control agents, respectively (Fig. 1). Accordingly, X-ray diffraction (XRD) was used to
characterize the structure of GO, PrGO, NH, CH, NCH, and NCH/PrGO (Fig. 2A,B). In the GO pattern, the peak at 2θ = 10.8° is attributed to the (001) plane that revealed the oxidation of
graphite to form GO43, while in the PrGO pattern, the appearance of two broad peaks at 23.1° (002) and 43.8° (101) and the disappearance of the peak at 10.8° indicated that GO was reduced
successfully30. Additionally, the broadening peaks of PrGO are due to a few layers of stacked graphene sheets forming the material's framework due to the poor stacking direction of
graphene sheets32. As can be seen, in the NCH pattern, the main diffraction peaks at 5.8°, 9.1°, 19.9°, 33.7°, 36°, 39.1°, 52.8°, 59.7°, 61.3°, and 63.4° suggesting the crystalline structure
of this MOF. Moreover, by comparing the XRD patterns of NCH with CH and NH, the diffraction peaks of both MOFs that appear in dual MOF can be concluded. According to the XRD pattern
obtained from NCH/PrGO, its index peaks are equal to the patterns of dual MOF and PrGO, so their presence of them in the final nanocomposite is confirmed. Many attempts were done to obtain
single crystal, but due to the very low solubility of this structure in most solvents, attempts were unsuccessful. Fourier-transform infrared spectroscopy (FT-IR) spectra of Hemin, NCH, and
NCH/PrGO are illustrated in Fig. 2C. In all spectra, the bands at 3451, 2927, and 1604 cm−1 are attributed to the O–H, C–H, and C=N/C=C of the protoporphyrin (IX) ring system44. The
band's intensity at 1703 cm−1 was attributed to the C=O stretching vibration that decreased in the NCH spectrum, compared with the Hemin spectrum. Furthermore, 1465, 1392, and 1004 cm−1
absorption bands are corresponded to the B3u vibration of porphyrin, –CH3 of Hemin, and =C–H deformation vibration of the olefin. All results confirm the successful coordination of the
metals and –COOH groups37,45,46. Besides, spectrum f reveals the NCH/PrGO is well composite. The soret (~ 400 nm) and Q (~ 630 nm) bands of Hemin appeared in the UV–Vis spectrum of NCH (Fig.
2D). Consequently, these results prove the MOF structure was successfully formed. Moreover, ICP-OES results reveal the molar ratio of elements in the NCH structure. Co, Ni, and Fe weight
percent equal 32.6, 10.4, and 1.2%, respectively. To characterize the chemical state of the bonded element and the phase composition of NCH/PrGO, X-ray photoelectron spectroscopy (XPS)
measurements were carried out. Figure 3A–F illustrates the high-resolution spectra of C 1s, O 1s, P 2p, Fe 2p, Co 2p, and Ni 2p, respectively. C 1s spectrum shows peaks at 285.01 (C–C bond),
285.7 (C–O bond), and 287.1 (C=O, C–P bonds) eV in PrGO47. The O 1s peaks at 529.8, 531.7, 532.9, 533.2, and 534.9 eV are associated with the P–OH, C=O/P=O, C–O/C–O–P, P–O–P, and
water/carboxylic groups respectively48. The high-resolution P 2p XPS spectrum showed two peaks at 133.2 (C–P bond) and 134.3 eV (P–O bond)48. Moreover, the P–O peak intensity is higher than
the P–C peak intensity revealing the GOs and phytic acids oxygen atoms led to the partial oxidation of P–C bonding in the thermal treatment30. Therefore, the results indicated that P had
been successfully incorporated into the graphene lattices. It was stated in the introduction that reversible redox reactions in the P–O and C–P groups increase the overall energy density
and, consequently, improve the electrochemical performance. The peaks at 700.1 (Fe pre-peak), 708.3 (Fe 2p3/2), 713 (Fe–O), and 734.1 (Fe 2p1/2) eV in a high-resolution scan of the Fe 2p
region are related to Fe3+ in Hemin49. The main peaks appeared in the high-resolution spectrum of the Co 2P attributed to Co 2p3/2 and Co 2p1/2, which indicates the existence50 of Co2+ and
Co3+. Likewise, the Ni 2p3/2 and Ni 2p1/2 spectra demonstrated four peaks that contribute to Ni2+ (related to Ni–O octahedral bonding) and Ni3+ in the nanocomposite45. Microscopy images were
used for the investigation of the morphology. Figure 4A–E illustrates field-emission scanning electron microscopy (FE-SEM) images of (A) PrGO, (B) CH, (C) NH, (D) NCH, and (E) NCH/PrGO.
PrGO image displays 3D porous network and GO sheets with an effective surface area to improve the fast diffusion of the ions51. The morphology of CH is illustrated in Fig. 4B. Moreover, the
NH MOF image showed a wrinkled nanoflakes shape with an average thickness of about 29 nm. On the other hand, the FE-SEM image of the nanocomposite proved both MOF structures that have been
successfully composited with the GO sheets. Additionally, NCH MOF structures may provide an extensive contact area between the electrolyte and the electrode due to having a large specific
surface area. Therefore, the path of OH− diffusion is reduced, and the nanocomposite has sufficient space to diminish deformation and improve the cycle life during charge and discharge. The
high-resolution TEM (HR-TEM) image of NCH MOF is shown in Fig. S2A–C revealing that this structure is well-crystallized and has uniformity. Moreover, the TEM image (Fig. S2D) of NCH/PrGO is
consistent with the FE-SEM images. Energy-dispersive X-ray spectroscopy (EDS) analysis and elemental mapping were used to study the nanocomposite structure elements and their distribution.
The EDS analysis of NCH/PrGO attests to the composition formation and the presence of the P element, which shows the successful doping of GO with this heteroatom. The results obtained from
Ni, Co, and Fe (Hemin) mapping show a uniform distribution in NCH. Moreover, the elemental mapping of C and P provide information about the uniform composition of NCH and PrGO (Fig. S3).
According to the results obtained from the reported analysis, it can be concluded that the NCH MOF is successfully synthesized, and the NCH/PrGO nanocomposite is formed. ELECTROCHEMICAL
PERFORMANCE OF THE NCH/PRGO The electrochemical performance of the different electrodes was investigated by cyclic voltammetry (CV), galvanostatic charge/discharge (GCD), and electrochemical
impedance spectroscopy (EIS) in 1.0 M KOH solution in a three-electrode system. Figure S4A illustrated CV curves of the NCH/PrGO/NF electrodes with different amounts of PrGO (0.2, 0.4, 0.6,
and 0.8 g L−1) at a scan rate of 20 mV s−1 in a potential window from 0.00 to 0.80 V vs. Hg/HgO. With increasing the amount of PrGO up to 0.6 g L−1, the area of the CV curve was increased;
after this amount, the area dramatically decreased. In addition, this claim was proven by the Brunauer–Emmett–Teller (BET) data. As a result, the specific surface area of CH, NH, NCH, and
NCH/PrGO was measured to be 40.21, 68.41, 71.44, and 92.54 m2 g−1, respectively. According to these observations, increasing the amount of P improves the capacitive behavior of the
nanomaterial because of the lattice distortions of GO. Still, over-doping with P may damage graphene's original structure and reduce its capacitance and conductivity30. Moreover, as
shown in Fig. S4B, the results of the GCD curves confirm the same trend; NCH/PrGO0.6 revealed the highest capacitance (963 C g−1), and this electrode was used for subsequent investigation.
On the other hand, comparing their FE-SEM images gives us similar results with electrochemical data. In the FE-SEM image of NCH/PrGO0.2, due to the low amount of PrGO, we cannot see the PrGO
sheets, and in the FE-SEM image of NCH/PrGO0.8, PrGO sheets cover the porosity of dual MOF structures (Fig. S5). In mathematics, computer science, and chemistry, an optimization process is
a problem of finding the best value of a parameter of all possible solutions52. In fact, in optimization problems, we are looking for the largest or smallest value that a function can take.
Many of these problems can be solved by finding the appropriate cost function and then using optimization problem-solving techniques to find the maximum or minimum required value of the
respective cost function. Hence, the effect of amounts of PrGO on the capacitance behavior is optimized with MATLAB R2008b. According to the numbers obtained from the practical experiments,
the cost function was estimated as follows: $$y = - 15667x^{3} + 20412x^{2} - 7550.8x + 1529$$ Figure S6 shows the estimated cost function. The next step is the optimal point to obtain the
maximum capacitance. To obtain the optimal point, we must take the cost function mentioned in Eq. (1) of the first-order derivative in terms of the amounts of PrGO and set it to zero.
$$\frac{dy}{{dx}} = - 47001x^{2} + 40820x - 7550.8 = 0$$ (1) $$x_{opt}^{(1)} = 0.26\quad and\quad x_{opt}^{(2)} = 0.6$$ The two optimal points (0.6 and 0.26) are obtained by zeroing the
first-order derivative of the cost function. According to the cost function estimated at 0.6, the maximum capacity is obtained, which is very consistent with the value obtained from
practical experiments. Figure 5A shows the comparative CV curves of (a) NH/NF, (b) CH/NF, (c) NCH/NF, (d) NCH/rGO/NF, and (e) NCH/PrGO/NF at a scan rate of 20 mV s−1 in the potential window
from 0.0 to 0.80 V vs. Hg/HgO. The NCH/PrGO/NF CV showed the highest current density, revealing excellent faradic behavior and a larger specific capacity. The results showed that a dual MOF
announced superior performance than a monometallic MOF due to the synergic effects of the metals. The peaks in the NCH/PrGO/NF CV curve are related to the overlaid peaks of two pairs of the
redox peaks of cobalt and nickel. Moreover, GO plays an important role in enhancing the electrochemical properties because of increasing the surface area and conductivity. P, as a dopant,
increased the charge density (the types of P groups, especially C–P and P–O groups, are responsible for augmenting the overall energy density through reversible redox reactions)53 and caused
structural distortions. Unlike the conventional methods, such as chemical vapor deposition, which produces hazardous gaseous for doping GO, the technique used in the present study for
doping GO is safe. Additionally, phytic acid is a natural and green P source compared with other sources. The peak currents enhanced by increasing the scan rates due to the diffusion of OH−
ions. The shape of the CVs shows the quasi-reversible reaction. Additionally, the cathodic peak potential was shifted to a negative potential, while the anodic peak potential was moved to a
positive value (Fig. S7A). As a result, Low reversible Faradic processes occur because of the active material's internal diffusion resistance at a high scan rate. Figure 5B illustrates
the relationships between the current densities and the square root of the scan rates that show OH− diffusion. Augmenting scan rates enhances the CV integrated area but decreases the charge
storage because of the difficulty of OH− into the electrode internal structures and pores and poor interaction between the electrode material and the electrolyte. Figure S7B depicts the
areal capacitance of NCH/PrGO. The Dunn method was utilized to examine the comprehensive performance of charge storage, encompassing both capacitive and diffusion-controlled processes54.
Through the investigation of capacitive and diffusion-controlled charge storage properties at different scan rates, as depicted in Fig. S8, it was determined that capacitive charge storage
prevails at high scan rates, whereas diffusion-controlled charge storage predominates at low scan rates. This trend illustrates that at scan rates of 2 and 50 mV s−1, the contribution of
capacitive charge storage accounts for 24% and 87% of the total stored charge, respectively. EIS is a perfect method to better study the charge-transfer behavior, interfacial properties, and
capacitive properties of the electrodes. The Nyquist plot of the NCH/NF, NCH/rGO/NF, and NCH/PrGO/NF electrode from 100 kHz to 100 mHz frequency range, with an equivalent circuit (inset)
plot for fitting the EIS data of NCH/PrGO/NF electrode at open circuit potential (OCP) is shown in Fig. 5C. The diameter of the semicircle part in the high-frequency region (that is related
to the charge transfer resistance (Rct) at the interface between the electrode surface/electrolyte) of NCH/PrGO/NF displays a lower Rct (1.05 Ω) than NCH/NF (1.16 Ω), and NCH/rGO/NF (2.05
Ω). Moreover, In the low-frequency region, the Warburg resistance (Rw) varies as a function of the ion diffusion resistance of the electrolyte inside the electrode materials; the steep slope
of the NCH/PrGO/NF curve shows the lower diffusive resistance and the pure capacitor behavior. Therefore, this electrode revealed fast electron transfer kinetics, lower internal resistance,
and good conductivity. The GCD curve of the NCH/PrGO/NF delivers the highest capacitance (963 C g−1) among other electrodes due to more active sites and high conductivity (Fig. 5D).
Besides, the GCD curve of the NCH/PrGO/NF electrode at different current densities (Fig. 5E); the specific capacities were 963, 956, 912, 894, 847, 756, 699, and 657 C g−1 at 1, 2, 4, 6, 8,
10, 15, and 20 A g−1, respectively. Thus, the specific capacity of the electrodes gradually decreased with increasing the current density due to diffusing the ions slowly through the
electrolyte during the GCD process. When the current density is increased 20 times, the specific capacitance of this electrode retains a 68.3% initial value (Fig. 5F). This result reveals a
high-rate capability for NCH/PrGO/NF. Cycle stability is one of the essential characteristics of evaluating supercapacitors. Figure S9 depicts that 86.63% of the initial specific capacitance
of NCH/PrGO/NF is maintained after 3000 successive GCDs performed at a current density of 10 A g−1. The result confirms its desirable stability, but a reduction in the specific capacitance
resulted from the destruction of NCH/PrGO and a loss of its active sites. Figure S10A displays the impedance spectra and FESEM images of NCH/PrGO/NF before and after cycling. The resistance
of the electrolyte and interface exhibited a modest increase from 1.05 to 2.64 Ω after 3000 cycles. This change in resistance is attributed to the maintained structural integrity of the
cross-linked structure and the favorable stability of the film formed in situ at the electrode–electrolyte interface during prolonged cycling. Moreover, FESEM image of the NCH/PrGO/NF
electrode after 3000 cycles reveals instances where the structure exhibits cohesion, resulting in reduced ion diffusion during GCD cycles. This observation is illustrated in Fig. S10B. A
significant shortcoming of supercapacitors is their low energy density and limited by the voltage window. The asymmetric hybrid supercapacitor (AHSC) was fabricated to improve the voltage
window; this configuration consists of two different kinds of electrodes. The NCH/PrGO/NF//AC/NF was assembled as an AHSC device to evaluate energy and power density performance. The
NCH/PrGO/NF and AC/NF were applied as positive and negative electrodes, respectively. Figure 6A depicts the CV curves of the NCH/PrGO/NF and AC/NF at the scan rate of 20 mV s−1 in the
three-electrode system for activation. CV (at scan rates of 20 mV s−1) and GCD (at 1.0 A g−1) curves of NCH/PrGO/NF//AC/NF AHSC device in different potential windows are illustrated in Fig.
6B,C. Since the limitation associated with oxygen evolution reaction at a voltage higher than 1.80 V, the operating voltage of the NCH/PrGO/NF//AC AHSC device was extended to 1.80 V. Having
a wide operating voltage allows this ASC device to be used in practical applications. Afterward, the CVs were performed at different scan rates (7–70 mV s−1) (Fig. 6D). Consequently, both
faradic behavior from NCH/PrGO/NF and electric double-layer capacitance from AC was confirmed for the charge storage. Moreover, as displayed in Fig. 6E (the GCD curves of the
NCH/PrGO/NF//AC/NF AHSC device at various current densities), the shape of the GCE curves is consistent with the CV curves in a term of charge storage. The specific capacitance of the
NCH/PrGO/NF//AC/NF AHSC device is calculated as 281, 257, 248, 230, 211, 201, 181, and 156 C g−1 at 1, 2, 4, 6, 8, 10, 15, and 20 A g−1, respectively. Hereupon, 55.5% of the initial specific
capacitance retains after increasing the current density 20 times (Fig. 6F). When the current densities increase due to the limited diffusion effect, the active material will be less
utilized, and the faradic process will be limited. Moreover, the carbon-based electrode has a higher ion exchange rate than a pseudocapacitive electrode, which causes a charge imbalance
between the electrodes. Therefore, the specific capacitances of the AHSC device decrease gently. This result reveals the excellent rate capability for NCH/PrGO/NF//AC/NF AHSC devices. The
long-term stability and coulombic efficiencies of the NCH/PrGO/NF//AC/NF AHSC device after 5000 successive GCD cycles at 10 A g−1 are shown in Fig. 7A. The specific capacitance and coulombic
efficiency of the NCH/PrGO/NF//AC/NF AHSC device after 5000 GCD cycles decrease by about 13.3% and 2.1% of its initial value, respectively. To further investigate the long-term stability of
NCH/PrGO/NF//AC/NF AHSC, a floating test was performed for 100 h. The potential of AHSC was maintained at a current density of 5 A g−1 at the potential of 1.75 V for 10 h and repeated ten
times. For every 10 h, 5 GCD test was performed, and capacity retention of 93.1% was maintained after 120 h (Fig. S11). Hence, NCH/PrGO/NF//AC/NF AHSC displays superb electrochemical
stability. The Ragone plot of the NCH/PrGO/NF//AC/NF AHSC device shows a maximum energy density of 70.3 Wh kg−1 at 0.9 kW kg−1, and the energy density remains at 38.9 Wh kg−1 at 18 kW kg−1
(Fig. 7B). Afterward, three NCH/PrGO/NF//AC/NF AHSC devices are connected in series to investigate a practical application. This device successfully ran the alarm clock (1.5 V) for 42 min
(Video 1 and Fig. 7C). The comparison between the NCH/PrGO as an active material for supercapacitors and some previous work in three-electrode and two-electrode systems reveal superior
performance (Table 1). METHODS SYNTHESIS OF PRGO Please see the supporting information. SYNTHESIS OF NCH/PRGO First, 0.2, 0.4, 0.6, and 0.8 mg mL−1 uniform suspension of PrGO was prepared.
Then, 0.64 mmol Ni(NO3)2·6H2O and 0.32 mmol Co(NO3)2·6H2O were added to 20 mL of each suspension and ultrasonic for 1 h. After that, 0.068 phytic acids and 0.15 mmol cetrimonium bromide
(CTAB) were added to the solution and stirred for 30 min (solution A). Besides, 20 mL (18 mL distilled water + 2.0 mL ammonium hydroxide solution) of 0.50 mM Hemin solution was prepared
(solution B). Afterward, solution B was gradually added to solution A and stirred for 15 min. The resulting solution was transferred into a 70 mL Teflon-lined stainless-steel autoclave, kept
at 150 °C for 8 h, cooled naturally to room temperature, and washed with distilled water several times. Finally, the dark slate grey sediments (NCH/PrGO0.2, NCH/PrGO0.4, NCH/PrGO0.6, and
NCH/PrGO0.8) were collected and dried at 60 °C overnight. Apparatus and methods are described in the supporting information. CONCLUSION To summarize, novel Ni/Co-Hemin MOF/PrGO nanocomposite
was synthesized as an active material for a high-performance hybrid supercapacitor. Afterward, this nanocomposite was characterized by different spectroscopic (XRD, XPS, and FT-IR) and
microscopic (FE-SEM and TEM) techniques. This nanocomposite demonstrates excellent electrochemical properties, including high specific capacity 963 C g−1 at 1.0 A g−1 (68.3%) retained after
increasing the current density 20 times), and good cycling life. The advantages of the composition of MOF with carbon-based material such as GO that is doped with heteroatom are (1) high
conductivity, (2) non-toxicity, (3) high porosity, (4) high internal and specific surface area, (5) facile synthesis, (6) good stability, (7) chemical and structural tunability, (8) superior
mechanical and electronic properties, (9) low cost, (10) enhance charge carriers, (11) increase pseudo-capacitance property, (12) synergistic effect of bimetallic and (13) prevent
agglomeration. Moreover, Hemin is a promising ligand for synthesizing MOF that is used in supercapacitor applications for the first time and has some advantages, such as low cost, non-toxic,
and highly conjugated structure. Furthermore, the AHSC was assembled on Ni/Co-Hemin MOF/PrGO/NF (as the positive electrode) and AC (as the negative electrode), resulting in a specific
capacity of 281 C g−1 with a high energy density of 70.3 Wh kg−1 and power density of 0.9 kW kg−1. The excellent electrochemical performance confirms Ni/Co-Hemin MOF/PrGO's potential as
an active material for AHSCs for future energy storage systems used in electronic devices and vehicles. Finally, this work aims to show the perspective of MOF-based Hemin (a natural
product) for energy storage systems. DATA AVAILABILITY The datasets supporting the conclusions of this article are included within the article. REFERENCES * Liu, C., Li, F., Lai-Peng, M.
& Cheng, H. M. Advanced materials for energy storage. _Adv. Mater._ 22, 28–62 (2010). Article CAS Google Scholar * Barazandeh, M. & Kazemi, S. H. High-performance freestanding
supercapacitor electrode based on polypyrrole coated nickel cobalt sulfide nanostructures. _Sci. Rep._ 12, 4628 (2022). Article ADS CAS PubMed PubMed Central Google Scholar * Dincer,
I. Renewable energy and sustainable development: A crucial review. _Renew. Sustain. Energy Rev._ 4, 157–175 (2000). Article Google Scholar * Shahsavari, A. & Akbari, M. Potential of
solar energy in developing countries for reducing energy-related emissions. _Renew. Sustain. Energy Rev._ 90, 275–291 (2018). Article CAS Google Scholar * Gu, C. & Li, H. Review on
deep learning research and applications in wind and wave energy. _Energies_ 15, 1510 (2022). Article Google Scholar * Olabi, A. G. & Abdelkareem, M. A. Renewable energy and climate
change. _Renew. Sustain. Energy Rev._ 158, 112111 (2022). Article CAS Google Scholar * Wang, L. _et al._ Metal–organic frameworks for energy storage: Batteries and supercapacitors.
_Coord. Chem. Rev._ 307, 361–381 (2016). Article ADS CAS Google Scholar * Raza, W. _et al._ Recent advancements in supercapacitor technology. _Nano Energy_ 52, 441–473 (2018). Article
CAS Google Scholar * Zhang, L. L. & Zhao, X. S. Carbon-based materials as supercapacitor electrodes. _Chem. Soc. Rev._ 38, 2520–2531 (2009). Article CAS PubMed Google Scholar *
Yan, J., Liu, T., Liu, X., Yan, Y. & Huang, Y. Metal-organic framework-based materials for flexible supercapacitor application. _Coord. Chem. Rev._ 452, 214300 (2022). Article CAS
Google Scholar * Sanati, S., Rezvani, Z., Abazari, R., Hou, Z. & Dai, H. Hierarchical CuAl-layered double hydroxide/CoWO 4 nanocomposites with enhanced efficiency for use in
supercapacitors with long cycling stability. _New J. Chem._ 43, 15240–15248 (2019). Article CAS Google Scholar * Simon, P. & Gogotsi, Y. Materials for electrochemical capacitors. In
_Nanoscience and Technology: A Collection of Reviews from Nature Journals_ 320–329 (World Scientific, 2010). Google Scholar * Zhi, M., Xiang, C., Li, J., Li, M. & Wu, N. Nanostructured
carbon–metal oxide composite electrodes for supercapacitors: A review. _Nanoscale_ 5, 72–88 (2013). Article ADS CAS PubMed Google Scholar * Sajjad, M., Khan, M. I., Cheng, F. & Lu,
W. A review on selection criteria of aqueous electrolytes performance evaluation for advanced asymmetric supercapacitors. _J. Energy Storage_ 40, 102729 (2021). Article Google Scholar *
Wang, Y. _et al._ Recent progress in carbon-based materials for supercapacitor electrodes: A review. _J. Mater. Sci._ 56, 173–200 (2021). Article ADS CAS Google Scholar * Sajjad, M. _et
al._ CdO nanocubes decorated on rGO sheets as novel high conductivity positive electrode material for hybrid supercapacitor. _J. Alloys Compd._ 938, 168462 (2023). Article CAS Google
Scholar * Iqbal, M. Z., Amjad, N. & Khan, M. W. Metal-organic-framework as novel electrode materials for hybrid battery-supercapacitor applications. _ChemElectroChem_ 9, e202200036
(2022). Google Scholar * Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal–organic frameworks. _Science_ 341, 1230444 (2013). Article
PubMed Google Scholar * Pan, Y. _et al._ Postsynthetic modification of NU-1000 for designing a polyoxometalate-containing nanocomposite with enhanced third-order nonlinear optical
performance. _Inorg. Chem._ 61, 18873–18882 (2022). Article CAS PubMed PubMed Central Google Scholar * Batten, S. R. _et al._ Terminology of metal–organic frameworks and coordination
polymers (IUPAC Recommendations 2013). _Pure Appl. Chem._ 85, 1715–1724 (2013). Article CAS Google Scholar * Li, H., Eddaoudi, M., O’Keeffe, M. & Yaghi, O. M. Design and synthesis of
an exceptionally stable and highly porous metal–organic framework. _Nature_ 402, 276–279 (1999). Article ADS CAS Google Scholar * Huang, S. _et al._ The application of metal–organic
frameworks and their derivatives for supercapacitors. _Nanomaterials_ 10, 2268 (2020). Article CAS PubMed PubMed Central Google Scholar * Abazari, R., Amani-Ghadim, A. R., Slawin, A. M.
Z., Carpenter-Warren, C. L. & Kirillov, A. M. Non-calcined layer-pillared Mn0.5Zn0.5 bimetallic–organic framework as a promising electrocatalyst for oxygen evolution reaction. _Inorg.
Chem._ 61, 9514–9522 (2022). Article CAS PubMed PubMed Central Google Scholar * Gao, H. _et al._ Review of pristine metal–organic frameworks for supercapacitors: Recent progress and
perspectives. _Energy Fuels_ 35, 12884–12901 (2021). Article CAS Google Scholar * Sajjad, M. & Lu, W. Regulating high specific capacitance NCS/α-MnO2 cathode and a wide potential
window α-Fe2O3/rGO anode for the construction of 2.7 V for high performance aqueous asymmetric supercapacitors. _J. Energy Storage_ 44, 103343 (2021). Article Google Scholar * Sajjad, M.
_et al._ Phosphine-based porous organic polymer/rGO composite anode and α-MnO2 nanowire cathode cooperatively enabling high-voltage aqueous asymmetric supercapacitors. _J. Energy Storage_
40, 102772 (2021). Article Google Scholar * Wang, G., Zhang, L. & Zhang, J. A review of electrode materials for electrochemical supercapacitors. _Chem. Soc. Rev._ 41, 797–828 (2012).
Article CAS PubMed Google Scholar * Pandolfo, A. G. & Hollenkamp, A. F. Carbon properties and their role in supercapacitors. _J. Power Sources_ 157, 11–27 (2006). Article ADS CAS
Google Scholar * Wang, H. & Cui, Y. Nanodiamonds for energy. _Carbon Energy_ 1, 13–18 (2019). Article Google Scholar * Fan, X. _et al._ Preparation and supercapacitive properties of
phosphorus-doped reduced graphene oxide hydrogel. _Electrochim. Acta_ 330, 135207 (2020). Article CAS Google Scholar * Sun, Z. _et al._ Hollow and yolk-shell iron oxide nanostructures on
few-layer graphene in Li-ion batteries. _Chem. Eur. J._ 20, 2022–2030 (2014). Article CAS PubMed Google Scholar * Xu, Y., Sheng, K., Li, C. & Shi, G. Self-assembled graphene hydrogel
via a one-step hydrothermal process. _ACS Nano_ 4, 4324–4330 (2010). Article CAS PubMed Google Scholar * Xu, H. _et al._ Synthesis of three-dimensional nitrogen-doped
graphene/polyaniline hydrogels for high performance supercapacitor applications. _J. Mater. Sci. Mater. Electron._ 28, 10674–10683 (2017). Article CAS Google Scholar * Tian, Y., Mei, R.,
Xue, D., Zhang, X. & Peng, W. Enhanced electrocatalytic hydrogen evolution in graphene via defect engineering and heteroatoms co-doping. _Electrochim. Acta_ 219, 781–789 (2016). Article
CAS Google Scholar * Li, R., Wei, Z., Gou, X. & Xu, W. Phosphorus-doped graphene nanosheets as efficient metal-free oxygen reduction electrocatalysts. _RSC Adv._ 3, 9978–9984 (2013).
Article ADS CAS Google Scholar * Lee, H., Paeng, K. & Kim, I. S. A review of doping modulation in graphene. _Synth. Met._ 244, 36–47 (2018). Article CAS Google Scholar * He, J.
_et al._ Smart nanocomposites of Cu-hemin metal–organic frameworks for electrochemical glucose biosensing. _Sci. Rep._ 6, 1–9 (2016). Article Google Scholar * Xie, S., Ye, J., Yuan, Y.,
Chai, Y. & Yuan, R. A multifunctional hemin@ metal–organic framework and its application to construct an electrochemical aptasensor for thrombin detection. _Nanoscale_ 7, 18232–18238
(2015). Article ADS CAS PubMed Google Scholar * Li, S. _et al._ Enhanced electronic interaction in hemin@ Ni(OH)2 composite for efficient electrocatalytic oxygen evolution. _J. Alloys
Compd._ 892, 161780 (2022). Article CAS Google Scholar * Yang, Z. _et al._ Facile synthesis of hemin-based Fe-NC catalyst by MgAl-LDH confinement effect for oxygen reduction reaction.
_Appl. Surf. Sci._ 573, 151505 (2022). Article CAS Google Scholar * Shen, H. & Gele, A. Facile synthesis of N-doped lignin-based carbon nanofibers decorated with iron oxides for
flexible supercapacitor electrodes. _Inorg. Chem. Commun._ 128, 108607 (2021). Article CAS Google Scholar * Lu, Y. _et al._ Hemin-based conjugated effect synthesis of Fe–N/CNT catalysts
for enhanced oxygen reduction. _New J. Chem._ 45, 6940–6949 (2021). Article CAS Google Scholar * Kigozi, M. _et al._ Synthesis and characterization of graphene oxide from locally mined
graphite flakes and its supercapacitor applications. _Results Mater._ 7, 100113 (2020). Article Google Scholar * Jahan, M., Bao, Q. & Loh, K. P. Electrocatalytically active
graphene–porphyrin MOF composite for oxygen reduction reaction. _J. Am. Chem. Soc._ 134, 6707–6713 (2012). Article CAS PubMed Google Scholar * Alizadeh, N., Salimi, A., Hallaj, R.,
Fathi, F. & Soleimani, F. Ni-hemin metal–organic framework with highly efficient peroxidase catalytic activity: Toward colorimetric cancer cell detection and targeted therapeutics. _J.
Nanobiotechnol._ 16, 1–14 (2018). Article Google Scholar * Gu, C.-J. _et al._ Reduced graphene oxide-Hemin-Au nanohybrids: Facile one-pot synthesis and enhanced electrocatalytic activity
towards the reduction of hydrogen peroxide. _Biosens. Bioelectron._ 78, 300–307 (2016). Article CAS PubMed Google Scholar * Yu, X., Kang, Y. & Park, H. S. Sulfur and phosphorus
co-doping of hierarchically porous graphene aerogels for enhancing supercapacitor performance. _Carbon_ 101, 49–56 (2016). Article CAS Google Scholar * Wen, Y., Wang, B., Huang, C., Wang,
L. & Hulicova-Jurcakova, D. Synthesis of phosphorus-doped graphene and its wide potential window in aqueous supercapacitors. _Chem. A Eur. J._ 21, 80–85 (2015). Article CAS Google
Scholar * Li, Y. _et al._ Graphene-hemin hybrid material as effective catalyst for selective oxidation of primary CH bond in toluene. _Sci. Rep._ 3, 1–7 (2013). Google Scholar * Du, H.,
Wang, C. & Lv, J. Controllable morphologies of Co3O4@MnO2 core-shell structure grown on nickel foam and their supercapacitor behavior. _Solid State Commun._ 277, 19–24 (2018). Article
ADS CAS Google Scholar * Liu, D., Fu, C., Zhang, N., Zhou, H. & Kuang, Y. Three-dimensional porous nitrogen doped graphene hydrogel for high energy density supercapacitors.
_Electrochim. Acta_ 213, 291–297 (2016). Article CAS Google Scholar * Naghsh, E., Sabahi, M. F. & Beheshti, S. Joint preprocessing of multiple datasets to enhance source separation.
_IEEE Signal Process. Lett._ 26, 1917–1921 (2019). Article ADS Google Scholar * Yu, X. _et al._ Elucidating surface redox charge storage of phosphorus-incorporated graphenes with
hierarchical architectures. _Nano Energy_ 15, 576–586 (2015). Article CAS Google Scholar * Sajjad, M. _et al._ Low-temperature synthesis of 3D copper selenide micro-flowers for
high-performance supercapacitors. _Mater. Lett._ 314, 131857 (2022). Article CAS Google Scholar * Dong, T., Zhang, X., Wang, P., Chen, H.-S. & Yang, P. Hierarchical nickel-cobalt
phosphide hollow spheres embedded in P-doped reduced graphene oxide towards superior electrochemistry activity. _Carbon_ 149, 222–233 (2019). Article CAS Google Scholar * Zhang, X. _et
al._ Nickel/cobalt bimetallic metal–organic frameworks ultrathin nanosheets with enhanced performance for supercapacitors. _J. Alloys Compd._ 825, 154069 (2020). Article CAS Google Scholar
* Ensafi, A. A., Heydari-Soureshjani, E., Taghipour-Jahromi, A. R. & Rezaei, B. Bimetallic metal organic framework-derived for both battery-like supercapacitor (electrolyte study) and
hydrogen evolution reaction. _Electrochim. Acta_ 395, 139192 (2021). Article CAS Google Scholar * Ye, C. _et al._ Coordination derived stable Ni–Co MOFs for foldable all-solid-state
supercapacitors with high specific energy. _J. Mater. Chem. A_ 7, 4998–5008 (2019). Article CAS Google Scholar * Cao, Y. _et al._ Interpenetrating network structures assembled by “string
of candied haws”-like PPY nanotube-interweaved NiCo-MOF-74 polyhedrons for high-performance supercapacitors. _Colloids Surf. Physicochem. Eng. Aspects_ 646, 128954 (2022). Article CAS
Google Scholar * Sun, S., Huang, M., Wang, P. & Lu, M. Controllable hydrothermal synthesis of Ni/Co MOF as hybrid advanced electrode materials for supercapacitor. _J. Electrochem. Soc._
166, A1799 (2019). Article CAS Google Scholar * Nie, G., Deng, H., Huang, J. & Wang, C. Phytic acid assisted formation of phosphorus-doped graphene aerogel as electrode material for
high-performance supercapacitor. _Int. J. Electrochem. Sci_ 15, 12578–12586 (2020). Article CAS Google Scholar * Ehrnst, Y. _et al._ Acoustotemplating: Rapid synthesis of freestanding
quasi-2D MOF/graphene oxide heterostructures for supercapacitor applications. _J. Mater. Chem. A_ 10, 7058–7072 (2022). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The
authors wish to thank Iran National Science Foundation, grant no. 98012523, to support this work. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Chemistry, Isfahan University
of Technology, Isfahan, 84156-83111, Iran Kimia Zarean Mousaabadi, Ali A. Ensafi & Behzad Rezaei * Department of Chemistry and Biochemistry, University of Arkansas, Fayetteville, AR,
72701, USA Ali A. Ensafi * Department of Electrical, Computer and Biomedical Engineering, Toronto Metropolitan University, Toronto, Canada Erfan Naghsh * Institute of Chemistry, Chinese
Academy of Sciences, Beijing, 100190, China Jin-Song Hu Authors * Kimia Zarean Mousaabadi View author publications You can also search for this author inPubMed Google Scholar * Ali A. Ensafi
View author publications You can also search for this author inPubMed Google Scholar * Erfan Naghsh View author publications You can also search for this author inPubMed Google Scholar *
Jin-Song Hu View author publications You can also search for this author inPubMed Google Scholar * Behzad Rezaei View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS K.Z.M.: Conceptualization, methodology, investigation, and writing—original draft. A.A.E.: supervision, conceptualization, data curation, validation, writing—review,
and editing. E.N.: conceptualization and methodology. J.-S.H.: validation, review, and editing; B.R.: advisor and validation. CORRESPONDING AUTHOR Correspondence to Ali A. Ensafi. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims
in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. Supplementary Video 1. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article
are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons
licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of
this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Mousaabadi, K.Z., Ensafi, A.A., Naghsh, E. _et al._ Dual
Ni/Co-hemin metal–organic framework-PrGO for high-performance asymmetric hybrid supercapacitor. _Sci Rep_ 13, 12422 (2023). https://doi.org/10.1038/s41598-023-39553-0 Download citation *
Received: 31 January 2023 * Accepted: 26 July 2023 * Published: 01 August 2023 * DOI: https://doi.org/10.1038/s41598-023-39553-0 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative







