- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT To share the experiences of organizing the epilepsy surgery program in Indonesia. This study was divided into two periods based on the presurgical evaluation method: the first
period (1999–2004), when interictal electroencephalogram (EEG) and magnetic resonance imaging (MRI) were used mainly for confirmation, and the second period (2005–2017), when long-term
non-invasive and invasive video-EEG was involved in the evaluation. Long-term outcomes were recorded up to December 2019 based on the Engel scale. All 65 surgical recruits in the first
period possessed temporal lobe epilepsy (TLE), while 524 patients were treated in the second period. In the first period, 76.8%, 16.1%, and 7.1% of patients with TLE achieved Classes I, II,
and III, respectively, and in the second period, 89.4%, 5.5%, and 4.9% achieved Classes I, II, and III, respectively, alongside Class IV, at 0.3%. The overall median survival times for
patients with focal impaired awareness seizures (FIAS), focal to bilateral tonic–clonic seizures and generalized tonic–clonic seizures were 9, 11 and 11 years (95% CI: 8.170–9.830,
10.170–11.830, and 7.265–14.735), respectively, with _p_ = 0.04. The utilization of stringent and selective criteria to reserve surgeries is important for a successful epilepsy program with
limited resources. SIMILAR CONTENT BEING VIEWED BY OTHERS THE PROGNOSTIC VALUE OF CORTICAL STIMULATION INDUCED SEIZURES USING STEREO EEG IN PRESURGICAL EVALUATION OF FOCAL EPILEPSIES Article
Open access 07 March 2025 TUMOR-RELATED EPILEPSY AND POST-SURGICAL OUTCOMES: TERTIARY HOSPITAL EXPERIENCE IN VIETNAM Article Open access 05 July 2023 EARLY POSTOPERATIVE SEIZURES (EPS) IN
PATIENTS UNDERGOING BRAIN TUMOUR SURGERY Article Open access 13 August 2020 INTRODUCTION Epilepsy is the most common neurological disorder affecting people of all ages and classes1.
Interestingly, the prevalence of epilepsy differs between developed and developing countries, in which the latter has a higher prevalence in both urban and rural settings2. Approximately 50
million people worldwide suffer from this disorder, and 80% among them reside in developing countries with limited available resources3. The remainder of these 40 million people are unable
to obtain appropriate treatment and, as a result, experience significant morbidity owing to seizures4. Furthermore, they still face psychosocial implications from stigma and prejudice due to
stereotypes and negative attitudes towards epilepsy, especially in developing countries5. Anti-seizure drug (ASD) intervention is the mainstay for care for people with epilepsy; however,
more than 30% of people with epilepsy experience frequent hallucinations through the use of sufficient ASDs6. Surgery is one of the essential treatment choices for patients whose ASD
medication fails to stop seizures and/or to achieve lasting relief from seizures. The effectiveness of epilepsy surgery relies on the successful selection of appropriate surgical candidates
based on available information and technologies. Nevertheless, resources are limited for the vast majority of patients with drug-resistant epilepsy (DRE), who live mostly in developing
countries. Epilepsy surgery in Indonesia started in 1999 under the direction of mentoring universities from Japan. We have now implemented intracranial electrode monitoring to the sum of 62
surgeries up until 2017. We share our experience in establishing an epilepsy surgery program in Indonesia, its challenges, and how we overcame those issues. METHODS SURGICAL INDICATION AND
PRESURGICAL EVALUATION We report our experience with patients who underwent epilepsy surgery in our neurosurgical centres in Semarang, Indonesia (Kariadi Hospital and the affiliated
Diponegoro Hospital) between July 1999 and December 2017. We divided this timespan into two periods based on resource availability: the first period (1999–2004) and the second period
(2005–2017). In the first period, the surgical indication for patients with drug-resistant temporal lobe epilepsy (TLE) was considered primarily based on whether they had a seizure-related
lesion. In addition to reviewing past treatment and a detailed history of medical and neurological examinations, neuropsychological tests, and psychiatric evaluations, all patients received
scalp electroencephalogram (EEG) while awake and asleep and magnetic resonance imaging (MRI) scans of at least 0.5 T or more. The MRI series included regular orientation of 5-mm slices at
T1WI and T2WI and fluid-attenuated inversion recovery (FLAIR). Invasive recordings and operations on patients without MRI confirmed lesions were not feasible during this time. In the second
period (2005–2017), the long-term monitoring of EEG recordings (Video EEG) was adopted for the presurgical evaluation, and intervention was aimed at different types of seizures. The phase
one study was basically the same as in the first period. As an advanced analysis, long-term EEG and PET investigations were performed in difficult cases using conventional methods7. The Wada
test was also performed on some patients. Phase two included invasive video EEG monitoring with subdural electrodes. Functional mapping was performed using subdural electrodes. A
reassessment was scheduled or alternative treatments were recommended for patients in cases where preoperative investigations were not consistent. During phase three (the intraoperation
phase), we analysed the electrocorticogram (ECoG) of interictal activity. Awake functional mapping and/or the somatosensory evoked potential test was performed if necessary. SURGICAL
PROCEDURES In the first period, most patients underwent anterior temporal lobectomy (ATL). In the second period, we introduced selective amygdalohippocampectomy (SAH) for patients with
unilateral dominant mesial temporal lobe surgery (TLE). At this point, we started to treat extratemporal lobe epilepsy, and we performed a variety of surgical procedures. Cortical resection
and multiple subpial transection (MST) were used for extratemporal neocortical epilepsy in non-eloquent and eloquent foci, respectively. Anterior callosotomy was indicated for infantile
hemiplegia, Lennox–Gastaut syndrome, multifocal bilateral epilepsy and drop attacks for palliative surgical procedures. POSTSURGICAL EVALUATION A pathological analysis of haematoxylin–eosin
(H&E)-stained resected samples was started in the second period. Several additional studies were applied to the hippocampus. Upon postoperative treatment, AEDs were gradually reduced
after a duration from six months to one year, in agreement with the patient who underwent follow-up EEG every six months to one year. Postoperative seizure results were measured using the
Engel Outcome Scale (EOS) for patients who underwent surgery at least 2 years after the procedure. STUDY DESIGN, PARTICIPANTS, AND ETHICAL DECLARATION We recorded the long-term outcomes and
patterns of seizure remission and relapse in 589 consecutive participants who underwent epilepsy surgery between July 1999 and December 2017. Based on the availability of resources, this
timespan was divided into two periods: the first period (1999–2004) and the second period (2005–2017). Two Indonesian consultant neurosurgeons specializing in epilepsy (Z.M. and M.T.A.)
conducted the operations with the direct supervision by Japanese mentors (K.A., R.H., K.K., and K.I.) for the first 15 cases in the first period. Clinical history, examination findings,
seizure semiology, interictal and ictal EEG, MRI results, neuropsychological and psychiatric assessments, and clarity of the epileptogenic zone location were examined as potential predictors
of neurological deficits. We also performed additional presurgical evaluations, i.e., intracarotid amytal, FDG-PET, ECoG, and subdural grid EEG. The optimum surgical approach was decided to
provide the best chance of freedom from seizures with the lowest risk of complications using the general principles of presurgical assessments previously mentioned. Furthermore, information
on the yearly seizure status was obtained from a review of contemporaneous notes from Kariadi Hospital and from the records of other hospitals attended by the patient. Thereafter,
postoperative seizure results were measured using the Engel Outcome Scale (EOS) for individuals who had undergone an operation 2 years prior to the assessment. All procedures performed in
studies involving human participants were in accordance with the ethical standards of the Medical Faculty, Diponegoro University and Kariadi Hospital Ethical Research Committee and with the
1964 Helsinki Declaration standards. This study was approved by the Joint Ethics Committee of the Kariadi General Hospital (number 122/EC/KEPK-RSDK/2019). Written informed consent was
obtained from all patients prior. For patients under the age of 18 years, informed consent was obtained from a parent and/or legal guardian. STATISTICAL ANALYSIS Statistical analysis was
performed using Statistical Package for the Social Sciences, version 22 (SPSS v22.0, IBM, USA). Differences between variables were tested with the non-parametric chi-square test. Survival
analysis with Cox proportional hazards regression was used to evaluate the total time-to-event outcomes and compare the postoperative seizure-free time (Engel 1a and 1b). Hazard ratios (HRs)
(and 95% CIs) were calculated for the operation type, aura, epileptogenic source and type of surgery during the first and second periods, as well as for the overall group. The Kaplan–Meier
method was used to estimate the proportion of individuals who remained seizure-free at various timepoints (years), while the median survival time was reported as the 95% CI, with a 3-, 5-
and 10-year chance of being seizure-free during follow-up. RESULTS Five hundred eighty-nine consecutive patients were treated between July 1999 and December 2017. Among the 65 patients
treated in the first period (Table 1), the most common seizure type was focal with impaired awareness and focal to bilateral tonic–clonic, with six cases of generalized seizures. The
majority of the aetiologies were associated with hippocampal sclerosis, with three tumours diagnosed through MRI findings: two dysembryoplastic neuroepithelial tumours (DNTs) and one of
unknown aetiology. Two non-lesional cases of TLE were recognized, demonstrating typical signs, although the interictal EEG was unable to identify lateralization. Therefore, subdural
electrodes were inserted under mentor supervision, and the EEG was recorded for a few hours. The resulting electrocorticography (ECoG) demonstrated the lateralization of interictal spikes in
one person and ictal spikes in the other. Most cases in the first period had an epileptogenic zone within the temporal lobes (Table 1). In the second period (Table 1), 524 patients were
treated: 428 with focal seizures, among whom 247 and 4 had impaired awareness and aware seizures, respectively. In addition, 177 patients had focal-to-bilateral tonic–clonic seizures, and 27
had generalized seizures, while the presurgical analysis identified 86.27% of the epileptogenic focus in the temporal lobe. This series recognized 90 patients with normal MRI findings
during first and second periods. There were also 443 and 4 patients with single and multiple abnormalities, respectively (Table 1). Scalp EEG, MRI and seizure semiology were the primary
presurgical evaluation tools used during the first period, while advanced assessments including long-term video EEG and PET were used during the second period (Table 2). The decision to
proceed with surgery was reserved in 74 patients with normal MRI TLE. Subdural grid was placed in 11 patients monitored with long-term video EEG and 46 patients decided on LTSV. Furthermore,
surgical outcome based on the multimodality evaluation was presented in Table 3. The underlying pathology was mostly mesial temporal sclerosis (MTS, 56.16%), as seen in Table 4. Within the
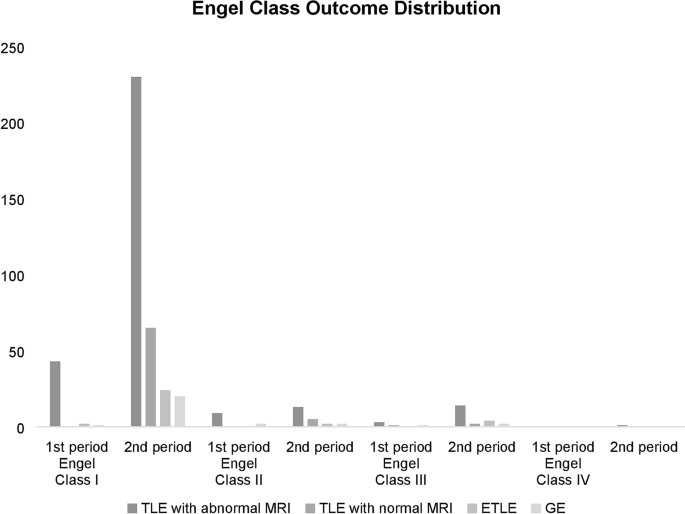
first period, 78.2%, 16.4%, and 5.5% of patients with TLE and abnormal MRI findings achieved Engel Classes I, II, and III, respectively. For the second period 84.1%, 5%, 5.4%, and 0.4% of
patients achieved Classes I, II, III and IV, respectively. During the first period, 76.8%, 16.1%, and 7.1% of patients with TLE achieved Classes I, II, and III, respectively, while during
the second period, 89.4%, 5.5%, 4.9% and 0.3% achieved Classes I, II, III and IV, respectively. The surgical outcomes of patients with TLE and abnormal or normal MRI findings as well as
extratemporal lobe epilepsy and general epilepsy are summarized in Fig. 1 and Table 5. Using the chi-square test, significant differences were observed between the seizure-free and
non-seizure-free groups based on seizure type, aura, epileptogenic source and type of surgery (Table 6). Based on seizure type, aura, epileptogenic source and type of surgery, the outcomes
in terms of seizures were not significantly different between the first and second periods (Table 6). Kaplan–Meier analysis confirmed significant differences in the overall survival time
amongst patients with FIAS, FBTCS and GTCS/GA, with respective medians of 9 (95% CI: 8.170–9.830), 11 (95% CI: 10.170–11.830), and 11 (95% CI: 7.265–14.735) years (p = 0.04), with GTCS/GA
patients exhibiting the greatest survival outcomes at the end of follow-up. The overall survival time also varied significantly based on surgery, with a median of 10 years for patients who
underwent ATL (95% CI: 9.364–10.636), 11 years for patients who underwent SAH (95% CI: 9.413–12.587), 20 years for patients who underwent callosotomy (95% CI: 0.000–43.958), and 9 years for
patients who underwent lesionectomy (95% CI: 6.129–11.871) (_p_ = 0.04), as shown in Table 6 and Fig. 2. In addition, outcomes were better for focal seizures than for general seizures, with
an HR of 0.801 (95% CI: 0.705–0.909; p < 0.05), as shown in Table 6. A comparison of HRs for each variable between the 1st and third periods is presented in Fig. 3. Postoperative outcomes
were analysed between the 1st and 2nd periods. At 5 years after surgery, 47/65 patients (72.3%) in the 1st period and 424/524 patients (80.9%) in the 2nd period were seizure free. Eighteen
of 65 patients in the 1st period (27.7%) and 100 of 524 patients (19.1%) still had seizures at the 5-year follow-up. There was no significant difference in the 5-year follow-up outcome
between the 1st period and 2nd period (p = 0.102) (Table 7). DISCUSSION This report presented long-term outcomes of a large cohort of epilepsy patients who underwent surgery (589 people)
with at least 2 years of follow-up, covering nearly three seizure-free quarters. In addition, this report strengthened our previous study epilepsy surgeries conducted in Indonesia, the most
populous country in Southeast Asia8. Previous authors have described the establishment of this surgical service as feasible and cost-effective in other developing countries, including India,
Lebanon, and Colombia, with comparable outcomes to those in developed countries9,10,11,12,13,14. However, during its initial development in 1999, epilepsy surgery was unknown to most
persons in Indonesia; hence, the staff worked hard to establish support from the medical community, especially the neurological society. Therefore, the first period (1999–2004) featured only
selected patients who required simple diagnostic procedures, ensuring the achievement of an excellent outcome and near-zero morbidity. Scalp EEG, MRI and seizure semiology were the primary
evaluation tools for surgery; hence, the criteria used reserved the surgery for remediable syndromes, characterized by well-understood pathophysiologies. Additionally, the natural history
was medically refractory or progressive, following failure of a major first-line AEDs. During this period, all preoperative evaluations were conducted non-invasively, and surgery was offered
as an excellent opportunity to accomplish the complete elimination of seizures. Furthermore, the eligible candidates for excisional procedures and surgery usually possessed localized and
generalized epilepsy syndromes, respectively, as mesial TLE features neocortical epilepsy caused by discrete, easily resectable lesions. SURGICAL OUTCOMES Engel Class I was achieved in 78.2%
and 84.1% of patients with TLE and abnormal MRI findings during the first and second periods, respectively. This result was similar to those of previous reports from studies conducted in
both adults and children, although freedom from seizures was more common after resection with MTS (60–90%)15,16,17. Meanwhile, ATL conducted by others within the past 15 years who attained
freedom from seizures on approximately 70–80% of adults and children with TLE18,19, and resected TLE surgery has been validated by class I evidence20. Among two randomized controlled trials,
the first reported that 58% of drug-resistant TLE patients who had undergone ATL were seizure free at 1 year compared to the 8% who had continued best drug therapy20. After 5 years of
postoperative follow-up, 47/65 patients in the 1st period (72,3%) and 424/524 patients in the 2nd period (80,9%) achieved a seizure-free status, with no statistically significant difference
between the 1st and 2nd period outcomes (Table 7 and Fig. 3). Furthermore, the limited resources available were used to achieve similar results. Engel’s classification was adopted to analyse
outcomes. One study21 reported a 60% response rate on average 7 years after temporal surgery, which showed the absence of seizures in 48% of patients from the date of surgery. This outcome
covers 40% of all seizure-free individuals in this cohort 10 years following anterior temporal resection based on the survival analysis estimation (Table 6). Another postsurgery study
reported that 43% of patients were seizure free after FIAS after a mean 7-year follow-up, while in other studies22,23, between 41 and 63% of patients with or without FIAS were seizure free
after being monitored for 5–10 years after temporal surgery. During this cohort study, the probability of being seizure free (no seizures with loss of awareness) was 92.1% within a 3-year
period, which decreased to 40% after a decade. A study on consecutive people who underwent temporal resection for hippocampal sclerosis estimated seizure-free success rates at the 2- and
5-year intervals that were lower than those in the current study24 (94.3% at 3 years and 80% at 5 years) (Table 6). Unsurprisingly, others25 associated the short interval between surgery and
recurrence of the first seizure with a longer term prognosis than the interval. It has been established that patients who undergo extratemporal resection have a greater probability of
recurrence than those who undergo anterior temporal resection. In addition, similar findings were reported in a long-term postsurgical follow-up study14, where individuals who underwent
temporal resection had an increased chance of being seizure free. This favourable outcome has been reported in previous studies26,27, as the overall seizure-free rate after anterior temporal
resection was 76.8% within the first period and 89.4% in the second period for patients with temporal lobe epilepsy or hippocampal sclerosis. A postoperative outcome study28 on patients
with pathological changes identified on preoperative MRI showed 2-year remission in 80% of respondents with tumours and vascular malformations, 58% of those with hippocampal sclerosis, and
29% with normal MRI findings. However, the assessment29 of people who underwent anterior temporal resection showed the inability for changes in the underlying pathology to determine the time
of subsequent seizures. Additionally, the presence of a ganglioglioma or dysembryoplastic neuroepithelial tumour and the absence of dysplasia have been associated with good postoperative
control30. Moreover, the majority of patients treated in the current study included those with hippocampal sclerosis (56.16%), while 17.37% had normal MRI findings, and 84.9% were free of
seizures, as shown in Table 4. The probability for patients to maintain this positive outcome increased with an increasing number of seizure-free years (Table 6). This finding is supported
by the results of a study on the outcome after temporal surgery, where individuals who received successful treatment for any 1 year or those with only SPS had a 90% probability of having no
seizures in the subsequent year23. However, those who achieved 2 successive years without seizures had a greater chance of having a seizure the subsequent year (94%). Intraoperative
monitoring in epilepsy surgery involves two aspects: monitoring cortical activity and identifying the epileptogenic zone (focus) minimal amount of tissues to be resected in a bid to control
seizures. Additionally, there is a need to screen for a functionally important cortex, which should be spared during the resection process. Furthermore, the use of an intraoperative
evaluation in the verification of epileptogenic zones is often based on electrocorticogram (ECoG) recordings, which were conducted in 45 patients. Additionally, the Wada test modified with
propofol was performed in one patient. This was not routinely administered because of the difficulty associated with the test and the availability of amobarbital in Indonesia. STRATEGIES TO
OVERCOME PROBLEMS IN CENTRES WITH LIMITED RESOURCES This study showed attempts to address several problems during the establishment of an epilepsy surgical centre. One of most challenging
was the poor availability and feasibility of the service, which consequently created an enormous "surgical treatment gap" between the number of potential beneficiaries and the
actual surgery performed, which was wide in a low-and middle income society31,32,33,34. Additionally, execution is difficult on an isolated basis, as the procedure should be performed within
an uninterrupted, well-defined program that uses a systematic approach with established criteria for patient selection, surgery, and follow-up7. This decision-making step requires a
multidisciplinary approach in which different investigators cooperate to understand epileptology35. Therefore, a successful outcome depends greatly on the teams’ willingness to identify
suitable candidates with an epileptogenic zone that is clearly established using locally available equipment and knowledge without risking patient safety36,37. This also involves acquiring
knowledge on when not to perform the operation and requesting additional evaluation, which is important in the identification of beneficiaries with the available resources38. Several core
strategies have been implemented to improve the services rendered. (1) Recruiting a trained epileptologist who is knowledgeable in all aspects of seizure control. Furthermore, the ability to
organize and manage a team is probably the most important element in establishing a successful epilepsy care centre. This is achievable by seeking assistance from well-established service
providers in developed countries due to the poor feasibility of self-training through trial and error within a limited setting. However, the epileptologist in our facility (Dr. Aris Catur
Bintoro) received training in the well-respected National Epilepsy Center, Shizuoka Institute of Epilepsy and Neurological Disorder, Japan. (2) Incorporating expert neurosurgeons, both
individually and as a group. The team in this study comprised two neurosurgeon, both trained in Japan. However, active enrolment in an epilepsy fellowship program within advanced centres and
obtaining appropriate training are assumed to be effective for long-term disease management. (3) Seeking EEG technicians to undergo relevant training; hence, the epileptologist always has
three qualified nurses. In places without access to EEG technician training programs, registered nurses with additional medical knowledge and motivation to participate are optimal training
recipients (under the epileptologist’s supervision). (4) Granting access to basic investigation modalities (i.e., MRI and EEG or, preferably, video-EEG) through public health support
systems. In addition, MRI for the evaluation of patients with refractory epilepsy should be performed using a special temporal lobe protocol that is then interpreted by an experienced
radiologist accustomed to identifying hippocampal sclerosis. Collaborations were established with the radiology department to create an epilepsy protocol that is appropriate for epilepsy
imaging, which led to the assignment of one expert to specifically work with the epilepsy surgery team. The centre was also equipped with a long-term video-EEG monitoring device in 2011,
alongside reasonable customer and technical support, followed by the separate use of a video tape recorder and time synchronization with the EEG machine. (5) Developing realistic
preoperative protocols featuring good history collection is key to selecting appropriate surgical candidates. This challenge was solved by developing and routinely using a protocol that was
designed to minimize the risk of missing important clinical details, including seizure type and frequency, associated disabilities, and the impacts of uncontrolled seizures. The next step
after collecting the relevant information was to provide the patient details to the epilepsy surgery committee, which comprised the epileptologist, two neurosurgeons and the
neuroradiologist. This multidisciplinary approach towards the management of individuals with medically refractory epilepsy was unique to the centre under investigation. Since 2010, these
programs have provided excellent care for patients with epilepsy, while more centres are currently in the developmental phase. This has been significantly hampered by limitations in
available resources and difficulties faced in attempts to access epileptologists, staff, and equipment. Following extensive discussions amongst all team members, a decision was reached
regarding three possible surgical scenarios in patients with medically refractory epilepsy. These include: a. Lesional epilepsy resections: performed in instances where a brain lesion is
identifiable by MRI, despite discordant semiology and interictal EEG evaluations with the lesion, and when the surgery procedure has no significant risks; b. Anterior temporal lobectomy in
temporal lobe epilepsy: conducted when there is a display of unilateral mesial temporal sclerosis and semiology as well as when interictal EEG is concordant with the MRI findings; and c.
Palliative surgery (corpus callosotomy): carried out when patients demonstrate intractable generalized seizures and are not considered candidates for focal resection. This is also
contemplated for those with refractory frontal lobe seizures and a tendency to be adequately localized39. CONCLUSIONS This report sought to share the experiences of organizing the epilepsy
surgery program in Indonesia. Our experience suggests that it is possible to initiate the surgical program even under imperfect circumstances, such as limited modalities for presurgical
evaluations. We emphasized the utilization of stringent and selective criteria to reserve surgeries for remediable syndromes characterized by well-understood pathophysiologies, such as
drug-resistant TLE with typical symptoms and the concordance of interictal EEG and MRI findings. These patients were identified as good candidates for the commencement of epilepsy surgery in
countries with limited resources based on positive results obtained from our centre. REFERENCES * Neligan, A. & Sander, J. W. The incidence and prevalence of epilepsy.
https://www.epilepsysociety.org.uk/sites/default/files/attachments/Chapter01Neligan-2015.pdf. * Ngugi, A. K., Bottomley, C., Kleinschmidt, I., Sander, J. W. & Newton, C. R. Estimation of
the burden of active and life-time epilepsy: A meta-analytic approach. _Epilepsia_ 51, 883–890 (2010). Article Google Scholar * Winkler, A., Schaffert, M. & Schmutzhard, E. Epilepsy
in resource poor countries–suggestion of an adjusted classification. _Epilepsia_ 48(5), 1029–1030 (2007). Article Google Scholar * Scott, R. A., Lhatoo, S. D. & Sander, J. W. The
treatment of epilepsy in developing countries: where do we go from here?. _Bull. World Health Org._ 79(4), 344–351 (2001). CAS PubMed Google Scholar * Asadi-pooya, A. A. &
Torabi-nami, M. Knowledge and attitude towards epilpesy among biology teachers in Fars province, Iran. _Iran J. Child Neurol._ 6, 13–18 (2012). Google Scholar * Kwan, P. _et al._ Definition
of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. _Epilepsia_ 51(6), 1069–1077 (2010). Article CAS Google Scholar
* Mehvari Habibabadi, J. _et al._ Outcome of lesional epilepsy surgery: Report of the first comprehensive epilepsy program in Iran. _Neurol. Clin. Pract._ 9(4), 286–295 (2019). Article
Google Scholar * Arifin, M. T. _et al._ Surgery for radiologically normal-appearing temporal lobe epilepsy in a Centre with limited resources. _Sci. Rep._ 10, 8144.
https://doi.org/10.1038/s41598-020-64968-4 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Sanyal, S. Relieving the burden of intractable epilepsy in India and other
developing countries: The case for two tier epilepsy centers. _Neurol. Asia_ 12, 23–28 (2007). Google Scholar * Dash, G. K. _et al._ An audit of the presurgical evaluation and patient
selection for extratemporal resective epilepsy surgery in a resource-poor country. _Seizure_ 21(5), 361–366 (2012). Article Google Scholar * Mikati, M. A. _et al._ Epilepsy surgery in a
developing country (Lebanon): Ten years experience and predictors of outcome. _Epileptic Disord._ 14(3), 267–274 (2012). Article Google Scholar * Fandino-Franky, J., Torres, M., Narino, D.
& Fandino, J. Corpus callosotomy in colombia and some reflections on care and research among the poor in developing countries. _Epilepsia_ 41, S22–S27 (2000). Article Google Scholar *
Rao, M. B. & Radhakrishnan, K. Is epilepsy surgery possible in countries with limited resources’. _Epilepsia_ 41, S31–S34 (2005). Article Google Scholar * Wieser, H.-G. &
Silfvenius, H. Overview: Epilepsy surgery in developing countries. _Epilepsia_ 41, S3–S9 (2000). Article Google Scholar * Englot, D. J., Breshears, J. D., Sun, P. P., Chang, E. F. &
Auguste, K. I. Seizure outcomes after resective surgery for extra-temporal lobe epilepsy in pediatric patients. _J. Neurosurg. Pediatr._ 12(2), 126–133.
https://doi.org/10.3171/2013.5.PEDS1336 (2013). Article PubMed Google Scholar * Englot, D. J. _et al._ Seizure outcomes after temporal lobectomy in pediatric patients. _J. Neurosurg.
Pediatr._ 12(2), 134–141. https://doi.org/10.3171/2013.5.PEDS12526 (2013). Article PubMed Google Scholar * Englot, D. J., Wang, D. D., Rolston, J. D., Shih, T. T. & Chang, E. F. Rates
and predictors of long-term seizure-freedom after frontal lobe epilepsy surgery: a systematic review and meta-analysis. _J. Neurosurg._ 116(5), 1042–1048.
https://doi.org/10.3171/2012.1.JNS111620 (2012). Article PubMed Google Scholar * Englot, D. J. _et al._ Seizure types and frequency in patients who “fail” temporal lobectomy for
intractable epilepsy. _Neurosurgery_ 73(5), 838–844. https://doi.org/10.1227/NEU.0000000000000120 (2013). Article PubMed Google Scholar * Englot, D. J. _et al._ Effects of temporal
lobectomy on consciousness-impairing and consciousness-sparing seizures in children. _Childs Nerv. Syst._ 29(10), 1915–1922. https://doi.org/10.1007/s00381-013-2168-7 (2013). Article PubMed
Google Scholar * Wiebe, S., Blume, W. T., Girvin, J. P. & Eliasziw, M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. _N. Engl. J. Med._ 45(5), 311–318.
https://doi.org/10.1056/NEJM200108023450501 (2001). Article Google Scholar * Dupont, S. _et al._ Long term prognosis and psychosocial outcomes after surgery for MTLE. _Epilepsia_ 47,
2115–2124 (2006). Article Google Scholar * Salanova, V., Markand, O. & Worth, R. Longitudinal follow-up in 145 patients with medically refractory temporal lobe epilepsy treated
surgically between 1984 and 1995. _Epilepsia_ 40, 1417–1423 (1999). Article CAS Google Scholar * Elwes, R. D., Dunn, G., Binnie, C. D. & Polkey, C. E. Outcome following resective
surgery for temporal lobe epilepsy: A prospective follow-up study of 102 consecutive cases. _J. Neurol. Neurosurg. Psychiatry_ 54, 949–952 (1991). Article CAS Google Scholar * Paglioli,
E. _et al._ Survival analysis of the surgical outcome of temporal lobe epilepsy due to hippocampal sclerosis. _Epilepsia_ 45, 1383–1391 (2004). Article Google Scholar * Buckingham, S. E.
_et al._ Latency to first seizure after temporal lobectomy predicts long-term outcome. _Epilepsia._ 51, 1987–1993 (2010). Article Google Scholar * Dunlea, O. _et al._ The Irish epilepsy
surgery experience: Long-term follow-up. _Seizure_ 19, 247–252 (2010). Article Google Scholar * Cohen-Gadol, A. A. _et al._ Long-term outcome of epilepsy surgery among 399 patients with
nonlesional seizure foci including mesial temporal lobe sclerosis. _J. Neurosurg._ 104, 513–524 (2006). Article Google Scholar * Berkovic, S. F. _et al._ Preoperative MRI predicts outcome
of temporal lobectomy: an actuarial analysis. _Neurology_ 45, 1358–1363 (1995). Article CAS Google Scholar * Burneo, J. G., Villanueva, V., Knowlton, R. C., Faught, R. E. & Kuzniecky,
R. I. Kaplan-Meier analysis on seizure outcome after epilepsy surgery: Do gender and race influence it?. _Seizure_ 17, 314–319 (2008). Article Google Scholar * Clusmann, H. _et al._
Prognostic factors and outcome after different types of resection for temporal lobe epilepsy. _J. Neurosurg._ 97, 1131–1141 (2002). Article Google Scholar * Mbuba, C. K., Ngugi, A. K.,
Newton, C. R. & Carter, J. A. The epilepsy treatment gap in developing countries: A systematic review of the magnitude, causes, and intervention strategies. _Epilepsia_ 49, 1491–1503
(2008). Article Google Scholar * Meinardi, H., Scott, R. A., Reis, R. & Sander, J. W. A. S. The treatment gap in epilepsy: The current situation and ways forward. _Epilepsia_ 42,
136–149 (2001). Article CAS Google Scholar * Espinosa-Jovel, C., Toledano, R., Aledo-Serrano, Á., García-Morales, I. & Gil-Nagel, A. Epidemiological profile of epilepsy in low income
populations. _Seizure_ 56, 67–72 (2018). Article Google Scholar * Qiu, J. Epilepsy surgery: Challenges for developing countries. _Lancet Neurol._ 8, 420–421 (2009). Article Google Scholar
* Li, W. _et al._ The experience of the multidisciplinary team in epilepsy management from a resource-limited country. _Epilepsia Open_ 4, 85–91 (2019). Article Google Scholar *
Asadi-Pooya, A. A. & Sperling, M. R. Strategies for surgical treatment of epilepsies in developing countries. _Epilepsia_ 49, 381–385 (2008). Article Google Scholar * Palmini, A.
Medical and surgical strategies for epilepsy care in developing countries. _Epilepsia_ 41, S10–S17 (2000). Article Google Scholar * Kawai, K. Epilepsy surgery: Current status and ongoing
challenges. _Neurol. Med. Chir._ 55, 357–366 (2015). Article Google Scholar * Cukiert, A. _et al._ Outcome after corticoamygdalohippocampectomy in patients with refractory temporal lobe
epilepsy and mesial temporal sclerosis without preoperative ictal recording. _Epilepsia_ 50(6), 1371–1376 (2009). Article Google Scholar Download references ACKNOWLEDGEMENTS We would like
to thank all medics, paramedics, staff, and patients involved in our epilepsy surgery program. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Neurosurgery, Faculty of Medicine,
Diponegoro University, Semarang City, Central Java Province, Indonesia Muhamad Thohar Arifin, Yuriz Bakhtiar, Jacob Bunyamin, Rofat Askoro, Surya Pratama Brilliantika, Novita Ikbar
Khairunnisa & Zainal Muttaqin * Department of Neurosurgery, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima City, Kagoshima Prefecture, Japan Ryosuke
Hanaya & Kazunori Arita * Department of Neurology, Faculty of Medicine, Diponegoro University, Semarang City, Central Java Province, Indonesia Aris Catur Bintoro * Department of
Neurosurgery, Graduate School of Biomedical and Health Sciences, Hiroshima University, Higashihiroshima City, Hiroshima Prefecture, Japan Koji Iida & Kaoru Kurisu Authors * Muhamad
Thohar Arifin View author publications You can also search for this author inPubMed Google Scholar * Ryosuke Hanaya View author publications You can also search for this author inPubMed
Google Scholar * Yuriz Bakhtiar View author publications You can also search for this author inPubMed Google Scholar * Aris Catur Bintoro View author publications You can also search for
this author inPubMed Google Scholar * Koji Iida View author publications You can also search for this author inPubMed Google Scholar * Kaoru Kurisu View author publications You can also
search for this author inPubMed Google Scholar * Kazunori Arita View author publications You can also search for this author inPubMed Google Scholar * Jacob Bunyamin View author publications
You can also search for this author inPubMed Google Scholar * Rofat Askoro View author publications You can also search for this author inPubMed Google Scholar * Surya Pratama Brilliantika
View author publications You can also search for this author inPubMed Google Scholar * Novita Ikbar Khairunnisa View author publications You can also search for this author inPubMed Google
Scholar * Zainal Muttaqin View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS R.H., K.A., A.C.B., K.I., K.K., and Z.M. conceived of the
presented idea. M.T.A., and Y.B. developed the theory and performed the computations. J.B., R.A., and S.P.B., N.I.K. verified the analytical methods. All authors discussed the results and
contributed to the final manuscript. CORRESPONDING AUTHOR Correspondence to Muhamad Thohar Arifin. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS
OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or
other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not
included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission
directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Thohar
Arifin, M., Hanaya, R., Bakhtiar, Y. _et al._ Initiating an epilepsy surgery program with limited resources in Indonesia. _Sci Rep_ 11, 5066 (2021).
https://doi.org/10.1038/s41598-021-84404-5 Download citation * Received: 23 June 2020 * Accepted: 16 February 2021 * Published: 03 March 2021 * DOI:
https://doi.org/10.1038/s41598-021-84404-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative