- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The clustered regularly interspaced short palindromic repeat (CRISPR)-associated protein 9 (Cas9) system represents a powerful gene-editing tool and could enable treatment of
blinding diseases of the retina. As a peptide of bacterial origin, we investigated the immunogenic potential of Cas9 in models of retinal immunocompetent cells: human microglia (IMhu) and
ARPE-19 cells. Transfection with _Streptococcus pyogenes_-Cas9 expression plasmids (_Sp_Cas9 plasmid) induced Cas9 protein expression in both cell lines. However, only ARPE-19 cells, not
IMhu cells, responded with pro-inflammatory immune responses as evidenced by the upregulation of IL-8, IL-6, and the cellular activation markers HLA-ABC and CD54 (ICAM). These
pro-inflammatory responses were also induced through transfection with equally sized non-coding control plasmids. Moreover, viability rates of ARPE-19 cells were reduced after transfection
with both the _Sp_Cas9 plasmids and the control plasmids. Although these results demonstrate cell type-specific responses to the DNA plasmid vector, they show no evidence of an immunogenic
effect due to the presence of Cas9 in models of human retinal pigment epithelial and microglia cells. These findings add another layer of confidence in the immunological safety of potential
future Cas9-mediated retinal gene therapies. SIMILAR CONTENT BEING VIEWED BY OTHERS LENTIVIRAL DELIVERY OF CO-PACKAGED _CAS9_ MRNA AND A _VEGFA_-TARGETING GUIDE RNA PREVENTS WET AGE-RELATED
MACULAR DEGENERATION IN MICE Article 04 January 2021 THE APPLICATION AND PROGRESSION OF CRISPR/CAS9 TECHNOLOGY IN OPHTHALMOLOGICAL DISEASES Article 01 August 2022 THE AAV2.7M8 CAPSID
PACKAGES A HIGHER DEGREE OF HETEROGENEOUS VECTOR GENOMES THAN AAV2 Article 12 August 2024 INTRODUCTION Inherited retinal dystrophies (IRDs) are a group of rare genetic disorders of the
retina that threaten vision and range from causing severe visual disability to the complete loss of light perception. Loss of function mutations can be addressed by supplementing the genetic
information with the coding sequence (e.g. _RPE65_ in _voretigene neparvovec_) of the disease gene, generally through the use of adeno-associated viral vectors (AAV)1. Gain-of-function
mutations, such as _RHO_ gene mutations causing retinitis pigmentosa (RP), can induce the production of cytotoxic proteins and dominant-negative mutations, like _RP1_ variants in RP, and can
lead to the expression of mutated proteins that impair the function of the wild type protein. Adding an episomal coding sequence of _RP1 _via AAV-mediated gene therapy would not address the
disease mechanism. However, genome editing may be used to disrupt the dominant allele causing the pathogenic mutation2. The clustered regularly interspaced short palindromic repeat
(CRISPR)-associated protein 9 (Cas9) system represents an important gene-editing tool for gene therapy. It consists of two components: the Cas9 protein, a bacterial RNA-guided DNA
endonuclease which induces double-stranded DNA breaks, and a single guide RNA (sgRNA) that forms a complex with Cas9 and guides it to a specific DNA target sequence3. Cas9 can be transferred
into a host cell as a preformed complex of Cas9 protein and sgRNA4,5, as an mRNA molecule encoding for the Cas9 transgene4,5, or via lentiviral delivery of Cas9 mRNA6 or AAV-mediated
delivery of Cas9 DNA44. Additionally, Cas9 gene editing can be achieved through plasmid-mediated gene transfer7. Cas9-encoding plasmids can be delivered into the cell via electroporation,
microinjection, and non-viral vectors such as nanoparticles and cationic lipids8. CRISPR-Cas9-based retinal gene therapy has already been analyzed in clinical settings
(www.clinicaltrials.gov; NCT03872479). DNA plasmid vectors for Cas9 gene transfer have successfully been tested in rodent studies of retinal gene therapy7,9,10. However, it was shown that
the transfer of DNA plasmids can up-regulate pro-inflammatory cytokines11,12 induce inflammatory cell infiltration11, and trigger cell death in various cell types11,12. Moreover,
CRISPR-Cas9-based gene therapies still contain unquantified risks, as Cas9 can induce innate and adaptive immune response in blood cells13,14,15,16,17. This is of notable relevance, as
immunity to Cas9 can lead to elimination of Cas9-expressing cells17. However, the potential immunogenicity of Cas9 in retinal cells remains to be determined. Immunocompetent cells in the
retina that could potentially react to Cas9 and/or the DNA of the plasmid vector include microglia and retinal pigment epithelium (RPE) cells. Microglia express major innate pattern
recognition receptors (PRRs)18. Upon stimulation, they are capable of producing various inflammatory cytokines and upregulating activation molecules including major histocompatibility
complex (MHC) class II molecules and intercellular adhesion molecule-1 (ICAM-1; CD54)18. Additionally, microglia play important roles in the defense against infectious diseases18 and become
activated and proliferate in response to retinal gene therapy19. They initiate retinal inflammation20 and essentially control the infiltration of immune cells into the retina20,21. Upon
stimulation they are activated within minutes22 and induce early inflammatory responses that precede responses of macroglia23 including Mueller cells24. Collectively, this suggests that
microglia might act as rapid immunological sensors and key inducers of initial innate immune responses in the retina, making them an interesting target in the study of initial innate immune
responses to Cas9. Similar to microglia, RPE cells have also been shown to upregulate MHC class II molecules and ICAM-1 after stimulation25. Due to their expression of major PRRs and their
responsiveness to stimulation of these receptors26,27 RPE cells are considered key players in the first-line innate immune response to microbial organisms27. Moreover, the RPE is involved in
the pathogenesis of several IRDs28. Accordingly, RPE cells not only represent an attractive gene therapy target for Cas9 gene therapy, but might also show immunological responses to Cas9.
Immune responses of retinal microglia and RPE cells to the DNA plasmid vector or to Cas9 DNA, RNA, and/or the transgene protein could potentially affect the Cas9 gene-editing efficacy and
impair cell viability. In this study, we analyzed whether plasmid-mediated gene transfer of Cas9 induces immune responses in models of human RPE and microglia cells using ARPE-19 cells and
the new immortalized human microglia cell line SV40 (IMhu). RESULTS CHARACTERIZATION OF THE IMMUNE-RESPONSIVENESS OF IMHU CELLS TO STIMULATION WITH PRR LIGANDS It has been shown that
inherited retinal diseases caused by gene mutations can be corrected via plasmid-mediated Cas9 gene editing7,10. However, there is evidence that either Cas9 or DNA plasmids can induce immune
responses4,11,14. Retinal microglia participate in inflammatory processes by secreting pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, and
IL-1818. Moreover, murine microglia have been shown to release pro-inflammatory cytokines like TNF-α, IL-1β, IL-6 and the type I interferons (IFN) IFN-α and IFN-β in response to ligands of
major PRRs29. This suggests that microglia could potentially also respond to the DNA plasmid vector and/or Cas9. IMhu cells were used to analyze microglial immune responses to
plasmid-mediated gene transfer of Cas9. This new microglia cell line is of validated human origin, as confirmed by sequencing and displays numerous similarities to primary human microglia in
terms of morphology, the expression of cell surface markers, and immune responses to stimulation with pro-inflammatory cytokines30,31. IMhu cells exhibit the same typical microglial
phenotype observed in primary and immortalized microglial cultures31. IMhu express surface markers specific for human microglia-macrophage lineage such as CD11b, TGFβR, and P2RY12; these
markers are also expressed on primary microglia30,31,32. IMhu also demonstrate phagocytic and migratory activity characteristic of primary microglia31,33. Additionally, it has been
demonstrated that IMhu respond to pro-inflammatory stimulation with the activation of an M1 phenotype, involving upregulation of several pro-inflammatory cytokines and chemokines, a response
also observed in primary human microglia30,34. The characterization of IMhu as a microglial cell model, though, has not yet been extended to an assessment of the cell line’s responsiveness
to the activation of major PRRs. Thus, before investigating the IMhu immune response to _Sp_Cas9 plasmid transfection, we tested the immune-competence of this cell line by stimulating it
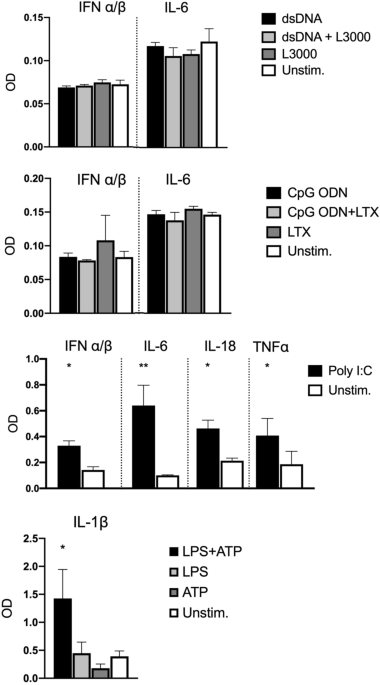
with ligands of various PRRs. After 24 h, levels of pro-inflammatory cytokines and type I IFNs in the supernatant were determined using HEK-Blue IFN-α/β, HEK-Blue IL-1β, HEK-Blue IL-6,
HEK-Blue IL-18, and HEK-Blue TNFα reporter cells (Fig. 1). Stimulation of intracellular DNA receptors with double-stranded DNA (dsDNA) or oligodeoxynucleotides containing unmethylated
cytosine-guanine dinucleotides (CpG ODNs) did not induce a detectable production of IFN-α/β or pro-inflammatory IL-6. In contrast, stimulation with the Toll-like receptor (TLR)3 ligand Poly
I:C induced significant releases of IFN-α/β, IL-6, IL-18, and TNF-α. Moreover, significantly elevated levels of IL-1β showed that IMhu also responded to inducers of inflammasome signaling
(LPS and ATP) (Fig. 1). This suggests that IMhu cells are capable of mounting inflammatory immune responses to ligands of TLR3 and inducers of inflammasome signaling, but have limited
immunoreactivity to ligands of intracellular DNA receptors. TRANSFECTION WITH THE SPCAS9 PLASMID RESULTS IN CAS9 PROTEIN EXPRESSION IN IMHU CELLS BUT DOES NOT TRIGGER CYTOKINE RESPONSES To
test whether plasmid-mediated gene transfer of Cas9 induces immune responses in cell models of human microglia cells, we designed an experimental plasmid coding for the _Streptococcus
pyogenes_ Cas9 (_Sp_Cas9) sequence (_Sp_Cas9 plasmid) (Fig. 2A). An identical non-coding plasmid (NC plasmid) without the Cas9 sequence served as negative control for Cas9 staining (Fig.
2A). To confirm that _Sp_Cas9 plasmid transfection results in Cas9 expression, IMhu cells were transfected with either the _Sp_Cas9 plasmid or NC plasmid using cationic lipid mediated
transfection. At 24 h post-transfection, cells were stained with an anti-_Sp_Cas9 antibody and analyzed via fluorescence microscopy (Fig. 2B). We found that approximately 30% of the IMhu
cells expressed Cas9 intracellularly. As expected, no Cas9 expression was detected in IMhu cells transfected with the NC plasmid (Fig. 2B). Next, we analyzed the immune responses of IMhu
cells to _Sp_Cas9 plasmid transfection. To this end, IMhu cells were transfected via cationic lipid mediated transfection with either the _Sp_Cas9 plasmid or the NC plasmid, or treated with
the transfection reagent Lipofectamine 3000 (L3000) alone. At 24 h post-transfection supernatant was harvested and the release of 105 cytokines was assessed using Proteome Profiler™ Antibody
Arrays. Heat map analysis of cytokine ratios of _Sp_Cas9 plasmid-transfected _versus_ L3000 treated cells, or NC plasmid-transfected _versus_ L3000 treated cells respectively showed that
neither transfection with the _Sp_Cas9 plasmid nor with the NC plasmid triggered a detectable change in the release of any of the measured immune mediators (Fig. 2C). To verify these
results, we repeated this experiment including IMhu cells which were either treated only with the _Sp_Cas9 plasmid (but no L3000) or not stimulated, as additional controls. At 6 h, 12 h, 24
h, and 48 h after stimulation, cytokine levels of IL-1β, IL-6, IL-18, and TNF-α were determined using HEK Blue™ cells. No differences in the levels of any of these cytokines were observed
between _Sp_Cas9 plasmid- or NC plasmid-transfected cells, or cells treated only with either the _Sp_Cas9 plasmid or L3000 compared to unstimulated controls (Figure S1A). To evaluate whether
IMhu cells responded to other plasmids of similar or larger size encoding for _Sp_Cas9 and/or different fluorescent proteins, IMhu cells were transfected with two additional plasmids
(mKate: 4.8 kbp and the EGFP _Sp_Cas9 plasmid: 9.3 kbp) (Figure S1B). Again, measurements of cytokine production from supernatant samples using HEK Blue™ IFN α/β-, IL-1β-, IL-6-, IL-18-, or
TFNα-cells revealed no significant changes in cytokine releases of the plasmid-stimulated cells compared to the non-stimulated controls (Figure S1C). These results indicate that neither
plasmid transfection in general, nor plasmid-mediated gene transfer of Cas9 triggers immune responses in IMhu microglia cells. SPCAS9 PLASMID TRANSFECTION INDUCES A VECTOR-RELATED RELEASE OF
IL-6 AND IL-8 IN ARPE-19 CELLS To evaluate whether plasmid-mediated gene transfer of Cas9 induces immune responses in human RPE cells, ARPE-19 cells were transfected with the _Sp_Cas9
plasmid and a non-coding control plasmid. As transfection efficacy35 and nuclear delivery of plasmids36,37 have been shown vary in relation to vector size35,36,37, the Cas9 sequence was
replaced by a non-coding stuffer sequence enlarging the plasmid to the size of the 8 kbp _Sp_Cas9 plasmid (stuffer plasmid) (Fig. 3A, upper panel) to create the control plasmid. Two
additional EGFP-encoding plasmids: an EGFP-_Sp_Cas9 plasmid (9.3 kbp) expressing Cas9 and EGFP under the same promoter and a respective equally sized non-coding EGFP-stuffer plasmid were
used (Fig. 3A, lower panel) to facilitate precise flow cytometric comparison of transfection rates. To assess Cas9 and/or EGFP protein expression following plasmid transfection, ARPE-19
cells were transfected with equal concentrations of the _Sp_Cas9 plasmid, the stuffer plasmid, the EGFP-_Sp_Cas9 plasmid, or the EGFP-stuffer plasmid. As the _Sp_Cas9 plasmids and their
respective stuffer control plasmids were of equal size and almost identical molar mass, the transfection at equal mass corresponded to an almost equimolar transfection of the _Sp_Cas9
plasmids and their respective control plasmids. After 24 h cells were stained with _Sp_Cas9 antibodies as described. Microscopic evaluation confirmed intracellular Cas9 expression
exclusively in _Sp_Cas9 plasmid and EGFP-_Sp_Cas9 plasmid-transfected cells and a comparable EGFP expression exclusively in EGFP-_Sp_Cas9 plasmid and EGFP-stuffer plasmid-transfected cells
(Fig. 3B) and demonstrated Cas9 and EGFP co-expression following transfection with the EGFP-_Sp_Cas9 plasmid as expected (Fig. 3B, third panel). For flow cytometric quantification of the
transfection rates, EGFP-_Sp_Cas9 plasmid or the EGFP-stuffer plasmid-transfected cells were harvested at 24 h post treatment, stained with the cell death marker 7-AAD, and subsequently
analyzed. Living ARPE-19 cells were gated and the percentage of EGFP positive cells was determined as shown in Fig. 3C,D. This analysis confirmed comparable transfection rates of the
EGFP-_Sp_Cas9 plasmid [16.13 ± 3.8% (mean ± SD)] and the EGFP-stuffer plasmid (20.92 ± 6.43%) (Fig. 3E) with no significant differences seen between groups (p = 0.557). To analyze immune
responses of ARPE-19 cells to plasmid-mediated gene transfer of Cas9, ARPE-19 cells were transfected with either the _Sp_Cas9 plasmid or treated with the transfection reagent Lipofectamine
LTX (LTX) alone. At 24 h following transfection, cytokine levels in the supernatant were determined using Proteome Profiler™ Antibody Arrays. Heat map analysis demonstrates that transfection
of ARPE-19 cells with the SpCas9 plasmid triggered an IL-8 response (Fig. 4A). IL-8 was increased 14-fold following _Sp_Cas9 plasmid-transfection when compared to LTX treatment only. To
strengthen the results of this semiquantitative analysis with a quantitative method, IL-8 concentrations in the supernatant of ARPE-19 cells transfected with the _Sp_Cas9 plasmid or the
EGFP-_Sp_Cas9 plasmid were measured at five time points after treatment (3 h, 12 h, 18 h, 24 h, and 48 h) using a sandwich ELISA. To determine whether the observed IL-8 response was
Cas9-related or plasmid-vector-related, this experiment also included ARPE-19 cells transfected with the stuffer plasmid and the EGFP-stuffer plasmid. ARPE-19 cells which were either treated
with only the _Sp_Cas9 plasmid, with LTX, or left unstimulated served as additional controls. Transfection with both _Sp_Cas9-encoding plasmids as well as with both stuffer plasmids induced
a strong IL-8 release, significant for all plasmid-transfected groups at 48 h after stimulation (48 h: SpCas9 plasmid + LTX vs unstim. control p < 0.001; stuffer plasmid + LTX vs unstim.
control p < 0.001; EGFP-SpCas9 plasmid vs unstim. control p = 0.014; EGFP-stuffer-plasmid vs unstim. control p < 0.001), whereas no significant differences in IL-8 levels were
observed between the additional controls (Fig. 4B). Comparison between plasmid-transfected ARPE-19 cells revealed no significant differences in the IL-8 response between cells treated with
_Sp_Cas9 encoding plasmids and stuffer plasmids (_Sp_Cas9 plasmid _vs_ stuffer plasmid, p = 0.368; EGFP-_Sp_Cas9 plasmid vs EGFP-stuffer-plasmid, p = 0.354) (Fig. 4B). Chen et al. have shown
that IL-8 secretion by ARPE-19 cells is mediated by the induction of NF-κB and MAPK signaling, and activation of these signaling pathways leads to the additional release of IL-638. To test
whether plasmid-mediated gene transfer of Cas9 in ARPE-19 cells triggers IL-6 secretion, we measured IL-6 in the supernatant of _Sp_Cas9 plasmid-transfected cells and the corresponding
control groups using a sandwich ELISA. This more sensitive cytokine analysis showed that transfection with both _Sp_Cas9-encoding plasmids as well as with both stuffer plasmids resulted in
an increase in IL-6 production which was significant for all groups at 48 h after stimulation (48 h: _Sp_Cas9 plasmid + LTX vs unstim. control p < 0.001; stuffer plasmid + LTX vs unstim.
control p < 0.001; EGFP-_Sp_Cas9 plasmid _vs_ unstim. control p < 0.001; EGFP-stuffer-plasmid vs unstim. control p < 0.001) (Fig. 4C). No differences in IL-6 concentrations were
observed between cells treated only with either the _Sp_Cas9 plasmid or LTX and unstimulated controls. Moreover, there were no significant differences in the IL-6 response between cells
treated with _Sp_Cas9 encoding plasmids and stuffer plasmids (_Sp_Cas9 plasmid _vs_ stuffer plasmid, p = 0.424; EGFP-_Sp_Cas9 plasmid _vs_ EGFP-stuffer-plasmid, p = 0.755) (Fig. 4C). Taken
together, plasmid transfection of ARPE-19 cells with either the _Sp_Cas9-encoding plasmids or the stuffer control plasmids triggered significant IL-8 and IL-6 secretion. These effects were
comparable between plasmids both coding and non-coding for Cas9, suggesting that in ARPE-19 cells, the immunogenic effect of Cas9 transfection is triggered by the transfected plasmid DNA
rather than by the presence or expression of the Cas9 transgene. PLASMID TRANSFECTION UPREGULATES IMMUNOLOGICAL SURFACE MARKERS ON ARPE-19 CELLS It has been shown that stimulated ARPE-19
cells upregulate immunological surface markers indicative of cell activation, including HLA-ABC and HLA-DR major histocompatibility (MHC) antigens, and CD54 (ICAM-1)25. To analyze whether
plasmid-mediated gene transfer of Cas9 leads to cell activation, ARPE-19 cells were stimulated with the _Sp_Cas9 plasmid and the stuffer plasmid. At 24 h post-stimulation, cells were
harvested, stained with fluorescent antibodies against HLA-ABC, HLA-DR and CD54, and the cell death marker 7-AAD, and subsequently analyzed via flow cytometry. Living ARPE-19 cells were
gated as shown in Fig. 3C and surface expression of HLA-DR, HLA-ABC, and CD54 was determined (Fig. 5A). We found that neither _Sp_Cas9 plasmid- and stuffer plasmid-transfection nor
stimulation of ARPE-19 cells with the Cas9 plasmid or LTX alone induced changes in the expression of HLA-DR (Fig. 5A,B; lower panels). However, both _Sp_Cas9 plasmid transfection and stuffer
plasmid transfection led to a significant upregulation of HLA-ABC (_Sp_Cas9 plasmid + LTX vs unstim. control p < 0.001; stuffer plasmid + LTX vs unstim. control p < 0.001) and CD54
(SpCas9 plasmid + LTX vs unstim. control p < 0.001; stuffer plasmid + LTX vs unstim. control p < 0.001), with no significant differences in the expression levels of these markers seen
between the two groups. No changes in HLA-ABC and CD54 expression were observed in cells treated only with either the _Sp_Cas9 plasmid or LTX (Fig. 5A,B; upper panels). Similar results were
observed following transfection with the EGFP-_Sp_Cas9 plasmid and the EGFP-stuffer-plasmid: here, transfection also did not lead to changes in the expression of HLA-DR (Fig. 5C,D; lower
panels), but caused increases in the expression of HLA-ABC and CD54. These were significant for HLA-ABC (EGFP-_Sp_Cas9 plasmid + LTX vs unstim. control p < 0.001; EGFP-stuffer plasmid +
LTX vs unstim. control p < 0.001). A similar trend was observed for CD54 (EGFP-_Sp_Cas9 plasmid + LTX vs unstim. control p = 0.008; EGFP-stuffer plasmid + LTX vs unstim. control p =
0.011) (Fig. 5A,B; upper panels). Overall, this suggests that the upregulation of HLA-ABC and CD54 expression on ARPE-19 cells was triggered by the transfection of the DNA plasmid rather
than by the presence or expression of the Cas9 transgene. PLASMID TRANSFECTION DECREASES THE VIABILITY RATE OF ARPE-19 CELLS It has been shown that cationic lipid transfection of DNA
plasmids can induce cell death12. To determine whether cationic lipid-mediated transfection of the _Sp_Cas9 plasmid influences cell viability, the transfected IMhu cells and ARPE-19 cells
were first examined microscopically. There was no evidence of increased cell death in _Sp_Cas9 plasmid-transfected and NC plasmid-transfected IMhu microglia up to 48 h after treatment
(Figure S2A). In contrast numerous floating spherical cells in the supernatant of ARPE-19 cultures suggested reduced cell viability from 24 h on after transfection with the _Sp_Cas9-encoding
plasmids or the corresponding stuffer plasmids (Figure S2B). To investigate the viability of transfected ARPE-19 cells in more detail, these cells were either transfected with the _Sp_Cas9
plasmid or stuffer plasmid, or treated with LTX or _Sp_Cas9 plasmid only and compared to unstimulated cells. After 24 h, 7-AAD negative living ARPE-19 cells were quantified by flow cytometry
(compare Fig. 3C). The analysis showed a minimal reduction in the viability percentages of _Sp_Cas9 plasmid-transfected and stuffer plasmid-transfected ARPE-19 cells when compared to
control groups (_Sp_Cas9 plasmid + LTX vs unstim. control p = 0.049; stuffer plasmid + LTX vs unstim. control p = 0.05) (Fig. 6A). Similar results were observed for EGFP-_Sp_Cas9
plasmid-transfected and EGFP-stuffer plasmid-transfected cells (EGFP-_Sp_Cas9 plasmid + LTX vs unstim. control p = 0.009; EGFP-stuffer plasmid + LTX vs unstim. control p = 0.02) (Fig. 6B).
The percentage of living cells did not differ between _Sp_Cas9 encoding plasmids or the corresponding stuffer plasmids, suggesting weak cytotoxic effects induced by plasmid transfection
rather than the presence or expression of the Cas9. DISCUSSION CRISPR/Cas9 technology has been successfully tested in animal studies of retinal gene therapy39,40 and a human trial of
CRISPR/Cas9 retinal gene editing is currently ongoing (www.clinicaltrials.gov; NCT03872479). Plasmids containing Cas9 or other transgenes have been used for gene transfer in a number of
translational efforts to develop genetic therapies for inherited retinal dystrophies (IRD)7,9,10,41,42. Moreover, plasmid-mediated gene transfer is applied in the development of cell-based
retinal therapies43,44, where target cells, such as RPE cells, are transfected ex vivo with the respective plasmid vector prior to subsequent transplantation into the retina. Interestingly,
the transfer of DNA plasmids can trigger strong innate immune responses in muscle cells11, demonstrating the potential immunogenicity of plasmid DNA. Moreover, Cas9 is a peptide of bacterial
origin and therefore carries the potential for non-self-recognition and immunogenicity. Such an effect could lead to adverse reactions and negate therapeutic efficacy in the target tissue.
Indeed, recent publications demonstrate that Cas9 can induce humoral and cell-specific immune responses in human blood cells13,14,15,16,17. On the other hand, no Cas9-specific immune
responses were observed in a pre-clinical study of CRISPR/Cas9 retinal gene editing45, suggesting a potential site- and cell-specific reaction to Cas9. We therefore investigated the innate
immune response to Cas9 in model systems relevant to ocular gene therapy. Specifically, we evaluated immune responses to plasmid-transfection of _Sp_Cas9 in human retinal cell models and
demonstrated cell type-specific immunogenicity of the DNA plasmid vector, but not of _Sp_Cas9, in a model of human RPE cells and a complete absence of plasmid-related or _Sp_Cas9-related
immunogenicity in a human microglia model. In plasmid-mediated gene transfer of Cas9, potential immunogenic components that could trigger innate immune responses consist of plasmid DNA, the
transgene mRNA, and the transgene protein. Additionally, immune responses observed following transfection of experimental plasmids lacking the Cas9 sequence might be induced by the
expression of vector encoded fluorescence proteins such as GFP46. It has been shown that transfection of non-coding plasmid DNA into muscle cells induced upregulation of the endosomally
expressed DNA sensor TLR9 and of other PRRs responding to cytoplasmic DNA and triggered pro-inflammatory cell infiltration11. Moreover, electrotransfer of _Sp_Cas9 transgene RNA stimulated
an immune response in human CD34+ hematopoietic stem cells4 and extracellular application of _Sp_Cas9 proteins induced a pro-inflammatory cytokine release in human monocytes14. Human RPE
cells have been shown to express intracellular receptors for DNA including TLR9 and the cytosolic DNA receptor cGAS27,47, and also exhibit PRRs capable of detecting RNA26,48 and
extracellular proteins48,49. Moreover, ARPE-19 cells were found to react to the TLR9 ligand CpG DNA and to intracellular mitochondrial DNA27,50. Additionally, human primary RPE cells
responded to ligands of the RNA sensing TLR348. Similarly, human microglia cells have been shown to express cGAS51 and innate PRRs responding to RNA and proteins52, but in contrast to RPE
cells, the expression of TLR9 is low or absent in primary human microglia52. In line with this we observed that although IMhu microglia cells reacted to ligands of a RNA sensing TLR (TLR3),
they did not respond to the TLR9 ligand CpG ODN. Additionally, we also found that they did not react to dsDNA. Collectively, this suggests that differences may exist in the capability of
microglia and RPE cells to sense intracellular DNA. Differences in the immune responsiveness to DNA provide a possible explanation for the discrepancy in the reactivity of IMhu cells and
ARPE-19 cells to _Sp_Cas9 plasmid transfection. While _Sp_Cas9 plasmid transfection did not elicit a cytokine response in IMhu cells, in ARPE-19 cells it caused a release of pro-inflammatory
IL-8 and IL-6 and an upregulation of the cellular activation markers HLA-ABC and CD54 (ICAM-1) in response to the _Sp_Cas9 plasmid as well as to the EGFP-_Sp_Cas9 plasmid. Similar responses
were also induced in cells transfected with the corresponding stuffer control plasmids. In the latter groups, the observed immune reaction could neither be attributed to the presence of
Cas9 mRNA nor to Cas9 protein. Moreover, no responses were detected in ARPE-19 cells when the Cas9 plasmid was applied extracellularly. Taken together, this suggests that intracellular
plasmid DNA vectors triggered inflammatory immune responses in ARPE-19 cells. In contrast, in IMhu cells, the absence of an immune response to plasmid transfection might have been related to
their reduced reactivity to DNA antigens and/or intracellularly expressed proteins. A detailed analysis of the immune responses to Cas9 plasmid transfection in ARPE-19 cells revealed no
significant differences in the IL-8 and IL-6 responses between cells treated with _Sp_Cas9 encoding plasmids and stuffer plasmids. There were also no significant differences seen in the
expression of HLA-ABC and CD54 between ARPE-19 cells transfected with _Sp_Cas9 encoding plasmids and stuffer plasmids. Collectively, this indicates that the inflammatory immune responses
were triggered by the intracellular plasmid DNA vector but not by the presence or the expression of the _Sp_Cas9 transgene. The cytokines and surface molecules upregulated in ARPE-19 cells
following plasmid transfection have been shown to play important roles in ocular inflammation. Pro-inflammatory IL-6 is a critical mediator of uveitis53 and induces the disruption of tight
junction complexes between RPE cells, leads to the VEGF-induced recruitment of retinal microglia to the RPE layer and thereby compromises the barrier function of the RPE54. IL-8 is also
associated with human retinal inflammatory diseases55,56,57 and has a chemotactic effect on neutrophil granulocytes58 as well as highly cytotoxic CD8+ T cells59. Moreover, ICAM-1, which is
also expressed by human primary RPE cells, is critically involved in the cross-migration of leukocytes across the blood-retinal barrier60, while antigen presentation via HLA-ABC molecules is
required for the activation of CD8+ cytotoxic T cells. This suggests that an upregulation of these immune molecules in the retina induced by cell or gene therapies might promote ocular
inflammatory processes. We observed that cationic lipid transfection of Cas9 plasmids not only induced pro-inflammatory immune responses in ARPE-19 cells but also resulted in a minor
decrease in cell viability. Interestingly, cell viability was also reduced in ARPE-19 cells transfected with the stuffer control plasmids, but not in cells that were only treated with either
transfection reagent or plasmid DNA. This suggests that increased cell death was not caused by the transfection reagent alone or by extracellular plasmids or the Cas9 transgene. Increased
cell death in cells transfected with DNA-cationic lipid complexes, but not in cells treated with uncomplexed material, was also observed by Nguyen et al.12 In this study the effect of
cationic lipid transfection of bacterial plasmids was studied in human HeLa cells. A detailed analysis revealed that cell death following transfection with plasmid DNA-cationic lipid
complexes was caused by apoptosis as shown by substantial DNA fragmentation and upregulation of genes involved in the ER stress-mediated apoptosis pathway12. Thus, it is possible that the
minor decrease in cell viability seen in our plasmid-transfected ARPE-19 cells was caused by apoptosis induced by plasmid DNA-cationic lipid complexes. Overall, this suggests that in vivo
approaches of plasmid-mediated retinal gene transfer should be monitored for the occurrence of inflammatory immune responses and cell death. In summary, we have shown that plasmid-mediated
gene transfer of _Sp_Cas9 induced an increased release of pro-inflammatory cytokines and an upregulation of cellular activation markers in a model of human RPE cells, but not in human
microglia. Importantly, this immune response was only induced by plasmid transfection but not specifically by Cas9. As the immune reactivity of retinal cells has been shown to depend on
their tissue context61, immune responses to retinal gene therapy might also depend on the ocular or cellular microenvironment of the transfected cells, indicating that in vitro immune
responses may differ from in vivo responses. Nevertheless, our results strongly suggest a generally low immunogenicity of Cas9 in microglia and RPE cells. Results demonstrating no or low
immunogenicity of Cas9 are also seen in vivo studies of CRISPR/Cas9 retinal gene editing. Thus, no peripheral adaptive immune responses to _Sa_Cas9 were detected in non-human primates
subretinally injected with AAV5-encoded CRISPR-SaCas9 to correct the common deep-intronic mutation in CEP290 associated with LCA10 (EDIT-101; Editas Medicine, Inc.)45. Additionally, initial
clinical data from the ongoing, open label Phase 1/2 BRILLIANCE clinical trial of EDIT-101 (www.clinicaltrials.gov; NCT03872479) also demonstrate the absence of peripheral Cas9-specific
antibody or T-cell responses in the treated patients62. However, detailed analyses of the immune response of retinal cells to Cas9 were still lacking. Now, our findings, showing for the
first time that Cas9 does not elicit immune responses in models of RPE and microglia cells, provide further evidence of the low/absent immunogenicity of Cas9 in and are therefore encouraging
for future studies of Cas9-mediated retinal gene therapies. MATERIALS AND METHODS PLASMIDS The EGFP-_Sp_Cas9 plasmid was obtained from Addgene (pSpCas9(BB)-2A-GFP plasmid, #48138, Addgene,
Watertown, MA, USA). To construct the _Sp_Cas9 plasmid, the EGFP-_Sp_Cas9 plasmid was used as template DNA for PCR amplification of the _Sp_Cas9 and CAG promoter gene sequences. PCR
amplification was performed using the primers: 5′-CCACGCGTGACGGCCTATTTCCCATGATTC-3′ and 5′-GGAATTCGGCAGTGGTCCGGACC-3′. The PCR product was digested with restriction enzymes MluI-HF (New
England Biolabs, Ipswich, MA, USA) and Nsil-HF (New England Biolabs, Ipswich, MA, USA) and inserted into the backbone of the _cj_Cas9 plasmid (#89752, Addgene, Watertown, MA, USA).
XL10-Gold® ultracompetent cells (Agilent, Santa Clara, CA, USA) were used in subsequent bacterial transformation. Plasmid isolation was performed with the EndoFree® Plasmid Mega Kit (Qiagen,
Hilden, DE) as per the manufacturer’s protocol. The _Sp_Cas9 plasmid was sequenced using the Mix2Seq Kit (Eurofins, Luxemburg, LU), successfully confirming the presence of the expected
plasmid sequence. Plasmid DNA concentration and purity were assessed using the Infinite M200 microplate reader (Tecan, Männedorf, CH) according to the manufacturer’s protocol. To design a
corresponding non-expression Cas9 plasmid (NC plasmid), the _Sp_Cas9 plasmid, excepting the _Sp_Cas9 sequence, was amplified using the KOD hot start DNA polymerase (Merck, Darmstadt, DE)
with the primers: 5′-GTCCGGAAAAAGGCCGGCGGCCAC-3′ and 5′- GTCCGGAGCTGGGACTCCGTGGAT-3′. The PCR fragment was subsequently digested using the restriction enzyme BspEI (New England Biolabs,
Ipswich, MA, USA) and was self-ligated using the T4 DNA ligase (New England Biolabs, Ipswich, MA, USA). Bacterial transformation, plasmid isolation, and sequencing were performed as describe
above. To create size-matched control plasmids for the _Sp_Cas9 plasmid (stuffer plasmid) and EGFP-_Sp_Cas9 plasmid (EGFP-stuffer plasmid), the _Sp_Cas9 sequence of both plasmids was
replaced by a stuffer sequence of the identical size. The generation of the stuffer plasmid and EGFP-stuffer plasmid was performed by VectorBuilder (Shenandoah, TX, USA). The mKate
fluorescent plasmid (4.8 kbp) was obtained from Addgene (#54826). CELL CULTURE ARPE-19 cells (ATCC, Manassas, VA, USA) were cultured in DMEM Glutamax containing 10% FBS and 1%
penicillin/streptomycin (all Thermo Fisher Scientific, Waltham, MA, USA). Immortalized Human Microglia SV40 (IMhu) (abm, Richmmond, BC, Canada) were cultured in flasks treated with 6–10
μg/cm2 Human Collagen type 1 in DMEM high glucose medium supplemented with 10% FBS and 1% penicillin/streptomycin (all Thermo Fisher Scientific, Waltham, MA, USA). HEK-Blue™ IFN α/β, IL-6,
TFNα, IL-1β, and IL-18 cells (InvivoGen, San Diego, CA, USA) were cultured in DMEM high glucose growth medium containing 1% penicillin/streptomycin, 30 μg/ml Blasticidin, 100 μg/ml Zeocin,
and 100 μg/ml Normocin (all InvivoGen, San Diego, CA, USA). All cell cultures were maintained at 37 °C and 5% CO2. Cell cultures were confirmed to be mycoplasma free through the use of the
MycoSEQ mycoplasma detection kit (Thermo Fisher Scientific, Waltham, MA, USA). STIMULATION OF IMHU WITH PRR LIGANDS IMhu were seeded in culture medium 24 h prior to stimulation. To stimulate
intracellular DNA receptors, or TLR3, or TLR7, the medium was replaced with OptiMEM reduced serum medium (Thermo Fisher Scientific, Waltham, MA, USA) containing either 7.5 µl/ml
Lipofectamine 3000, 8.35 µl/ml Plus Reagent (both Thermo Fisher Scientific, Waltham, MA, USA), and 1 μg/ml dsDNA (InvivoGen, San Diego, CA, USA), or 5 µl/ml Lipofectamine LTX, 3.5 µl/ml Plus
reagent (both Thermo Fisher Scientific, Waltham, MA, USA) and 1 µM CpG ODN 2216 (InvivoGen, San Diego, CA, USA), or 10 μg/ml Poly (I:C) (InvivoGen, San Diego, CA, USA), or 5 μg/ml Imiquimod
(InvivoGen, San Diego, CA, USA) respectively. To stimulate the inflammasome, cells were incubated with 5 μg/ml LPS O55:B5 (Sigma Aldrich, St Louis, MO, USA) in OptiMEM for 6 h, before
removal of the LPS medium and replacement with 5 mM ATP (InvivoGen, San Diego, CA, USA) in OptiMEM. Cells were incubated at 37 °C and supernatant was collected after 24 h. PLASMID
TRANSFECTION OF IMHU AND ARPE-19 Cationic lipid mediated transfection of IMhu cells with _Sp_Cas9 plasmids and NC plasmids and of ARPE-19 cells with EGFP-_Sp_Cas9 plasmids, EGFP-stuffer
plasmids, _Sp_Cas9 plasmids and stuffer plasmids was performed using Lipofectamine 3000 or Lipofectamine LTX with Plus reagent respectively (both Thermo Fisher Scientific, Waltham, MA, USA).
IMhu cells were seeded at 2 × 105 cells per well of a 24-well plate. ARPE-19 cells were seeded at 9 × 105 cells per well of a 24-well plate and at 2.5 × 104 cells per well of a 96-well
plate, respectively. Both cell types were incubated in cell culture medium overnight prior to transfection with Cas9 or control plasmids at optimized plasmid-reagent ratios. Additionally,
IMhu were transfected with an mKate fluorescent plasmid and the EGFP-_Sp_Cas9 plasmid. IMhu were transfected with 1.5 μl/ml Lipofectamine 3000, 1 μl/ml Plus reagent and 1.5 μg/ml plasmid
DNA. ARPE-19 cells were transfected with 1.5 µl/ml Lipofectamine LTX, 0.375 µl/ml Plus reagent and 375 ng/ml plasmid DNA. Stimulation with 10 µg/ml Poly (I:C) served as positive control.
Cells were incubated for 24 h at 37 °C prior to collection of supernatant and fixation of cells for immunohistochemistry staining. To assess the time point after plasmid transfection at
which the Cas9 protein started to be expressed by IMhu cells and ARPE-19 cells, the cells were transfected with the EGFP-_Sp_Cas9 plasmid and EGFP fluorescence was analyzed using
fluorescence microscopy. MEASUREMENT OF CYTOKINES Semiquantitative immune-detection of human cytokines, chemokines, growth factors, and angiogenesis markers in the supernatant of IMhu and
ARPE-19 cells was performed using the Proteome Profiler Human XL Cytokine Array Kit (R&D Systems, Minneapolis, MN, USA). A near-infrared fluorescence signal corresponding to the amount
of cytokine bound was generated utilizing IRDye 800CW followed by LI-COR detection. A LI-COR Odyssey® Infrared Imaging System (LI-COR, Bad Homburg, Germany) was used to detect near-infrared
fluorescence. The Odyssey scan was run with a resolution of 84 μm, an intensity of 5, 800 nm, an absorbance of 774 nm, and an emission of 789 nm. Signal intensity, measured in pixel density,
was analyzed using ImageStudioLite Software (LI-COR Biosciences, Lincoln, NE, USA). The average signal of the duplicate spots was measured for each analyte and determined and normalized to
the average signal of the reference spots after correction with the background signal. Concentrations of IL-8 and IL-6 in the supernatants of ARPE-19 cells were determined by sandwich ELISA
using the Human IL-8 DuoSet ELISA and the Human IL-6 DuoSet ELISA (both R&D systems, Minneapolis, MN, USA) according to the manufacturer’s protocol. Cytokine concentrations in the
supernatants of IMhu cells were measured using HEK-Blue™ IFN α/β, IL-6, TFNα, IL-1β, and IL-18 reporter cells (all InvivoGen, San Diego, CA, USA). These cells allow the detection of the
respective cytokine through the activation of an NF-κB-inducible promoter and the production of secreted embryonic alkaline phosphatase (SEAP). Concentrations of SEAP in the supernatant can
be assessed by a SEAP detection assay (InvivoGen, San Diego, CA, USA) using QUANTI-Blue™, a reagent that turns blue in the presence of SEAP. HEK-Blue™ cell measurement of cytokines was
performed as per manufacturer’s protocol. In brief, HEK-Blue™ cells were incubated with supernatant from stimulation experiments at 37 °C for 24 h. Induced HEK-Blue™ supernatant was then
collected and incubated with QUANTI-Blue™ at 37 °C and SEAP concentrations were assessed using Infinite M200 microplate reader (Tecan, Männedorf, CH) at 640 nm. IMMUNOHISTOCHEMISTRY IMhu and
ARPE-19 cells were seeded onto 18 mm diameter round coverslips (Fisher Scientific, Waltham, MA, USA) and transfected as described above. Then, cells were washed twice in a wash buffer (WB)
of 1% NDS (abcam, Cambridge, UK) and 0.05% Tween20 (Sigma Aldrich, St Louis, MO, USA) in PBS (Thermo Fisher Scientific, Waltham, MA, USA) and fixed in 4% formaldehyde (Sigma Aldrich, St
Louis, MO, USA) in PBS for 10 min. Following fixing, cells were washed three times with WB and permealized for ten minutes using 0.05% Triton X-100 (Thermo Fisher Scientific, Waltham, MA,
USA) in PBS. Following three washes with WB, the cells were blocked with 10% NDS and 0.05% Tween20 in PBS for one hour. The cells were then washed twice in WB before overnight incubation at
8 °C in the presence of the primary antibody, Cas9 (7A9-3A3) Mouse mAb (Cell Signaling Technology, Danvers, MA, USA), at a concentration of 1:600. After three washes in WB, the cells were
incubated with the secondary antibody, Donkey Anti-Mouse IgG (Alexa Fluor 568) (abcam, Cambridge, UK) at 1:500 in Dako antibody diluent (Agilent, Santa Clara, CA, USA) for two hours. Cells
were washed three times with WB prior to incubation with DAPI (Sigma Aldrich, St Louis, MO, USA) in WB for 2 min in the dark. Coverslips were mounted using Fluor Save Reagent (Merk
Millipore, Burlington, MA, USA). Staining was assessed and images were captured using fluorescence microscopy. FLOW CYTOMETRY For flow cytometric characterization of cells, the following
antibodies, peptides or reagents were used: 7-aminoactinomycin D (7-AAD), Anti-HLA-DR, APC-Cy™7 (1:1; AB_2868692, clone L243), APC Mouse Anti-Human HLA-ABC (1:100; AB_398603, clone G46-2.6),
PE Mouse Anti-Human CD54 (1:100; AB_395901, clone HA58), Human BD Fc Block™ (1:40; AB_2869554) and Anti-Mouse Ig, κ/Negative Control Compensation Particles Set (all BD Biosciences,
Heidelberg, Germany). As positive control, cells were stimulated with 1000 IU/ml IFN-γ (Bio-Techne, Wiesbaden, Germany). Flow cytometry measurements were performed on a FACSCanto™ II (BD
Biosciences) and data was evaluated using FlowJo software. STATISTICAL ANALYSIS JMP version 14.2 (SAS Institute) and SPSS version 25.0 (SPSS Inc. Chicago) were used for statistical analysis.
Data was initially assessed for normality using the Shapiro–Wilk test and normally distributed data was further assessed using Student’s _t_-tests or One-way ANOVA. Post hoc analysis of
One-way ANOVAs was performed using Tukey’s tests or Bonferroni adjustment. Non-normally distributed data was assessed using Kruskal–Wallis tests or non-parametric Mann–Whitney tests. Post
hoc assessment of Data analyzed with Kruskal–Wallis tests was performed using pairwise comparisons or Dunn’s test with control for joint ranks and Bonferroni adjustment. GraphPad PRISM
version 8 (GraphPad Software Inc.) was used to create graphs. DATA AVAILABILITY The datasets generated and analyzed during the current study are available from the corresponding author on
reasonable request. The plasmid sequences generated in the present study can be accessed at the NCBI databank, GenBank, under the following accession numbers: ON886908 (SpCas9-plasmid);
ON886909 (NC-plasmid); ON886911 (EGFP-Stuffer plasmid); ON886910 (Stuffer plasmid). REFERENCES * Bucher, K., Rodríguez-Bocanegra, E., Dauletbekov, D. & Fischer, M. D. Immune responses to
retinal gene therapy using adeno-associated viral vectors—implications for treatment success and safety. _Prog. Retin. Eye Res._ 83, 100915 (2021). Article CAS PubMed Google Scholar *
Diakatou, M., Manes, G., Bocquet, B., Meunier, I. & Kalatzis, V. Genome editing as a treatment for the most prevalent causative genes of autosomal dominant retinitis pigmentosa. _Int. J.
Mol. Sci._ 20, 1–22 (2019). Article CAS Google Scholar * Peddle, C. F. & Maclaren, R. E. The application of CRISPR/CAS9 for the treatment of retinal diseases. _Yale J. Biol. Med._
90, 533–541 (2017). CAS PubMed PubMed Central Google Scholar * Cromer, M. K. _et al._ Global transcriptional response to CRISPR/Cas9-AAV6-based genome editing in CD34+ hematopoietic stem
and progenitor cells. _Mol. Ther._ 26, 2431–2442 (2018). Article CAS PubMed PubMed Central Google Scholar * Hendel, A. _et al._ Chemically modified guide RNAs enhance CRISPR-Cas genome
editing in human primary cells. _Nat. Biotechnol._ 33, 985–989 (2015). Article CAS PubMed PubMed Central Google Scholar * Ling, S. _et al._ Lentiviral delivery of co-packaged Cas9 mRNA
and a Vegfa-targeting guide RNA prevents wet age-related macular degeneration in mice. _Nat. Biomed. Eng._ 5, 144–156 (2021). Article CAS PubMed Google Scholar * Bakondi, B. _et al._ In
vivo CRISPR/Cas9 gene editing corrects retinal dystrophy in the S334ter-3 rat model of autosomal dominant retinitis pigmentosa. _Mol. Ther._ 24, 556–563 (2016). Article CAS PubMed PubMed
Central Google Scholar * Lino, C. A., Harper, J. C., Carney, J. P. & Timlin, J. A. Delivering CRISPR: A review of the challenges and approaches. _Drug Deliv._ 25, 1234–1257 (2018).
Article CAS PubMed PubMed Central Google Scholar * Cai, Y. _et al._ In vivo genome editing rescues photoreceptor degeneration via a Cas9/RecA-mediated homology-directed repair pathway.
_Sci. Adv._ 5, 1–12 (2019). Article CAS Google Scholar * Vagni, P. _et al._ Gene editing preserves visual functions in a mouse model of retinal degeneration. _Front. Neurosci._ 13, 1–18
(2019). Article Google Scholar * Mann, C. J. _et al._ Molecular signature of the immune and tissue response to non-coding plasmid DNA in skeletal muscle after electrotransfer. _Gene Ther._
19, 1177–1186 (2012). Article CAS PubMed Google Scholar * Nguyen, L. T., Atobe, K., Barichello, J. M., Ishida, T. & Kiwada, H. Complex formation with plasmid DNA increases the
cytotoxicity of cationic liposomes. _Biol. Pharm. Bull._ 30, 751–757 (2007). Article CAS PubMed Google Scholar * Charlesworth, C. T. _et al._ Identification of preexisting adaptive
immunity to Cas9 proteins in humans. _Nat. Med._ 25, 249–254 (2019). Article CAS PubMed PubMed Central Google Scholar * Kang, R., Zhu, S., Zeh, H. & Tang, D. The STING-STAT6 pathway
drives Cas9-induced host response in human monocytes. _Biochem. Biophys. Res. Commun._ 506, 278–283 (2018). Article CAS PubMed PubMed Central Google Scholar * Moreno, A. M. _et al._
Immune-orthogonal orthologues of AAV capsids and of Cas9 circumvent the immune response to the administration of gene therapy. _Nat. Biomed. Eng._ 3, 806–816 (2019). Article CAS PubMed
PubMed Central Google Scholar * Simhadri, V. L. _et al._ Prevalence of pre-existing antibodies to CRISPR-associated nuclease Cas9 in the USA population. _Mol. Ther. Methods Clin. Dev._ 10,
105–112 (2018). Article CAS PubMed PubMed Central Google Scholar * Wagner, D. L. _et al._ High prevalence of _Streptococcus pyogenes_ Cas9-reactive T cells within the adult human
population. _Nat. Med._ 25, 242–248 (2019). Article CAS PubMed Google Scholar * Rathnasamy, G., Fould, W. S., Ling, E. & Kaur, C. Retinal microglia—a key player in healthy and
diseased retina. _Prog. Neurobiol._ 173, 18–40 (2018). Article PubMed Google Scholar * Reichel, F. F. _et al._ AAV8 can induce innate and adaptive immune response in the primate eye.
_Mol. Ther._ 25, 2648–2660 (2017). Article CAS PubMed PubMed Central Google Scholar * Okunuki, Y. _et al._ Retinal microglia initiate neuroinflammation in ocular autoimmunity. _Proc.
Natl. Acad. Sci._ 116, 9989–9998 (2019). Article CAS PubMed PubMed Central Google Scholar * Okunuki, Y. _et al._ Microglia inhibit photoreceptor cell death and regulate immune cell
infiltration in response to retinal detachment. _Proc. Natl. Acad. Sci. USA_ 115, E6264–E6273 (2018). Article CAS PubMed PubMed Central Google Scholar * Nimmerjahn, A., Kirchhoff, F.
& Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. _Neuroforum_ 11, 95–96 (2005). Article Google Scholar * Wang, M. & Wong, W. T.
Microglia-Müller cell interactions in the retina. _Adv. Exp. Med. Biol._ 801, 333–338 (2014). Article PubMed PubMed Central Google Scholar * Wang, M., Ma, W., Zhao, L., Fariss, R. N.
& Wong, W. T. Adaptive Müller cell responses to microglial activation mediate neuroprotection and coordinate inflammation in the retina. _J. Neuroinflammation_ 8, 173 (2011). Article
CAS PubMed PubMed Central Google Scholar * Kanuga, N. _et al._ Characterization of genetically modified human retinal pigment epithelial cells developed for in vitro and transplantation
studies. _Investig. Ophthalmol. Vis. Sci._ 43, 546–555 (2002). Google Scholar * Wörnle, M. _et al._ Inhibition of TLR3-mediated proinflammatory effects by alkylphosphocholines in human
retinal pigment epithelial cells. _Investig. Ophthalmol. Vis. Sci._ 52, 6536–6544 (2011). Article CAS Google Scholar * Ebihara, N. _et al._ Distinct functions between toll-like receptors
3 and 9 in retinal pigment epithelial cells. _Ophthalm. Res._ 39, 155–163 (2007). Article CAS Google Scholar * von Lintig, J., Kiser, P. D., Golczak, M. & Palczewski, K. The
biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. _Trends Biochem. Sci._ 35, 400–410 (2010). Article CAS Google Scholar * Olson, J.
K. & Miller, S. D. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. _J. Immunol._ 173, 3916–3924 (2004). Article CAS PubMed Google
Scholar * Chiavari, M., Ciotti, G. M. P. & Navarra, P. L. L. Pro-inflammatory activation of a new immortalized human microglia cell line. _Brain Sci._ 9, 111 (2019). Article CAS
PubMed Central Google Scholar * Garcia-Mesa, Y. _et al._ Immortalization of primary microglia: A new platform to study HIV regulation in the central nervous system. _J. Neurovirol._ 23,
47–66 (2017). Article CAS PubMed Google Scholar * Butovsky, O. _et al._ Identification of a unique TGF-b-dependent molecular and functional signature in microglia. _Nat. Neurosci._ 17,
131–143 (2013). Article PubMed PubMed Central CAS Google Scholar * Rawat, P. & Spector, S. A. Development and characterization of a human microglia cell model of HIV-1 infection.
_J. Neurovirol._ https://doi.org/10.1007/s13365-016-0472-1 (2016). Article PubMed PubMed Central Google Scholar * Orihuela, R., McPherson, C. A. & Harry, G. J. Microglial M1/M2
polarization and metabolic states. _Br. J. Pharmacol._ 173, 649–665 (2016). Article CAS PubMed Google Scholar * Kreiss, P. _et al._ Plasmid DNA size does not affect the physicochemical
properties of lipoplexes but modulates gene transfer efficiency. _Nucleic Acids Res._ 27, 3792–3798 (1999). Article CAS PubMed PubMed Central Google Scholar * Lukacs, G. L. _et al._
Size-dependent DNA mobility in cytoplasm and nucleus. _J. Biol. Chem._ 275, 1625–1629 (2000). Article CAS PubMed Google Scholar * McLenachan, S., Sarsero, J. P. & Ioannou, P. A.
Flow-cytometric analysis of mouse embryonic stem cell lipofection using small and large DNA constructs. _Genomics_ 89, 708–720 (2007). Article CAS PubMed Google Scholar * Chen, X. _et
al._ Nepetin inhibits IL-1β induced inflammation via NF-κB and MAPKs signaling pathways in ARPE-19 cells. _Biomed. Pharmacother._ 101, 87–93 (2018). Article CAS PubMed Google Scholar *
Hung, S. S. C. _et al._ AAV-mediated CRISPR/Cas gene editing of retinal cells in vivo. _Investig. Ophthalmol. Vis. Sci._ 57, 3470–3476 (2016). Article CAS Google Scholar * Jo, D. H. _et
al._ CRISPR-Cas9-mediated therapeutic editing of Rpe65 ameliorates the disease phenotypes in a mouse model of Leber congenital amaurosis. _Sci. Adv._ 5, 1–9 (2019). Article ADS Google
Scholar * Farjo, R., Skaggs, J., Quiambao, A. B., Cooper, M. J. & Naash, M. I. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. _PLoS One_ 1, 1–8 (2006).
Google Scholar * Chalberg, T. W. _et al._ Gene transfer to rabbit retina with electron avalanche transfection. _Investig. Ophthalmol. Vis. Sci._ 47, 4083–4090 (2006). Article Google
Scholar * Thumann, G. _et al._ Engineering of PEDF-expressing primary pigment epithelial cells by the SB transposon system delivered by pFAR4 plasmids. _Mol. Ther. Nucleic Acids_ 6, 302–314
(2017). Article CAS PubMed PubMed Central Google Scholar * Hernandez, M. _et al._ Preclinical evaluation of a cell-based gene therapy using the sleeping beauty transposon system in
choroidal neovascularization. _Mol. Ther. Methods Clin. Dev._ 15, 403–417 (2019). Article CAS PubMed PubMed Central Google Scholar * Maeder, M. L. _et al._ Development of a gene-editing
approach to restore vision loss in Leber congenital amaurosis type 10. _Nat. Med._ 25, 229–233 (2019). Article CAS PubMed Google Scholar * Ansari, A. M. _et al._ Cellular GFP toxicity
and immunogenicity: Potential confounders in in vivo cell tracking experiments. _Stem Cell Rev. Rep._ 12, 553–559 (2016). Article CAS PubMed Google Scholar * Kerur, N. _et al._ cGAS
drives non-canonical inflammasome activation in age-related macular degeneration. _Nat. Med._ 24, 50–61 (2018). Article CAS PubMed Google Scholar * Kumar, M. V., Nagineni, C. N., Chin,
M. S., Hooks, J. J. & Detrick, B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. _J. Neuroimmunol._ 153, 7–15 (2004).
Article CAS PubMed PubMed Central Google Scholar * Chang, Y. C. _et al._ High mobility group B1 up-regulates angiogenic and fibrogenic factors in human retinal pigment epithelial
ARPE-19 cells. _Cell. Signal._ 40, 248–257 (2017). Article CAS PubMed Google Scholar * Dib, B. _et al._ Mitochondrial DNA has a pro-inflammatory role in AMD. _Biochim. Biophys. Acta Mol.
Cell Res._ 1853, 2897–2906 (2015). Article CAS Google Scholar * Jeffries, A. M., Nitika, X., Truman, A. W. & Marriott, I. The intracellular DNA sensors cGAS and IFI16 do not mediate
effective antiviral immune responses to HSV-1 in human microglial cells. _J. Neurovirol._ 26, 544–555 (2020). Article CAS PubMed PubMed Central Google Scholar * Bsibsi, M., Ravid, R.,
Gveric, D. & Van Noort, J. M. Broad expression of Toll-like receptors in the human central nervous system. _J. Neuropathol. Exp. Neurol._ 61, 1013–1021 (2002). Article CAS PubMed
Google Scholar * Karkhur, S. _et al._ Interleukin-6 inhibition in the management of non-infectious uveitis and beyond. _J. Ophthalm. Inflamm. Infect._ 9, 17 (2019). Article CAS Google
Scholar * Jo, D. H. _et al._ Interaction between microglia and retinal pigment epithelial cells determines the integrity of outer blood-retinal barrier in diabetic retinopathy. _Glia_ 67,
321–331 (2019). Article PubMed Google Scholar * Aksünger, A., Or, M., Okur, H., Hasanreisoğlu, B. & Akbatur, H. Role of interleukin 8 in the pathogenesis of proliferative
vitreoretinopathy. _Ophthalmologica_ 211, 223–225 (1997). Article PubMed Google Scholar * Jonas, J. B., Tao, Y., Neumaier, M. & Findeisen, P. Cytokine concentration in aqueous humour
of eyes with exudative age-related macular degeneration. _Acta Ophthalmol._ 90, e381–e388 (2012). Article PubMed Google Scholar * Petrovič, G. M., Korošec, P., Košnik, M. & Hawlina,
M. Vitreous levels of interleukin-8 in patients with proliferative diabetic retinopathy. _Am. J. Ophthalmol._ 143, 175–176 (2007). Article PubMed CAS Google Scholar * Baggiolini, M.,
Walz, A. & Kunkel, S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. _J. Clin. Investig._ 84, 1045–1049 (1989). Article CAS PubMed
PubMed Central Google Scholar * Hess, C. _et al._ IL-8 responsiveness defines a subset of CD8 T cells poised to kill. _Blood_ 104, 3463–3471 (2004). Article CAS PubMed Google Scholar *
Holtkamp, G. M., Kijlstra, A., Peek, R. & De Vos, A. F. Retinal pigment epithelium-immune system interactions: Cytokine production and cytokine-induced changes. _Prog. Retin. Eye Res._
20, 29–48 (2001). Article CAS PubMed Google Scholar * Abreu, C. M. _et al._ Microglia increase inflammatory responses in iPSC-derived human brainSpheres. _Front. Microbiol._ 9, 1–12
(2018). Article CAS Google Scholar * Editas Medicine. Editas Medicine Announces Positive Initial Clinical Data from Ongoing Phase 1/2 BRILLIANCE Clinical Trial of EDIT-101 for LCA10.
https://ir.editasmedicine.com/node/10671/pdf (2021). Download references ACKNOWLEDGEMENTS The authors wish to thank Prof. T. Langmann (Department of Ophthalmology, Faculty of Medicine and
University Hospital Cologne, University of Cologne, Cologne, Germany) for the kind gift of the microglia SV40 (IMhu) cells, Dr. Kristin Bieber (Core Facility Flow Cytometry, University
Hospital Tübingen, Tübingen, Germany) and Oksana Faul (Institute for Ophthalmic Research, Centre for Ophthalmology, University of Tübingen) for excellent technical support and Prof. P.
Martus (Department of Clinical Epidemiology and Applied Biostatistics, Eberhard Karls Universität Tübingen, Tübingen, Germany) for advice regarding the statistical analysis of the ARPE-19
cell experiments. We gratefully acknowledge Prof. S.J. Clark (Institute for Ophthalmic Research, Centre for Ophthalmology, University Hospital Tübingen, Germany) for helpful discussion and
critical review of this manuscript. FUNDING Open Access funding enabled and organized by Projekt DEAL. This research was funded by the German Research Foundation (FI 2336/1-1). AUTHOR
INFORMATION Author notes * These authors contributed equally: Julia K. Pfromm and Mario Bonillo. * These authors jointly supervised this work: Kirsten Bucher and M. Dominik Fischer. AUTHORS
AND AFFILIATIONS * University Eye Hospital, Centre for Ophthalmology, University Hospital Tübingen, Tübingen, Germany Julia K. Pfromm, Mario Bonillo, Daniyar Dauletbekov, Kirsten Bucher
& M. Dominik Fischer * Institute for Ophthalmic Research, Centre for Ophthalmology, University Hospital Tübingen, Elfriede-Aulhorn-Strasse 7, 72076, Tübingen, Germany Julia K. Pfromm,
Mario Bonillo, Daniyar Dauletbekov, Kirsten Bucher & M. Dominik Fischer * Oxford Eye Hospital, Oxford University Hospitals NHS Foundation Trust, Oxford, UK M. Dominik Fischer * Nuffield
Laboratory of Ophthalmology, Department of Clinical Neurosciences, University of Oxford, Oxford, UK M. Dominik Fischer Authors * Julia K. Pfromm View author publications You can also search
for this author inPubMed Google Scholar * Mario Bonillo View author publications You can also search for this author inPubMed Google Scholar * Daniyar Dauletbekov View author publications
You can also search for this author inPubMed Google Scholar * Kirsten Bucher View author publications You can also search for this author inPubMed Google Scholar * M. Dominik Fischer View
author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.B. and J.K.P. designed and executed experiments, analyzed data and wrote manuscript. D.D.
designed experiments. K.B. designed experiments, analyzed data, wrote manuscript. M.D.F. designed experiments and edited manuscript. CORRESPONDING AUTHOR Correspondence to Kirsten Bucher.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative
Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the
original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in
the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your
intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence,
visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Pfromm, J.K., Bonillo, M., Dauletbekov, D. _et al._ Plasmid-mediated gene
transfer of Cas9 induces vector-related but not _Sp_Cas9-related immune responses in human retinal pigment epithelial cells. _Sci Rep_ 12, 13202 (2022).
https://doi.org/10.1038/s41598-022-17269-x Download citation * Received: 28 April 2022 * Accepted: 22 July 2022 * Published: 01 August 2022 * DOI: https://doi.org/10.1038/s41598-022-17269-x
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative