- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Plasmalemmal ATP sensitive potassium (KATP) channels are recognized metabolic sensors, yet their cellular reach is less well understood. Here, transgenic Kir6.2 null hearts devoid
of the KATP channel pore underwent multiomics surveillance and systems interrogation versus wildtype counterparts. Despite maintained organ performance, the knockout proteome deviated beyond
a discrete loss of constitutive KATP channel subunits. Multidimensional nano-flow liquid chromatography tandem mass spectrometry resolved 111 differentially expressed proteins and their
expanded network neighborhood, dominated by metabolic process engagement. Independent multimodal chemometric gas and liquid chromatography mass spectrometry unveiled differential expression
of over one quarter of measured metabolites discriminating the Kir6.2 deficient heart metabolome. Supervised class analogy ranking and unsupervised enrichment analysis prioritized
nicotinamide adenine dinucleotide (NAD+), affirmed by extensive overrepresentation of NAD+ associated circuitry. The remodeled metabolome and proteome revealed functional convergence and an
integrated signature of disease susceptibility. Deciphered cardiac patterns were traceable in the corresponding plasma metabolome, with tissue concordant plasma changes offering surrogate
metabolite markers of myocardial latent vulnerability. Thus, Kir6.2 deficit precipitates multiome reorganization, mapping a comprehensive atlas of the KATP channel dependent landscape.
SIMILAR CONTENT BEING VIEWED BY OTHERS DECIPHERING METABOLOMICS AND LIPIDOMICS LANDSCAPE IN ZEBRAFISH HYPERTROPHIC CARDIOMYOPATHY MODEL Article Open access 19 September 2024 A MULTI-OMICS
APPROACH IDENTIFIES THE KEY ROLE OF DISORDERS OF SPHINGOLIPID METABOLISM IN ANG II-INDUCED HYPERTENSIVE CARDIOMYOPATHY MYOCARDIAL REMODELING Article Open access 05 December 2024 OUTLINING
CARDIAC ION CHANNEL PROTEIN INTERACTORS AND THEIR SIGNATURE IN THE HUMAN ELECTROCARDIOGRAM Article Open access 13 July 2023 INTRODUCTION ATP sensitive potassium (KATP) channels operate as
high fidelity rheostats in response to metabolic stress1,2,3,4,5. Abundant in the cardiomyocyte sarcolemma, where originally discovered6, KATP channels adjust membrane electrical activity to
match cellular energetic demand7,8. Channel opening under diverse stressor challenges is a recognized cardioprotective event, with channel deficiency associated with poor
outcome9,10,11,12,13,14,15. The KATP channel dependent molecular landscape, however, remains only partially understood. Myocardial KATP channels assemble into a heteromeric complex of the
_KCNJ11_ encoded Kir6.2 potassium conductive pore and the regulatory ATP binding cassette sulfonylurea receptor 2A (SUR2A) partner16,17,18. Channel metabolic sensing relies on intrinsic ATP
mediated gating of Kir6.2, governed by ATP/ADP dependent conformations of tandem SUR2A nucleotide binding domains19,20,21. Under physiological workload, hearts lacking KATP channels exhibit
a switch in metabolic substrate and an augmented oxygen consumption, indicating excessive energy cost compared to hearts containing intact channels22,23. Channel linkage to the cellular
bioenergetic machinery involves communication with energy shuttles facilitated by near equilibrium enzymatic transfer24,25. Messaging with NAD+/NADH interconverting enzymes (lactate
dehydrogenase), phosphotransferring enzymes (creatine kinase and adenylate kinase), and glycolytic enzymes (glyceraldehyde-3-phosphate dehydrogenase, triose-phosphate isomerase, and pyruvate
kinase) have been implicated in KATP channel contribution to cellular metabolism26,27,28,29,30,31. Comprehensive molecular profiling would enable decoding the full extent of the cardiac
KATP channel interactome. In this regard, systems biology approaches provide unbiased means of resolving the complex cellular milieu32,33. Downstream from genetic and epigenetic inputs,
proteomic surveillance captures infrastructure constituents while metabolomic assessment provides a readout of functional activity34,35,36. These complementary approaches facilitate
expression analysis and function prioritization based on objective dataset interrogation37, and when used in conjunction provide greater insight into complex biological processes than can be
achieved from either approach alone. Multiomics data offer extraction of distilled biological signatures, identification of cross-strata common denominators, and merged interpretation.
Integrated consideration mitigates misinterpretation due to potential single perspective idiosyncrasy and can alleviate the risk of overlooking pertinent information. These attributes help
address the molecular intricacy of the cardiovascular system38. The present study drafts an integrated map of the cardiac plasmalemmal KATP channel dependent multiome, leveraging a systems
strategy applied to a transgenic model lacking the channel pore. Parallel application of proteomics and metabolomics deciphered differential molecular expression segregating Kir6.2 knockout
from wildtype hearts. Molecular reorganization induced by KATP channel loss prioritized a metabo-centric adaptation, handicapped by risk of compromised cardiac resilience. Corroborated in
the corresponding plasma metabolome, the resolved multilevel cartography provides an expanded omics guide of KATP channel reliant cardiac homeostasis. RESULTS KIR6.2 KNOCKOUT DEVIATES BEYOND
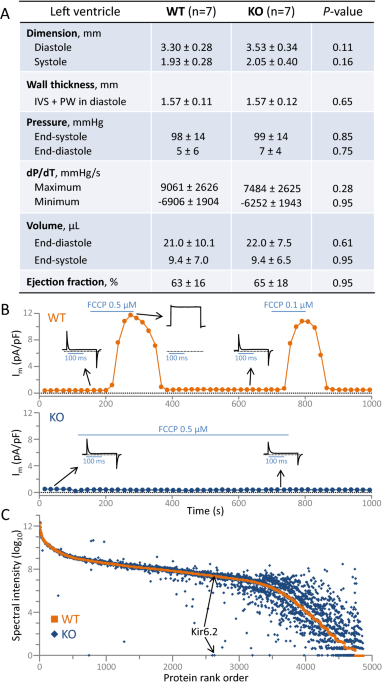
KATP CHANNELS _Kcnj11_ ablation produced viable offspring that reached adulthood with no apparent adverse cardiac phenotype at the organ level (Fig. 1A). Adult (3–4 months) Kir6.2 KATP
channel knockout hearts (KO; n = 7) did not differ from age, sex, and environment matched wildtype (WT; n = 7) counterparts on echocardiography and catheterization. Left ventricular
end-diastolic/end-systolic dimensions, pressures, volumes, and ejection fraction were all comparable in WT and KO (Fig. 1A). Concordant with KATP channel ablation, under whole-cell patch
clamp, metabolic stress-induced outward current was evident in WT but not in KO cardiomyocytes (Fig. 1B). Mean current density provoked by the oxidative phosphorylation uncoupler
2-[2-[4-(trifluoromethoxy)phenyl] hydrazinylidene]-propanedinitrile was 14.4 ± 1.5 pA/pF in WT (n = 7) versus 0.09 ± 0.08 pA/pF in KO (n = 6) cardiomyocytes (_P_ = 0.0002). At the molecular
level, high mass accuracy nano-flow liquid chromatography tandem mass spectrometry (LC–MS/MS) of ventricular tissue homogenates (WT, n = 10; KO, n = 10) identified 56,086 peptides assigned
to 4846 proteins of which 4205 were quantifiable (Supplementary Table 1). Resolved by label-free relative quantitation (median coefficient of variance: WT = 2.3%, KO = 2.4%), Kir6.2 protein
was found abundant in WT but absent in KO (Fig. 1C). In addition to the discrete loss of channel pore expression, extensive KO proteome deviation away from WT was prominent (Fig. 1C). Kir6.2
deletion, while apparently phenotypically silent, causes molecular departure beyond the KATP channel proper. KIR6.2 ABLATION RESTRUCTURES MYOCARDIAL PROTEOME Cardiac proteome remodeling
imposed by Kir6.2 deletion segregated KO (n = 10) from WT (n = 10) hearts, as visualized by 3-D principal component analysis (PCA, Fig. 2A). Contrasting WT, cardiac plasmalemmal KATP channel
subunits were absent (Kir6.2) or significantly reduced (SUR2A, false discovery rate [FDR] _P_ = 0.016) in KO (Fig. 2B). The distinct mitochondrial KATP channel subunits, Mitok (_Ccdc51_)
and Mitosur (_Abcb8_), remained equivalent in WT and KO (see Supplementary Table 1). Of the 4205 quantifiable proteins, 111 were differentially expressed in KO versus WT (limma FDR corrected
_P_ < 0.05; Fig. 2C). The 68 upregulated and 43 downregulated proteins demarcated a distinct KO molecular substrate delineated by PCA loading plot (Fig. 2C). The resulting agglomerative
heatmap distinguished the cohorts based on the differential proteome (Fig. 2D). The Kir6.2 dependent proteome changes spanned 11 primary biological process categories (Fig. 3A). Metabolic or
catabolic processes harbored the greatest change, accounting for over 25% of all proteins (28 of 111, with 16 upregulated, 12 downregulated), followed by: signaling, transport, and motility
(23%, 12 up, 14 down); immunity or inflammation (13%, 14 up); morphology or structure (9%, 9 up, 1 down); stress or stimulus response (7%, 3 up, 5 down); protein post-translational
modification (PTM) or processing (5%, 4 up, 2 down); transcription, epigenetics, or DNA related processes (5%, 3 up, 3 down); differentiation or development (5%, 1 up, 4 down); biosynthesis
(4%, 2 up, 2 down); cell cycle (1%, 1 up); apoptosis or cell death (1%, 1 up); with 2 upregulated proteins uncharacterized (Fig. 3A). The spectrum of associated biological processes was
validated at the network level, upon integration of the differential proteome within an expanded 239 node neighborhood composed of molecules with known interactions (Supplementary Figure,
left). Gene ontology analysis of the network specified 223 associated biological processes enriched at _P_ < 0.001 (Supplementary Figure, right, and Supplementary Table 2). Grouping of
these processes further highlighted the prioritization of ‘Metabolism, Catabolism’, which harbored the largest proportion of enriched processes (> 27%) and exhibited the greatest extent
of significance (−log harmonic mean _P_-value = 20.97) compared to other enriched clusters (Fig. 3B). Thus, metabolism-centric processes dominated the proteome makeover engendered by Kir6.2
ablation. REORGANIZED CARDIAC METABOLOME DISTINGUISHES KIR6.2 ABSENCE From the WT (n = 10) and KO (n = 10) hearts, distinct metabotypes were independently resolved by high throughput
chemometric surveillance using multimodal untargeted mass spectrometry, with prominent cohort segregation evident by 3-D PCA (Fig. 4A). Over one quarter of the measured cardiac metabolome
(Supplementary Table 3) was significantly altered by Kir6.2 deletion (59/219 metabolites, _P_ < 0.05), with 73% of changing metabolites upregulated and 27% downregulated (Fig. 4B),
underscored by differential metabolite loading plots in WT and KO (Fig. 4C). The KATP channel dependent metabolome, arrayed by unsupervised agglomerative clustering, spanned 6 of the 7
pathway macroclusters encompassing all measured metabolites. Downregulated metabolites contributed to 4 and upregulated metabolites to all 6 pathway macroclusters (Fig. 4D). Kir6.2 deletion
precipitated a distinct pattern of change. The percent of metabolites changed in each pathway macrocluster ranged from 17 to 35% (Fig. 4D, upper inset). Specifically, the number of
metabolites significantly changed were: 16 (12 up, 4 down) out of 53 in the amino acid cluster; 6 (up) out of 27 in the carbohydrate cluster; 2 (1 up, 1 down) out of 6 in the
cofactor/vitamin cluster; 1 (up) out of 6 in the energy cluster; 26 (18 up, 8 down) out of 100 in the lipid cluster; and 8 (5 up, 3 down) out of 23 in the nucleotide cluster. Notably, 100%
predictive classification accuracy across cohorts was achieved in Random Forest modeling using the top 30 differential metabolites (Fig. 4D, lower inset). Thus, the resolved chemometric
fingerprint mapping the extent and diversity of metabolite changes readily distinguished KO from WT hearts, underscoring the impact of KATP channel deficiency on the cardiac metabolome.
KIR6.2 DEPENDENT METABOLIC PRIORITIZATION Supervised classification of the metabolome by soft independent modeling of class analogy (SIMCA) validated KO and WT intra-group consistency and
inter-cohort separation, as evident by partial least squares—discriminant analysis (PLS-DA; Fig. 5A). Systems modeling by SIMCA identified 28 metabolites with variable importance in
projection (VIP) scores exceeding 1.5, affirming their prominence in group segregation (Fig. 5B). The top scoring metabolite was nicotinamide adenine dinucleotide (NAD+; reduced in KO by ≈
30% from WT levels). In parallel, nicotinate and nicotinamide metabolism was the top pathway for cohort discrimination. The Kir6.2 dependent differential metabolome was expanded to a 135
node scale-free interactome (Fig. 5C). Unsupervised classification by Metabolite Pathway Analysis (MetPA) of the interactome corroborated the preeminence of NAD+ and the nicotinate and
nicotinamide pathway (Fig. 5D), with 75% of the most significant MetPA pathways confirmed among the top pathways modeled by VIP scoring (Fig. 5D, bold italicized font). While NAD+ levels
were significantly reduced in response to Kir6.2 ablation (_P_ = 1.37 × 10−7; Fig. 5E, left), flavin adenine dinucleotide (the other primary electron acceptor) did not differ between WT and
KO cohorts (_P_ = 0.55; Fig. 5E, right). Consistent with NAD+ prioritization by unsupervised and supervised systems interrogation, NAD+ was associated with the greatest number of metabolic
and signaling pathways enriched in KO hearts (Fig. 6A,B). Notably, 61% (22/36) of enriched Ingenuity Pathway Analysis (IPA) canonical pathways were NAD+ related (Fig. 6A). Less preeminent
was glycine linked to 12 enriched pathways, followed by l-glutamine (7 pathways), xanthine (6), l-tyrosine (5), and 4 or fewer IPA enriched pathways for the remaining 22 metabolites.
Likewise, 95% (60/63) of enriched Metabolite Set Enrichment Analysis (MSEA) pathways were associated with NAD+ (Fig. 6B). In contrast, second-ranked glycine was associated with only 9 of the
63 pathways. Additional metabolites linking to MSEA enriched pathways included l-glutamine (7 pathways), glycerol-3-phosphate (6), and β-alanine (4), with 3 or fewer enriched pathways
linking to each of the remaining 21 differential metabolites. Concordant with an NAD+-centric KO metabotype, the corresponding Kir6.2 dependent proteome displayed altered expression of 9
proteins associated with NAD+ biosynthesis, consumption, or utilization (Fig. 6C). Complementary interrogation thus identified altered metabolites prioritizing key pathways delineating the
metabolic identity of the Kir6.2 deficient state. CARDIAC SUSCEPTIBILITY IMPRINTED IN THE REMODELED MULTIOME Integrated multiomics analysis was used to query the influence of the remodeled
metabolome and proteome in the setting of Kir6.2 deficiency. Metabolome enrichment profiling in response to Kir6.2 ablation revealed 36 overrepresented functions, prioritizing metabolism (11
functions), followed by development (7), homeostasis and survival (6), signaling, transport, and motility (5), morphology and structure (4), as well as functions (3) involved in cell cycle,
DNA, and gene expression (Fig. 7A, left). Of note, 97% of proteome-enriched functions (35/37) matched the metabolome-enriched functions, revealing synonymity across platform readouts (Fig.
7A, Venn diagram). Collective analysis of metabolome and proteome datasets unmasked disease and adverse outcome susceptibility in response to Kir6.2 ablation. Specifically, multiomics
interrogation demonstrated an enrichment of metabolic disease, developmental and hereditary disorders, organismal injury, inflammatory and immunological dysfunction, and muscle-related
disorder including cardiovascular disease (Fig. 7B). Moreover, an array of cardiac adverse outcomes was overrepresented, with predicted susceptibility to enlargement, dysfunction,
arrhythmia, dilation, tachycardia, necrosis/cell death, congenital heart anomaly, and damage (Fig. 7C). Thus, Kir6.2 deficit induces congruent remodeling of the proteome and metabolome,
yielding a multiome imprint of cardiac compromise. PLASMA METABOLOME DISTINCT IN KIR6.2 KNOCKOUT To assess the utility of peripheral plasma in distinguishing Kir6.2 KO, plasma metabolites
from corresponding WT (n = 10) and KO (n = 10) mice were isolated and analyzed. Of the 257 measured plasma metabolites (Supplementary Table 4), a quarter (or 61 metabolites) were
significantly altered (_P_ < 0.05) in response to Kir6.2 ablation. Supervised classification of the plasma metabolome by PLS-DA documented separation of KO from WT (Fig. 8A), with
_p_-cresol sulfate and N-acetylornithine the top metabolites in predicting cohort discrimination. Unsupervised agglomerative clustering documented 34 elevated and 27 decreased metabolites,
segregating WT and KO cohorts (Fig. 8B). Random Forest modeling achieved 95% predictive classification across cohorts (i.e., correctly allocating 10/10 WT and 9/10 KO; Fig. 8C, upper), and
specified _p_-cresol sulfate and N-acetylornithine as top ranked discriminatory metabolites (Fig. 8C, lower). Rank ordered by mean decrease accuracy scores, the top 30 differential plasma
metabolites used for classification spanned metabolic pathways (Fig. 8C, lower), with inter-group separation articulated by 3-D PCA (Fig. 8D). Thus, plasma profiling discriminated KO from WT
at the metabolome level. DISTINCT KIR6.2 KNOCKOUT PLASMA REFLECTS HEART METABOLOME Functional enrichment analysis of the resolved differential plasma metabolome recapitulated 94% of the 36
functional traits enriched in the corresponding heart metabolome (Supplementary Table 5). Over one quarter of Kir6.2 dependent tissue metabolome changes (16/59) were also detected as
differentially expressed in plasma (Fig. 9A, upper). Of these common changes, 94% (15/16) exhibited concordant direction of change in response to Kir6.2 deletion, with 10 upregulated and 5
downregulated metabolites spanning metabolic pathways (Fig. 9A, lower). This shared core included the metabolites prioritized by both SIMCA VIP scoring and Random Forest modeling, namely
_p_-cresol sulfate and N-acetylornithine (see also Fig. 8A,C), offering a plasma readout of tissue level change (Fig. 9B). The differential plasma metabolome reproduced the disease and
disorder enrichment associations prioritized in the corresponding heart tissue (Fig. 9C). Matching the extent of heart damage susceptibility predicted from the tissue metabolome, the plasma
metabolome prognosticated cardiovascular adverse outcome (Fig. 9D). Tissue concordant differential metabolites within the plasma metabolome thus represent potential reporter molecules of
latent cardiac susceptibility associated with Kir6.2 deficiency. DISCUSSION The present study demonstrates that hearts deprived of the Kir6.2 KATP channel pore undergo a proteomic and
metabolomic overhaul beyond constitutive channel subunits. The distinct proteome and metabolome conversion underpinned adaptation in hearts lacking functional KATP channels. Deep phenotyping
characterized a metabo-centric metamorphosis across the molecular infrastructure and biochemical output of Kir6.2 devoid hearts, compromised by an imprint of disease susceptibility. The
resolved Kir6.2 dependent interactome highlights the centrality of intact KATP channels in proteome and metabolome maintenance ensuring heart resilience. A systems biology strategy was here
employed to acquire and interpret molecular information sampled in vivo across complementary proteomic and metabolomic dimensions39 (Fig. 10). Proteomic surveillance of the myocardium
identified over 56,000 peptides representing 4846 proteins, enabling untargeted capture of the Kir6.2 dependent expression change spectrum. The high stringency design pinpointed 111 altered
proteins across a range of vital cellular processes, demonstrating metabolic primacy of the remodeled KATP channel deficient heart proteome. Comprehensive protein cataloging extended the
findings of more targeted approaches linking metabolism with the cardiac KATP channel at local partner, associated pathway, or subproteome levels40,41,42,43,44,45. Specificity of observed
changes attributed to plasmalemmal KATP channel integrity was supported by unaltered expression of Mitok and Mitosur, in line with a distinct, non-redundant, channel identity in subcellular
compartments46. Underpinnings of metabolic prioritization were further mined by unbiased evaluation of the cardiac KATP channel dependent metabolome. Multidimensional chemometric profiling
revealed that 27% of ventricular metabolites were altered in response to Kir6.2 ablation, spanning metabolic families. The metabolomic changes provoked by Kir6.2 ablation are comparable in
magnitude to those characterizing hearts with compromised energy regulators or failing hearts47,48. Notably, Kir6.2 dependent metabolome and proteome enriched functions exhibited remarkable
overlap (97% for the metabolome and 95% for the proteome), revealing convergence across platform readouts. Screening multiple omics layers from the same source, in conjunction with data
inclusivity free of selection and interpretation bias, supports the validity and utility of considering unique yet interrelated datasets49,50. Taken together, the congruent interrogation
over multiple molecular strata underscored the impact of KATP channels as an influential nexus in cardiac metabolism. Across the breadth of KATP channel dependent reorganization, systems
deconvolution prioritized the multivalent coenzyme NAD+ and its associated metabolic pathways. The decrease in NAD+ in Kir6.2 deficient hearts was paralleled by change in NAD+ associated
proteins, including upregulation of NAD+ salvage enzymes, namely the metazoan spot homologue 1 (_Hddc3_)51 and renalase (_Rnls_)52. Maintenance of NAD+ is vital to tissue homeostasis53,54,
with myocardial NAD+ pool derangement associated with metabolic remodeling in heart failure and supplementation preserving cardiac performance55,56,57. Notably, NAD+ at physiological
concentrations regulates KATP channel activity58, and a nicotinamide-rich diet upregulates KATP channel expression and increases myocardial resilience59. In this context, the present
findings support a reciprocal relationship of KATP channels and metabolism, and reveal that the Kir6.2 null heart is typified by NAD+ deficit, a prominent feature of cardiomyopathy prone
environments60. Indeed, dual metabolome and proteome assessment of the Kir6.2 knockout heart exposed an acquired predisposition to disease susceptibility. This vulnerability signature was
herein evident in the young adult at an age apparently free from Kir6.2 dependent extracardiac confounders such as altered insulin secretion, glucose tolerance, and muscle properties61. The
molecular imprint of heart disease susceptibility was present in advance of overt physiological dysfunction, suggesting that molecular reorganization in response to Kir6.2 deletion is a
compensatory adaptation in the young adult animal. Documented independently or collectively across profiling modalities, the current multiomics findings build on initial single omic
exploration of Kir6.2 loss62. The predictive imprint of disease risk is further reinforced by overt organ failure compromising KATP channel deficient hearts subjected to
stress63,64,65,66,67,68,69. KATP channels are implicated in the maintenance of cellular homeostasis, recognized as early responders to metabolic challenge70. The mechanism by which Kir6.2
ablation mediates subcellular adaptation needs further study. In principle the observed proteome and metabolome remodelling could be related to the energetically costly KO heart’s propensity
for exaggerated Ca2+ loading9,11,12,22. Calcium overload has been directly implicated in cellular transformation at the protein and metabolite level71. Here none of the identified proteins
involved in Ca2+ handling, regulation, or homeostasis differed in expression between WT and KO (see Supplemental Table 1). This would suggest that omic alterations could be mediated by a
proclivity for Ca2+ loading on a beat-to-beat basis, rather than a structural change across the Ca2+ regulatory proteome. Corroborating the cardiac disease risk exposed at the tissue level,
the resolved KATP channel dependent plasma metabolome independently reflected myocardial susceptibility. Diverse pathological processes associated with organ failure can be monitored by
blood biomarkers, serving as molecular surrogates for early disease diagnosis, stratification, and detection at an asymptomatic state72. Among concordant differential metabolites shared
between tissue and plasma, _p_-cresol sulfate and N-acetylornithine were consistently prioritized across applied modeling algorithms. Upregulation of _p_-cresol sulfate and downregulation of
N-acetylornithine have been associated with cardiovascular disease, namely in (a)symptomatic cardiac dysfunction and incident heart failure73,74,75,76. These candidate biomarkers offer a
clinically applicable and readily accessible source for detecting KATP channel dependent vulnerability. Limitations in proteomic and metabolomic analyses may arise from small sample number,
restricted data inclusivity, absence of cross-validation, or inadequate application of interrogation resources77,78,79,80. Here, quality control ensured that the extended cohort size used
was adequately powered to capture distinct patterns at high resolution. Moreover, high throughput screening was applied without imposed constraints for inclusive data input, avoiding
inadvertent biases. Examining datasets with, and extracting common signatures from, multiple algorithms here provided added confidence in interpretation. Accordingly, supervised and
unsupervised approaches were systematically employed following best practices, generating matching output across platforms. Additionally, examination of the heart and plasma in a global
deletion model must account for potential confounding effects arising from extracardiac influences. To mitigate this possibility in the present study where Kir6.2 expression in pancreas and
skeletal muscle was also impacted, young adult mice (< 4 months of age) were chosen for analysis at an age when insulin secretion, glucose tolerance, and skeletal muscle properties are
known to be equivalent between WT animals and those with Kir6.2 deletion61. In conclusion, an atlas of KATP channel dependent interactome was here constructed using an unbiased systems
strategy integrating proteome and metabolome strata. Multiomics surveillance of Kir6.2 null hearts mapped a metabo-centric landscape, exposing latent vulnerability further traceable in the
plasma metabolome. The captured multidimensionality of the KATP channel reliant bioenergetic system offers a broadened perspective on a vital contributor to cardiac homeostasis. METHODS
ETHICS APPROVAL Protocols were approved by the Mayo Clinic Institutional Animal Care and Use Committee, following National Institutes of Health guidelines. Reporting of animal studies here
follows the recommendations in the ARRIVE guidelines81. Mice were young adult (up to 4 month-old) male WT (C57BL6) and age-, sex-, environment-matched Kir6.2 null KATP channel KO
counterparts. Of note, up to this age, KO mice maintain insulin secretion, glucose tolerance, and skeletal muscle properties within a normal range61. IN VIVO PHYSIOLOGY Group-housed
sedentary mice (≤ 5 siblings per cage) received standard chow, with WT and KO exhibiting equivalent glycemic levels61. Cardiac structure and function were evaluated under 1–2% isoflurane
anesthesia (n = 14). Left ventricular (LV) dimension and wall thickness were measured by echocardiography M-mode parasternal long-axis view (MX400 transducer, Vevo3100 system; MS-400
transducer, Vevo2100; FUJIFILM VisualSonics, Toronto, Canada)82,83. Hemodynamics was assessed by LV catheterization (PVR-1045 catheter, MPVS-400; PowerLab 8/30; Miller Instruments, Houston,
TX; ADInstruments, Colorado Springs, CO). LV ejection fraction (EF) was calculated as EF% = 100 × (LVEDV − LVESV)/LVEDV, where LVEDV and LVESV are end‐diastolic and end‐systolic
volumes84,85. First derivatives (dP/dT maximum and minimum) evaluated LV systolic and diastolic pressure86. Difference between groups was assessed by Mann‐Whitney _U_ test (JMP Pro 14.1.0,
SAS Institute Inc., Cary, NC). Data are presented as mean ± standard deviation with _P_ < 0.05 significant. CELL ELECTROPHYSIOLOGY Cardiomyocytes were isolated by enzymatic
dissociation87. Under anesthesia, following thoracotomy, the right ventricle was perfused with 7 mL of HEPES buffer (in mM: 10 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid [HEPES], pH
7.8; 130 NaCl; 5 KCl; 0.5 NaH2PO4; 10 glucose; 10 2,3-butanedione monoxime; 10 taurine) containing 5 mM EDTA. Following aortic clamping, the coronary arteries were perfused through the left
ventricle with 10 mL HEPES buffer + 5 mM EDTA, followed by 3 mL HEPES buffer + 1 mM MgCl2, and 30–40 mL HEPES collagenase buffer + 1 mM MgCl2 (with 0.5 mg/ml collagenase II, 0.5 mg/ml
collagenase IV, and 0.05 mg/ml protease XIV). Released cells were filtered through 100 μm nylon mesh and gravity settled, with CaCl2 increased to 1.2 mM. Whole cell voltage-clamp was
conducted by patch-clamp amplifier (Axopatch 200B, Molecular Devices, San Jose, CA) for cardiomyocytes bathed in (in mM) 136.5 NaCl, 5.4 KCl, 1.0 MgCl2, 5.5 glucose, and 10 HEPES–NaOH (pH
7.3) at 31 ± 0.5 °C using an HCC-100A temperature controller (Dagan Corp., Minneapolis, MN). Pipettes (resistance: 4–5 MΩ) contained (in mM) 140 KCl, 1 MgCl2, 5 EGTA-KOH, 5 HEPES–KOH, and 5
MgATP (pH 7.3). Stimulation protocol, data acquisition, and cell parameter determination were performed using BioQuest software88. MULTIOMICS SAMPLING For molecular profile sampling, excised
hearts were rinsed with ice-cold phosphate buffered saline. For proteomics, ventricular apex was placed in cryovials, snap frozen in liquid N2, and stored at −80 °C, with remaining
ventricle snap frozen and stored at −80 °C for tissue metabolomics. For plasma metabolomics, blood collected in cryovials containing 5 µL of 0.5 M EDTA was centrifuged at 2000×_g_ (10 min at
4 °C), with supernatant transferred to fresh cryovials, frozen in liquid N2, and stored at −80 °C. PROTEOMICS PROTEIN EXTRACTION Ventricular proteins were extracted by 3 rounds of
homogenization and centrifugation in 150 µL of 25 mM HEPES, pH 7.4, Mini-Complete™ protease inhibitor (−)EDTA cocktail (Roche Applied Science, Indianapolis, IN), and 1% phosphatase inhibitor
cocktails 2 and 3 (Sigma, St. Louis, MO) at 4 °C, followed by 3 rounds of pellet extraction in 150 µL of 7 M urea, 2 M thiourea, and 2% 3-((3-cholamidopropyl)
dimethylammonio)-1-propanesulfonic acid89. Extracts were quantified by Bio-Rad protein assay (Bio-Rad, Hercules, CA) using bovine γ-globulin standard. Samples (30 µg per extract) were
resolved by 10.5–14% gradient Criterion Tris–HCL precast (Bio-Rad) sodium dodecyl sulfate–polyacrylamide gel electrophoresis and stained with Coomassie blue R-250, with gel lanes sectioned
for individual mass spectrometry runs. NANO-FLOW LIQUID CHROMATOGRAPHY TANDEM MASS SPECTROMETRY Gel tranches were de-stained, with protein reduced, alkylated, digested with trypsin, and
peptides extracted and dried89. Peptides were resuspended in 0.2% formic acid, 0.1% trifluoroacetic acid, and 0.002% zwittergent 3–16 (Calbiochem, San Diego, CA), and analyzed by nano-flow
LC–MS/MS using a Q-Exactive Hybrid Quadrupole Orbitrap mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) coupled to a Thermo UltiMate 3000 RSLCnano HPLC system. Peptides were
loaded onto a 250 nL OPTI-PAK trap (Optimize Technologies, Oregon City, OR) packed with Michrom Magic C8, 5 µm solid phase (Michrom Bioresources, Auburn, CA). Chromatography was performed
using 0.2% formic acid in solvents A (98% water, 2% acetonitrile) and B (80% acetonitrile, 10% isopropanol, 10% water), over a 2–45% B gradient for 60 min at 400 nL/min through a 100 µm × 35
cm PicoFrit column (New Objective, Woburn, MA) packed with Agilent Poroshell 120 EC-C18 (Agilent Scientific Instruments, Santa Clara, CA). MS1 survey scans 350–2000 m/z were acquired at
70,000 resolution targeting 3 × 106 ions and 60 ms maximum inject time, followed by data dependent high energy collisional dissociation MS2 on the top 15 ions at 17,500 resolution targeting
2 × 105 ions with 60 ms maximum inject time, using dynamic exclusion of measured ions for 60 s. MASS SPECTROMETRY DATA ANALYSIS Raw files consisting of 10 LC–MS/MS runs per sample were
processed in MaxQuant 1.6.7.090, using Andromeda search engine for label-free quantification (LFQ), with applied fastLFQ settings. Spectra were searched against UniProt mouse entries,
combining forward and reverse peptides as decoys to estimate FDR, with peptide match and protein assignment FDR set at 0.01. Search parameters included trypsin/P digestion, cysteine
carbamido-methylation, and variable modifications of amino-terminal protein acetylation, glutamate to pyro-glutamate, and methionine oxidation. Maximum charge was + 7, with up to 3 dynamic
modifications, maximum of 2 missed cleavages, and minimum of 7 amino acids. Mass tolerance was 20 and 10 ppm for first and main searches. LFQ identification was maximized by MaxQuant’s
‘Match Between Runs’ feature, assigning identified spectra from one LC–MS/MS run to corresponding aligned mass and retention time spectra in other runs. Peptides were rolled into protein
assignments, requiring ≥ 2 peptides per protein. DIFFERENTIAL EXPRESSION Relative protein abundance was calculated in R (cran.r-project.org) using Proteus91, for limma analysis92 of
label-free MaxQuant data. Peptide information acquired from MaxQuant evidence files was filtered for contaminants and reverse peptides without imputing missing values. Peptides were rolled
into corresponding proteins, data median normalized, and the high-flyer method applied to calculate relative protein abundance. Proteins with FDR corrected _P_ < 0.05 were considered
differentially expressed. METABOLOMICS Tissue (> 50 mg) and plasma (> 150 µL) metabolites were processed for untargeted gas chromatography (GC)/MS, and for positive and negative ion
mode liquid chromatography (+ LC and − LC)/MS (Metabolon, Research Triangle Park, NC). Protein was removed using organic and aqueous buffers, placed on a TurboVap® (Zymark, Hopkinton, MA),
frozen, and small molecules dried under vacuum. GAS CHROMATOGRAPHY MASS SPECTROMETRY For GC/MS of volatile metabolites, samples were re-dried under vacuum prior to derivatization under N2
using bistrimethyl-silyl-triflouroacetamide. Samples were analyzed on a Thermo-Finnigan Trace DSQ single-quadrupole MS by electron impact ionization using a 5% phenyl GC column with a 40–300
°C ramp over 16 min. LIQUID CHROMATOGRAPHY MASS SPECTROMETRY LC/MS samples were resolved on a Waters ACQUITY UPLC and Thermo-Finnigan LTQ-FT mass spectrometer. For + LC/MS and − LC/MS,
extracts were gradient eluted using water and methanol buffers containing 0.1% formic acid or 6.5 mM ammonium bicarbonate, respectively, alternating between MS1 and MS2 injection scans using
dynamic exclusion. IDENTITY AND EXPRESSION Metabolites were identified by matching spectral chromatographic elution properties to Metabolon’s curated library. Expression values were log
transformed, and imputation applied using the minimum measured value. Random Forest classification was carried out to model individual cohort allocation, generating decision tree ensembles
of the top 30 predictive metabolites. Boxplots of metabolite expression were generated with JMP 14.1.0 (SAS Institute Inc., Cary, NC). Statistical analysis was performed in R, using Welch’s
Two-Sample t-test with _P_ < 0.05 significant. SYSTEMS BIOINFORMATICS CLUSTERING Hierarchical agglomerative clustering (with z-score transformed normalization) and PCA visualization were
conducted using ClustVis93. For 3-D PCA, principal components were plotted in Spotfire 10.0.0 (TIBCO, Palo Alto, CA). SOFT INDEPENDENT MODELING OF CLASS ANALOGY Metabolome grouping and class
membership was predicted by soft independent modeling of class analogy (SIMCA 15.0.2, Sartorius, Bohemia, NY) using PLS-DA. Individual metabolite PLS-DA contributions were rank ordered by
VIP scores. FUNCTIONAL ENRICHMENT Differential metabolome interrogation was carried out by MSEA and MetPA within MetaboAnalyst 4.0 (metaboanalyst.ca)94. Using Human Metabolome Database
(HMDB) identifiers, normalized expression values were analyzed by MSEA, screening Small Molecule Pathway Database (smpdb.ca) libraries. For pathway analysis, the HMDB identifier expression
matrix was uploaded in MetPA, surveying the Kyoto Encyclopedia of Genes and Genomes _Mus musculus_ metabolic pathway library. In MetPA a global enrichment test was applied, with calculation
of the relative betweenness centrality, a network topological parameter of metabolite contribution to shortest paths within the enriched pathway. The entire library served as reference for
MSEA and MetPA calculations. PATHWAY AND NETWORK ANALYSIS Proteins and metabolites were submitted to IPA (QIAGEN Bioinformatics, Hilden, Germany), prescribing cutoffs of corrected _P_ <
0.05 for proteins, _P_ < 0.05 for metabolites. IPA output included: enriched canonical pathways; molecular, cellular, and physiological functions; diseases and disorders; cardiac adverse
outcomes; and network interactions. Significance was calculated using Fisher’s Exact Test, screening proteins against the gene background and metabolites against the compound library, or
both when interpreting merged data. Merged pairwise interactions generated composite networks, exported to Cytoscape 3.8.295. In Cytoscape, NetworkAnalyzer yielded degree distributions to
evaluate network topology96, with Gene Ontology (GO) Biological Process enrichment assessed in BiNGO (Biological Network Gene Ontology), applying a hypergeometric distribution and
Benjamini–Hochberg FDR correction97. Enriched processes were clustered and visualized as a bubble plot, with bubble diameters proportional to the number of annotations and vertically
centered at the harmonic mean _P_-value98. DATA AVAILABILITY Data supporting findings are available in article/supplementary material. REFERENCES * Nichols, C. G. KATP channels as molecular
sensors of cellular metabolism. _Nature_ 440, 470–476. https://doi.org/10.1038/nature04711 (2006). Article ADS CAS PubMed Google Scholar * Ashcroft, F. M. ATP-sensitive K+ channels and
disease: From molecule to malady. _Am. J. Physiol. Endocrinol. Metab._ 293, E880–E889. https://doi.org/10.1152/ajpendo.00348.2007 (2007). Article CAS PubMed Google Scholar * Olson, T. M.
& Terzic, A. Human KATP channelopathies: Diseases of metabolic homeostasis. _Pflugers Arch._ 460, 295–306. https://doi.org/10.1007/s00424-009-0771-y (2010). Article CAS PubMed Google
Scholar * Foster, M. N. & Coetzee, W. A. KATP channels in the cardiovascular system. _Physiol. Rev._ 96, 177–252. https://doi.org/10.1152/physrev.00003.2015 (2016). Article CAS
PubMed Google Scholar * Tinker, A., Aziz, Q., Li, Y. & Specterman, M. ATP-sensitive potassium channels and their physiological and pathophysiological roles. _Compr. Physiol._ 8,
1463–1511. https://doi.org/10.1002/cphy.c170048 (2018). Article PubMed Google Scholar * Noma, A. ATP-regulated K+ channels in cardiac muscle. _Nature_ 305, 147–148.
https://doi.org/10.1038/305147a0 (1983). Article ADS CAS PubMed Google Scholar * Flagg, T. P., Enkvetchakul, D., Koster, J. C. & Nichols, C. G. Muscle KATP channels: Recent insights
to energy sensing and myoprotection. _Physiol. Rev._ 90, 799–829. https://doi.org/10.1152/physrev.00027.2009 (2010). Article CAS PubMed Google Scholar * Terzic, A., Alekseev, A. E.,
Yamada, S., Reyes, S. & Olson, T. M. Advances in cardiac ATP-sensitive K+ channelopathies from molecules to populations. _Circ. Arrhythm. Electrophysiol._ 4, 577–585.
https://doi.org/10.1161/CIRCEP.110.957662 (2011). Article CAS PubMed PubMed Central Google Scholar * Zingman, L. V. _et al._ Kir6.2 is required for adaptation to stress. _Proc. Natl.
Acad. Sci. USA_ 99, 13278–13283. https://doi.org/10.1073/pnas.212315199 (2002). Article ADS CAS PubMed PubMed Central Google Scholar * Hodgson, D. M. _et al._ Cellular remodeling in
heart failure disrupts KATP channel-dependent stress tolerance. _EMBO J._ 22, 1732–1742. https://doi.org/10.1093/emboj/cdg192 (2003). Article CAS PubMed PubMed Central Google Scholar *
Kane, G. C. _et al._ _KCNJ11_ gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. _Hum. Mol. Genet._ 15, 2285–2297.
https://doi.org/10.1093/hmg/ddl154mcga (2006). Article CAS PubMed Google Scholar * Yamada, S. _et al._ Protection conferred by myocardial ATP-sensitive K+ channels in pressure
overload-induced congestive heart failure revealed in _KCNJ11_ Kir6.2-null mutant. _J. Physiol._ 577, 1053–1065. https://doi.org/10.1113/jphysiol.2006.119511 (2006). Article CAS PubMed
PubMed Central Google Scholar * Stoller, D. A. _et al._ Cardiomyocyte sulfonylurea receptor 2-KATP channel mediates cardioprotection and ST segment elevation. _Am. J. Physiol. Heart Circ.
Physiol._ 299, H1100–H1108. https://doi.org/10.1152/ajpheart.00084.2010 (2010). Article CAS PubMed PubMed Central Google Scholar * Storey, N. M., Stratton, R. C., Rainbow, R. D.,
Standen, N. B. & Lodwick, D. Kir6.2 limits Ca2+ overload and mitochondrial oscillations of ventricular myocytes in response to metabolic stress. _Am. J. Physiol. Heart Circ. Physiol._
305, H1508–H1518. https://doi.org/10.1152/ajpheart.00540.2013 (2013). Article CAS PubMed PubMed Central Google Scholar * Nichols, C. G. Adenosine triphosphate-sensitive potassium
currents in heart disease and cardioprotection. _Card. Electrophysiol. Clin._ 8, 323–335. https://doi.org/10.1016/j.ccep.2016.01.005 (2016). Article PubMed PubMed Central Google Scholar
* Inagaki, N. _et al._ A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. _Neuron_ 16, 1011–1017.
https://doi.org/10.1016/s0896-6273(00)80124-5 (1996). Article CAS PubMed Google Scholar * Babenko, A. P., Gonzalez, G., Aguilar-Bryan, L. & Bryan, J. Reconstituted human cardiac KATP
channels: Functional identity with the native channels from the sarcolemma of human ventricular cells. _Circ. Res._ 83, 1132–1143. https://doi.org/10.1161/01.res.83.11.1132 (1998). Article
CAS PubMed Google Scholar * Lorenz, E. & Terzic, A. Physical association between recombinant cardiac ATP-sensitive K+ channel subunits Kir6.2 and SUR2A. _J. Mol. Cell Cardiol._ 31,
425–434. https://doi.org/10.1006/jmcc.1998.0876 (1999). Article CAS PubMed Google Scholar * Zingman, L. V. _et al._ Tandem function of nucleotide binding domains confers competence to
sulfonylurea receptor in gating ATP-sensitive K+ channels. _J. Biol. Chem._ 277, 14206–14210. https://doi.org/10.1074/jbc.M109452200 (2002). Article CAS PubMed Google Scholar * Alekseev,
A. E. _et al._ ATP-sensitive K+ channel channel/enzyme multimer: Metabolic gating in the heart. _J. Mol. Cell Cardiol._ 38, 895–905. https://doi.org/10.1016/j.yjmcc.2005.02.022 (2005).
Article CAS PubMed PubMed Central Google Scholar * Zingman, L. V., Alekseev, A. E., Hodgson-Zingman, D. M. & Terzic, A. ATP-sensitive potassium channels: Metabolic sensing and
cardioprotection. _J. Appl. Physiol._ 103, 1888–1893. https://doi.org/10.1152/japplphysiol.00747.2007 (2007). Article CAS PubMed Google Scholar * Alekseev, A. E. _et al._ Sarcolemmal
ATP-sensitive K+ channels control energy expenditure determining body weight. _Cell Metab._ 11, 58–69. https://doi.org/10.1016/j.cmet.2009.11.009 (2010). Article CAS PubMed PubMed Central
Google Scholar * Youssef, N. _et al._ Hearts lacking plasma membrane KATP channels display changes in basal aerobic metabolic substrate preference and AMPK activity. _Am. J. Physiol.
Heart Circ. Physiol._ 313, H469–H478. https://doi.org/10.1152/ajpheart.00612.2016 (2017). Article PubMed Google Scholar * Abraham, M. R. _et al._ Coupling of cell energetics with membrane
metabolic sensing. Integrative signaling through creatine kinase phosphotransfer disrupted by M-CK gene knock-out. _J. Biol. Chem._ 277, 24427–24434. https://doi.org/10.1074/jbc.M201777200
(2002). Article CAS PubMed Google Scholar * Alekseev, A. E., Reyes, S., Selivanov, V. A., Dzeja, P. P. & Terzic, A. Compartmentation of membrane processes and nucleotide dynamics in
diffusion-restricted cardiac cell microenvironment. _J. Mol. Cell Cardiol._ 52, 401–409. https://doi.org/10.1016/j.yjmcc.2011.06.007 (2012). Article CAS PubMed Google Scholar * Carrasco,
A. J. _et al._ Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. _Proc. Natl. Acad. Sci. USA_ 98, 7623–7628.
https://doi.org/10.1073/pnas.121038198 (2001). Article ADS CAS PubMed PubMed Central Google Scholar * Crawford, R. M. _et al._ M-LDH serves as a sarcolemmal KATP channel subunit
essential for cell protection against ischemia. _EMBO J._ 21, 3936–3948. https://doi.org/10.1093/emboj/cdf388 (2002). Article CAS PubMed PubMed Central Google Scholar * Selivanov, V.
A., Alekseev, A. E., Hodgson, D. M., Dzeja, P. P. & Terzic, A. Nucleotide-gated KATP channels integrated with creatine and adenylate kinases: Amplification, tuning and sensing of
energetic signals in the compartmentalized cellular environment. _Mol. Cell Biochem._ 256–257, 243–256. https://doi.org/10.1023/b:mcbi.0000009872.35940.7d (2004). Article PubMed PubMed
Central Google Scholar * Dhar-Chowdhury, P. _et al._ The glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase, triose-phosphate isomerase, and pyruvate kinase are components of the
KATP channel macromolecular complex and regulate its function. _J. Biol. Chem._ 280, 38464–38470. https://doi.org/10.1074/jbc.M508744200 (2005). Article CAS PubMed Google Scholar *
Jovanović, S. _et al._ Glyceraldehyde 3-phosphate dehydrogenase serves as an accessory protein of the cardiac sarcolemmal KATP channel. _EMBO Rep._ 6, 848–852.
https://doi.org/10.1038/sj.embor.7400489 (2005). Article CAS PubMed PubMed Central Google Scholar * Hong, M. _et al._ Cardiac ATP-sensitive K+ channel associates with the glycolytic
enzyme complex. _FASEB J._ 25, 2456–2467. https://doi.org/10.1096/fj.10-176669 (2011). Article CAS PubMed PubMed Central Google Scholar * Arrell, D. K. & Terzic, A. Interpreting
networks in systems biology. _Clin. Pharmacol. Ther._ 93, 389–392. https://doi.org/10.1038/clpt.2013.28 (2013). Article CAS PubMed Google Scholar * Trachana, K. _et al._ Taking systems
medicine to heart. _Circ. R.es_ 122, 1276–1289. https://doi.org/10.1161/CIRCRESAHA.117.310999 (2018). Article CAS Google Scholar * Lindsey, M. L. _et al._ Transformative impact of
proteomics on cardiovascular health and disease: A scientific statement from the American Heart Association. _Circulation_ 132, 852–872. https://doi.org/10.1161/CIR.0000000000000226 (2015).
Article CAS PubMed Google Scholar * McGarrah, R. W., Crown, S. B., Zhang, G. F., Shah, S. H. & Newgard, C. B. Cardiovascular metabolomics. _Circ. Res._ 122, 1238–1258.
https://doi.org/10.1161/CIRCRESAHA.117.311002 (2018). Article CAS PubMed PubMed Central Google Scholar * Diz, A. P., Martínez-Fernández, M. & Rolán-Alvarez, E. Proteomics in
evolutionary ecology: Linking the genotype with the phenotype. _Mol. Ecol._ 21, 1060–1080. https://doi.org/10.1111/j.1365-294X.2011.05426.x (2012). Article CAS PubMed Google Scholar *
Vakili, D., Radenkovic, D., Chawla, S. & Bhatt, D. L. Panomics: New databases for advancing cardiology. _Front. Cardiovasc. Med._ 8, 587768. https://doi.org/10.3389/fcvm.2021.587768
(2021). Article PubMed PubMed Central Google Scholar * Joshi, A., Rienks, M., Theofilatos, K. & Mayr, M. Systems biology in cardiovascular disease: A multiomics approach. _Nat. Rev.
Cardiol._ 18, 313–330. https://doi.org/10.1038/s41569-020-00477-1 (2021). Article PubMed Google Scholar * Bäckhed, F. _et al._ The next decade of metabolism. _Nat. Metab._ 1, 2–4.
https://doi.org/10.1038/s42255-018-0022-7 (2019). Article PubMed Google Scholar * Park, S., Lim, B. B. C., Perez-Terzic, C., Mer, G. & Terzic, A. Interaction of asymmetric
ABCC9-encoded nucleotide binding domains determines KATP channel SUR2A catalytic activity. _J. Proteome Res._ 7, 1721–1728. https://doi.org/10.1021/pr7007847 (2008). Article CAS PubMed
PubMed Central Google Scholar * Arrell, D. K., Zlatkovic, J., Kane, G. C., Yamada, S. & Terzic, A. ATP-sensitive K+ channel knockout induces cardiac proteome remodeling predictive of
heart disease susceptibility. _J. Proteome Res._ 8, 4823–4834. https://doi.org/10.1021/pr900561g (2009). Article CAS PubMed PubMed Central Google Scholar * Jovanović, S., Du, Q.,
Sukhodub, A. & Jovanović, A. M-LDH physically associated with sarcolemmal KATP channels mediates cytoprotection in heart embryonic H9C2 cells. _Int. J. Biochem. Cell Biol._ 41,
2295–2301. https://doi.org/10.1016/j.biocel.2009.05.012 (2009). Article CAS PubMed PubMed Central Google Scholar * Zlatkovic, J. _et al._ Proteomic profiling of KATP channel-deficient
hypertensive heart maps risk for maladaptive cardiomyopathic outcome. _Proteomics_ 9, 1314–1325. https://doi.org/10.1002/pmic.200800718 (2009). Article CAS PubMed PubMed Central Google
Scholar * Yoshida, H. _et al._ AMP-activated protein kinase connects cellular energy metabolism to KATP channel function. _J. Mol. Cell Cardiol._ 52, 410–418.
https://doi.org/10.1016/j.yjmcc.2011.08.013 (2012). Article CAS PubMed Google Scholar * Kefaloyianni, E. _et al._ Comparative proteomic analysis of the ATP-sensitive K+ channel complex
in different tissue types. _Proteomics_ 13, 368–378. https://doi.org/10.1002/pmic.201200324 (2013). Article CAS PubMed PubMed Central Google Scholar * Paggio, A. _et al._ Identification
of an ATP-sensitive potassium channel in mitochondria. _Nature_ 572, 609–613. https://doi.org/10.1038/s41586-019-1498-3 (2019). Article ADS CAS PubMed PubMed Central Google Scholar *
Sansbury, B. E. _et al._ Metabolomic analysis of pressure-overloaded and infarcted mouse hearts. _Circ. Heart Fail._ 7, 634–642. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001151 (2014).
Article CAS PubMed PubMed Central Google Scholar * Warren, J. S. _et al._ Histone methyltransferase Smyd1 regulates mitochondrial energetics in the heart. _Proc. Natl. Acad. Sci. USA_
115, E7871–E7880. https://doi.org/10.1073/pnas.1800680115 (2018). Article CAS PubMed PubMed Central Google Scholar * Arrell, D. K. & Terzic, A. Proteomic network systems analysis.
In _Manual of Cardiovascular Proteomics_ (eds Agnetti, G. _et al._), 321–342. ISBN: 978-3-319-31826-4 https://doi.org/10.1007/978-3-319-31828-8_14 (Springer, 2016). * Hasin, Y., Seldin, M.
& Lusis, A. Multi-omics approaches to disease. _Genome Biol._ 18, 83. https://doi.org/10.1186/s13059-017-1215-1 (2017). Article CAS PubMed PubMed Central Google Scholar * Ding,
C.-K.C. _et al._ MESH1 is a cytosolic NADPH phosphatase that regulates ferroptosis. _Nat. Metab._ 2, 270–277. https://doi.org/10.1038/s42255-020-0181-1 (2020). Article CAS PubMed PubMed
Central Google Scholar * Beaupre, B. A., Hoag, M. R., Roman, J., Försterling, F. H. & Moran, G. R. Metabolic function for human renalase: Oxidation of isomeric forms of β-NAD(P)H that
are inhibitory to primary metabolism. _Biochemistry_ 54, 795–806. https://doi.org/10.1021/bi5013436 (2015). Article CAS PubMed Google Scholar * Katsyuba, E., Romani, M., Hofer, D. &
Auwerx, J. NAD+ homeostasis in health and disease. _Nat. Metab._ 2, 9–31. https://doi.org/10.1038/s42255-019-0161-5 (2020). Article CAS PubMed Google Scholar * Lopaschuk, G. D., Karwi,
Q. G., Tian, R., Wende, A. R. & Abel, E. D. Cardiac energy metabolism in heart failure. _Circ. Res._ 128, 1487–1513. https://doi.org/10.1161/CIRCRESAHA.121.318241 (2021). Article CAS
PubMed Google Scholar * Diguet, N. _et al._ Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. _Circulation_ 137, 2256–2273.
https://doi.org/10.1161/CIRCULATIONAHA.116.026099 (2018). Article CAS PubMed Google Scholar * Walker, M. A. & Tian, R. Raising NAD in heart failure: Time to translate?. _Circulation_
137, 2274–2277. https://doi.org/10.1161/CIRCULATIONAHA.117.032626 (2018). Article PubMed PubMed Central Google Scholar * Abdellatif, M. _et al._ Nicotinamide for the treatment of heart
failure with preserved ejection fraction. _Sci. Transl. Med._ 13, eabd7064. https://doi.org/10.1126/scitranslmed.abd7064 (2021). Article CAS PubMed PubMed Central Google Scholar *
Dabrowski, M., Trapp, S. & Ashcroft, F. M. Pyridine nucleotide regulation of the KATP channel Kir6.2/SUR1 expressed in Xenopus oocytes. _J. Physiol._ 550(Pt 2), 357–363.
https://doi.org/10.1113/jphysiol.2003.041715 (2003). Article CAS PubMed PubMed Central Google Scholar * Sukhodub, A., Du, Q., Jovanović, S. & Jovanović, A. Nicotinamide-rich diet
protects the heart against ischaemia-reperfusion in mice: A crucial role for cardiac SUR2A. _Pharmacol. Res._ 61, 564–570. https://doi.org/10.1016/j.phrs.2010.01.008 (2010). Article CAS
PubMed PubMed Central Google Scholar * Abdellatif, M., Sedej, S. & Kroemer, G. NAD+ metabolism in cardiac health, aging, and disease. _Circulation_ 144, 1795–1817.
https://doi.org/10.1161/CIRCULATIONAHA.121.056589 (2021). Article CAS PubMed Google Scholar * Miki, T. _et al._ Defective insulin secretion and enhanced insulin action in KATP
channel-deficient mice. _Proc. Natl. Acad. Sci. USA_ 95, 10402–10406. https://doi.org/10.1073/pnas.95.18.10402 (1998). Article ADS CAS PubMed PubMed Central Google Scholar * Arrell, D.
K., Zlatkovic Lindor, J., Yamada, S. & Terzic, A. KATP channel-dependent metaboproteome decoded: Systems approaches to heart failure prediction, diagnosis, and therapy. _Cardiovasc.
Res._ 90, 258–266. https://doi.org/10.1093/cvr/cvr046 (2011). Article CAS PubMed PubMed Central Google Scholar * Suzuki, M. _et al._ Role of sarcolemmal KATP channels in
cardioprotection against ischemia/reperfusion injury in mice. _J. Clin. Investig._ 109, 509–516. https://doi.org/10.1172/JCI14270 (2002). Article CAS PubMed PubMed Central Google Scholar
* Kane, G. C. _et al._ ATP-sensitive K+ channel knockout compromises the metabolic benefit of exercise training, resulting in cardiac deficits. _Diabetes_ 53(Suppl 3), S169-175.
https://doi.org/10.2337/diabetes.53.suppl_3.s169 (2004). Article CAS PubMed Google Scholar * Gumina, R. J. _et al._ KATP channel knockout worsens myocardial calcium stress load in vivo
and impairs recovery in stunned heart. _Am. J. Physiol. Heart Circ. Physiol._ 292, H1706–H1713. https://doi.org/10.1152/ajpheart.01305.2006 (2007). Article CAS PubMed Google Scholar *
Hu, X. _et al._ Disruption of sarcolemmal ATP-sensitive potassium channel activity impairs the cardiac response to systolic overload. _Circ. Res._ 103, 1009–1017.
https://doi.org/10.1161/CIRCRESAHA.107.170795 (2008). Article CAS PubMed PubMed Central Google Scholar * Tinker, A., Aziz, Q. & Thomas, A. The role of ATP-sensitive potassium
channels in cellular function and protection in the cardiovascular system. _Br. J. Pharmacol._ 171, 12–23. https://doi.org/10.1111/bph.12407 (2014). Article CAS PubMed Google Scholar *
Fatehi, M., Carter, C. C., Youssef, N. & Light, P. E. The mechano-sensitivity of cardiac ATP-sensitive potassium channels is mediated by intrinsic MgATPase activity. _J. Mol. Cell
Cardiol._ 108, 34–41. https://doi.org/10.1016/j.yjmcc.2017.05.004 (2017). Article CAS PubMed Google Scholar * Zhang, B. _et al._ _Kcnj11_ ablation is associated with increased
nitro-oxidative stress during ischemia-reperfusion injury: Implications for human ischemic cardiomyopathy. _Circ. Heart Fail_ 10, e003523. https://doi.org/10.1161/CIRCHEARTFAILURE.116.003523
(2017). Article CAS PubMed PubMed Central Google Scholar * Mayr, M. _et al._ Metabolic homeostasis is maintained in myocardial hibernation by adaptive changes in the transcriptome and
proteome. _J. Mol. Cell Cardiol._ 50, 982–990. https://doi.org/10.1016/j.yjmcc.2011.02.010 (2011). Article CAS PubMed PubMed Central Google Scholar * Chaanine, A. H. Metabolic
remodeling and implicated calcium and signal transduction pathways in the pathogenesis of heart failure. _Int. J. Mol. Sci._ 22, 10579. https://doi.org/10.3390/ijms221910579 (2021). Article
CAS PubMed PubMed Central Google Scholar * Chow, S. L. _et al._ Role of biomarkers for the prevention, assessment, and management of heart failure: A scientific statement from the
American Heart Association. _Circulation_ 135, e1054–e1091. https://doi.org/10.1161/CIR.0000000000000490 (2017). Article CAS PubMed Google Scholar * Meijers, B. K. _et al._ Free
_p_-cresol is associated with cardiovascular disease in hemodialysis patients. _Kidney Int._ 73, 1174–1180. https://doi.org/10.1038/ki.2008.31 (2008). Article CAS PubMed Google Scholar *
Zheng, Y. _et al._ Associations between metabolomic compounds and incident heart failure among African Americans: The ARIC study. _Am. J. Epidemiol._ 178, 534–542.
https://doi.org/10.1093/aje/kwt004 (2013). Article PubMed PubMed Central Google Scholar * Chinnappa, S. _et al._ Association between protein-bound uremic toxins and asymptomatic cardiac
dysfunction in patients with chronic kidney disease. _Toxins_ 10, 520. https://doi.org/10.3390/toxins10120520 (2018). Article CAS PubMed Central Google Scholar * Aa, N. _et al._ Plasma
metabolites alert patients with chest pain to occurrence of myocardial infarction. _Front. Cardiovasc. Med._ 8, 652746. https://doi.org/10.3389/fcvm.2021.652746 (2021). Article PubMed
PubMed Central Google Scholar * Arrell, D. K. & Terzic, A. Network systems biology for drug discovery. _Clin. Pharmacol. Ther._ 88, 120–125. https://doi.org/10.1038/clpt.2010.91
(2010). Article CAS PubMed Google Scholar * Arrell, D. K. & Terzic, A. Systems proteomics for translational network medicine. _Circ. Cardiovasc. Genet._ 5, o8–o16.
https://doi.org/10.1161/CIRCGENETICS.110.958991 (2012). Article Google Scholar * Adhikari, S. _et al._ A high-stringency blueprint of the human proteome. _Nat. Commun._ 11, 5301.
https://doi.org/10.1038/s41467-020-19045-9 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Wieder, C. _et al._ Pathway analysis in metabolomics: Recommendations for the
use of over-representation analysis. _PLoS Comput. Biol._ 17, e1009105. https://doi.org/10.1371/journal.pcbi.1009105 (2021). Article ADS CAS PubMed PubMed Central Google Scholar *
Percie du Sert, N. _et al._ The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. _PLoS Biol._ 18, e3000410. https://doi.org/10.1371/journal.pbio.3000410 (2020).
Article CAS PubMed PubMed Central Google Scholar * Zacchigna, S. _et al._ Towards standardization of echocardiography for the evaluation of left ventricular function in adult rodents: A
position paper of the ESC Working Group on Myocardial Function. _Cardiovasc. Res._ 117, 43–59. https://doi.org/10.1093/cvr/cvaa110 (2021). Article CAS PubMed Google Scholar * Zhang, S.
_et al._ Adenylate kinase AK2 isoform integral in embryo and adult heart homeostasis. _Biochem. Biophys. Res. Commun._ 546, 59–64. https://doi.org/10.1016/j.bbrc.2021.01.097 (2021). Article
CAS PubMed PubMed Central Google Scholar * Lang, R. M. _et al._ Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of
Echocardiography and the European Association of Cardiovascular Imaging. _J. Am. Soc. Echocardiogr._ 28, 1-39.e14. https://doi.org/10.1016/j.echo.2014.10.003 (2015). Article PubMed Google
Scholar * Yamada, S. _et al._ Ventricular remodeling in ischemic heart failure stratifies responders to stem cell therapy. _Stem Cells Transl. Med._ 9, 74–79.
https://doi.org/10.1002/sctm.19-0149 (2020). Article PubMed Google Scholar * Yamada, S. _et al._ Regenerative therapy prevents heart failure progression in dyssynchronous nonischemic
narrow QRS cardiomyopathy. _J. Am. Heart Assoc._ 4, e001614. https://doi.org/10.1161/JAHA.114.001614 (2015). Article CAS PubMed PubMed Central Google Scholar * Ackers-Johnson, M. _et
al._ A simplified, Langendorff-free method for concomitant isolation of viable cardiac myocytes and nonmyocytes from the adult mouse heart. _Circ. Res._ 119, 909–920.
https://doi.org/10.1161/CIRCRESAHA.116.309202 (2016). Article CAS PubMed PubMed Central Google Scholar * Alekseev, A. E., Gomez, L. A., Aleksandrova, L. A., Brady, P. A. & Terzic,
A. Opening of cardiac sarcolemmal KATP channels by dinitrophenol separate from metabolic inhibition. _J. Membr. Biol._ 157, 203–214. https://doi.org/10.1007/s002329900229 (1997). Article
CAS PubMed Google Scholar * Arrell, D. K., Rosenow, C. S., Yamada, S., Behfar, A. & Terzic, A. Cardiopoietic stem cell therapy restores infarction-altered cardiac proteome. _NPJ
Regen. Med._ 5, 5. https://doi.org/10.1038/s41536-020-0091-6 (2020). Article CAS PubMed PubMed Central Google Scholar * Tyanova, S., Temu, T. & Cox, J. The MaxQuant computational
platform for mass spectrometry-based shotgun proteomics. _Nat. Protoc._ 11, 2301–2319. https://doi.org/10.1038/nprot.2016.136 (2016). Article CAS PubMed Google Scholar * Gierlinski, M.,
Gastaldello, F., Cole, C. & Barton, G. Proteus: An R package for downstream analysis of MaxQuant output. _BioRxiv_ https://doi.org/10.1101/416511v2 (2018). Article Google Scholar *
Ritchie, M. E. _et al._ limma powers differential expression analyses for RNA-sequencing and microarray studies. _Nucleic Acids Res._ 43, e47. https://doi.org/10.1093/nar/gkv007 (2015).
Article CAS PubMed PubMed Central Google Scholar * Metsalu, T. & Vilo, J. Clustvis: A web tool for visualizing clustering of multivariate data using principal component analysis and
heatmap. _Nucleic Acids Res._ 43, W566-570. https://doi.org/10.1093/nar/gkv468 (2015). Article CAS PubMed PubMed Central Google Scholar * Chong, J. _et al._ MetaboAnalyst 4.0: Towards
more transparent and integrative metabolomics analysis. _Nucleic Acids Res._ 46, W486–W494. https://doi.org/10.1093/nar/gky310 (2018). Article CAS PubMed PubMed Central Google Scholar *
Shannon, P. _et al._ Cytoscape: A software environment for integrated models of biomolecular interaction networks. _Genome Res._ 13, 2498–2504. https://doi.org/10.1101/gr.1239303 (2003).
Article CAS PubMed PubMed Central Google Scholar * Doncheva, N. T., Assenov, Y., Domingues, F. S. & Albrecht, M. Topological analysis and interactive visualization of biological
networks and protein structures. _Nat. Protoc._ 7, 670–685. https://doi.org/10.1038/nprot.2012.004 (2012). Article CAS PubMed Google Scholar * Maere, S., Heymans, K. & Kuiper, M.
BiNGO: A Cytoscape plugin to assess overrepresentation of Gene Ontology categories in biological networks. _Bioinformatics_ 21, 3448–3449. https://doi.org/10.1093/bioinformatics/bti551
(2005). Article CAS PubMed Google Scholar * Wilson, D. J. The harmonic mean p-value for combining dependent tests. _Proc. Natl. Acad. Sci. USA_ 116, 1195–1200.
https://doi.org/10.1073/pnas.1814092116 (2019). Article MathSciNet CAS PubMed PubMed Central MATH Google Scholar Download references ACKNOWLEDGEMENTS The authors recognize the expert
contributions of Lois A. Rowe for sample collections, Jonathan J. Nesbitt for cardiac catheterization, Diane M. Jech and Katrina M. Tollefsrud for echocardiography analysis, and Carrie Jo
Holtz Heppelman (Mayo Proteomics Core) for peptide mass spectrometry. The authors are grateful to Drs. Takashi Miki and Susumu Seino for initial derivation of the Kir6.2 knockout. FUNDING
Authors are funded by National Institutes of Health (R01 HL134664), National Institute of General Medical Sciences (T32 GM 65841), Marriott Family Foundation, Mayo Clinic Center for
Regenerative Medicine, Gerstner Family Foundation, Mayo Clinic Center for Individualized Medicine, and Medical Scientist Training Program. A.T. recognizes tenure as Michael S. and Mary Sue
Shannon Family Director, Center for Regenerative Medicine, Marriott Family Director, Comprehensive Cardiac Regenerative Medicine, and Marriott Family Professorship at Mayo Clinic. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Marriott Heart Disease Research Program, Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA D. Kent Arrell, Sungjo Park, Satsuki
Yamada, Alexey E. Alekseev, Armin Garmany, Ryounghoon Jeon, Jelena Zlatkovic Lindor & Andre Terzic * Marriott Family Comprehensive Cardiac Regenerative Medicine, Center for Regenerative
Medicine, Mayo Clinic, Rochester, MN, USA D. Kent Arrell, Sungjo Park, Satsuki Yamada, Alexey E. Alekseev, Armin Garmany, Ryounghoon Jeon & Andre Terzic * Department of Molecular
Pharmacology & Experimental Therapeutics, Mayo Clinic, Rochester, MN, USA D. Kent Arrell, Sungjo Park, Satsuki Yamada, Alexey E. Alekseev, Armin Garmany, Ryounghoon Jeon, Jelena
Zlatkovic Lindor & Andre Terzic * Department of Biochemistry & Molecular Biology, Mayo Clinic, Rochester, MN, USA Sungjo Park & Ivan Vuckovic * Division of Geriatric Medicine
& Gerontology, Department of Medicine, Mayo Clinic, Rochester, MN, USA Satsuki Yamada * Institute of Theoretical and Experimental Biophysics, Russian Academy of Science, Pushchino,
Moscow Region, Russia Alexey E. Alekseev * Mayo Clinic Alix School of Medicine, Regenerative Sciences Track, Mayo Clinic Graduate School of Biomedical Sciences, Mayo Clinic, Rochester, MN,
USA Armin Garmany * Metabolomics Core, Mayo Clinic, Rochester, MN, USA Ivan Vuckovic * Department of Clinical Genomics, Mayo Clinic, Rochester, MN, USA Andre Terzic Authors * D. Kent Arrell
View author publications You can also search for this author inPubMed Google Scholar * Sungjo Park View author publications You can also search for this author inPubMed Google Scholar *
Satsuki Yamada View author publications You can also search for this author inPubMed Google Scholar * Alexey E. Alekseev View author publications You can also search for this author inPubMed
Google Scholar * Armin Garmany View author publications You can also search for this author inPubMed Google Scholar * Ryounghoon Jeon View author publications You can also search for this
author inPubMed Google Scholar * Ivan Vuckovic View author publications You can also search for this author inPubMed Google Scholar * Jelena Zlatkovic Lindor View author publications You can
also search for this author inPubMed Google Scholar * Andre Terzic View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS D.K.A., A.T. conception
and design; D.K.A., S.P., S.Y., A.E.A., A.G., R.J., J.Z.L. data collection and assembly; D.K.A., S.P., S.Y., A.E.A., A.G., I.V., A.T. data analysis and interpretation; D.K.A., S.P., S.Y.,
A.E.A., A.G., A.T. manuscript writing; D.K.A., S.P., S.Y., A.E.A., A.G., R.J., I.V., J.Z.L., A.T. final manuscript approval; D.K.A., S.Y., A.T. financial support; A.T. administrative care.
CORRESPONDING AUTHOR Correspondence to Andre Terzic. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1. SUPPLEMENTARY TABLE 1.
SUPPLEMENTARY TABLE 2. SUPPLEMENTARY TABLE 3. SUPPLEMENTARY TABLE 4. SUPPLEMENTARY TABLE 5. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution
4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and
the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's
Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Arrell, D.K., Park, S., Yamada, S. _et al._ KATP channel dependent heart multiome
atlas. _Sci Rep_ 12, 7314 (2022). https://doi.org/10.1038/s41598-022-11323-4 Download citation * Received: 15 December 2021 * Accepted: 21 April 2022 * Published: 05 May 2022 * DOI:
https://doi.org/10.1038/s41598-022-11323-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative