- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The human estrogen receptor has been used for about thirty years, in the yeast _S. cerevisiae_, as a component of chimeric transcription factors. Its ligand, β-estradiol, permits to
control the protein translocation into the nucleus and, as a consequence, the expression of the gene(s) targeted by the synthetic transcription factor. Activators that are orthogonal to the
yeast genome have been realized by fusing the human estrogen receptor to an activation and a DNA-binding domain from bacteria, viruses, or higher eukaryotes. In this work, we optimized the
working of a β-estradiol-sensing device—in terms of detection range and maximal output signal—where the human estrogen receptor is flanked by the bacterial protein LexA and either the strong
VP64 (from herpes simplex virus) or the weaker B42 (from _E. coli_) activation domain. We enhanced the biosensor performance by thoroughly engineering both the chimeric activator and the
reporter protein expression cassette. In particular, we constructed a synthetic promoter—where transcription is induced by the chimeric activators—based on the core sequence of the yeast
_CYC1_ promoter, by tuning parameters such as the length of the 5′ UTR, the distance between adjacent LexA binding sites (operators), and the spacing between the whole operator region and
the main promoter TATA box. We found a configuration that works both as a highly sensitive biosensor and a sharp switch depending on the concentration of the chimeric activator and the
strength of its activation domain. SIMILAR CONTENT BEING VIEWED BY OTHERS EFFICIENT SEX HORMONE BIOSENSORS IN _SACCHAROMYCES CEREVISIAE_ CELLS TO EVALUATE HUMAN AROMATASE ACTIVITY AND
INHIBITION Article Open access 03 January 2025 ENGINEERING A SYNTHETIC GENE CIRCUIT FOR HIGH-PERFORMANCE INDUCIBLE EXPRESSION IN MAMMALIAN SYSTEMS Article Open access 17 April 2024
COMBINATORIAL PROTEIN DIMERIZATION ENABLES PRECISE MULTI-INPUT SYNTHETIC COMPUTATIONS Article Open access 09 March 2023 INTRODUCTION Synthetic Biology is a new branch of life science that
aims at reengineering living cells such that they carry out new, precise functions1. Its origin can be traced back to the first two synthetic gene circuits published in January 20002,3.
Among the circuits that followed, biosensors became the object of many research works due to their numerous possible applications4. Biosensors have been engineered to respond to several
different inputs, e.g., heavy metals5,6, metabolites7, organophosphates8 2,4-dinitrotoluene9, intracellular xylose10, and arsenic11. In yeast Synthetic Biology, the β-estradiol biosensor has
been engineered in various configurations and used, mainly, as a tool to control the synthesis of endogenous and synthetic genes. β-estradiol diffuses through the yeast membrane and does
not provoke, per se, toxic effects unless its concentration becomes much higher than 1000 nM12. In the Eighties of the last century, Ma and Ptashne13 realized a collection of yeast chimeric
activators that combined either GAL4 or LexA DNA-binding domain (DBD) with one of 15 bacterial activation domains (ADs)—among which there was also B42 that we used in this work. The chimeric
activator library was later extended with the inclusion of more bacterial ADs (e.g., B112)14. In 1993, Louvion et al_._12 engineered the first β-estradiol-inducible chimeric activator
(later referred to as GEV) by merging GAL4 DBD with the hormone binding domain of the human estrogen receptor—HBD(hER), where β-estradiol binds—and the strong VP16 AD from the herpes simplex
virus type 115. GEV permitted to activate any galactose-inducible promoter in glucose-containing media, where the growth of _S. cerevisiae_ cells is faster. More recently, Mclsaac et
al_._16 turned GEV into Z3EV by replacing GAL4 DBD with the zinc finger protein Zif268 DBD. Ottoz et al_._17, in contrast, modified GEV by using LexA as a DBD and tested four ADs, among
which B112 was the most performant. Both works aimed at constructing a system for the control of gene expression in _S. cerevisiae_ without interfering with the original molecular processes
that take place in the cells. In Synthetic Biology, such a system is termed _orthogonal_ to the host cell. Finally, Dossani et al_._18 built a large library of hybrid promoters consisting of
core yeast promoters preceded by a variable number of LexA binding site (operators). Each promoter was activated by LexA-HBD(hER)-VP16 at low (up to 100 nM) concentrations of β-estradiol. A
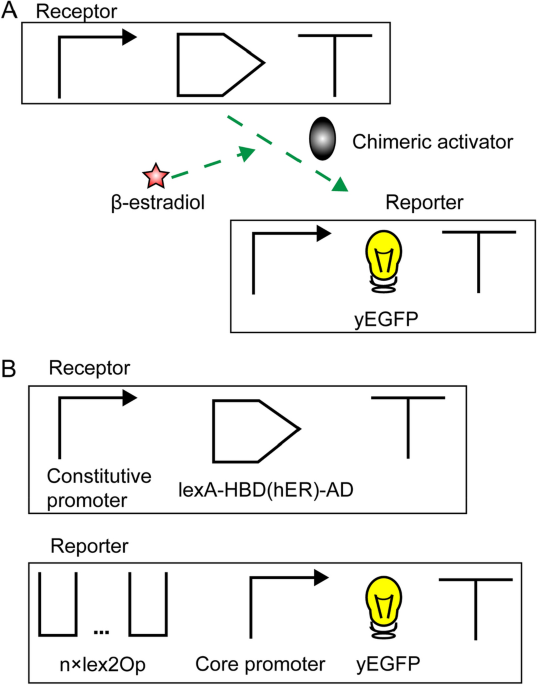
complete β-estradiol biosensor is divided into two components: the receptor and the reporter19. They are transcription units (TUs), i.e., DNA sequences made of a promoter, a coding region,
and a terminator. The receptor expresses constitutively a chimeric activator hosting HBD(hER). The reporter produces a fluorescent protein only in the presence of β-estradiol (see Fig. 1A).
Despite all the implementations mentioned above, a common way to characterize a β-estradiol biosensor has not been established yet. In16,17, important parameters are the basal fluorescence
(i.e., the fluorescence expressed by the biosensor in the absence of β-estradiol) and the maximal fluorescence reached by the circuit—which quantifies how strongly a gene can be expressed
under β-estradiol induction. Basal fluorescence shall be as close as possible to zero, whereas the maximal fluorescence should equal or even overcome that of the strongest yeast constitutive
promoter (i.e., the _GPD_ promoter). In18, these two quantities are merged into a single one, the inducibility, that roughly corresponds to the ON/OFF ratio. In order to obtain both low
basal fluorescence and high response from the same β-estradiol biosensor, its components shall be balanced carefully. In the receptor part, the strength of promoter and AD should be chosen
to avoid the overexpression of the chimeric activator, which would likely cause toxicity effects20. The reporter shall host a reasonably weak core promoter preceded by a thoroughly
engineered operator cassette. Here, several parameters shall be adjusted (e.g., operator number, distance between two adjacent operators, separation from the TATA box) to achieve a good
trade-off between basal and maximal fluorescence level. As a sensing device, however, a β-estradiol biosensor should be also characterized by its sensitivity and tolerance to the input,
which permit to define the detection range over which the biosensor works reliably. None of the β-estradiol biosensors in the literature was evaluated according to these parameters, as if
its original function (detecting β-estradiol) had been forgotten. In this work, we propose a general way to assess the performance of a β-estradiol biosensor. This requires estimating: (1)
the detectivity (D), i.e., the minimal β-estradiol concentration that is unequivocally detected by the biosensor; (2) the tolerance, i.e., the maximal concentration of β-estradiol at which
the biosensor activity is not damaged by toxicity effects; (3) the detection range, which goes from D to T; and (4) the maximal output (usually fluorescence) level, which tells us if the
biosensor can be used to enhance the expression of a target gene up to a desired level. Taking inspiration from17, we engineered deeply both receptor and reporter in order to find out
criteria to optimize the working of the β-estradiol (and potentially other) biosensor(s). Like in17, also in our work the receptor includes the full LexA bacterial protein as a DBD. LexA
binds, as a dimer, a 41-nt-long operator that is organized in two halves of 20 nt separated by a single base pair. Thus, the lex operator is usually referred to as lex2Op. We fused LexA to
HBD(hER) and, separately, four different ADs. In the absence of β-estradiol, the heat shock protein 90 (Hsp90) sequesters the activator in the cytoplasm upon binding HBD(hER). In contrast,
β-estradiol—when present in the cell culture—docks to HBD(hER) and prevents any further interactions with Hsp90, such that the activator translocates into the nucleus19 (see Fig. S1). The
performance of the whole biosensor depends on three main components: (1) the constitutive promoter upstream of the activator; (2) the synthetic promoter upstream of the fluorescent protein;
and (3) the AD at the C-terminus of the chimeric activator, which recruits RNA polymerase II to the activated promoter. We engineered, first, yeast strains for the expression of yEGFP (yeast
enhanced green fluorescent protein). They contained different weak promoters, based on variants of the _CYC1_ core promoter21, preceded by lex2Op sites in a variable number. On these
strains, we constructed overall ten types of β-estradiol biosensors. The initial configuration performed poorly by returning a low output signal in the presence of β-estradiol. We improved
and, finally, optimized the biosensor design by making several changes on the promoter of the reporter part. We modified the number of lex2Op, their reciprocal distance, the distance between
the complete operator cassette and the main TATA box, and the length of the 5′UTR. Moreover, we tested three constitutive promoters and four ADs of different strength on the receptor side,
in order to tune the expression both of the chimeric activator and yEGFP. This work of deep engineering led us to formulate criteria to optimize the β-estradiol biosensor and convert it into
a switch responding to the same hormone. RESULTS AND DISCUSSION We engineered, overall, ten kinds of β-estradiol biosensors organized in a receptor and a reporter TU. The receptor encoded
for the chimeric activator LexA-HBD(hER)-AD, where AD was one among: VP16, VP64 (i.e., the fusion of four VP16 units), mDR521-805—from the mouse dioxin receptor protein22—and B42. The
reporter expressed yEGFP upon activation of the synthetic promoter that combined a fragment of the core yeast constitutive _CYC1_ promoter and a variable quantity of lex2Op sites (see Fig.
1B). We carried out a deep engineering work to understand which features were essential either to enhance the output signal or to detect very low concentrations of the input. There are
various ways to evaluate how a biosensor works. Since it represents a YES (or buffer) Boolean gate, the most straightforward parameter would be the gain (i.e., the ON/OFF ratio) or the
signal separation (i.e., the difference between the one and the zero output). However, each biosensor configuration was characterized via a titration curve, i.e., the fluorescence output was
measured for twelve concentrations of β-estradiol (from 0.98 to 2000 nM—a range over which β-estradiol is not toxic, per se, to the yeast cells) and in the absence of the hormone.
Therefore, other quantities appeared more significative: (1) the basal fluorescence, i.e., the fluorescence expressed in the absence of β-estradiol, which quantifies the biosensor leakage;
(2) the detectivity (D), that corresponds to the minimal concentration of β-estradiol that is sensed by a biosensor, i.e., the smallest concentration of the input that induces an output
signal at least twofold higher than the noise—where the noise is the highest between the background fluorescence (i.e., the mean fluorescence of the empty chassis—byMM2: 52.45 ± 1.86 A.U.)
and the basal fluorescence, as defined previously. It should be noted, though, that the background fluorescence has been subtracted from all fluorescence values reported in this work such
that, to determine D, we should look for the concentration of β-estradiol that induced an output signal either bigger than (or equal to) 52.45 A.U. or twice as high as the basal
fluorescence; (3) the tolerance, which represents the maximal concentration of β-estradiol that is detected unambiguously before the occurrence of toxicity effects; (4) the detection range,
which is the interval in the concentration of β-estradiol delimited by the detectivity and the tolerance; (5) the maximal fluorescence signal—which we used as a reference to quantify the
effects on the biosensor due to changes in one (or more) of its elements. MINIMIZING THE BASAL FLUORESCENCE BY EMPLOYING THE WEAK PROMOTER _TRUNCATED_PCYC1MIN_ IN THE REPORTER. MAXIMIZING
THE EXPRESSION OF THE CHIMERIC ACTIVATOR FROM THE RECEPTOR TO ENHANCE FLUORESCENCE IN THE PRESENCE OF Β-ESTRADIOL Biosensor 1 design: pGPD-LexA-HBD(hER)-AD and 7×lex2Op(2,
37-TATA-52)-truncated_pCYC1min (see Fig. S6). Our initial target was a weak variant of the _CYC1_ core promoter, where the TATA box starting at -106 (TATA-106) was removed and the 5′UTR
shortened from 71 to 24 nt (see Fig. 2). This promoter, termed _truncated_pCYC1min_, was characterized by a very low fluorescence expression (79.34 A.U.)23,24 corresponding to 2.44% of that
of pCYC1min (and 0.43% of that of pGPD—see Table S1). To turn it into an activated promoter, we placed seven copies of lex2Op upstream of its sequence, realizing the topology: 7×lex2Op(2,
37-TATA-52)-truncated_pCYC1min. The first number within the brackets indicates that adjacent lex2Op were separated by two nucleotides (always GT in this case), whereas the second notation
means that the whole operator cassette was placed 37 nt upstream of the TATA box starting at position -52 (TATA-52). We expressed the chimeric activator in high amount via the strong _GPD_
promoter (pGPD) and tested four ADs: the strong V16, VP64, and mDR521-805 together with the weak B42 (in the rest of this section, we will use the AD name to refer to the whole biosensor).
These initial configurations highlighted some interesting features (see Fig. 3A). First, pGPD produced too many chimeric activators such that a fraction of them probably escaped the
interaction with Hsp90 in the cytoplasm and diffused to the nucleus in the absence of β-estradiol. This caused a very high basal fluorescence (1237.13 A.U.) when the activator carried VP64
(byMM109). Both VP16 (byMM335) and mDR521-805 (byMM125) provoked a moderate basal fluorescence (372.19 and 228.32 A.U., respectively), whereas an extremely low value (1.58 A.U.) was due to
B42 (byMM187). VP64 determined both the lowest tolerance—since fluorescence dropped after reaching its peak (3142.85 A.U.) at 7.81 nM β-estradiol—and the shortest detection range (from 3.91
to 7.81 nM). byMM335 (VP16) worked from 1.95 to 62.5 nM β-estradiol and expressed its highest fluorescence (2797.11) at 15.62 nM of the inducer. byMM125 (mDR521-805) further broadened the
detection range (1.95 to 250 nM β-estradiol) and returned the highest fluorescence (3088.08 A.U.) at 125 nM β-estradiol. It should be noted that there was no statistically significant
difference among the maximal fluorescence levels of these three biosensors hosting a strong AD (one-way ANOVA, p-value = 0.4843). Furthermore, the mean value of the highest fluorescence here
measured corresponded to only 17.09% of that constitutively expressed by pGPD (18,318.37 ± 1318.32 A.U.—see Table S1). As for B42, all fluorescence values between 0 and 15.62 nM β-estradiol
were lower than the background fluorescence. Hence its detection range went from 31.25 to 2000 nM β-estradiol, i.e., the maximal concentration used in this work. This first biosensors
pointed out a general pattern: strong ADs cause high D (i.e., they permit to detect small concentrations of β-estradiol) and low tolerance. In contrast, weak ADs determine a lower D and a
higher tolerance. Overall, none of the four biosensors was able to detect 0.98 nM β-estradiol. Moreover, as already mentioned above, the highest fluorescence levels were rather modest. USING
WEAKER PROMOTERS IN THE RECEPTOR TO DECREASE BASAL FLUORESCENCE AND AVOID TOXICITY EFFECTS Biosensor 2 design: constitutive promoter-LexA-HBD(hER)-VP64 and 7×lex2Op(2,
37-TATA-52)-truncated_pCYC1min (see Fig. S7). In biosensors 1, pGPD appeared to be too strong if the chimeric activator carried a potent AD. High basal fluorescence and low tolerance were
the drawbacks of this configuration. Thus, we modified the receptor part of biosensors 1 by employing two weaker promoters to express the chimeric activator (still carrying VP64). We chose
the synthetic promoter DEG1t_pCYC1noTATA (19.75% as strong as pGPD25) and the viral _CMV_ promoter (pCMV, 4.74% as pGPD26—see Table S1). With respect to pGPD, the expression of the synthetic
activator decreased drastically (see Fig. S2). Both DEG1t_pCYC1noTATA and pCMV reduced the basal fluorescence due to VP64 (458 ± 38.97 A.U. and 326 ± 76.01 A.U., respectively). Moreover,
they did not show any toxicity effects up to the maximal concentration of β-estradiol. DEG1t_pCYC1noTATA turned out to be a better choice than pCMV since it guaranteed a larger detection
interval (3.91–2000 nM β-estradiol) and reached a maximal fluorescence level (3242.78 A.U.) comparable to that of biosensors 1 (p-value = 0.5650; two-tailed Welch’s t-test), though at a much
higher concentration of β-estradiol (2000 nM—see Fig. 3B). ENHANCING FLUORESCENCE EXPRESSION BY RESTORING THE FULL 5′UTR OF THE TARGET PROMOTER IN THE REPORTER Biosensor 3 design:
pGPD-LexA-HBD(hER)-B42 and 8×lex2Op(2, variable distance-TATA-52)-truncated_pCYC1min/core (see Fig. S8). In order to increase the maximal fluorescence signal, we restored, in the reporter
part, the full 5′UTR of the _CYC1_ promoter, which permitted us to design three new synthetic promoters where transcription was activated by LexA-HBD(hER)-AD. They contained a cassette of
eight lex2Op (separated by 2 nt—GT) that was followed by either pCYC1min (in two cases) or pCYC1core (as defined in Fig. 2). Each promoter was also characterized by a different distance
between the 8×lex2Op cassette and TATA-52. On the receptor side, we chose the combination of pGPD and B42 AD in order to avoid toxicity. The three biosensors behaved quite similarly and
pointed out that the full 5′UTR is necessary to achieve high fluorescence levels upon transcription activation (see Fig. 3C). Only one biosensor (byMM1376) had a basal fluorescence higher
than the background signal. The maximal fluorescence outputs did not show any statistically significant difference among the three biosensors (see Fig. 3C caption). However, pCYC1core
(byMM1378), which also contains TATA-106, reached the fluorescence peak (9312.6 A.U) at a lower β-estradiol concentration (500 nM) than that of the two pCYC1min-based biosensors (byMM1377:
1000 nM; byMM1376: 2000 nM). It should also be noted that the mean maximal fluorescence of byMM1378 turned out to be 5.55-fold higher than that of biosensor 1 byMM187 (our main reference,
here) and 2.87-fold greater than that of biosensor 2 byMM198, corresponding to 50.64% of that of pGPD. Furthermore, both byMM1376 and byMM1378 showed a higher detectivity than that of
byMM187 (31.25 nM), i.e., D = 7.81 nM and 15.62 nM, respectively. ENHANCING FLUORESCENCE EXPRESSION BY VARYING THE DISTANCE BETWEEN A SINGLE LEX2OP AND TATA-52 IN THE PROMOTER OF THE
REPORTER Biosensor 4 design: DEG1t_pCYC1noTATA-LexA-HBD(hER)-VP64 and lex2Op(variable distance-TATA-52)_pCYC1core (see Fig. S9). From biosensors 3, it was apparent that we needed to keep the
full _CYC1_ 5′UTR in order to increase the maximal fluorescence level. Moreover, pCYC1core provided a higher detectivity than pCYC1min. In order to further improve the performance of our
biosensors, we started making changes on the location of lex2Op. Initially, we replaced the operator cassette with a single lex2Op inside pCYC1core and we varied its distance from TATA-52.
In the receptor part, we adopted the configuration DEG1t_pCYC1noTATA-VP64, i.e., the most promising from biosensors 2. In general, in order to have activation of transcription, the operator,
where the activator binds, shall be upstream of, but not too close to, the TATA box. Otherwise, the activator, once bound to the DNA, will prevent RNA polymerase II from getting access to
the promoter27. The lowest lex2Op-TATA-52 distance that we tested corresponded to 6 nt, which led to both a poor maximal fluorescence value (1933.79 A.U.) and a low detectivity (D = 62.5 nM
β-estradiol). By increasing the distance between lex2Op and TATA-52, the maximal fluorescence grew steadily: 5222.21 A.U. (10 nt); 8186.36 A.U. (37 nt); and 14,170.70 A.U. (60
nt—byMM382—corresponding to 77.05% of pGPD fluorescence; see Fig. 4A). It should be noted that, when lex2Op is 60 nt far from TATA-52, it is also 6 nt upstream of TATA-106, which could give
a (probably modest) contribution to the final fluorescence level. Interestingly, the detectivity improved with the distance as well. However, the best value (D = 7.81 nM β-estradiol) was
reached already at 37 nt. The basal fluorescence was, in general, rather high. However, no specific relation with the lex2Op-TATA-52 distance could be found. CONFIRMING THE BEST
CONFIGURATION FOR THE RECEPTOR BY TESTING TWO MORE CONSTITUTIVE PROMOTERS Biosensor 5 design: constitutive promoter-LexA-HBD(hER)-AD and lex2Op(60-TATA-52)_ pCYC1core (see Fig. S10). After
selecting lex2Op(60-TATA-52)_pCYC1core as a reporter part, we constructed two new circuits where the receptor TU consisted of configurations tried in previous experiments, namely pGPD-B42
and pCMV-VP64 (i.e., a strong and a week element together). As reported in Fig. 4B, byMM368 (pGPD-B42) outperformed byMM371 (pCMV-VP64) in terms of both maximal and basal fluorescence
(numerical values for all biosensor parameters are given in Table 1). However, both designs did not approach the performance of byMM382 and were not considered in further analysis.
INCREASING FLUORESCENCE EXPRESSION BY ENGINEERING THE OPERATOR CASSETTE OF THE PROMOTER IN THE REPORTER. VARYING THE DISTANCE BETWEEN TWO LEX2OP Biosensor 6 design:
DEG1t_pCYC1noTATA-LexA-HBD(hER)-VP64 and 2×lex2Op(variable distance, 60/138-TATA-52)_ pCYC1core (see Fig. S11). A way to further increase transcriptional activation demands to place several
copies of the same operator upstream of the promoter TATA box19,23,24. Moreover, the distance between adjacent promoters can be used to modulate the transcription initiation rate28. We
constructed three more promoters for the reporter TU. They were characterized by the presence of two lex2Op (2×lex2Op) far upstream of TATA-52 (i.e., 60 or 138 nt) of pCYC1core. They were
spaced with 2, 15, and 42 nt. The receptor made use of the DEG1t_pCYC1noTATA-VP64 configuration. As shown in Fig. 4C and Table 1, 15 nt turned out to be an adequate distance between the two
lex2Op (byMM482), whereas both 2 and 42 nt performed poorly, especially concerning the detectivity. Remarkably, the maximal fluorescence of byMM482 reached 17,707.27 A.U., i.e., 96.28% of
that of pGPD. The detection range was also reasonably wide, from 7.81 up to 2000 nM β-estradiol. ADDING A THIRD LEX2OP TO THE ACTIVATED PROMOTER Biosensor 7 design:
DEG1t_pCYC1noTATA-LexA-HBD(hER)-VP64 and 3×lex2Op(15, 60-TATA-52)_ pCYC1core (see Fig. S12). By merging all our previous results, we were able to construct a highly performant biosensor just
by adding a third lex2Op 15 nt upstream of the 2×lex2Op(15,60-TATA-52)_pCYC1core employed in byMM482. The new device returned a maximal fluorescence (23,114.96 A.U.) even 1.26-fold higher
than that of pGPD and the overall broadest detection range (from 1.95 to 2000 nM β-estradiol). Only the basal fluorescence showed a rather high value (1010.08 A.U.), comparable to those of
the circuits with only one or two lex2Op (see Fig. 4D). VARYING THE DISTANCE BETWEEN ADJACENT LEX2OP IN THE 3×LEX2OP CONFIGURATION OF THE ACTIVATED PROMOTER Biosensor 8 design:
DEG1t_pCYC1noTATA-LexA-HBD(hER)-VP64 and 3×lex2Op(variable distance, 60-TATA-52)_ pCYC1core (see Fig. S13). Even though biosensor 7 turned out to work remarkably well, it was built on an
approximative analysis of biosensors 6. We considered only three possible distances between the two lex2Op (2, 15, and 42 nt). Moreover, byMM1369 (2 nt) had a much longer distance from
TATA-52 (138 nt) than the other two biosensors 6 (60 nt). Since 3×lex2Op permitted to achieve both a fluorescence level greater than that of pGPD and a high detectability, we decided to
investigate if the performance of biosensor 7 could be further improved by varying the separation between two contiguous lex2Op. Thus, we built five more 3×lex2Op where two consecutive
lex2Op were separated by 2, 6, 9, 21, and 42 nt with the overall cassette placed 60 nt upstream of TATA-52. Furthermore, for a comparison with biosensor 6 byMM1369, we constructed another
biosensor where the three operators were separated by GT and the whole 3×lex2Op cassette lied 138 nt upstream of TATA-52 (byMM1396, we will refer to it as 2-GT). As for the basal
fluorescence, there was no statistically significant difference among the 6, 15, and 21 nt configuration (ns: p-value = 0.4907, one-way ANOVA—fluorescence oscillated around 1000 A.U.),
whereas 9 nt expressed over 2200 A.U. of fluorescence in the absence of β-estradiol. Forty-two and two nt returned the highest (3934.32 A.U.) and the lowest (595.63 A.U.) value,
respectively, whereas 2-GT expressed 1663.96 A.U. Compared to the 2×lex2Op configuration, the basal fluorescence increased in a statistically significant way only for 2-GT and 42 nt (p-value
< 0.0001; two-sided Welch’s t-test), whereas the increment in 15 nt was not significant (p-value = 0.4507; two-sided Welch’s t-test—see Table 1). The maximal fluorescence was reached at
2000 nM β-estradiol and overcame 120% of that of pGPD in the majority of the biosensor configurations. Exceptions were 2-GT (53.94%, at 250 nM) and 42 nt (98.33% at 125 nM). Higher basal
fluorescence and lower maximal output made the titration curves of 2-GT and 42 nt (byMM1397) clearly different from that of biosensor 7 (byMM381), as apparent in Fig. 5A. By looking at the
three biosensors containing 2×lex2Op, the addition of the third operator was beneficial both for the maximal fluorescence and the detectivity: 2-GT maximal fluorescence showed a 2.07-fold
increase and D went from 250 to 7.81 nM β-estradiol. The improvements on 42 nt performance were even more evident: a 4.22-fold increment in the maximal fluorescence and D from 1000 to 7.81
nM β-estradiol. Less apparent were the improvements on the already highly efficient 15 nt: 1.30-fold augment in the maximal fluorescence and D from 7.81 to 1.95 nM β-estradiol. Therefore,
despite causing an increase in basal fluorescence, a third lex2Op provided an overall advancement in the biosensors’ performance (see Fig. 5A). A striking result from our analysis was that
by varying the distance between adjacent lex2Op from 2 to 21 nt, no big change arose in the circuit behavior. More precisely, we took into account five distances: 2, 6, 9, 15, and 21 nt; 6,
15, and 21 nt showed statistically significant difference only at 31.25 nM β-estradiol (*: p-value = 0.0153; one-way ANOVA), otherwise their titration curves were basically equivalent. Two
nt differed from the previous three configuration in four cases only: 0, 0.98, 1.95 and 31.25 nM β-estradiol. Nine nt reached a remarkable maximal output, corresponding to 120.83% of pGPD
fluorescence, at only 250 nM β-estradiol. It should be noted, though, that the high error associated with 9 nt measurements made the fluorescence levels between 31.25 and 2000 nM β-estradiol
undistinguishable in statistical terms (p-value = 0.6899; one-way ANOVA). Finally, there was no significant difference among the five biosensors in the range 62.5–2000 nM (more than one
third of the whole measurements), i.e., at the concentrations of β-estradiol where the highest fluorescence values were reported. Thus, the maximal fluorescence values of the five circuits
turned out not to be significantly different in statistical terms (p-value = 0.6974; one-way ANOVA). Furthermore, each configuration showed high detectability: 1.95 (2, 9, 15, and 21 nt) and
3.91 (6 nt) nM β-estradiol (see Fig. 5B and Table 1). Taken together, we realized a highly efficient β-estradiol biosensor via the combination of a receptor characterized by the synthetic
promoter DEG1t_pCYC1noTATA and the VP64 AD together with a reporter containing a 3×lex2Op cassette 60 nt upstream of TATA-52 of the _CYC1_ core promoter (which retains its full natural
5′UTR). When the distance between adjacent lex2Op was between 2 and 21 nt, every biosensor was characterized by a very broad detection range (from, at least, 3.91 to 2000 nM β-estradiol no
toxicity effects were present) and a mean maximal fluorescence value at least 1.20-fold higher than that produced by the strong _GPD_ promoter. Therefore, our device can also be adopted to
upregulate gene expression. TURNING THE BIOSENSOR INTO A SWITCH BY CHANGING THE RECEPTOR CONFIGURATION Biosensor 9 design: constitutive promoter-LexA-HBD(hER)-AD and 3×lex2Op(15,
60-TATA-52)_pCYC1core (see Fig. S14). After finding five efficient designs for the promoter in the reporter part, we chose the configuration in biosensor
7—3×lex2Op(15,60-TATA-52)_pCYC1core—and checked if it could lead to the construction of more performant biosensors where pGPD-B42 and pCMV-VP64 took in the receptor part the place of
DEG1t_pCYC1noTATA-VP64. Previously, in biosensors 5, both configurations did not work very well by acting on a single lex2Op placed 60 nt upstream of TATA-52. pCMV-VP64 together with an
optimized 3×lex2Op cassette (byMM369) increased both the maximal fluorescence value (from 26.32 to 65.89% of pGPD one) and the detectivity (from 125 to 3.91 nM β-estradiol) of byMM371.
However, it remained overall far from the performance of most of the biosensors 8. The results obtained with pGPD-B42 appeared more interesting. The new biosensor byMM367 had an extremely
low basal fluorescence (11.32 A.U.) but needed 31.25 nM β-estradiol to overcome the background fluorescence, which limited its detection range. At 250 nM β-estradiol, byMM367 fluorescence
level was comparable to that of byMM369, thus clearly lower (44.15%) that than of byMM381 but, then, increased sharply and reached a remarkable maximal value (122.17% of pGPD one) at 2000 nM
β-estradiol (see Fig. 5C). Overall, the circuit based on pGPD-B42 guarantees a strong gene expression at 2000 nM concentration of β-estradiol and almost no leakage when the hormone is
absent. It should be noted, though, that higher concentrations of β-estradiol (from 4000 nM up to 16,000 nM) spoil the working of byMM367 with the appearance of toxicity, whereas they have
no relevant effects on biosensors containing the configuration DEG1t_pCYC1noTATA-VP64 in the receptor and one up to three lex2Op in the reporter (byMM382, byMM482, and byMM381—see Figs. S4,
S5). On the whole, byMM367 shall be used as a switch (rather than a biosensor) between 0 and 2000 nM β-estradiol to turn OFF and ON effectively—without any problems for the cells—the
expression of a target gene. CONCLUSIONS Chimeric proteins made of domains that are not expressed in yeast are likely to be orthogonal to the yeast genome and, therefore, represent good
candidates for wiring the TUs present in a synthetic circuit29. Bi- or tripartite fusion proteins hosting the docking site for a small molecule permit, furthermore, to control the circuit
working from the outside. HBD(hER) has allowed the construction of several chimeric transcription factors responding to β-estradiol, a hormone well-tolerated by the yeast _S. cerevisiae_. In
this work, we focused on a set of synthetic activators where the bacterial LexA had the function of DBD and it was fused to HBD(hER) and an AD of viral, bacterial, or even mammalian origin.
As previously mentioned, this chimeric protein is not new and several variants—together with their target promoters built ad hoc—have been adopted16,18,30,31,32,33. We constructed ten
different types of β-estradiol biosensors in order to find an optimal configuration that combined a very high fluorescence expression (higher than that reached by the strong _GPD_ promoter)
and a broad detection range. To this aim, we had to assess different configurations of the receptor part (the constitutive promoter and the AD) and engineer target promoters in the reporter
part. In our analysis, the best promoter, in the reporter, was made by the core _CYC1_ sequence (which keeps the three TATA boxes and the full 5′UTR) preceded by three lex2Op operators.
Adjacent lex2Op shall be separated by 2 up to 21 nt and the 3×lex2Op cassette is placed 60 nt upstream of TATA-52. On the receptor side, we found two useful configurations. The best
biosensor configuration demands to fuse LexA-HBD(hER) to the strong VP64 AD and express it under the synthetic constitutive DEG1t_pCYC1noTATA promoter. In this way, the maximal fluorescence
level overcomes that of the _GPD_ promoter (1.38-fold higher when 6 nt separate two lex2Op) and the detection range goes from 1.95 to 2000 nM (2, 9, 15, and 21 nt). In contrast, by replacing
VP64 with B42 and DEG1t_pCYC1noTATA with pGPD, we obtain a perfect switch that expresses, at 2000 nM β-estradiol, more fluorescence than pGPD and shows a negligible leakage in the absence
of β-estradiol. By comparing our results to those by Ottoz et al_._17, our main reference, we can see that our promoter leads to a stronger gene expression with a lower number of lex2Op
(three instead of eight). Hence, we might achieve a further enhancement in gene expression by adding more lex2Op. The core promoter in17 was a minimal version of pCYC1 that retained a single
TATA box starting at position -46 with respect to the TSS (not at -52 because TATA-22 was deleted) and had a long 5′UTR (77 nt of which the last 41 nt came from the plasmid MCS—multiple
cloning sequence). The distance between two adjacent lex2Op was 6 nt (in the optimal range for high gene expression, according to our analysis), whereas the lex2Op cassette lied only 22 nt
upstream of TATA-46, i.e., much less than in our target promoter. On the receptor side, Ottoz et al_._ obtained their best results by using B112 AD and the pACT1 promoter. When using VP16,
they reported toxicity effects between 8 and 31 nM β-estradiol. Interestingly, our optimized biosensors did not show any toxicity despite the fact that VP64 was expressed under
DEG1t_pCYC1noTATA that is 1.37-fold stronger than pACT1. This seems to point out that toxicity is not caused only by the kind and the amount of the ADs expressed in the cells. Strong ADs in
high concentration are, indeed, supposed to prevent the synthesis of vital genes—and lead to cell death—by reducing the availability of RNA polymerase II molecules20. However, different
chimeric activators that are made of dCas9:gRNA (guide RNA) or dCas12a:crRNA (CRISPR RNA) fused to VP64 or the stronger VPR have been expressed in _S. cerevisiae_ under pGPD without inducing
any toxicity effects23,24,27. Therefore, we think that we still have to fully understand what causes toxicity, in yeast cells, in the presence of β-estradiol and LexA-HBD(hER)-AD. Only this
knowledge will permit us to include, in a safe and reliable way, this system inside more complex synthetic gene networks that respond to a combination of multiple inputs. MATERIALS AND
METHODS PLASMID CONSTRUCTION The plasmids realized in this study (see Table S2) are based on the yeast-integrative shuttle vector pRSII405 (receptor part) and pRSII406 (reporter part). Both
backbones are available at Addgene (number 35440 and 35442, respectively; a gift from Steven Haase34). Every plasmid, which hosted a different transcription unit, was assembled with the
Gibson method35. To this aim, the integrative backbone was cut-open (5 µg in a 30 µl solution) with the restriction endonucleases Acc65I (NEB-R0599S) and SacI (NEB-R0156S) to remove the MCS.
At the end of the digestion, the two enzymes were heat-inactivated (20 min. at 65 °C). Every biological part (promoters, coding regions, and terminators) was amplified from their original
plasmid via touchdown PCR36 that was carried out by using Q5 Hot Start high-fidelity DNA polymerase (NEB-M0493S). Primers were designed to guarantee a 40-nt-overlap between adjacent DNA
sequences (see Table S4). PCR products were eluted from the agarose gel by means of the AxiPrep DNA extraction kit (Axigen-AP-GX-250). Biological parts and the cut-open backbone were mixed
in equimolar amount in a 5 µl solution, which was added later to 15 µl of Gibson master mixture solution (main components: T5 exonuclease (NEB-M0363), Phusion High-Fidelity DNA polymerase
(Thermo Scientific-F530L), and Taq DNA ligase (NEB-M0208L)). The overall 20 µl solution was kept in a Thermal Cycler for 1 h at 50 °C. _Escherichia coli_ competent cells (strains DH5α—Life
Technology, 18263-012) were transformed with the plasmids that resulted from the Gibson assembly via a thermal shock (30 s at 42 °C)36. All plasmids were sequenced at Genewiz Inc., Suzhou
(China), to check the correctness of the DNA sequences corresponding to the different TUs. YEAST STRAIN CONSTRUCTION Our plasmids were integrated into the genome of the yeast _S. cerevisiae_
strain FY1679-08A (MATa; ura3-52; leu2D1; trp1D63; his3D200; GAL2)—EUROSCARF 10000M, Johann Wolfgang Goethe University, Frankfurt, Germany. Genomic integration was carried out according to
the lithium-acetate protocol, as described in37, and required to linearize 5 µg of plasmid DNA with either the enzyme StuI (NEB-R0187V), to cut inside the URA3 marker, or BstEII
(NEB-R0162S), to cleave the LEU2 marker. Transformed cells were grown for two days at 30 °C on plates (2% glucose, 2% agar) containing a synthetic defined selective medium: SD-URA or SD-LEU.
All yeast strains engineered in this work are listed in Table S3. RT-QPCR RNA extraction and purification from yeast cells (strains: byMM357, byMM367, byMM369, and byMM381) were carried via
the YeaStar RNA kit (Zymo Research-R1002). cDNA was synthesized via the HiFiScript cDNA Synthesis Kit (CWBIO-CW2569M). The primers used to amplify a portion of LexA, Hsp90, and the
reference ACT1 transcript are reported in Table S5. A qPCR solution had a 10 μL volume divided in: 5 μL 2xSYBR qPCR Mix (SparkJade AH0104); 0.2 μL 10 μM forward and reverse primers; 0.2 μL
ROX(II); cDNA (from 20 to 50 ng); and RNase-free water. On a Roche LightCycler96 machine, we run the program: (1) hold stage: 2 min at 50 °C followed by 10 min at 95 °C; (2) PCR stage: 15 s
at 95 °C, followed by 34 s at 55 °C. The PCR stage was cycled 45 times. Each sample was present in three replicates. Relative mean mRNA expression levels were calculated via the Pfaffl
formula38. The standard deviation was determined through the error propagation formula. GROWTH CURVE Yeast strains (byMM2 and byMM367) were grown in complete synthetic defined medium (SDC)
at 30 °C and 240 RPM for 14 h. Cell concentration (OD600) was then measured such that cell solutions could be diluted to OD600 ~ 0.2 in 30 mL SDC. Yeast cells were grown for 20 h, and OD600
was measured every 2 h with an Eppendorf BioPhotometer device. FLOW CYTOMETRY Yeast cells were grown, first, for 14 h at 30 °C and 240 RPM in SDC. Cells were then 1:100 diluted in 2 ml SDC
supplemented with varying concentrations of β-estradiol (Sigma-Aldrich—E8875; 10 mM stock solution in ethanol). They grew for 20 h at the same conditions described above. Before the FACS
experiment, cells were 1:20 diluted (in SDC). To measure fluorescence intensity, we used a BD FACSVerse (blue laser 488 nm, emission filter 527/32). The FACS machine set-up was checked via
the QC (quality check) program using fluorescent beads (BD FACS uiteTM CS&T Research Beads—17495). Every yeast strain was measured three times in different days. During each experiment,
10,000 events were collected. DATA ANALYSIS The flowcore R-Bioconductor package was used to analyze the data from BD FACSVerse39. The mean background fluorescence, measured on the chassis
strain (byMM2) that does not contain any fluorescence source, was subtracted from the fluorescence intensity associated with each engineered strain. DATA AVAILABILITY FACS data (fcs files)
relating to the results in this work have been uploaded to FlowRepository (http://flowrepository.org)
http://flowrepository.org/id/RvFrJsV75hjylPqY4nRk5jbOptOaYbA8XwUqGlylxSmEjZysGDGGHpXIfcHbnUpy. REFERENCES * Endy, D. Foundations for engineering biology. _Nature_ 438, 449–453.
https://doi.org/10.1038/nature04342 (2005). Article ADS CAS Google Scholar * Gardner, T. S., Cantor, C. R. & Collins, J. J. Construction of a genetic toggle switch in _Escherichia
coli_. _Nature_ 403, 339–342. https://doi.org/10.1038/35002131 (2000). Article ADS CAS Google Scholar * Elowitz, M. B. & Leibler, S. A synthetic oscillatory network of
transcriptional regulators. _Nature_ 403, 335–338. https://doi.org/10.1038/35002125 (2000). Article ADS CAS Google Scholar * Mehrotra, P. Biosensors and their applications—A review. _J.
Oral Biol. Craniofac. Res._ 6, 153–159. https://doi.org/10.1016/j.jobcr.2015.12.002 (2016). Article Google Scholar * Akboğa, D., Saltepe, B., Bozkurt, E. U. & Şeker, U. A
recombinase-based genetic circuit for heavy metal monitoring. _Biosensors_ https://doi.org/10.3390/bios12020122 (2022). Article Google Scholar * Zhang, G., Hu, S. & Jia, X. Highly
sensitive whole-cell biosensor for cadmium detection based on a negative feedback circuit. _Front. Bioeng. Biotechnol._ 9, 799781. https://doi.org/10.3389/fbioe.2021.799781 (2021). Article
Google Scholar * Skjoedt, M. L. _et al._ Engineering prokaryotic transcriptional activators as metabolite biosensors in yeast. _Nat. Chem. Biol._ 12, 951–958.
https://doi.org/10.1038/nchembio.2177 (2016). Article CAS Google Scholar * He, J., Zhang, X., Qian, Y., Wang, Q. & Bai, Y. An engineered quorum-sensing-based whole-cell biosensor for
active degradation of organophosphates. _Biosens. Bioelectron._ 206, 114085. https://doi.org/10.1016/j.bios.2022.114085 (2022). Article CAS Google Scholar * Zhang, Y. _et al._ Design and
optimization of _E. coli_ artificial genetic circuits for detection of explosive composition 2,4-dinitrotoluene. _Biosens. Bioelectron._ 207, 114205.
https://doi.org/10.1016/j.bios.2022.114205 (2022). Article CAS Google Scholar * Wang, M., Li, S. & Zhao, H. Design and engineering of intracellular-metabolite-sensing/regulation gene
circuits in _Saccharomyces cerevisiae_. _Biotechnol. Bioeng._ 113, 206–215. https://doi.org/10.1002/bit.25676 (2016). Article CAS Google Scholar * Chen, S.-Y., Zhang, Y., Li, R., Wang, B.
& Ye, B.-C. De novo design of the ArsR regulated Pars promoter enables a highly sensitive whole-cell biosensor for arsenic contamination. _Anal. Chem._ 94, 7210–7218.
https://doi.org/10.1021/acs.analchem.2c00055 (2022). Article CAS Google Scholar * Louvion, J. F., Havaux-Copf, B. & Picard, D. Fusion of GAL4-VP16 to a steroid-binding domain provides
a tool for gratuitous induction of galactose-responsive genes in yeast. _Gene_ 131, 129–134. https://doi.org/10.1016/0378-1119(93)90681-r (1993). Article CAS Google Scholar * Ma, J.
& Ptashne, M. A new class of yeast transcriptional activators. _Cell_ 51, 113–119. https://doi.org/10.1016/0092-8674(87)90015-8 (1987). Article CAS Google Scholar * Ruden, D. M., Ma,
J., Li, Y., Wood, K. & Ptashne, M. Generating yeast transcriptional activators containing no yeast protein sequences. _Nature_ 350, 250–252. https://doi.org/10.1038/350250a0 (1991).
Article ADS CAS Google Scholar * Triezenberg, S. J., Kingsbury, R. C. & McKnight, S. L. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early
gene expression. _Genes Dev._ 2, 718–729. https://doi.org/10.1101/gad.2.6.718 (1988). Article CAS Google Scholar * McIsaac, R. S., Gibney, P. A., Chandran, S. S., Benjamin, K. R. &
Botstein, D. Synthetic biology tools for programming gene expression without nutritional perturbations in _Saccharomyces cerevisiae_. _Nucleic Acids Res._ 42, e48.
https://doi.org/10.1093/nar/gkt1402 (2014). Article CAS Google Scholar * Ottoz, D. S., Rudolf, F. & Stelling, J. Inducible, tightly regulated and growth condition-independent
transcription factor in _Saccharomyces cerevisiae_. _Nucleic Acids Res._ 42, e130. https://doi.org/10.1093/nar/gku616 (2014). Article CAS Google Scholar * Dossani, Z. Y. _et al._ A
combinatorial approach to synthetic transcription factor-promoter combinations for yeast strain engineering. _Yeast_ 35, 273–280. https://doi.org/10.1002/yea.3292 (2018). Article CAS
Google Scholar * McIsaac, R. S. _et al._ Fast-acting and nearly gratuitous induction of gene expression and protein depletion in _Saccharomyces cerevisiae_. _Mol. Biol. Cell_ 22, 4447–4459.
https://doi.org/10.1091/mbc.E11-05-0466 (2011). Article CAS Google Scholar * Gill, G. & Ptashne, M. Negative effect of the transcriptional activator GAL4. _Nature_ 334, 721–724.
https://doi.org/10.1038/334721a0 (1988). Article ADS CAS Google Scholar * Hahn, S., Hoar, E. T. & Guarente, L. Each of three “TATA elements” specifies a subset of the transcription
initiation sites at the CYC-1 promoter of _Saccharomyces cerevisiae_. _Proc. Natl. Acad. Sci. USA_ 82, 8562–8566. https://doi.org/10.1073/pnas.82.24.8562 (1985). Article ADS CAS Google
Scholar * Whitelaw, M. L., McGuire, J., Picard, D., Gustafsson, J. A. & Poellinger, L. Heat shock protein hsp90 regulates dioxin receptor function in vivo. _Proc. Natl. Acad. Sci. USA_
92, 4437–4441. https://doi.org/10.1073/pnas.92.10.4437 (1995). Article ADS CAS Google Scholar * Yu, L. & Marchisio, M. A. _Saccharomyces cerevisiae_ synthetic transcriptional
networks harnessing dCas12a and type V-A anti-CRISPR proteins. _ACS Synth. Biol._ 10, 870–883. https://doi.org/10.1021/acssynbio.1c00006 (2021). Article CAS Google Scholar * Zhang, Y.
& Marchisio, M. A. Interaction of bare dSpCas9, scaffold gRNA, and type II anti-CRISPR proteins highly favors the control of gene expression in the yeast _S. cerevisiae_. _ACS Synth.
Biol._ 11, 176–190. https://doi.org/10.1021/acssynbio.1c00352 (2022). Article CAS Google Scholar * Song, W., Li, J., Liang, Q. & Marchisio, M. A. Can terminators be used as insulators
into yeast synthetic gene circuits?. _J. Biol. Eng._ 10, 19. https://doi.org/10.1186/s13036-016-0040-5 (2016). Article CAS Google Scholar * Feng, X. & Marchisio, M. A. Novel _S.
cerevisiae_ hybrid synthetic promoters based on foreign core promoter sequences. _Int. J. Mol. Sci._ https://doi.org/10.3390/ijms22115704 (2021). Article Google Scholar * Farzadfard, F.,
Perli, S. D. & Lu, T. K. Tunable and multifunctional eukaryotic transcription factors based on CRISPR/Cas. _ACS Synth. Biol._ 2, 604–613. https://doi.org/10.1021/sb400081r (2013).
Article CAS Google Scholar * Murphy, K. F., Balazsi, G. & Collins, J. J. Combinatorial promoter design for engineering noisy gene expression. _Proc. Natl. Acad. Sci. USA_ 104,
12726–12731. https://doi.org/10.1073/pnas.0608451104 (2007). Article ADS CAS Google Scholar * Abraha, B. W. & Marchisio, M. A. NOT gates based on protein degradation as a case study
for a new modular modeling via SBML level 3-comp package. _Front. Bioeng. Biotechnol._ 10, 845240. https://doi.org/10.3389/fbioe.2022.845240 (2022). Article Google Scholar * Ajo-Franklin,
C. M. _et al._ Rational design of memory in eukaryotic cells. _Genes Dev._ 21, 2271–2276. https://doi.org/10.1101/gad.1586107 (2007). Article CAS Google Scholar * Marchisio, M. A. In
silico design and in vivo implementation of yeast gene Boolean gates. _J. Biol. Eng._ 8, 6. https://doi.org/10.1186/1754-1611-8-6 (2014). Article CAS Google Scholar * Rantasalo, A. _et
al._ Synthetic transcription amplifier system for orthogonal control of gene expression in _Saccharomyces cerevisiae_. _PLoS ONE_ 11, e0148320. https://doi.org/10.1371/journal.pone.0148320
(2016). Article CAS Google Scholar * Rantasalo, A., Kuivanen, J., Penttila, M., Jantti, J. & Mojzita, D. Synthetic toolkit for complex genetic circuit engineering in _Saccharomyces
cerevisiae_. _ACS Synth. Biol._ 7, 1573–1587. https://doi.org/10.1021/acssynbio.8b00076 (2018). Article CAS Google Scholar * Chee, M. K. & Haase, S. B. New and redesigned pRS plasmid
shuttle vectors for genetic manipulation of _Saccharomyces cerevisiae_. _G3_ 2, 515–526. https://doi.org/10.1534/g3.111.001917 (2012). Article CAS Google Scholar * Gibson, D. G. _et al._
Enzymatic assembly of DNA molecules up to several hundred kilobases. _Nat. Methods_ 6, 343–345. https://doi.org/10.1038/nmeth.1318 (2009). Article CAS Google Scholar * Green, M. R. &
Sambrook, J. Touchdown polymerase chain reaction (PCR). _Cold Spring Harb. Protoc._ https://doi.org/10.1101/pdb.prot095133 (2018). Article Google Scholar * Gietz, R. D. & Woods, R. A.
Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. _Methods Enzymol._ 350, 87–96. https://doi.org/10.1016/s0076-6879(02)50957-5 (2002).
Article CAS Google Scholar * Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT-PCR. _Nucleic Acids Res._ 29, e45. https://doi.org/10.1093/nar/29.9.e45
(2001). Article CAS Google Scholar * Hahne, F. _et al._ flowCore: A bioconductor package for high throughput flow cytometry. _BMC Bioinform._ 10, 106.
https://doi.org/10.1186/1471-2105-10-106 (2009). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We want to thank all the students of the Synthetic Biology lab-SPST
(Tianjin University) for their generous help. Moreover, we express our gratitude to Li Zhi and Xiangyang Zhang for their assistance in the FACS experiments. AUTHOR INFORMATION Author notes *
These authors contributed equally: Tian Zhou and Zhiying Liang. AUTHORS AND AFFILIATIONS * School of Pharmaceutical Science and Technology, Tianjin University, 92 Weijin Road, Tianjin,
300072, China Tian Zhou & Mario Andrea Marchisio * School of Life Science and Technology, Harbin Institute of Technology, 2 Yikuang Street, Harbin, 150080, China Zhiying Liang Authors *
Tian Zhou View author publications You can also search for this author inPubMed Google Scholar * Zhiying Liang View author publications You can also search for this author inPubMed Google
Scholar * Mario Andrea Marchisio View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Experiments: T.Z. and Z.L. Data analysis: T.Z., L.Z., and
M.A.M. Manuscript writing: T.Z. and M.A.M. Conceptualization and supervision: M.A.M. CORRESPONDING AUTHOR Correspondence to Mario Andrea Marchisio. ETHICS DECLARATIONS COMPETING INTERESTS
The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's
Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhou, T., Liang, Z. & Marchisio, M.A. Engineering a two-gene system to operate
as a highly sensitive biosensor or a sharp switch upon induction with β-estradiol. _Sci Rep_ 12, 21791 (2022). https://doi.org/10.1038/s41598-022-26195-x Download citation * Received: 28
September 2022 * Accepted: 12 December 2022 * Published: 16 December 2022 * DOI: https://doi.org/10.1038/s41598-022-26195-x SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative










