- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Timing mechanisms play a key role in the biology of coral reef fish. Typically, fish larvae leave their reef after hatching, stay for a period in the open ocean before returning to
the reef for settlement. During this dispersal, larvae use a time-compensated sun compass for orientation. However, the timing of settlement and how coral reef fish keep track of time via
endogenous timing mechanisms is poorly understood. Here, we have studied the behavioural and genetic basis of diel rhythms in the clown anemonefish _Amphiprion ocellaris_. We document a
behavioural shift from nocturnal larvae to diurnal adults, while juveniles show an intermediate pattern of activity which potentially indicates flexibility in the timing of settlement on a
host anemone. qRTPCR analysis of six core circadian clock genes (_bmal1_, _clocka_, _cry1b_, _per1b_, _per2_, _per3_) reveals rhythmic gene expression patterns that are comparable in larvae
and juveniles, and so do not reflect the corresponding activity changes. By establishing an embryonic cell line, we demonstrate that clown anemonefish possess an endogenous clock with
similar properties to that of the zebrafish circadian clock. Furthermore, our study provides a first basis to study the multi-layered interaction of clocks from fish, anemones and their
zooxanthellae endosymbionts. SIMILAR CONTENT BEING VIEWED BY OTHERS PLASTICITY OF CIRCADIAN AND CIRCATIDAL RHYTHMS IN ACTIVITY AND TRANSCRIPTOMIC DYNAMICS IN A FRESHWATER SNAIL Article Open
access 27 March 2024 BIOLOGICAL RHYTHMS IN THE DEEP-SEA HYDROTHERMAL MUSSEL _BATHYMODIOLUS AZORICUS_ Article Open access 10 July 2020 CELLULAR PATHWAYS DURING SPAWNING INDUCTION IN THE
STARLET SEA ANEMONE _NEMATOSTELLA VECTENSIS_ Article Open access 29 July 2021 INTRODUCTION Diel, lunar and annual rhythms in the marine environment dominate the behaviour and physiology of
numerous fish species. For example, in intricate coral reef environments, diel and lunar rhythms are major determinants of the timing of spawning, hatching and dispersal for many resident
species. While adult coral reef fish are mostly sedentary, larvae are highly mobile and leave their natal reef directly after hatching. The time that larvae stay in the open ocean before
settlement varies between species1. Dispersal sets the spatial scale for population connectivity and geographic population size (reviewed by2) and therefore influences replenishment of fish
stocks in coral reefs. While dispersal is a very important life history trait, our knowledge about what determines dispersal duration remains incomplete. Importantly, larvae are not just
transported passively through water currents. Instead, they exhibit impressive swimming abilities before settlement3 and can sustain speeds faster than average currents for days4. This may
well enable them to return to their natal reef within a given time frame. Up to 60 % of new settlers of the coral reef fishes _Ostorhinchus doederleini_ and _A. percula_ were found to
originate from the respective reef of settlement5,6. Remarkably, 32 % of _A. polymnus_ larvae settled within two hectares of their birth site7. Coral reef fish larvae may use a wide range of
cues to find the way back to their natal reef8. For example, celestial cues in combination with a time-compensated sun compass are used by the cardinal fish _O. doederleini_ larvae9.
Anemonefish may use similar mechanisms for orientation, which underlines the necessity for them to have a functional timing system. Not only dispersal but also settlement involves the
function of an endogenous timekeeping system. Reef fish larvae enter the coral reef during dusk and night10 and settlement takes place during the night11. The available data indicate that it
is vital for the fish to enter the reef at a particular time of day12. The mechanisms whereby coral reef animals adapt to and anticipate daily changes in their environment remain poorly
understood. The fascinating clown anemonefish _A. ocellaris_ (family: Pomacentridae) represents an ideal model organism to study the contribution of the circadian clock to shaping coral reef
fish biology. Adult anemonefish participate in a remarkably close, three-way mutualism with anemones and their zooxanthellae endosymbionts13. They are therefore restricted to the photic and
mesophotic zones due to their obligate association with anemones. Host anemones, like _E. quadricolor_ together with the inhabiting anemonefish predominantly occur in shallow water14.
Furthermore, like most coral reef fish, clown anemonefish have a larval dispersal stage with larvae hatching at night15, leaving the natal reef immediately after hatching and staying for up
to 12 days in the pelagic zone. One major time-keeping mechanism is the circadian clock, which most organisms, including plants, fungi, bacteria and animals possess16. The circadian clock
allows organisms to anticipate daily environmental changes and consequently, many physiological and behavioural processes are temporally organized and synchronized with the daily 24 hours
cycle17,18. The use of fish as models for studying the circadian clock, most notably the zebrafish _Danio rerio_, has provided new insight into the function and evolution of the clock19,20.
In contrast to mammals, almost all fish tissue clocks are directly light-entrainable. Exploiting this special feature, it has been possible to study clock regulation by light even in
fish-derived cell cultures. The circadian clock in zebrafish like all other vertebrates, consists of transcriptional/translational feedback loops. The core circadian clock consists of
positively and negatively acting elements: Heterodimers comprised of CLOCK and BMAL proteins which activate the transcription of _period_ and _cryptochrome_ genes. PERIOD and CRYPTOCHROME in
turn suppress the transcriptional activation driven by the CLOCK and BMAL transcription factors, thereby reducing _period_ and _cryptochrome_ expression levels and so ultimately enabling a
new cycle of activation driven by CLOCK and BMAL. This gene regulatory circuit thereby directs circadian rhythms of gene expression21. While sunlight is one of the most important timing cues
and fundamentally enables photosynthesis for coral reefs, it is also associated with exposure to damaging UV radiation. The ozone layer is at its thinnest near the equator and water
turbidity is also low, leading to very high UV irradiance in coral reefs22. Both reef fish and anemones have developed multiple adaptation strategies to minimize UV induced damage. These
include behavioural avoidance strategies such as occupying shady areas as well as the production of UV light absorbing protective substances23,24. Additionally, DNA damage induced by UV
light is repaired by the efficient photoreactivation DNA repair system via light dependent catalysis by the photolyase enzymes, close relatives of the cryptochromes25. Here we have
investigated the diel rhythms of clown anemonefish at different stages of development to better understand the timing of dispersal and settlement in this species. We reveal a striking shift
from nocturnal to diurnal locomotor activity during the development of larval clownfish into the juvenile forms. In turn, we document rhythmic circadian clock gene expression in this species
and show that its phase is not altered during the shift in behaviour. We also demonstrate light-inducible _photolyase_ gene expression in the clown anemonefish which is comparable between
larval and juvenile fish and closely resembles that previously observed in zebrafish. In order to minimize the need to sacrifice extremely valuable adult clown anemonefish for studying
temporal changes in gene expression at high resolution and under different lighting regimes, we have established an embryonic _A. ocellaris_ cell line and used this to study circadian clock
function. This confirmed that the properties of the clown anemonefish circadian clock closely resemble those of the zebrafish. Therefore, we reveal important changes in activity patterns
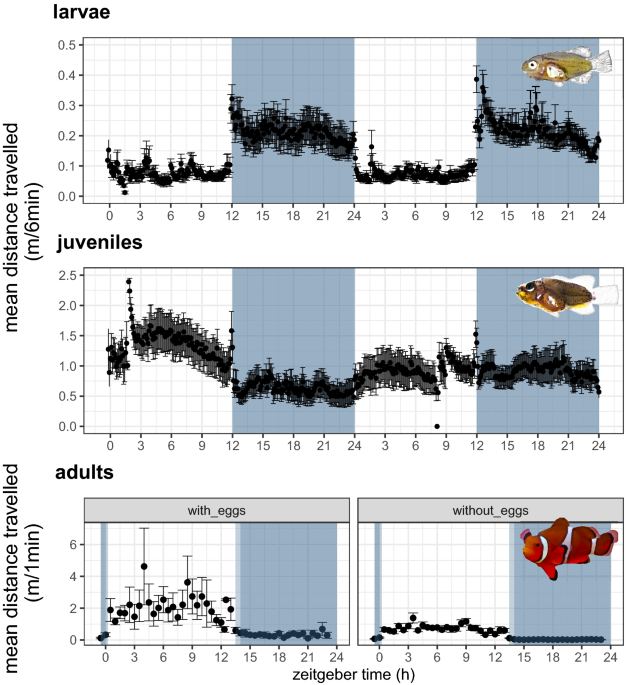
during development, while rhythmic clock gene expression remains relatively unchanged. RESULTS ACTIVITY PATTERNS IN LARVAL, JUVENILE AND ADULT CLOWN ANEMONEFISH The habitat of very young
larvae and adult animals is extremely different - young larvae are dispersing in the open ocean, while adults are highly sedentary in close mutualism with their host anemone. The locomotor
activity of _A. ocellaris_ larvae ranging from 7 to 23 days post hatching (dph), juveniles from 52 to 56 dph, 86 to 91 dph and 98 to 106 dph and breeding clownfish pairs (several years old)
was observed during exposure to a Light:Dark (LD) cycle under laboratory conditions. We tested for rhythmic locomotor activity and also calculated the diurnality index (D)26 which represents
a quantitative measure of the degree of nocturnal or diurnal activity, ranging from -1 (all animals are nocturnal) to +1 (all animals are diurnal). Larval and juvenile clownfish (> 96%)
showed daily activity rhythms (_p_ < 0_._05, Fig. 1). All larvae were nocturnal (Fig. 1, D = -1). However, the nocturnality of animals diminished with increasing age (juveniles 52–91 dph:
-0.1 < D < -0.5). Older juveniles (98–106 dph) were mostly diurnal (D = +0.38) and all adult clownfish were exclusively diurnal (D = +1), irrespective of breeding status.
Importantly, locomotor activity levels tended to increase just before the onset or offset of the light period showing one of the most characteristic features of circadian clock regulated
behaviour, the anticipation of the daily light–dark cycle (Fig. 1). GENE EXPRESSION OF CLOCK AND PHOTOREACTIVATION DNA REPAIR GENES IN LARVAL AND JUVENILE CLOWNFISH Does the shift from
nocturnal to diurnal activity in _A. ocellaris_ reflect a corresponding shift in the function of the core circadian clock mechanism? To address this question we compared the mRNA expression
profile of a set of six core clock genes in whole body RNA extracts prepared from larval and juvenile clownfish exposed to a 12:12 h LD cycle. Specifically, we examined the temporal
expression profile of six core clock genes (_bmal1_, _clocka_, _cry1b_, _per1b_, _per2_, _per3_ sequences derived from an existing transcriptome database27) with a relatively low resolution
of four timepoints over the course of one day-night cycle. The mean normalized gene expression is shown in Fig. 2. The peak of RNA expression of _per2_, _per3_, _cry1b_ was at zeitgeber time
(ZT) 3 and of _per1b_ at ZT21 (_p _< 0_._05), while genes encoding the positive elements of the core clock regulatory loop, _bmal1_ and _clocka_ were instead expressed highest at the
day-night transition in both larvae and juveniles (a peak at ZT9 for _clocka_ in larvae and juveniles and for _bmal1_ at ZT9 in larvae and at ZT15 in juveniles (_p _< 0_._05)). Thus, the
rhythmic expression pattern for each gene was equivalent in nocturnal larvae and diurnal juveniles. Given the importance of an efficient DNA repair system in the coral reef environment, we
also investigated the expression of three genes of the photoreactivation DNA repair mechanism (_cpd photolyase_, _cry dash_ and _6-4-photolyase_) (Fig. 2). They were most highly expressed
during the day with a peak at ZT3 (_p _< 0_._05) in larvae and juveniles, consistent with the predominantly light driven expression observed in other fish species, such as zebrafish and
goldfish. CLOCK AND LIGHT REGULATED TRANSCRIPTIONAL RHYTHMS IN CLOWNFISH In order to explore in more detail the molecular mechanisms directing rhythmic gene expression in _A. ocellaris_, our
next goal was to study in depth the regulation of clock gene expression under a range of lighting conditions. Such an experiment would be entirely impractical using fish, particularly at
high resolution, due to the large numbers of animals required which would exceed the capacity of our animal facility. Furthermore, such a costly experiment would be objectionable purely
based on ethical considerations. Therefore, we chose to establish an _A. ocellaris_ cell line from enzymatically dissociated embryos, the embryonic _A. ocellaris_ (EAO) cell line. Based on
our previous studies of zebrafish and cavefish cell lines28,29, such a clownfish cell line should possess a directly light-entrainable circadian clock mechanism, comparable to that observed
_in vivo_. To test this prediction, we compared rhythmic gene expression in the EAO cell line with that of larvae and juveniles as described above (see Fig. 2). In the EAO cell line, RAIN
analysis revealed two rhythmic genes, _per2_ and _per3_ with a peak at ZT3 (_p _< 0_._05), similar to larval and juvenile clownfish. Expression of _cry1b_ seemed to be similar, compared
to that of the fish with the highest expression at ZT3, a decrease until ZT15 and a small increase until ZT21. _Per1b_ was most highly expressed during the day, but not at ZT21, as seen in
clownfish larvae and juveniles. Thus, the rhythmic expression pattern for the _cryptochrome_ and _period_ genes was equivalent in the EAO cells, larvae and juveniles. Furthermore, consistent
with our results in larvae and juveniles, in EAO cells, the _photolyase_ genes were expressed at higher levels during the day than during the night, but without the clear peak at ZT3.
However, interestingly, rhythmic mRNA expression of _bmal1_ and _clocka_ in larvae and juveniles did not resemble the non-cycling expression pattern in the clownfish cell line. We next
wished to study at higher temporal resolution, clock and light-regulated transcription in _A. ocellaris_ cells. With this aim we examined the expression of transiently transfected luciferase
reporter gene constructs based on zebrafish _cryptochrome_ and _period_ gene promotors which we have previously characterized extensively in zebrafish cell lines. Specifically,
zf_Per1b_-Luc has been previously reported to contain multiple E-box enhancer elements and so is strongly regulated by the core circadian clock elements CLOCK and BMAL21. Instead,
D-box_Cry1a_-Luc contains multimerized copies of the D-box enhancer element which we have revealed serves as a light responsive enhancer element in most fish species analysed so far30,31. In
particular, this enhancer directs light induced expression of _photolyase_ genes32. EAO cells transfected with both constructs were exposed for four days to a 12:12 h LD cycle followed by
one day in constant darkness (DD) and during the entire period, levels of bioluminescence generated by the reporters was measured at 48 min resolution by an _in vivo_ bioluminescence assay
(Fig. 3). With the D-box_Cry1a_-Luc construct, both transfected EAO and control zebrafish PAC-2 cells showed a robust bioluminescence rhythm in LD cycle, which was immediately absent in DD
(Fig. 3, above), in agreement with zebrafish and _A. ocellaris_ sharing light-regulated D-box function. This is also consistent with the light inducible expression observed for the _A.
ocellaris photolyase_ genes (Fig. 2). On the contrary, EAO and PAC-2 cells, transfected with the zf_Per1b_-Luc construct, showed a robust cycle of bioluminescence in LD, which persisted even
in DD with a peak in the subjective day (Fig. 3, below), consistent with the presence of a robustly light-entrainable clock in both EAO and PAC-2 cell lines. DISCUSSION In our study we have
revealed a considerable change in locomotor activity during developmental maturation of _A. ocellaris_ from nocturnal larvae to diurnal adults. In juveniles we found both patterns of
activity with an increase of diurnal activity with increasing age. Interestingly, the phase of rhythmic expression of the core clock genes did not change during ontogeny. We provide new
insight about locomotor activity of _A. ocellaris_ larvae over a range from 7 to 23 dph. Larvae and juveniles up to 90 dph were mostly nocturnal (D < -0.1) and did not change their
behaviour during the natural settlement period at the reef (around 12 dph). In the wild, undisturbed locomotor activity has not been observed in dispersing coral reef fish larvae. There is
only indirect evidence for nocturnal locomotor activity e.g. that settlement occurs at dusk and night10. Thus, during the night, but not during the day, pomacentrid larvae prefer reef sound
which might guide them to a reef for settlement33. Also, under laboratory conditions, larvae of the closely related species _A. melanopus_ and _A. percula_ moved at higher speeds during the
night compared to the day when they were 7 and 9 dph old34. Nocturnal settlement of another pomacentrid species _Dascyllus trimaculatus_ was observed in the field11, with these fish
switching from nocturnal to diurnal behaviour directly after settlement. Larvae post metamorphosis at an age of 7–14 dph were protected by anemones, so they were able to settle at this
age35. Anemonefish have been assumed to settle after 6–10 days in order to seek out an anemone15. Interestingly, during our experiments, the switch from nocturnal to diurnal behaviour did
not occur during the natural settlement time when clownfish arrive at a reef, but instead much later. In our experimental setup we could not provide anemones, so the absence of a potential
host anemone for settling might have delayed the behavioural switch in the larvae. In the wild, clownfish are always found in close proximity to an anemone; indeed, not being able to find a
suitable anemone is lethal36. It is possible that clownfish which initially cannot find an anemone, hide during the day to avoid predation and delay their settlement in an anemone. Future
studies are required to explore whether clownfish larvae might exhibit wide temporal plasticity in this regard. However, we should be cautious not to overinterpret the exact timepoint when
clownfish switch from nocturnal to diurnal behaviour since the laboratory conditions might have influenced this process. Nevertheless, it is important to emphasize that the switch from
nocturnal to diurnal behaviour was not evoked by feeding or handling. All animals, from larvae and juveniles to adult clownfish were fed during the day, therefore it is likely that this
change of activity is innate. We analysed the rhythmic gene expression of _A. ocellaris_ circadian clocks. Rhythmic clock gene expression overall is very similar to that described for
zebrafish, the most studied fish model regarding circadian clock gene expression37,38 as well as other fishes including the migrating _Oncorhynchus mykiss_39 and the marine _Sparus
aurata_40. The _bmal1_ and _clock_ genes serve as positive elements within the core clock mechanism, activating the transcription of the _cryptochrome_ and _period_ clock genes. The
expression of _A. ocellaris clocka_ and _bmal1_ is comparable with the expression of zebrafish _clock_28,37 and _bmal_37 and _O. mykiss clock_ and _bmal1_39 with a peak at around ZT12.
Heterodimers of CLOCK and BMAL activate the transcription of _period_ and _cryptochrome_ genes including _per1b_ via binding to E-box promotor elements29. Expression of _per1b_ was highest
at late night in _A. ocellaris_, similar to _per1_ expression in the retina of the nocturnal _Solea senegalensis_41, the reef fish _Siganus guttatus_42 and zebrafish larvae37. In zebrafish
the expression of period gene _per2_ is light driven and therefore important for entrainment of the clock by light30. In the clown anemonefish, _per2_ is expressed at highest levels during
the day, similar to what is found in zebrafish28 and _S. senegalensis_41,43. We also found highest expression of _cry1b_ at ZT3 and also elevated expression at ZT9, which is comparable with
the peak of expression of _cry1b_ in zebrafish during the middle of the light phase21. All of our behavioural analysis together with the assays for clock gene expression in clownfish larvae
and juveniles were performed under LD cycle conditions. Therefore, it is theoretically conceivable that the observed rhythms are driven acutely by the lighting conditions rather than being
driven by a self-sustaining circadian clock. Definitive proof of circadian clock regulation _in vivo_ is the persistence of behavioural or clock gene rhythmicity even under prolonged periods
of DD. However, due to the high cost in terms of number of animals required as well as the small size and sensitivity of clownfish larvae combined with the need for daily feeding and water
changing, we could not analyse behaviour or clock gene expression during DD in whole clownfish. Instead, we used a newly established clownfish cell line that gave us the possibility to
analyse the circadian clock over several days with high temporal resolution. The zebrafish zf_Per1b_-Luc reporter is clock driven29 and EAO cells showed a robust bioluminescence rhythm with
this construct which persisted in DD. This provides important evidence for clown anemonefish possessing an endogenous and cell autonomous, directly light-entrainable clock. We also tested
expression of a D-box_Cry1a_-Luc reporter to analyse whether light drives rhythmic gene expression in these clownfish cells. The bioluminescence was robustly rhythmic in LD cycle conditions,
with reporter expression increasing exclusively during the light period, before descending to lower basal levels following return to darkness. This rhythmicity was immediately abolished
under DD, which provides additional evidence the zebrafish and clown anemonefish circadian clock share similar molecular mechanisms whereby light regulates expression and clock function.
Furthermore, consistent with this conclusion, in our behavioural analysis we observed a characteristic upward tendency in locomotor activity occurring just before the onset or offset of the
light period. This anticipatory activity is entirely consistent with regulation by a functional clock mechanism that would provide the necessary basis for timing such as that observed
time-compensated sun compass orientation, which was found in coral reef fish _O. doederleini_9. Interestingly, while robust rhythmic mRNA expression of the _clocka_ and _bmal1_ genes was
observed in clownfish juveniles and larvae, these genes appeared to be arrhythmically expressed in EAO cells. Differences in the amplitude of rhythmic _clock_ gene expression have been
frequently reported between species and may reflect differences in the relative contributions of transcriptional and post-transcriptional mechanisms to driving circadian rhythms in _clock_-
and _bmal_-mediated transcriptional activation44. Furthermore, within a single species, tissue and cell type-specific differences in the rhythmic expression pattern for individual clock
genes have been frequently observed45. For example, rhythmic clock gene expression is absent in mouse embryonic stem cells46. Given that the EAO cell line was generated from a complex pool
of cells obtained from enzymatically dissociated entire _A. ocellaris_ embryos, it is problematic to determine precisely which original cell type represented the source of this cell line.
Furthermore, cell phenotypes may be influenced by the inevitable selection process involved in repeated propagation of the cells under artificial cell culture conditions. Nevertheless, based
on our previous comparison of the zebrafish circadian clock mechanism _in vivo_ with that in zebrafish embryo-derived cell lines29 the observed differences in clock gene expression between
EAO cells and _A. ocellaris_ larvae and juveniles are unlikely to indicate radical differences in the clock mechanism. Interestingly, although the locomotor activity pattern changed
dramatically during maturation, the clock gene expression pattern remains similar in larvae and juvenile clown anemonefish. There are theoretically a number of possible interpretations for
this observation. Inaccuracies in our analysis seem unlikely given that our experimental approaches for behavioural and gene expression analysis follow well established protocols that have
been applied to many other fish species, including the well-studied zebrafish37. Furthermore, given that the kinetics of clock gene expression has been firmly linked with the timing of clock
regulatory targets38 it also seems unlikely that the phase of rhythmic clock gene expression observed _in vivo_ in clownfish larvae and juveniles has no functional consequence. However,
given that our analysis of gene expression patterns was performed using whole fish extracts and thus represented a mixture of different peripheral tissues, it is possible that changes in the
phase of rhythmic clock gene expression did occur in small collections of cells in the central nervous system, that might be important for directing these changes in locomotor activity. In
this regard, our results are consistent with other studies comparing clock gene expression in diurnal and nocturnal species. No general shift in clock gene expression was found between
diurnal and nocturnal mammals (reviewed in47). Furthermore, in the case of fish, depending on the precise timing of regular feeding time, the gilthead sea bream (_S. aurata_) can be
nocturnal or diurnal, but importantly, clock gene expression within the brain remains identical40. Similar patterns were also found in _Dicentrarchus labrax_48. Thus, our findings provide
additional evidence that the switch between nocturnal and diurnal behaviour might be controlled downstream of the core clock or alternatively might result from switches in the phase of the
core clock rhythmicity occurring in a subregion of the brain. The light-driven, rhythmic expression of genes involved in repair of UV-damaged DNA also show a very similar expression pattern
over ontogeny. While UV irradiance is very high in coral reefs causing damage (e.g. decreased growth rate, DNA damage and oxidative stress)22, UV light might be used e.g. by reef fish larvae
for navigation during foraging49. Adult clownfish are closely linked to anemones, which are located in the photic zone of coral reefs. We found higher expression of genes of the
photoreactivation DNA repair mechanism during the day, compared to night in both, larval and juvenile clown anemonefish. This expression pattern was similar in our embryonic, clownfish
derived cell line. These findings provide basic evidence that these fish might rely on photoreactivation DNA repair as a major strategy to survive DNA damage, beside other methods such as
the production of UV-absorbing substances22,23. During the night, anemones close with clownfish hiding inside. Anemones and their photosynthetic active zooxanthellae are dependent on
sunlight and open up during the day and thereby facilitate diurnal activity in adult clownfish. The mutualism is highly advantageous for all three partners, fish, anemone and zooxanthellae.
On the one hand, anemones provide a safe habitat for the fish where they can live and breed36, which might facilitate remarkable longevity of anemonefish in comparison to other
pomacentrids50. On the other hand, anemones are protected from predators by anemonefishes51 and they perform worse if they do not have a symbiotic partner52. Part of the three-way mutualism
is a nitrogen and carbon flux circulating between fish, anemone and its embedded zooxanthellae53,54. Our study provides a first basis to study a fascinating, complex and multi-layered
interaction between clocks from organisms which evolved together over 10 million years55. METHODS All animal procedures were approved by the Animal Care and Use Committees of the
Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit (LAVES, Oldenburg, Germany), Az.: 33.19-42502-04-20/3338 and performed in accordance with the relevant guidelines
and regulations and are reported in compliance with the ARRIVE guidelines. HUSBANDRY OF CLOWN ANEMONEFISH Adult clownfish were kept for breeding and observation in a circulating 800 l water
system. Each pair was separated in 40–60 l aquaria with at least one anemone _E. quadricolor_, one living rock and one terracotta pot. The salinity of water was approximately 33, the water
temperature was 28 °C. Aquaria were illuminated with a 13:11 h LD cycle by a Fluval Marine & Reef 2.0 LED light system (HAGEN Deutschland, Holm, Germany) with 33.3 W/m2. Light intensity
was measured using X1-3 optometer with XD-45-HB sensor (Gigahertz Optik, Türkenfeld, Germany) directly under the light source. Clownfish were fed with frozen food once per day. Eggs from two
breeding pairs, attached to a terracotta pot, were transferred before hatching into a 25 l tank at 28 °C where eggs were aerated. Tanks were illuminated using a white LED system (185 mW/m2)
with a LD cycle of 12:12 h. Larvae hatched within two days. Thereafter, the terracotta pot was removed. Larvae were fed with _Brachionus_ sp. until 6 dph and artemia nauplii (_Artemia_ sp.
(Sanders, South Ogden, USA)), starting from 3 dph, enriched with _Nannochloropsis salina_ and Easy DHA Selco (Inve, Salt Lake City, USA). Larvae were used for behavioural tests and gene
expression analysis. ESTABLISHMENT AND CULTIVATION OF THE EMBRYONIC _A. OCELLARIS_ CELL LINE We established an embryonic _A. ocellaris_ (EAO) cell line to analyse clock gene expression in
living cells and facilitate further analysis, so not being dependent on sacrificing valuable adults. The EAO cell line was prepared using a method previously applied with zebrafish56. Eggs
were scraped from one terracotta pot three days post fertilization and disrupted mechanically followed by 15 min incubation with Trypsin/EDTA (Gibco by Thermo Fisher Scientific, Paisley,
United Kingdom). The cell suspension was transferred to a 6-well plate (Cellstar by Greiner Bio-One, Frickenhausen, Germany) and cultivated in Leibovitz’s L-15 medium supplemented with 15%
fetal bovine serum (FBS), 100 U/ml penicillin/100 µg/ml streptomycin and 25 µg/ml gentamicin (all Gibco) at 27 °C. After one week, cell debris was removed and cells were washed with 1× PBS
(Gibco) and were further incubated in culture medium. For passaging, a confluent cell monolayer was washed with 1× PBS, detached from the culture substrate by incubation with Trypsin/EDTA at
37 °C and then reseeded in culture medium in 25 cm2 flasks (Cellstar by Greiner Bio-One). BEHAVIOURAL EXPERIMENTS To analyse potential differences in activity patterns during development,
we studied the diel activity of _A. ocellaris_ larvae (7–23 dph), juveniles (ca. 52–106 dph), and breeding pairs (several years old). We observed activity of 45 larvae and 36 juveniles and 4
adults. Animals which were observed for less than 24 h were not further analysed (three larvae). For recording behaviour, all clownfish tanks were illuminated with infrared light at 940 nm
wavelength, which is not seen by fish57 and activity was recorded using webcams (Logitech c920) adapted by removing the infrared cut-off filter. ACTIVITY RECORDINGS OF CLOWNFISH LARVAE AND
JUVENILES Clownfish larvae were observed individually in 6-well plates with 15 ml water volume and within 30 ml tanks with squared base area, where larvae behaved similarly. Juvenile
clownfish were observed in 150 ml tanks with a square base area. Water temperature was maintained at 28 °C. Visual light was supplied by CCFL backlight from a striped TFT monitor (787 mW/m2)
with a 12:12 h LD cycle. Every day at a different time, one third of the water was exchanged and the animals were fed with live artemia. The activity of larvae and juveniles was recorded
with Multiviewer (resolution: 640:480 pixel, one frame per second) (Computer System Department, University of Murcia) and compressed using VirtualDub 1.10.4 and xvid codec. The movement of
larvae was tracked with FishTracker (Computer System Department, University of Murcia). To verify reliable tracking, data was also analysed with EthoVision XT (Noldus Information
Technology). ACTIVITY RECORDINGS OF ADULT CLOWNFISH Activity of adult clownfish was recorded with two cameras using Yawcam 0.6.2 (one picture per second with a resolution of 1920:1080
pixel), one camera from above and one at the front. The position of fish was tracked manually per second every 30 min for 1 min with ImageJ58 to calculate their three-dimensional movement.
_A. ocellaris_ undertake intensive egg care, therefore breeding pairs were observed with as well as without a clutch off eggs in their keeping aquarium to avoid handling stress. During video
recording, the room was entered only once for daily fish care. Fish were not fed during recording. DATA ANALYSIS Data analysis was performed with R 3.6.159. The distance larval and juvenile
clownfish moved per second was calculated from x- and y-coordinates, obtained from FishTracker using Pythagorean theorem. The distance covered was summed up individually for each fish per 6
min and translated from pixel to centimetres. The mean distance covered was calculated for animals grouped according to age for graphical representation with ggplot260. For adult clownfish
the three-dimensional movement was calculated based on x-, y- and z-coordinates within Excel (Microsoft) and the movement per second was summed up per analysed one minute. Data was
statistically analysed with RAIN (Rhythmicity Analysis Incorporating Nonparametric methods)61 to identify significant rhythmicity. Therefore, distance moved was summed up per hour. Animals
were counted as diurnal for ZT between 0 and 11 and as nocturnal for ZT between 12 and 23 h. The diurnality was calculated as an index, based on26. This is an index from +1 (all animals are
diurnal) to -1 (all animals are nocturnal) and was calculated with this formula: \({\text{D}} = \frac{{N_{diurnal} {-} N_{nocturnal} }}{{N_{diurnal} + N_{nocturnal} }}\). ANALYSIS OF CORE
CLOCK AND PHOTOREACTIVATION DNA REPAIR GENES To analyse the core clock mechanism of _A. ocellaris_, gene expression of six genes was analysed by qRTPCR: _bmal1_, _clocka_, _cry1b_, _per1b_,
_per2_, _per3_. Additionally, we analysed the expression of three genes of the photoreactivation DNA repair mechanism: _cpd photolyase_, _cry dash_, _6-4-photolyase_ (also known as _cry5_).
Primers were designed using Primer362,63,64 for an annealing temperature of 60 °C and checked for specificity with Primer-BLAST65. Sequences for all genes were obtained from a genome hybrid
assembly of an _A. ocellaris_ genome using Oxford Nanopore and Illumina27. See table 1 for primer sequences and accession numbers. GENE EXPRESSION ANALYSIS USING QRTPCR Larval (10 dph) and
juvenile clownfish (105 dph) were sampled at 3, 9, 15 and 21 h after light was switched on from two separate tanks. Before sampling, animals were left undisturbed for 12 h. Larval and
juvenile clownfish were euthanized by an overdose of MS-222 (Pharmaq, Hampshire, UK) in 4 °C cold salt water according to the ethical protocol standards. Samples were stored in
RNA-stabilising buffer66 at −80 °C until RNA extraction. To analyse gene expression of the EAO cell line, cells were seeded in 25 cm2 flasks and incubated for one week under a 12:12 h LD
cycle (452 mW/m2). Confluent cell monolayers were harvested, by scraping in culture medium using a cell scraper. The cell suspension was then centrifuged at 1500 g for 5 min at 4 °C. The
pellet was washed twice with 1× PBS and stored at −80 °C until RNA extraction. Total RNA was extracted using Monarch Total RNA Miniprep Kit (New England Biolabs, Frankfurt am Main, Germany)
following the manufacturer’s instructions for cultured mammalian cells and tissue including DNase treatment. Deviating from that protocol, whole animals were homogenized in 1× DNA/RNA
Protection Reagent using a bead beater (MagNa Lyser, Roche Diagnostics, Mannheim, Germany) with glass beads (diameter 1.25–1.65 mm, Carl Roth, Karlsruhe, Germany) at 5000 rpm for up to 6
min. The amount of RNA and its purity was determined using an optical spectrometer (BioSpectrometer basic, Eppendorf, Hamburg, Germany) at 230, 260 and 280 nm. Samples were treated using
TURBO DNA-free Kit (Invitrogen by Thermo Fisher Scientific Baltics, Vilnius, Lithuania) following the manufacturer’s instructions. Extracted RNA was reverse transcribed to cDNA using iScript
cDNA Synthesis Kit (Bio-Rad Laboratories, Feldkirchen, Germany). Quantitative PCR was conducted with GoTaq qPCR Master Mix (Promega, Madison, USA) in a 10 µl reaction volume on a
LightCycler 480 Instrument II (Roche). Three technical replicates per gene and timepoint were performed. Efficiency was calculated using a dilution series ensuring efficiencies between 1.86
and 2.09. Data were analysed with LightCycler 480 Software, Version 1.5 (Roche). Cycle of quantification (C_q_) was calculated using the 2nd derivative maximum method. Relative gene
expression was calculated using expression of _rpl13a_ and _ef1a_ as housekeeping genes. Each experimental group, consisting of one animal per timepoint, which were handled together during
the whole experimental procedure, was normalized separately using Excel (Microsoft). The mean was calculated with the normalized data for larvae, juveniles and cells. Rhythmicity was
analysed using RAIN61. IN VIVO_ LUCIFERASE ASSAY_ EAO cells were transiently transfected using FuGene HD (Promega) transfection reagent according to the manufacturer’s instructions. One day
before transfection, 2 × 104 cells per well were seeded in a white 96-well plate (Nunc by Thermo Fisher Scientific, Roskilde, Denmark) and incubated at 27 °C for 24 h. Each well was
transfected independently with a 4:1 ratio of reagent:DNA with 100 ng plasmid DNA. The cells were transfected with the reporter construct zf_Per1b_-Luc29 or D-box_Cry1a_-Luc based on31. As a
control, zebrafish PAC-2 cells30 were transiently transfected in the same 96-well plate. Non-transfected cells were used as negative control for each cell line. After incubation for 24 h,
the transfection solution was replaced by culture medium supplemented with 2 mM D-Luciferin (Biosynth, Bratislava, Slovakia). Cells were exposed to four 12:12 h LD cycles followed by 24 h
DD. The bioluminescence was measured with a Topcount NXT counter (PerkinElmer, Shelton, USA) as previously described29. We imported the data into Excel (Microsoft) by using the "Import
and Analysis" macro (S. Kay, Scripps Research Institute). Mean and standard deviation of eight independently transfected wells were calculated with R 3.6.159 and shown using ggplot260.
DATA AVAILABILITY All data generated and analysed during the current study, including the used R-script, are included in this published article and its Supplementary Information files. Raw
video files are available on request. REFERENCES * Thresher, R. E., Colin, P. L. & Bell, L. J. Planktonic duration, distribution and population structure of western and Central Pacific
Damselfishes (Pomacentridae). _Copeia_ 420–434, 1989. https://doi.org/10.2307/1445439 (1989). Article Google Scholar * Leis, J. M. Behaviour as input for modelling dispersal of fish
larvae: Behaviour, biogeography, hydrodynamics, ontogeny, physiology and phylogeny meet hydrography. _Mar. Ecol. Prog. Ser._ 347, 185–194. https://doi.org/10.3354/meps06977 (2007). Article
ADS Google Scholar * Fisher, R., Leis, J. M., Clark, D. L. & Wilson, S. K. Critical swimming speeds of late-stage coral reef fish larvae: Variation within species, among species and
between locations. _Mar. Biol._ 147, 1201–1212. https://doi.org/10.1007/s00227-005-0001-x (2005). Article Google Scholar * Stobutzki, I. & Bellwood, D. Sustained swimming abilities of
the late pelagic stages of coral reef fishes. _Mar. Ecol. Prog. Ser._ 149, 35–41. https://doi.org/10.3354/meps149035 (1997). Article ADS Google Scholar * Gerlach, G., Atema, J.,
Kingsford, M. J., Black, K. P. & Miller-Sims, V. Smelling home can prevent dispersal of reef fish larvae. _Proc. Natl. Acad. Sci._ 104, 858–863. https://doi.org/10.1073/pnas.0606777104
(2007). Article CAS PubMed ADS Google Scholar * Almany, G. R., Berumen, M. L., Thorrold, S. R., Planes, S. & Jones, G. P. Local Replenishment of Coral Reef fish populations in a
Marine Reserve. _Science_ 316, 742–744. https://doi.org/10.1126/science.1140597 (2007). Article CAS PubMed ADS Google Scholar * Jones, G. P., Planes, S. & Thorrold, S. R. Coral Reef
Fish Larvae Settle Close to Home. _Curr. Biol._ 15, 1314–1318. https://doi.org/10.1016/j.cub.2005.06.061 (2005). Article CAS PubMed Google Scholar * Kingsford, M. J. _et al._ Sensory
environments, larval abilities and local self-recruitment. _Bull. Mar. Sci._ 70, 309–340 (2002). Google Scholar * Mouritsen, H., Atema, J., Kingsford, M. J. & Gerlach, G. Sun compass
orientation helps coral reef fish larvae return to their natal reef. _PLoS ONE. _https://doi.org/10.1371/journal.pone.0066039 (2013). * Dufour, V. & Galzin, R. Colonization patterns of
reef fish larvae to the lagoon at Moorea Island, French Polynesia. _Mar. Ecol. Prog. Ser._ 102, 143–152. https://doi.org/10.3354/meps102143 (1993). Article ADS Google Scholar * Holbrook,
S. & Schmitt, R. Settlement patterns and process in a coral reef damselfish: In situ nocturnal observations using infrared video. In _Proceedings of the 8th International Coral Reef
Symposium_, Vol. 2, 1143–1148 (1997). * Leis, J. M. & Carson-Ewart, B. M. Complex behaviour by coral-reef fish larvae in open-water and near-reef pelagic environments. _Environ. Biol.
Fishes_ 53, 259–266. https://doi.org/10.1023/A:1007424719764 (1998). Article Google Scholar * Litsios, G. _et al._ Mutualism with sea anemones triggered the adaptive radiation of
clownfishes. _BMC Evol. Biol._ 12, 212. https://doi.org/10.1186/1471-2148-12-212 (2012). Article PubMed PubMed Central Google Scholar * Bridge, T., Scott, A. & Steinberg, D.
Abundance and diversity of anemonefishes and their host sea anemones at two mesophotic sites on the Great Barrier Reef, Australia. _Coral Reefs_ 31, 1057–1062.
https://doi.org/10.1007/s00338-012-0916-x (2012). Article ADS Google Scholar * Mariscal, R. N. Behavior of symbiotic fishes and sea anemones. In Winn, H. E. & Olla, B. L. (eds.)
_Behavior of Marine Animals_, 327–360 (Springer US, 1972). https://doi.org/10.1007/978-1-4684-0910-9_4. * Tauber, E., Last, K. S., Olive, P. J. & Kyriacou, C. P. Clock gene evolution and
functional divergence. _J. Biol. Rhythm._ 19, 445–458. https://doi.org/10.1177/0748730404268775 (2004). Article CAS Google Scholar * Emran, F., Rihel, J., Adolph, A. R. & Dowling, J.
E. Zebrafish larvae lose vision at night. _Proc. Natl. Acad. Sci._ 107, 6034–6039. https://doi.org/10.1073/pnas.0914718107 (2010). Article PubMed ADS Google Scholar * Cahill, G. M.,
Hurd, M. W. & Batchelor, M. M. Circadian rhythmicity in the locomotor activity of larval zebrafish. _NeuroReport_ 9, 3445–3449. https://doi.org/10.1097/00001756-199810260-00020 (1998).
Article CAS PubMed Google Scholar * Ceinos, R. M. _et al._ Mutations in blind cavefish target the light-regulated circadian clock gene, period 2. _Sci. Rep._ 8, 8754.
https://doi.org/10.1038/s41598-018-27080-2 (2018). Article CAS PubMed PubMed Central ADS Google Scholar * Frøland Steindal, I. & Whitmore, D. Circadian clocks in fish—What have we
learned so far?. _Biology_ 8, 17. https://doi.org/10.3390/biology8010017 (2019). Article CAS PubMed Central Google Scholar * Vatine, G., Vallone, D., Gothilf, Y. & Foulkes, N. S.
It’s time to swim! Zebrafish and the circadian clock. _FEBS Lett._ 585, 1485–1494. https://doi.org/10.1016/j.febslet.2011.04.007 (2011). Article CAS PubMed Google Scholar * Banaszak, A.
T. & Lesser, M. P. Effects of solar ultraviolet radiation on coral reef organisms. _Photochem. Photobiol. Sci._ 8, 1276. https://doi.org/10.1039/b902763g (2009). Article CAS PubMed
Google Scholar * Häder, D.-P., Kumar, H. D., Smith, R. C. & Worrest, R. C. Effects of solar UV radiation on aquatic ecosystems and interactions with climate change. _Photochem.
Photobiol. Sci._ 6, 267–285. https://doi.org/10.1039/B700020K (2007). Article PubMed Google Scholar * Eckes, M., Siebeck, U., Dove, S. & Grutter, A. Ultraviolet sunscreens in reef
fish mucus. _Mar. Ecol. Prog. Ser._ 353, 203–211. https://doi.org/10.3354/meps07210 (2008). Article CAS ADS Google Scholar * Kienzler, A., Bony, S. & Devaux, A. DNA repair activity
in fish and interest in ecotoxicology: A review. _Aquat. Toxicol._ 134–135, 47–56. https://doi.org/10.1016/j.aquatox.2013.03.005 (2013). Article CAS PubMed Google Scholar * Hoogenboom,
I., Daan, S., Dallinga, J. H. & Schoenmakers, M. Seasonal change in the daily timing of behaviour of the common vole, _Microtus arvalis_. _Oecologia_ 61, 18–31.
https://doi.org/10.1007/BF00379084 (1984). * Tan, M. H. _et al._ Finding Nemo: Hybrid assembly with Oxford Nanopore and Illumina reads greatly improves the clownfish (_Amphiprion ocellaris_)
genome assembly. _GigaScience._ https://doi.org/10.1093/gigascience/gix137 (2018). Article PubMed PubMed Central Google Scholar * Cavallari, N. _et al._ A blind circadian clock in
cavefish reveals that opsins mediate peripheral clock photoreception. _PLoS Biol._ https://doi.org/10.1371/journal.pbio.1001142 (2011). Article PubMed PubMed Central Google Scholar *
Vallone, D., Gondi, S. B., Whitmore, D. & Foulkes, N. S. E-box function in a _period_ gene repressed by light. _Proc. Natl. Acad. Sci._ 101, 4106–4111.
https://doi.org/10.1073/pnas.0305436101 (2004). Article CAS PubMed ADS Google Scholar * Vatine, G. _et al._ Light directs Zebrafish _period2_ expression via conserved D and E boxes.
_PLOS Biol._ https://doi.org/10.1371/journal.pbio.1000223 (2009). Article PubMed PubMed Central Google Scholar * Mracek, P. _et al._ Regulation of _per_ and _cry_ Genes Reveals a Central
Role for the D-Box Enhancer in Light-Dependent Gene Expression. _PLOS ONE_ https://doi.org/10.1371/journal.pone.0051278 (2012). Article PubMed PubMed Central Google Scholar * Zhao, H.
_et al._ Modulation of DNA repair systems in blind cavefish during evolution in constant darkness. _Curr. Biol._ 28, 3229-3243.e4. https://doi.org/10.1016/j.cub.2018.08.039 (2018). Article
CAS PubMed Google Scholar * Tolimieri, N., Haine, O., Jeffs, A., McCauley, R. & Montgomery, J. Directional orientation of pomacentrid larvae to ambient reef sound. _Coral Reefs_ 23,
184–191. https://doi.org/10.1007/s00338-004-0383-0 (2004). Article Google Scholar * Fisher, R. & Bellwood, D. Undisturbed swimming behaviour and nocturnal activity of coral reef fish
larvae. _Mar. Ecol. Prog. Ser._ 263, 177–188. https://doi.org/10.3354/meps263177 (2003). Article ADS Google Scholar * Elliott, J. K. & Mariscal, R. N. Ontogenetic and interspecific
variation in the protection of anemonefishes from sea anemones. _J. Exp. Mar. Biol. Ecol._ 208, 57–72. https://doi.org/10.1016/S0022-0981(96)02629-9 (1997). Article Google Scholar *
Fautin, D. G. The anemonefish symbiosis: What is known and what is not. _Symbiosis_ 10, 23–46 (1991). Google Scholar * Di Rosa, V., Frigato, E., López-Olmeda, J. F., Sánchez-Vázquez, F. J.
& Bertolucci, C. The light wavelength affects the ontogeny of clock gene expression and activity rhythms in zebrafish larvae. _PLOS ONE_ https://doi.org/10.1371/journal.pone.0132235
(2015). Article PubMed PubMed Central Google Scholar * Idda, M. L. _et al._ Chapter 3—Circadian clocks: Lessons from fish. In Kalsbeek, A., Merrow, M., Roenneberg, T. & Foster, R. G.
(eds.) _The Neurobiology of Circadian Timing_, vol. 199 of _Progress in Brain Research_, 41–57, DOI: https://doi.org/10.1016/B978-0-444-59427-3.00003-4 (Elsevier, 2012). * Patiño, M. A. L.,
Rodríguez-Illamola, A., Conde-Sieira, M., Soengas, J. L. & Míguez, J. M. Daily rhythmic expression patterns of _Clock1a_, _Bmal1_, and _Per1_ genes in retina and hypothalamus of the
rainbow trout, Oncorhynchus Mykiss. _Chronobiol. Int._ 28, 381–389. https://doi.org/10.3109/07420528.2011.566398 (2011). Article CAS PubMed Google Scholar * Vera, L. M. _et al._ Light
and feeding entrainment of the molecular circadian clock in a marine teleost (_Sparus aurata_). _Chronobiol. Int._ 30, 649–661. https://doi.org/10.3109/07420528.2013.775143 (2013). Article
CAS PubMed Google Scholar * Martín-Robles, A. J., Whitmore, D., Sánchez-Vázquez, F. J., Pendón, C. & Muñoz-Cueto, J. A. Cloning, tissue expression pattern and daily rhythms of
_Period1_, _Period2_, and _Clock_ transcripts in the flatfish Senegalese sole,Solea senegalensis. _J. Comp. Physiol. B_ 182, 673–685. https://doi.org/10.1007/s00360-012-0653-z (2012).
Article CAS PubMed Google Scholar * Park, J.-G., Park, Y.-J., Sugama, N., Kim, S.-J. & Takemura, A. Molecular cloning and daily variations of the _Period_ gene in a reef fish
_Siganus guttatus_. _J. Comp. Physiol. A_ 193, 403–411. https://doi.org/10.1007/s00359-006-0194-6 (2007). Article CAS Google Scholar * Martín-Robles, A. J., Isorna, E., Whitmore, D.,
Muñoz-Cueto, J. A. & Pendón, C. The clock gene _Period3_ in the nocturnal flatfish _Solea senegalensis_: Molecular cloning, tissue expression and daily rhythms in central areas. _Comp.
Biochem. Physiol. A Mol. Integr. Physiol._ 159, 7–15. https://doi.org/10.1016/j.cbpa.2011.01.015 (2011). Article CAS PubMed Google Scholar * Whitmore, D., Foulkes, N. S., Strähle, U.
& Sassone-Corsi, P. Zebrafish _Clock_ rhythmic expression reveals independent peripheral circadian oscillators. _Nat. Neurosci._ 1, 701–707. https://doi.org/10.1038/3703 (1998). Article
CAS PubMed Google Scholar * Yamazaki, S. _et al._ Resetting central and peripheral circadian oscillators in transgenic rats. _Science_ 288, 682–685.
https://doi.org/10.1126/science.288.5466.682 (2000). Article CAS PubMed ADS Google Scholar * Yagita, K. _et al._ Development of the circadian oscillator during differentiation of mouse
embryonic stem cells in vitro. _Proc. Natl. Acad. Sci._ 107, 3846–3851. https://doi.org/10.1073/pnas.0913256107 (2010). Article PubMed ADS Google Scholar * Challet, E. Minireview:
Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. _Endocrinology_ 148, 5648–5655. https://doi.org/10.1210/en.2007-0804 (2007). Article CAS PubMed Google
Scholar * del Pozo, A., Montoya, A., Vera, L. M. & Sánchez-Vázquez, F. J. Daily rhythms of clock gene expression, glycaemia and digestive physiology in diurnal/nocturnal European
seabass. _Physiol. Behav._ 106, 446–450. https://doi.org/10.1016/j.physbeh.2012.03.006 (2012). Article CAS PubMed Google Scholar * Job, S. & Shand, J. Spectral sensitivity of larval
and juvenile coral reef fishes: Implications for feeding in a variable light environment. _Mar. Ecol. Prog. Ser._ 214, 267–277. https://doi.org/10.3354/meps214267 (2001). Article ADS
Google Scholar * Buston, P. M. & García, M. B. An extraordinary life span estimate for the clown anemonefish _Amphiprion percula_. _J. Fish Biol._ 70, 1710–1719.
https://doi.org/10.1111/j.1095-8649.2007.01445.x (2007). Article Google Scholar * Godwin, J. & Fautin, D. G. Defense of host actinians by anemonefishes. _Copeia_ 902–908, 1992.
https://doi.org/10.2307/1446171 (1992). Article Google Scholar * Roopin, M. & Chadwick, N. E. Benefits to host sea anemones from ammonia contributions of resident anemonefish. _J. Exp.
Mar. Biol. Ecol._ 370, 27–34. https://doi.org/10.1016/j.jembe.2008.11.006 (2009). Article CAS Google Scholar * Cleveland, A., Verde, E. A. & Lee, R. W. Nutritional exchange in a
tropical tripartite symbiosis: Direct evidence for the transfer of nutrients from anemonefish to host anemone and zooxanthellae. _Mar. Biol._ 158, 589–602.
https://doi.org/10.1007/s00227-010-1583-5 (2011). Article Google Scholar * Verde, E. A., Cleveland, A. & Lee, R. W. Nutritional exchange in a tropical tripartite symbiosis II: Direct
evidence for the transfer of nutrients from host anemone and zooxanthellae to anemonefish. _Mar. Biol._ 162, 2409–2429. https://doi.org/10.1007/s00227-015-2768-8 (2015). Article CAS Google
Scholar * da Silva, K. B. & Nedosyko, A. Sea Anemones and Anemonefish: A Match Made in Heaven. In Goffredo, S. & Dubinsky, Z. (eds.) _The Cnidaria, Past, Present and Future_,
425–438 (Springer International Publishing, 2016). https://doi.org/10.1007/978-3-319-31305-4_27. * Vallone, D., Santoriello, C., Gondi, S. B. & Foulkes, N. S. Basic protocols for
zebrafish cell lines. In Rosato, E. (ed.) _Circadian Rhythms: Methods and Protocols_, 429–441. https://doi.org/10.1007/978-1-59745-257-1_35 (Humana Press, 2007). * Dekens, M. P. S., Foulkes,
N. S. & Tessmar-Raible, K. Instrument design and protocol for the study of light controlled processes in aquatic organisms, and its application to examine the effect of infrared light
on zebrafish. _PLoS ONE_ 12. https://doi.org/10.1371/journal.pone.0172038 (2017). * Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis.
_Nat. Methods_ 9, 671–675. https://doi.org/10.1038/nmeth.2089 (2012). Article CAS PubMed PubMed Central Google Scholar * R Core Team. _R: A Language and Environment for Statistical
Computing_. R Foundation for Statistical Computing, Vienna, Austria (2020). * Wickham, H. _Ggplot2: Elegant Graphics for Data Analysis_. Use R! (Springer, 2009). * Thaben, P. F. &
Westermark, P. O. Detecting rhythms in time series with RAIN. _J. Biol. Rhythm._ 29, 391–400. https://doi.org/10.1177/0748730414553029 (2014). Article Google Scholar * Kõressaar, T. _et
al._ Primer3_masker: Integrating masking of template sequence with primer design software. _Bioinformatics_ 34, 1937–1938. https://doi.org/10.1093/bioinformatics/bty036 (2018). Article CAS
PubMed Google Scholar * Untergasser, A. _et al._ Primer3—new capabilities and interfaces. _Nucleic Acids Res._ 40, e115. https://doi.org/10.1093/nar/gks596 (2012). * Kõressaar, T. &
Remm, M. Enhancements and modifications of primer design program Primer3. _Bioinformatics_ 23, 1289–1291. https://doi.org/10.1093/bioinformatics/btm091 (2007). Article CAS PubMed Google
Scholar * Ye, J. _et al._ Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. _BMC Bioinforma_ 13, 134. https://doi.org/10.1186/1471-2105-13-134 (2012).
Article CAS Google Scholar * Wit, P. D. _et al._ The simple fool’s guide to population genomics via RNA-Seq: An introduction to high-throughput sequencing data analysis. _Mol. Ecol.
Resour._ 12, 1058–1067. https://doi.org/10.1111/1755-0998.12003 (2012). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank the workshop of the
University of Oldenburg for the construction of our experimental setups and people involved in fish care. We especially thank Susanne Wallenstein for excellent technical assistance and
Daniela Vallone for advice in cell culture. FUNDING This work was supported by the ministry of science and culture (MWK) of Lower Saxony, Germany (Research Training Group
"Interdisciplinary approach to functional biodiversity research" (IBR)) to G.S. and the Research Training Group 1885 "Molecular Basis of Sensory Biology" (MSB) to K.B.
and the SFB1372 to G.G. by the German Science Foundation (DFG). C.B. is supported by research grants from the University of Ferrara (FAR2019-2020 and FIR2020). N.S.F. and N.G. are supported
by the Helmholtz funding programme BIFTM. Open Access funding enabled and organized by Projekt DEAL. AUTHOR INFORMATION Author notes * These authors contributed equally: Gregor Schalm and
Kristina Bruns. AUTHORS AND AFFILIATIONS * Institute of Biology and Environmental Sciences, Carl von Ossietzky University Oldenburg, Ammerländer Heerstr. 114-118, 26129, Oldenburg, Germany
Gregor Schalm, Kristina Bruns, Nina Drachenberg & Gabriele Gerlach * Institute of Biological and Chemical Systems (IBCS), Karlsruhe Institute of Technology (KIT),
Hermann-von-Helmholtz-Platz 1, 76344, Eggenstein-Leopoldshafen, Germany Nathalie Geyer & Nicholas S. Foulkes * Department of Life Sciences and Biotechnology, University of Ferrara, Via
Luigi Borsari 46, 44121, Ferrara, Italy Cristiano Bertolucci * Biology and Evolution of Marine Organisms, Stazione Zoologica Anton Dohrn Napoli, Villa Comunale, 80121, Naples, Italy
Cristiano Bertolucci * Helmholtz Institute for Functional Marine Biodiversity (HIFMB), Ammerländer Heerstr. 231, 26129, Oldenburg, Germany Gabriele Gerlach * Centre of Excellence for Coral
Reef Studies and School of Marine and Tropical Biology, James Cook University, Townsville, QLD, 4811, Australia Gabriele Gerlach Authors * Gregor Schalm View author publications You can also
search for this author inPubMed Google Scholar * Kristina Bruns View author publications You can also search for this author inPubMed Google Scholar * Nina Drachenberg View author
publications You can also search for this author inPubMed Google Scholar * Nathalie Geyer View author publications You can also search for this author inPubMed Google Scholar * Nicholas S.
Foulkes View author publications You can also search for this author inPubMed Google Scholar * Cristiano Bertolucci View author publications You can also search for this author inPubMed
Google Scholar * Gabriele Gerlach View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS G.S., K.B. and N.G. carried out the molecular lab work,
G.S., K.B. and N.D. carried out behavioural experiments. G.S. and K.B. drafted the manuscript. G.S. and C.B. carried out the statistical analyses. G.S., K.B., N.S.F., G.G., C.B. conceived
and designed the study. N.S.F., G.G., C.B. critically conceived the study and helped draft the manuscript. All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Gregor
Schalm. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under
a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article
are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons
licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of
this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Schalm, G., Bruns, K., Drachenberg, N. _et al._ Finding Nemo’s
clock reveals switch from nocturnal to diurnal activity. _Sci Rep_ 11, 6801 (2021). https://doi.org/10.1038/s41598-021-86244-9 Download citation * Received: 28 September 2020 * Accepted: 12
March 2021 * Published: 24 March 2021 * DOI: https://doi.org/10.1038/s41598-021-86244-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative








.jpg?w=1200&ar=40%3A21&auto=format%2Ccompress&ogImage=true&mode=crop&enlarge=true&overlay=false&overlay_position=bottom&overlay_width=100)

