- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Download PDF Article Open access Published: 18 March 2021 Salivary cortisol as a non-invasive approach to assess stress in dystocic dairy calves Levente Kovács1,2, Fruzsina Luca Kézér2,
Szilárd Bodó1, Ferenc Ruff3, Rupert Palme4 & …Ottó Szenci5 Show authors Scientific Reports volume 11, Article number: 6200 (2021) Cite this article
5261 Accesses
19 Citations
Metrics details
Subjects Environmental sciencesPhysiologyZoology AbstractThe intensity and the magnitude of saliva cortisol responses were investigated during the first 48 h following birth in newborn dairy calves which underwent normal (eutocic, EUT, n = 88) and
difficult (dystocic, DYS, n = 70) calvings. The effects of parity and body condition of the dam, the duration of parturition, the time spent licking the calf, the sex and birth weight of
the calf were also analyzed. Neonatal salivary cortisol concentrations were influenced neither by factors related to the dam (parity, body condition) nor the calf (sex, birth weight). The
duration of parturition and the time spent licking the calf also had no effect on salivary cortisol levels. Salivary cortisol concentrations increased rapidly after delivery in both groups
to reach their peak levels at 45 and 60 min after delivery in EUT and DYS calves, respectively supporting that the birth process means considerable stress for calves and the immediate
postnatal period also appears to be stressful for newborn calves. DYS calves exhibited higher salivary cortisol concentrations compared to EUT ones for 0 (P = 0.022), 15 (P = 0.016), 30 (P =
0.007), 45 (P = 0.003), 60 (P = 0.001) and 120 min (P = 0.001), and for 24 h (P = 0.040), respectively. Peak levels of salivary cortisol and the cortisol release into saliva calculated as
AUC were higher in DYS than in EUT calves for the 48-h of the sampling period (P = 0.009 and P = 0.003, respectively). The greater magnitude of saliva cortisol levels in DYS calves compared
to EUT ones suggest that difficult parturition means severe stress for bovine neonates and salivary cortisol could be an opportunity for non-invasive assessment of stress during the early
neonatal period in cattle.
Similar content being viewed by others Effects of routine procedures on salivary cortisol in mechanically ventilated neonates Article Open access 21 March 2023Salivary cortisol is an unreliable correlate of serum cortisol in adult pet dogs and assistance dog puppies Article Open access 08 May 2025 Salivary analysis to unveil the paradigma of
stress of domestic horses reared in the wild Article Open access 17 May 2024 Introduction
Bovine parturition is initiated by rising cortisol levels in the fetus that provoke a cascade of endocrine activity in the dam1. This increase in fetal cortisol is a result of increased
adrenocorticotropic hormone production by the maturing fetal pituitary caused by fetal stressors such as hypoxia and hypercapnia. However, the process of parturition may also be a stressful
event for the fetus, especially during the stage of expulsion, if difficulties at calving occur2.
Acute responses to stressful stimuli include activation of the hypothalamic–pituitary–adrenal (HPA) axis and the autonomic nervous system. Pain biomarkers related to HPA axis are often
measured in biological samples (e.g. blood). Plasma cortisol concentrations have been widely used to evaluate the HPA axis activity in painful procedures in calves3 and in mature cattle4,5.
However, the process of taking blood samples is accompanied by additional stress, which can affect the test results6. According to human studies, psychobiological mechanisms, which trigger
the HPA axis, can be assessed by salivary cortisol concentrations7,8 that reflect unbound (free) cortisol9,10. Saliva samples can be easily taken at fixed time intervals after an imposed
stress11 and it is a minimally invasive6 and appropriate method to assess HPA axis reactivity in cattle. Furthermore, salivary cortisol correlates well with plasma cortisol with 012 or with
a 10 min time lag13,14.
Although dystocia is a growing problem on dairy farms15, it is not known how it influences the stress level of calves during the first 48 h of life which is the most critical period in terms
of survival16. The present paper attempts to look at the effects of dystocia and some calving-related factors (i.e. parity and body condition of the dam, the duration of parturition, the
time spent with licking the calf, and the sex and birth weight of the calf) on salivary cortisol as noninvasive measure of HPA activity in newborn calves.
MethodsExperimental farm andanimals
All methods and the applied procedures on the animals were performed in accordance with the relevant guidelines and regulations of the Pest County Government Office, Department of Animal
Health (Permit Number: PE/EA/1973-6/2016) that approved the study. A total of 168 calvings were enrolled on a large-scale dairy farm in Hungary consisting of 1,200 lactating
Holstein–Friesian cows. The farm was visited for an 8-month period in the spring (between February and May) and autumn (September and December) of 2016 to exclude the possible effects of
heat stress on the trial results. Cows calved in the prepartum group pen or, if assistance was required, in a separate maternity pen. Calves were removed from the dams 2 h after birth and
received colostrum by nipple bottle and then fed four times a day with 1.65 L of fresh-cow colostrum per feeding during the first 48 h of life. Newborns were housed individually until 60 d
of age in 1.65 × 1.20 m plastic calf hutches with a 1.60 m2 exercise pen, both bedded with straw.
Observation of calvingsCalvings occurring in the group pen were observed with two day/night outdoor network bullet cameras (Vivotek IP8331, VIVOTEK Inc., Taipei, Taiwan), while individual calvings were observed
with two portable video cameras (Legria HF M36, CANON Inc., Japan, Tokyo).
Dystocia (DYS, n = 70) was defined as calving difficulty resulting from prolonged spontaneous calving (> 2 h from the appearance of hooves to delivery) or prolonged or severe assisted
extraction by one or more people with considerable force with a calving rope or with a calving jack17. Normal calving (eutocia; EUT, n = 98) was regarded as a combination of ‘no assistance’
and ‘slight assistance’ (where assistance was brief, and traction was slight) by one person17.
The condition of the dam was scored using the 5-point BCS system18 following calving. Sex and birth weight of the calves were also recorded immediately after delivery. Since during stage 1
of labor significant stress was found in dairy cows19, and thus, possibly for newborn calves, the duration of parturition was considered as the time lag between the onset of stage 1 (the
onset of calving restlessness) and the completion of stage 2 of labor (delivery). The onset of calving restlessness was determined based on accepted behavioral predictors such as lying down
frequency, tail raising and walking20 and was observed by two trained experimenters through the above-mentioned camera system. The time spent licking the calf’s head or body was recorded
during the first 2 h following calving according to the recommendation of Jensen21.
Salivary cortisolUsing a synthetic swab (Salivette Cortisol, Sarstedt, Nümbrecht-Rommelsdorf, Germany), saliva samples were taken 0, 15, 30, 45, 60, 120 min, 24 and 48 h after delivery. Without retain of the
animals, the swabs were placed loosely onto the tongue of the calf until it was well soaked with saliva. This procedure required up to 10 s, and the animals tolerated saliva samplings well.
The swabs were then inserted into Salivette polypropylene tubes, which were placed on ice immediately after sampling and stored at 4 °C until centrifugation (within 10 min after sampling)
at 1000 g for 10 min. At least 1.5 ml saliva per sample was obtained and frozen at − 20 °C until analysis. After a further dilution step (1:10) with assay buffer, salivary cortisol
concentrations were determined in an aliquot (10 µl) with a competitive cortisol enzyme immunoassay (EIA). For the details of the EIA, including cross-reactions and its application in calves
refer to Palme and Möstl22 and Wagner et al.23. Interassay coefficients of variation of high and low concentration pool samples from saliva of the calves of this study were 9.2% and 12.8%,
respectively. The detection limit of the assay was 0.02 ng/ml.
Statistical analysisStatistical analyses were performed in the R–3.3.1 statistical environment and language24. All results are expressed as mean plus SEM values.
Multivariable linear regression models were fit to the data25 for each sampling time point to test the effects of independent variables on salivary cortisol concentrations. Independent
variables were parity and BCS of the dam, sex and birth weight of the calf, the duration of parturition, calving ease (dystocia or eutocia), and the time spent licking the calf. Salivary
cortisol concentrations were inserted into the models as response (dependent) variables. Log-transformation of saliva cortisol concentrations was applied to satisfy the normality and
variance homogeneity assumptions of the models.
Based on the results of the linear models (only calving ease had a significant effect on cortisol levels; see Results section), for reducing the number of statistical comparisons between
groups during the 48-h postnatal period, salivary cortisol concentrations of EUT and DYS calves were calculated as area under the curve (AUC) and cortisol responses were compared. The AUC
represents both the magnitude and the changes over time of the response26. Response parameters included peak values of salivary cortisol concentrations, baseline (48 h sample), and AUCs that
were determined for the first 48 h of life following delivery utilizing a trapezoid method described by Lay et al.27 as follows:
$$ {\text{AUC}}_{{{\text{RESP}}}} = \, \Sigma \left[{\left( {{\text{P}}_{{\text{n}}} + {\text{P}}_{{{\text{n}} + {1}}} } \right)/{2 } \times {\text{ m }}{-}{\text{ BASELINE}}} \right], $$
where ‘P’ is salivary cortisol concentration at a given time point, ‘m’ is the time in minutes between the two P values and ‘baseline’ is the mean value of cortisol concentrations in saliva
48 h after delivery. Data were tested for constant variance (Levene’s test) and the Shapiro–Wilk test was used for testing the equality of error variances. Comparisons between EUT and DYS
groups for peak salivary cortisol levels and AUCs were made by a Wilcoxon rank-sum test. Significance was set at the level of 0.05 in case of both parameters.
Non-significant variables on salivary cortisol concentrations were compared between EUT and DYS groups with the Welch’s two-sample t test (parity and BCS of the dam, sex and birth weight of
the calf, the duration of parturition, and the time spent licking the calf) and with the Pearson’s Chi-squared test (proportions of male and female calves) at the significance level of 0.05
in both cases.
ResultsFrom the 168 calvings, 49, 56 and 63 calves were born to first, second and third parity cows, respectively. Comparison of independent variables between EUT and DYS groups is shown in Table
1. Salivary cortisol concentrations determined within 48 h after delivery were neither influenced by factors related to the dam (parity, body condition score, BCS) nor the calf (sex, birth
weight). Although the duration of parturition (range: 1.3–8.2 h) and the time spent linking the calf (5.5–86.5 min) differed significantly between EUT and DYS calves (Table 1) none of these
factors influenced salivary cortisol levels.
Table 1 Characteristics of calvings involved in this study (means ± SEM).Full size tableExcept for 48 h after delivery, linear models (df = 7; 150) indicated higher salivary cortisol concentrations in DYS calves compared to EUT ones for 0 (P = 0.022), 15 (P = 0.016), 30 (P =
0.007), 45 (P = 0.003), 60 (P = 0.001) and 120 min (P = 0.001), and for 24 h (P = 0.040) after birth, respectively. The evolution of salivary cortisol concentrations after delivery are shown
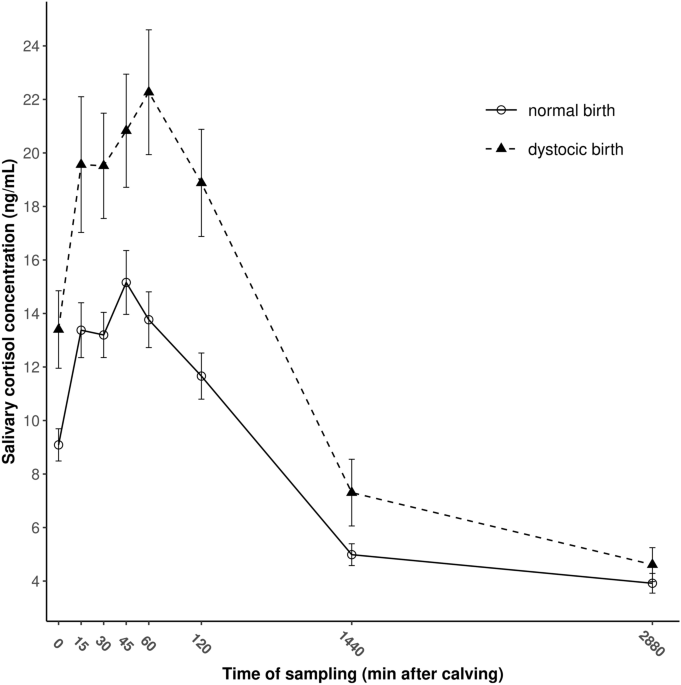
in Fig. 1 for EUT and DYS calves. The HPA response showed a similar pattern in both groups. Salivary cortisol concentrations increased rapidly after delivery in both groups to reach their
peak levels at 45 and 60 min after delivery in EUT and DYS calves, respectively. Afterward, a gradual decrease in cortisol concentrations was observed in both groups (Fig. 1). Twenty-four h
post-calving, salivary cortisol decreased to 32.9% and 33.7% of the peak levels in EUT and DYS calves, respectively and for the 48-h samples, similar circulating cortisol concentrations were
observed in both groups in saliva (P = 0.245).
Figure 1The evolution of salivary cortisol concentrations during the first 48 h of life in dairy calves born to eutocic (n = 98) and dystocic (n = 70) dams. Data are given in means ± SEM.
Fullsize image
DYS calves exhibited significantly higher peak levels and AUC of salivary cortisol than EUT calves (with 45.6% and 92.1%, respectively) for the 48-h of the sampling period (Table
2).
Table 2 Peak levels of salivary cortisol concentrations and salivary cortisol concentrations calculated as area under the curve (AUC) of newborn calves born from eutocic (EUT, n = 98)and dystocic (DYS, n = 70) deliveries.Full size tableDiscussion
This is the first study which investigates both the intensity and magnitude of the postnatal HPA response to birth of EUT and DYS dairy calves using AUC analyses based on high sampling
frequencies of saliva. The present findings demonstrate that the birth process induces significant elevation in HPA axis activity in newborn calves, even if no difficulties during
parturition occur. Differences between cortisol levels measured at 0 and 48 min in EUT (106.4%) and DYS calves (175.2%) found in the present study suggest that calves experienced stress
before delivery irrespective of obstetrical conditions. However, calves experiencing DYS births exhibited greater saliva cortisol levels, thus higher stress after calving compared to EUT
calves. Our results support earlier findings on serum28, plasma29 and salivary cortisol levels30 of EUT and DYS calves. In general, peak cortisol levels found in saliva in the present study
were similar to those observed by Stewart et al.31 in plasma 40 min after administration of adrenocorticotropic hormone (34.5 ng/mL), or after castration without local anesthetic (28.7
ng/mL) in Holstein–Friesian heifer calves32.
An earlier study found lower peak levels of salivary cortisol (14.8 ng/mL) in newborn calves after induced parturitions33, whereas others reported 6 ng/mL concentrations in calves born from
assisted deliveries30; however, authors collected saliva once within 24 h of birth, therefore they were not able to determine peak levels.
Gradually increasing cortisol levels after delivery in both groups support that the birth process means considerable stress for calves34 and the immediate postnatal period also appears to be
stressful for the newborn calf. In the present study, peak cortisol levels at 45 and 60 min after delivery in EUT and DYS calves, respectively, may be a delayed increase due to cortisol
transfer from the serum to the saliva13,14 or even reflect additional stress experienced by the calves during transition from the fetal to the extrauterine life.
Similar to our findings, Nagel et al.33 reported peak saliva cortisol levels at 60 min after birth. Others found peak levels immediately after birth in serum28, and 3 h after delivery in
plasma35. Salivary cortisol peak levels found in the present study in DYS calves was 12.2% of those observed by the latter authors in plasma35, which support field12 and laboratory
observations on cattle36 indicating that salivary cortisol levels yield around 10% of plasma cortisol levels.
Although Hoyer et al.37 found that reversal of stress occurs rapidly during the first hours of neonatal life, the results presented here suggest that newborn calves appear to adapt to the
extrauterine environment by 24 h of age. In line with our results, cortisol concentrations measured from saliva33 and plasma35,38,39 decreased gradually for 24 and 48 h after parturition.
As calving means significant stress also for the dam even from the onset of stage 1 of labor19, it came into question if naturally occurring cortisol in cows before delivery had a
significant influence on the amount of cortisol levels of the neonatal calf. It has been shown in goats40 and ewes41 that cortisol can partly cross the placenta, from the mother to the fetus
and may lead to hypercortisolism in situations of prolonged stress experienced by the dam during parturition42. However, results of Wooley35 indicate that maternal cortisol concentrations
in plasma do not influence calf cortisol concentration in cattle.
It should be noted that within the early neonatal life, other factors might also affect HPA axis functioning. Similar to recent observations33, birth weight and duration of parturition had
no effect on neonatal salivary cortisol concentrations. As calves were removed from the dams only 2 h after delivery to receive colostrum by farmhand, the only factor would have been the
dam-offspring contact. According to our earlier findings, the duration of licking the calf is a prominent factor in the thermal and metabolic adaptation of newborn calves to extrauterine
life43. Although the time spent licking the calf had no effect on salivary cortisol concentrations in this study, it can be assumed that maternal grooming might have caused a positive stress
for the calves by increasing cortisol levels between 15 and 60 min after birth, irrespectively for calving ease.
As a progressive maturation and activation of the fetal HPA axis during late gestation results in a considerably increased cortisol release from the fetal adrenals starting between 7 and 3
days before parturition44,45 it is thus questionable whether this initial fetal cortisol would affect cortisol levels measured from saliva after delivery. According to our assumption it
could not have been significantly present in the saliva of newborns, as fetal cortisol is proven to be absorbed by the maternal unit causing initiation of the preparation stage of labor46
and the gradual prepartum rise in fetal plasma cortisol during the last week of gestation was found to be much less marked even in spontaneously born calves than the abrupt increase
immediately after birth47.
Glucocorticoids can have a significant influence on the amount of immunoglobulins in colostrum and also on the amount of immunoglobulins absorbed by the neonate. Decreased cortisol
concentrations may reduce the ability or time available for the calf to absorb colostral immunoglobulins, whereas increased serum cortisol concentrations increase IgG concentrations48.
However, it has been proposed that the increased susceptibility to bacterial infection in calves may be enhanced by high plasma cortisol concentrations at birth and their effects upon the
lymphocytes49. This is a limitation of the present study that we did not measure immune parameters or followed-up calves to examine the longer-term effects of dystocia-related stress either
on growth, behavior, or overall welfare.
The greater magnitude of saliva cortisol responses in DYS calves compared to EUT ones suggest that difficult calving is more stressful for bovine neonates than a normal birth due to
prolonged parturition and/or forced extraction, and salivary cortisol could be an opportunity for non-invasive assessment of stress during the early neonatal period in cattle. The findings
of the present study should be integrated in further investigations with data from behavioral observations, production, and pathology records in a comprehensive approach of bovine neonatal
well-being.
Data availabilityAll materials, data that support the findings of this study and associated protocols are available from the corresponding author upon reasonable request.
References Hunter, J. T., Fairclough, R. J., Peterson, A. J. & Welch, R. A. Foetal and maternal hormonal changes preceding normal bovine parturition. Acta Endocrinol. 84, 653–662 (1977).
CAS Google Scholar
Kovács, L., Kézér, F. L. & Szenci, O. Effect of calving process on the outcomes of delivery and postpartum health of dairy cows with unassisted and assisted calvings. J. Dairy Sci. 99,
7568–7573 (2016).
Article PubMed CAS Google Scholar
Stafford, K. J. & Mellor, D. J. Addressing the pain associated with disbudding and dehorning in cattle. Appl. Anim. Behav. Sci. 135, 226–231 (2011).
Article Google Scholar
Lay, D. C. Jr. et al. Behavioral and physiological effects of freeze or hot-iron branding on crossbred cattle. J. Anim. Sci. 70, 330–336 (1992).
Article PubMed Google Scholar
Fidan, A., Pamuk, F. K., Ozdemir, A., Saritas, Z. K. & Tarakci, U. Effects of dehorning by amputation on oxidant-antioxidant status in mature cattle. Rev. Med. Vet. 161, 502–508 (2010).
CAS Google Scholar
Cook, N. J. Review: Minimally invasive sampling media and the measurement of corticosteroids as biomarkers of stress in animals. Can. J. Anim. Sci. 92, 227–259 (2012).
Article CAS Google Scholar
Hellhammer, D. H., Wüst, S. & Kudielka, B. M. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 34, 163–171 (2009).
Article CAS PubMed Google Scholar
Kudielka, B. M., Hellhammer, D. H. & Wüst, S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology 34, 2–18
(2009).
Article CAS PubMed Google Scholar
Kirschbaum, C. Salivary cortisol. In Encyclopedia of Stress (ed. Fink, G.) 379–383 (Academic Press, San Diego, 2000).
Google Scholar
Möstl, E. & Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 23, 67–74 (2002).
Article PubMed Google Scholar
Mormède, P. et al. Exploration of the hypothalamic-pituitary-adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 92, 317–339 (2007).
Article PubMed CAS Google Scholar
Kovács, L. et al. Hypothalamic–pituitary–adrenal and cardiac autonomic responses to transrectal examination differ with behavioral reactivity in dairy cows. J. Dairy Sci. 99, 7444–7457
(2016).
Article PubMed CAS Google Scholar
Negrao, J. A., Porcionato, M. A., de Passille, A. M. & Rushen, J. Cortisol in saliva and plasma of cattle after ACTH administration and milking. J. Dairy Sci. 87, 1713–1718 (2004).
Article CAS PubMed Google Scholar
Hernandez, C. E. et al. Time lag between peak concentrations of plasma and salivary cortisol following a stressful procedure in dairy cattle. Acta Vet. Scand. 56, 61 (2014).
Article PubMed PubMed Central CAS Google Scholar
Mee, J. F. Newborn dairy calf management. Vet. Clin. N. Am. Food Anim. Pract. 24, 1–17 (2008).
Article Google Scholar
Schuijt, G. Iatrogenic fractures of ribs and vertebrae during delivery in perinataly dying calves: 235 cases (1978–1988). J. Am. Vet. Med. Assoc. 197, 1196–1202 (1990).
CAS PubMed Google Scholar
Mee, J. F., Berry, D. P. & Cromie, A. R. Risk factors for calving assistance and dystocia in pasture-based Holstein-Friesian heifers and cows in Ireland. Vet. J. 187, 189–194 (2011).
Article CAS PubMed Google Scholar
Hady, P. J., Domecq, J. J. & Kaneene, J. B. Frequency and precision of body condition scoring in dairy cattle. J. Dairy Sci. 77, 1543–1547 (1994).
Article CAS PubMed Google Scholar
Kovács, L. et al. Heart rate and heart rate variability in multiparous dairy cows with unassisted calvings in the periparturient period. Physiol. Behav. 139, 281–289 (2015).
Article PubMed CAS Google Scholar
Miedema, H. M., Cockram, M. S., Dwyer, C. M. & Macrae, A. I. Behavioural predictors of the start of normal and dystocic calving in dairy cows and heifers. Appl. Anim. Behav. Sci. 132, 14–19
(2012).
Article Google Scholar
Jensen, M. B. Behaviour around the time of calving in dairy cows. Appl. Anim. Behav. Sci. 139, 195–202 (2012).
Article Google Scholar
Palme, R. & Möstl, E. Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. Z. Saugetierkd. Int. J. Mammal. Biol. 62(Suppl. 2), 192–197
(1997).
Google Scholar
Wagner, K. et al. Mother rearing of dairy calves: Reactions to isolation and to confrontation with an unfamiliar conspecific in a new environment. Appl. Anim. Behav. Sci. 147, 43–54 (2013).
Article Google Scholar
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. http://www.r-project.org/ (2017).
Pinheiro, J. C. & Bates, D. M. Mixed-Effects Models in S and S-PLUS 1st edn. (Springer, 2000).
Book MATH Google Scholar
Fekedulegn, D. B. et al. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom. Med. 69, 651–659 (2017).
Article CAS Google Scholar
Lay, D. C. Jr. et al. Adrenocorticotropic hormone dose response and some physiological effects of transportation on pregnant Brahman cattle. J. Anim. Sci. 74, 1806–1811 (1996).
Article CAS PubMed Google Scholar
Vannucchi, C. I. et al. Association between birth conditions and glucose and cortisol profiles of periparturient dairy cows and neonatal calves. Vet. Rec. 176, 358 (2015).
Article CAS PubMed Google Scholar
Civelek, T., Celik, H. A., Avci, G. & Cingi, C. C. Effects of dystocia on plasma cortisol and cholesterol levels in holstein heifers and their newborn calves. Bull. Vet. Inst. Pulawy 52,
649–654 (2008).
Google Scholar
Barrier, A. C. et al. The impact of dystocia on dairy calf health, welfare, performance and survival. Vet. J. 195, 86–90 (2013).
Article CAS PubMed Google Scholar
Stewart, M., Stafford, K. J., Dowling, S. K., Schaefer, A. L. & Webster, J. R. Eye temperature and heart rate variability of calves disbudded with or without local anaesthetic. Physiol.
Behav. 93, 789–797 (2008).
Article CAS PubMed Google Scholar
Stewart, M., Verkerk, G. A., Stafford, K. J., Schaefer, A. L. & Webster, J. R. Noninvasive assessment of autonomic activity for evaluation of pain in calves, using surgical castration as a
model. J. Dairy Sci. 93, 3602–3609 (2010).
Article CAS PubMed Google Scholar
Nagel, C. et al. Stress response and cardiac activity of term and preterm calves in the perinatal period. Theriogenology 86, 1498–1505 (2016).
Article PubMed Google Scholar
Aurich, J. E. et al. Influence of labor and neonatal hypoxia on sympathoadrenal activation and methionine enkephalin release in calves. Am. J. Vet. Res. 54, 1333–1338 (1993).
CAS PubMed Google Scholar
Wooley, D. N. Prepartum maternal cortisol concentrations on postnatal cortisol concentration and immunoglobulin absorption in neonatal dairy calves. LSU Master's Theses. 4124.
https://digitalcommons.lsu.edu/gradschool_theses/4124 (2010).
Chacón, G., Laita, S.G.-B., del Portal, J. C. I. & Liesa, J. P. Validation of an EIA technique for the determination of salivary cortisol in cattle. Spanish J. Agric. Res. 2, 45–52 (2004).
Article Google Scholar
Hoyer, C., Grunert, E. & Jochle, W. Plasma glucocorticoid concentrations in calves as an indicator of stress during parturition. Am. J. Vet. Res. 51, 1882–1884 (1990).
CAS PubMed Google Scholar
Hadorn, U., Hammon, H., Bruckmaier, R. M. & Blum, J. W. Delaying colostrum intake by one day has important effects on metabolic traits and on gastrointestinal and metabolic hormones in
neonatal calves. J. Nutr. 127, 2011–2023 (1997).
Article CAS PubMed Google Scholar
Hammon, H. M. & Blum, J. W. Metabolic and endocrine traits of neonatal calves are influenced by feeding colostrum for different durations or only milk replacer. J. Nutr. 128, 624–632 (1998).
Article CAS PubMed Google Scholar
Thorburn, G. D., Nicol, D. H., Bassett, J. M., Shutt, D. A. & Cox, R. I. Parturition in the goat and sheep: changes in corticosteroids, progesterone, oestrogens and prostaglandin F. J.
Reprod. Fert. 16(suppl), 61–84 (1972).
Google Scholar
Dixon, R. et al. Feto-maternal transfer and production of cortisol in the sheep. Steroids 16, 771–789 (1970).
Article CAS PubMed Google Scholar
Jones, C. T., Robinson, R. O., Luther, E., Ritchie, J. W. K. & Worthington, D. Control of adrenocorticotrophin secretion by catecholamines in the pregnant and foetal sheep. J. Endocr. 73,
11–20 (1977).
Article CAS PubMed Google Scholar
Kovács, L., Kézér, F. L., Albert, E., Ruff, F. & Szenci, O. Seasonal and maternal effects on acid-base, lactate, electrolyte and hematological status of 205 dairy calves born to eutocic
dams. J. Dairy Sci. 100, 7534–7543 (2017).
Article PubMed CAS Google Scholar
Schuler, G., Fürbass, R. & Klisch, K. Placental hormones in gestation and parturition. Anim. Reprod. 15(Suppl. 1), 822–842 (2018).
Article Google Scholar
Taverne, M. A. M., Bevers, M. M., van der Weyden, G. C., Dieleman, S. J. & Fontijne, P. Concentration of growth hormone, prolactin, and cortisol in fetal and maternal blood and amniotic
fluid during late pregnancy and parturition in cows with cannulated fetuses. Anim. Reprod. Sci. 17, 51–59 (1988).
Article CAS Google Scholar
Hoffmann, B., Wagner, W. C., Rattenberger, E. & Schmidt, J. Endocrine relationships during late gestation and parturition in the cow. Ciba Found. Symp. 47, 107–125 (1977).
CAS Google Scholar
Comline, R. S., Hall, L. W., Lavelle, R. B., Nathanielsz, P. W. & Silver, M. Parturition in the cow: endocrine changes in animals with chronically implanted catheters in the foetal and
maternal circulations. J. Endocrinol. 63, 451–472 (1974).
Article CAS PubMed Google Scholar
Johnston, N. E. & Oxender, W. D. Effect of altered serum glucocorticoid concentrations on the ability of the newborn calf to absorb colostral immunoglobulin. Am. J. Vet. Res. 40, 32–34
(1979).
CAS PubMed Google Scholar
Eberhart, R. J. & Patt, J. A. Plasma cortisol concentrations in newborn calves. Am. J. Vet. Res. 32, 1921–1927 (1971).
CAS PubMed Google Scholar
Download references
AcknowledgementsThe authors would like to thank Ferenc Bodó, farm owner, and Ágoston Bodó, farm manager for supporting the study, and the farm staff of Prograg Agrárcentrum Ltd. at Ráckeresztúr,
Lászlópuszta for taking care of the animals during the experiment. Levente Kovács was supported by the OTKA Research Scholarship of the National Research, Development and Innovation Office
(Budapest, Hungary; K-134204) and by the NTP-NFTÖ-20 project by the Human Capacities Grant Management Office and the Hungarian Ministry of Human Capacities, Budapest, Hungary
(NTP-NFTÖ-20-B-0030). Thanks to Edith Klobetz-Rassam for cortisol analysis.
Author informationAuthors and Affiliations Institute of Animal Sciences, Hungarian University of Agriculture and Life Sciences, Guba Sándor utca 40, Kaposvár, 7400, Hungary
Levente Kovács & Szilárd Bodó
Bovine Research Division, Bona Adventure Ltd, Peres utca 44, Gödöllő, 2100, Hungary
Levente Kovács & Fruzsina Luca Kézér
Department of Methodology, Hungarian Central Statistical Office, Keleti Károly utca 5-7, Budapest, 1024, Hungary
Ferenc Ruff
Unit of Physiology, Pathophysiology, and Experimental Endocrinology, University of Veterinary Medicine, Veterinärplatz 1, 1210, Vienna, Austria
Rupert Palme
Department of Obstetrics and Food Animal Medicine Clinic, University of Veterinary Medicine, István utca 2, Budapest, 1078, Hungary
Ottó Szenci
AuthorsLevente KovácsView author publications You can also search for this author inPubMed Google Scholar
Fruzsina Luca KézérView author publications You can also search for this author inPubMed Google Scholar
Szilárd BodóView author publications You can also search for this author inPubMed Google Scholar
Ferenc RuffView author publications You can also search for this author inPubMed Google Scholar
Rupert PalmeView author publications You can also search for this author inPubMed Google Scholar
Ottó SzenciView author publications You can also search for this author inPubMed Google Scholar
ContributionsPlan of the experiment: L.K., O.S.Z. Performing the experiment: L.K., F.L.K. Salivary cortisol assessment: R.P. Manuscript writing: L.K. Statistical analysis: F.R. Supervision: S.Z.B.,
O.S.Z.
Corresponding author Correspondence to Levente Kovács.
Ethics declarations Competing interestsThe authors declare no competing interests.
Additional informationPublisher's noteSpringer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissionsOpen Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or
other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in
the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and permissions
About this articleCite this article Kovács, L., Kézér, F.L., Bodó, S. et al. Salivary cortisol as a non-invasive approach to assess stress in dystocic dairy calves. Sci Rep 11, 6200 (2021).
https://doi.org/10.1038/s41598-021-85666-9
Download citation
Received: 07 July 2020
Accepted: 03 March 2021
Published: 18 March 2021
DOI: https://doi.org/10.1038/s41598-021-85666-9
Share this article Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article.
Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative




:max_bytes(150000):strip_icc():focal(119x0:121x2)/tori-spelling-240-13-da9d4c7732454004aed71e77a291f0a8.jpg)




