- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT We developed and validated a deep learning (DL)-based model using the segmentation method and assessed its ability to detect lung cancer on chest radiographs. Chest radiographs for
use as a training dataset and a test dataset were collected separately from January 2006 to June 2018 at our hospital. The training dataset was used to train and validate the DL-based model
with five-fold cross-validation. The model sensitivity and mean false positive indications per image (mFPI) were assessed with the independent test dataset. The training dataset included 629
radiographs with 652 nodules/masses and the test dataset included 151 radiographs with 159 nodules/masses. The DL-based model had a sensitivity of 0.73 with 0.13 mFPI in the test dataset.
Sensitivity was lower in lung cancers that overlapped with blind spots such as pulmonary apices, pulmonary hila, chest wall, heart, and sub-diaphragmatic space (0.50–0.64) compared with
those in non-overlapped locations (0.87). The dice coefficient for the 159 malignant lesions was on average 0.52. The DL-based model was able to detect lung cancers on chest radiographs,
with low mFPI. SIMILAR CONTENT BEING VIEWED BY OTHERS EVALUATION OF THE FEASIBILITY OF EXPLAINABLE COMPUTER-AIDED DETECTION OF CARDIOMEGALY ON CHEST RADIOGRAPHS USING DEEP LEARNING Article
Open access 19 August 2021 BETTER PERFORMANCE OF DEEP LEARNING PULMONARY NODULE DETECTION USING CHEST RADIOGRAPHY WITH PIXEL LEVEL LABELS IN REFERENCE TO COMPUTED TOMOGRAPHY: DATA QUALITY
MATTERS Article Open access 10 July 2024 LOCALIZATION-ADJUSTED DIAGNOSTIC PERFORMANCE AND ASSISTANCE EFFECT OF A COMPUTER-AIDED DETECTION SYSTEM FOR PNEUMOTHORAX AND CONSOLIDATION Article
Open access 30 July 2022 INTRODUCTION Lung cancer is the primary cause of cancer death worldwide, with 2.09 million new cases and 1.76 million people dying from lung cancer in 20181. Four
case-controlled studies from Japan reported in the early 2000s that the combined use of chest radiographs and sputum cytology in screening was effective for reducing lung cancer mortality2.
In contrast, two randomized controlled trials conducted from 1980 to 1990 concluded that screening with chest radiographs was not effective in reducing mortality in lung cancer3,4. Although
the efficacy of chest radiographs in lung cancer screening remains controversial, chest radiographs are more cost-effective, easier to access, and deliver lower radiation dose compared with
low-dose computed tomography (CT). A further disadvantage of chest CT is excessive false positive (FP) results. It has been reported that 96% of nodules detected by low-dose CT screening are
FPs, which commonly leads to unnecessary follow-up and invasive examinations5. Chest radiography is inferior to chest CT in terms of sensitivity but superior in terms of specificity. Taking
these characteristics into consideration, the development of a computer-aided diagnosis (CAD) model for chest radiograph would have value by improving sensitivity while maintaining low FP
results. The recent application of convolutional neural networks (CNN), a field of deep learning (DL)6,7, has led to dramatic, state-of-the-art improvements in radiology8. DL-based models
have also shown promise for nodule/mass detection on chest radiographs9,10,11,12,13, which have reported sensitivities in the range of 0.51–0.84 and mean number of FP indications per image
(mFPI) of 0.02–0.34. In addition, radiologist performance for detecting nodules was better with these CAD models than without them9. In clinical practice, it is often challenging for
radiologists to detect nodules and to differentiate between benign and malignant nodules. Normal anatomical structures often appear as if they are nodules, which is why radiologists must pay
careful attention to the shape and marginal properties of nodules. As these problems are caused by the conditions rather than the ability of the radiologist, even skillful radiologists can
misdiagnose14,15. There are two main methods for detecting lesions using DL: detection and segmentation. The detection method is a region-level classification, whereas the segmentation
method is a pixel-level classification. The segmentation method can provide more detailed information than the detection method. In clinical practice, classifying the size of a lesion at the
pixel-level increases the likelihood of making a correct diagnosis. Pixel-level classification also makes it easier to follow up on changes in lesion size and shape, since the shape can be
used as a reference during detection. It also makes it possible to consider not only the long and short diameters but also the area of the lesion when determining the effect of treatment16.
However, to our knowledge, there are no studies using the segmentation method to detect pathologically proven lung cancer on chest radiographs. The purpose of this study was to train and
validate a DL-based model capable of detecting lung cancer on chest radiographs using the segmentation method, and to evaluate the characteristics of this DL-based model to improve
sensitivity while maintaining low FP results. The following points summarize the contributions of this article: * This study developed a deep learning-based model for detection and
segmentation of lung cancer on chest radiographs. * Our dataset is high quality because all the nodules/masses were pathologically proven lung cancers, and these lesions were pixel-level
annotated by two radiologists. * The segmentation method was more informative than the classification or detection methods, which is useful not only for the detection of lung cancer but also
for follow-up and treatment efficacy. MATERIALS AND METHODS STUDY DESIGN We retrospectively collected consecutive chest radiographs from patients who had been pathologically diagnosed with
lung cancer at our hospital. Radiologists annotated the lung cancer lesions on these chest radiographs. A DL-based model for detecting lung cancer on radiographs was trained and validated
with the annotated radiographs. The model was then tested with an independent dataset for detecting lung cancers. The protocol for this study was comprehensively reviewed and approved by the
Ethical Committee of Osaka City University Graduate School of Medicine (No. 4349). Because the radiographs had been acquired during daily clinical practice and informed consent for their
use in research had been obtained from patients, the Ethical Committee of Osaka City University Graduate School of Medicine waived the need for further informed consent. All methods were
performed in accordance with the relevant guidelines and regulations. ELIGIBILITY AND GROUND TRUTH LABELLING Two datasets were used to train and test the DL-based model, a training dataset
and a test dataset. We retrospectively collected consecutive chest radiographs from patients pathologically diagnosed with lung cancer at our hospital. The training dataset was comprised of
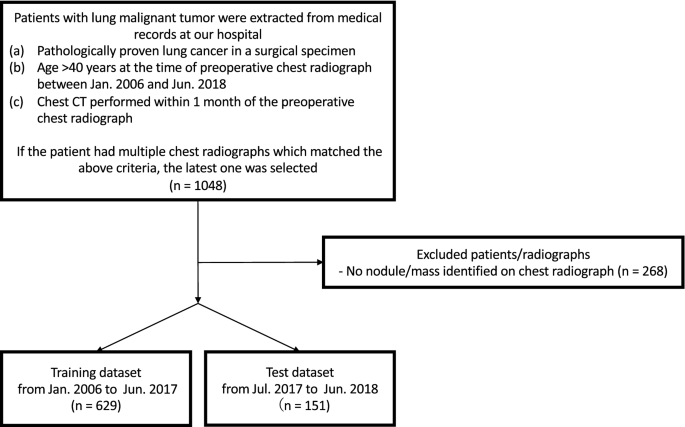
chest radiographs obtained between January 2006 and June 2017, and the test dataset contained those obtained between July 2017 and June 2018. The inclusion criteria were as follows: (a)
pathologically proven lung cancer in a surgical specimen; (b) age > 40 years at the time of the preoperative chest radiograph; (c) chest CT performed within 1 month of the preoperative
chest radiograph. If the patient had multiple chest radiographs that matched the above criteria, the latest radiograph was selected. Most of these chest radiographs were taken as per routine
before hospitalization and were not intended to detect lung cancer. Chest radiographs on which radiologists could not identify the lesion, even with reference to CT, were excluded from
analysis. For eligible radiographs, the lesions were annotated by two general radiologists (A.S. and D.U.), with 6 and 7 years of experience in chest radiography, using ITK-SNAP version
3.6.0 (http://www.itksnap.org/)_._ These annotations were defined as ground truths. The radiologists had access to the chest CT and surgical reports and evaluated the lesion characteristics
including size, location, and edge. If > 50% of the edge of the nodule was traceable, the nodule was considered to have a “traceable edge”; if not, it was termed an “untraceable edge”.
MODEL DEVELOPMENT We adopted the CNN architecture using segmentation method. The segmentation method outputs more information than the detection method (which present a bounding box) or the
classification method (which determine the malignancy from a single image). Maximal diameter of the tumor is particularly important in clinical practice. Since the largest diameter of the
tumor often coincides with an oblique direction, not the horizontal nor the vertical direction, it is difficult to measure with detection methods which present a bounding box. Our CNN
architecture was based on the encoder-decoder architecture to output segmentation17. The encoder-decoder architecture has a bottleneck structure, which reduces the resolution of the feature
map and improves the model robustness to noise and overfitting18. In addition, one characteristic of this DL-based model is that it used both a normal chest radiograph and a black-and-white
inversion of a chest radiograph. This is an augmentation that makes use of the experience of radiologists19. It is known that black-and-white inversion makes it easier to confirm the
presence of lung lesions overlapping blind spots. We considered that this augmentation could be effective for this model as well, so we applied a CNN architecture to each of the normal and
inverted images and then an ensemble model using these two architectures20. Supplementary Fig. S1 online shows detailed information of the model. Using chest radiographs from the training
dataset, the model was trained and validated from scratch, utilizing five-fold cross-validation. The model when the value of the loss function was the smallest within 100 epochs using Adam
(learning rate = 0.001, beta_1 = 0.9, beta_2 = 0.999, epsilon = 0.00000001, decay = 0.0) was adopted as the best-performing. MODEL ASSESSMENT A detection performance test was performed on a
per-lesion basis using the test dataset to evaluate whether the model could identify malignant lesions on radiographs. The model calculated the probability of malignancy in a lesion detected
on chest radiographs as an integer between 0 and 255. If the center of output generated by the model was within the ground truth, it was considered true positive (TP). All other outputs
were FPs. When two or more TPs were proposed by the model for one ground truth, they were considered as one TP. If there was no output from the model for one ground truth, it was one FN. Two
radiologists (A.S. and D.U.) retrospectively referred to the radiograph and CT to evaluate what structures were detected by the FP output. The dice coefficient was also used to evaluate
segmentation performance. STATISTICAL ANALYSIS In the detection performance test, metrics were evaluated on a per-lesion basis. We used the free-response receiver-operating characteristic
(FROC) curve to evaluate whether the bounding boxes proposed by the model accurately identified malignant cancers in radiographs21. The vertical axis of the FROC curve is sensitivity and the
horizontal axis is mFPI. Sensitivity is the number of TPs that the model was able to identify divided by the number of ground truths. The mFPI is the number of FPs that the model mistakenly
presented divided by the number of radiographs in the dataset. Thus, the FROC curve shows sensitivity as a function of the number of FPs shown on the image. One of the authors (D.U.)
performed all analyses, using R version 3.6.0 (https://www.r-project.org/). The FROC curves were plotted by R software. All statistical inferences were performed with two-sided 5%
significance level. RESULTS DATASETS Figure 1 shows a flowchart of the eligibility criteria for the chest radiographs. For the training dataset, 629 radiographs with 652 nodules/masses were
collected from 629 patients (age range 40–91 years, mean age 70 ± 9.0 years, 221 women). For the test dataset, 151 radiographs with 159 nodules/masses were collected from 151 patients (age
range 43–84 years, mean age 70 ± 9.0 years, 57 women) (Table 1). MODEL TEST The DL-based model had sensitivity of 0.73 with 0.13 mFPI in the test dataset (Table 2). The FROC curve is shown
in Fig. 2. The highest sensitivity the model attained was 1.00 for cancers with a diameter of 31–50 mm, and the second highest sensitivity was 0.85 for those with a diameter > 50 mm. For
lung cancers that overlapped with blind spots such as the pulmonary apices, pulmonary hila, chest wall, heart, or sub-diaphragmatic space, sensitivity was 0.52, 0.64, 0.52, 0.56, and 0.50,
respectively. The sensitivity of lesions with traceable edges on radiographs was 0.87, and that for untraceable edges was 0.21. Detailed results are shown in Table 2. The dice coefficient
for all 159 lesions was on average 0.52 ± 0.37 (standard deviation, SD). For 116 lesions detected by the model, the dice coefficient was on average 0.71 ± 0.24 (SD). The dice coefficient for
all 71 lesions overlapping blind spots was 0.34 ± 0.38 (SD). For 39 lesions detected by the model that overlapped with blind spots, the dice coefficient was 0.62 ± 0.29 (SD). Of the 20 FPs,
19 could be identified as some kind of structure on the chest radiograph by radiologists (Table 3). In these 20 FPs, 13 overlapped with blind spots. There were 43 FNs, ranging in size from
9 to 72 mm (mean 21 ± 15 mm), 32 of which overlapped with blind spots (Table 4). There were four FNs > 50 mm, all of which overlapped with blind spots. Figure 3 shows representative cases
of our model. Figure 4 shows overlapping of a FP output with normal anatomical structures and Fig. 5 shows a FN lung cancer that overlapped with a blind spot. Supplementary Fig. S2 online
shows visualized images of the first and last layers. An ablation study to use black-and-white inversion images is shown in Supplementary Data online. DISCUSSION In this study, we developed
a model for detecting lung cancer on chest radiographs and evaluated its performance. Adding pixel-level classification of lesions in the proposed DL-based model resulted in sensitivity of
0.73 with 0.13 mFPI in the test dataset. To our knowledge, ours is the first study to use the segmentation method to detect pathologically proven lung cancer on chest radiographs. We found
several studies that used classification or detection methods to detect lung cancer on chest radiographs, but not the segmentation method. Since the segmentation method has more information
about the detected lesions than the classification or detection methods, it has advantages not only in the detection of lung cancer but also in follow-up and treatment efficacy. We achieved
performance as high as that in similar previous studies9,10,11,12,13 using DL-based lung nodule detection models, with fewer training data. It is particularly noteworthy that the present
method achieved low mFPI. In previous studies, sensitivity and mFPI were 0.51–0.84 and 0.02–0.34, respectively, and used 3,500–13,326 radiographs with nodules or masses as the training data,
compared with the 629 radiographs used in the present study. Although comparisons to these studies are difficult because the test datasets were different, our accuracy was similar to that
of the detection models employed in most of the previous studies. We performed pixel-level classification of the lesions based on the segmentation method and included for analysis only
lesions that were pathologically proven to be malignant, based on examination of surgically resected specimens. All previous studies9,10,11,12,13 have included potentially benign lesions,
clinically malignant lesions, or pathologically malignant lesions by biopsy in their training data. Therefore, our model may be able to analyze the features of the malignant lesions in more
detail. In regard with the CNN, we created this model based on Inception-ResNet-v217, which combines the Inception structure and the Residual connection. In the Inception-ResNet block,
convolutional filters of multiple sizes are combined with residual connections. The use of residual connections not only avoids the degradation problem caused by deep structures but also
reduces the training time. In theory, the combination of these features further improves the recognition accuracy and learning efficiency17. By using this model with combining normal and
black-white-inversion images, our results achieved comparable or better performance with fewer training data than previous studies. In regard with the robustness of the model, we consider
this model to be relatively robust against imaging conditions or body shape because we consecutively collected the dataset and did not set any exclusion criteria based on imaging conditions
or body shape. The dice coefficient for 159 malignant lesions was on average 0.52. On the other hand, for the 116 lesions detected by the model, the dice coefficient was on average 0.71.
These values provide a benchmark for the segmentation performance of lung cancer on chest radiograph. The 71 lesions which overlapped with blind spots tended to have a low dice coefficient
with an average of 0.34, but for 39 lesions detected by the model that overlapped with blind spots, the average dice coefficient was 0.62. This means that lesions overlapping blind spots
were not only difficult to detect, but also had low accuracy in segmentation. On the other hand, the segmentation accuracy was relatively high for lesions that were detected by the model
even if they overlapped with the blind spots. Two interesting tendencies were found after retrospectively examining the characteristics of FP outputs. First, 95% (19/20) FPs could be
visually recognized on chest radiographs as nodule/mass-like structures. The model identified some nodule-like structures (FPs), which overlapped with vascular shadows and ribs. This is also
the case for radiologists in daily practice. Second, nodules with calcification overlapped with normal anatomical structures tended to be misdiagnosed by the model (FPs). Five FPs were
non-malignant calcified lung nodules on CT and also overlapped with the heart, clavicle or ribs. As the model was trained only on malignant nodules without calcification in the training
dataset, calcified nodules should not be identified in theory. Most calcified nodules are actually not identified by the model, however, this was not the case for calcified nodules that
overlapped with normal anatomical structures. In other word, there is a possibility that the model could misidentify the lesion as a malignant if the features of calcification that should
signal a benign lesion are masked by normal anatomical structures. When we investigated FNs, we found that nodules in blind spots and metastatic nodules tended to be FNs. With regard to
blind spots, our model showed a decrease in sensitivity for lesions that overlapped with normal anatomical structures. It was difficult for the model to identify lung cancers that overlapped
with blind spots even when the tumor size was large (Fig. 5). In all FNs larger than 50 mm, there was wide overlap with normal anatomical structures, for the possible reason that it becomes
difficult for the model to detect subtle density differences in lesions that overlapped with large structures such as the heart. With regard to metastatic nodules, 33% (14/43) metastatic
lung cancers were FNs. These metastatic nodules ranged in size from 10 to 20 mm (mean 14 ± 3.8 mm) and were difficult to visually identify on radiographs, even with reference to CT. In fact,
the radiologists had overlooked most of the small metastatic nodules at first and could only identify them retrospectively, with knowledge of the type of lung cancer and their locations.
There are some limitations of this study. The model was developed using a dataset collected from a single hospital. Although our model achieved high sensitivity with low FPs, the number of
FPs may be higher in a screening cohort and the impact of this should be considered. Furthermore, an observer’s performance study is needed to evaluate the clinical utility of the model. In
this study, we included only chest radiographs containing malignant nodules/masses. The fact that we used only pathologically proven lung cancers and pixel-level annotations by two
radiologists in our dataset is a strength of our study, on the other hand, it may reduce the detection rate of benign nodules/masses. This is often not a problem in clinical practice.
Technically, all areas other than the malignant nodules/masses could be trained as normal areas. However, normal images should be mixed in and tested to evaluate the model for detailed
examination in clinical practice. In conclusion, a DL-based model developed using the segmentation method showed high performance in the detection of lung cancer on chest radiographs.
Compared with CT, chest radiographs have advantages in terms of accessibility, cost effectiveness, and low radiation dose. However, the known effectiveness of the model for lung cancer
detection is limited. We believe that a CAD model with higher performance can support clinical detection and interpretation of malignant lesions on chest radiographs and offers additive
value in lung cancer detection. REFERENCES * Bray, F. _et al._ Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. _CA
Cancer J. Clin._ 68, 394–424. https://doi.org/10.3322/caac.21492 (2018). Article PubMed Google Scholar * Sagawa, M. _et al._ The efficacy of lung cancer screening conducted in 1990s: Four
case–control studies in Japan. _Lung Cancer_ 41, 29–36. https://doi.org/10.1016/s0169-5002(03)00197-1 (2003). Article PubMed Google Scholar * Fontana, R. S. _et al._ Lung cancer
screening: The Mayo program. _J. Occup. Med._ 28, 746–750. https://doi.org/10.1097/00043764-198608000-00038 (1986). Article CAS PubMed Google Scholar * Kubik, A. _et al._ Lack of benefit
from semi-annual screening for cancer of the lung: Follow-up report of a randomized controlled trial on a population of high-risk males in Czechoslovakia. _Int. J. Cancer_ 45, 26–33.
https://doi.org/10.1002/ijc.2910450107 (1990). Article CAS PubMed Google Scholar * Raghu, V. K. _et al._ Feasibility of lung cancer prediction from low-dose CT scan and smoking factors
using causal models. _Thorax_ 74, 643–649. https://doi.org/10.1136/thoraxjnl-2018-212638 (2019). Article PubMed Google Scholar * Hinton, G. Deep learning—a technology with the potential
to transform health care. _JAMA_ 320, 1101–1102. https://doi.org/10.1001/jama.2018.11100 (2018). Article PubMed Google Scholar * LeCun, Y., Bengio, Y. & Hinton, G. Deep learning.
_Nature_ 521, 436–444. https://doi.org/10.1038/nature14539 (2015). Article ADS CAS PubMed Google Scholar * Ueda, D., Shimazaki, A. & Miki, Y. Technical and clinical overview of deep
learning in radiology. _Jpn. J. Radiol._ 37, 15–33. https://doi.org/10.1007/s11604-018-0795-3 (2019). Article PubMed Google Scholar * Nam, J. G. _et al._ Development and validation of
deep learning–based automatic detection algorithm for malignant pulmonary nodules on chest radiographs. _Radiology_ 290, 218–228. https://doi.org/10.1148/radiol.2018180237 (2019). Article
PubMed Google Scholar * Park, S. _et al._ Deep learning-based detection system for multiclass lesions on chest radiographs: Comparison with observer readings. _Eur. Radiol._ 30, 1359–1368.
https://doi.org/10.1007/s00330-019-06532-x (2020). Article PubMed Google Scholar * Yoo, H., Kim, K. H., Singh, R., Digumarthy, S. R. & Kalra, M. K. Validation of a deep learning
algorithm for the detection of malignant pulmonary nodules in chest radiographs. _JAMA Netw. Open_ 3, e2017135. https://doi.org/10.1001/jamanetworkopen.2020.17135 (2020). Article PubMed
PubMed Central Google Scholar * Sim, Y. _et al._ Deep convolutional neural network–based software improves radiologist detection of malignant lung nodules on chest radiographs. _Radiology_
294, 199–209. https://doi.org/10.1148/radiol.2019182465 (2020). Article PubMed Google Scholar * Hwang, E. J. _et al._ Development and validation of a deep learning-based automated
detection algorithm for major thoracic diseases on chest radiographs. _JAMA Netw. Open_ 2, e191095. https://doi.org/10.1001/jamanetworkopen.2019.1095 (2019). Article PubMed PubMed Central
Google Scholar * Manser, R. _et al._ Screening for lung cancer. _Cochrane Database Syst Rev_ CD001991. https://doi.org/10.1002/14651858.CD001991.pub3 (2013). * Berlin, L. Radiologic
errors, past, present and future. _Diagnosis (Berl)_ 1, 79–84. https://doi.org/10.1515/dx-2013-0012 (2014). Article Google Scholar * From the RECIST committee. Schwartz, L.H. et al. RECIST
1.1-Update and clarification. _Eur. J. Cancer._ 62, 132–137. https://doi.org/10.1016/j.ejca.2016.03.081 (2016). Article Google Scholar * Szegedy, C., Ioffe, S., Vanhoucke, V. & Alemi,
A. A. Inception-v4, Inception-ResNet and the Impact of Residual Connections on Learning_. In Proceedings of the Thirty-First AAAI Conference on Artificial Intelligence_, 4278–4284 (AAAI
Press, San Francisco, California, USA, 2017). * Matějka P. et al. Neural Network Bottleneck Features for Language Identification. _In Proceedings of Odyssey 2014. vol. 2014. International
Speech Communication Association_, 299–304 (2014). * Sheline, M. E. _et al._ The diagnosis of pulmonary nodules: Comparison between standard and inverse digitized images and conventional
chest radiographs. _Am. J. Roentgenol._ 152(2), 261–263. https://doi.org/10.2214/ajr.152.2.261 (1989). Article CAS Google Scholar * Wang, G., Hao, J., Ma, J. & Jiang, H. A comparative
assessment of ensemble learning for credit scoring. _Expert. Syst. Appl._ 38(1), 223–230 (2011). Article Google Scholar * Bunch, P., Hamilton, J., Sanderson, G. & Simmons, A. A free
response approach to the measurement and characterization of radiographic observer performance. _Proc. SPIE_ 127, 124–135. https://doi.org/10.1117/12.955926 (1977). Article ADS Google
Scholar Download references ACKNOWLEDGEMENTS We are grateful to LPIXEL Inc. for joining this study. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Diagnostic and Interventional
Radiology, Graduate School of Medicine, Osaka City University, Osaka, Japan Akitoshi Shimazaki, Daiju Ueda, Akira Yamamoto, Takashi Honjo & Yukio Miki * Smart Life Science Lab, Center
for Health Science Innovation, Osaka City University, Osaka, Japan Daiju Ueda * LPIXEL Inc, Tokyo, Japan Antoine Choppin & Yuki Shimahara Authors * Akitoshi Shimazaki View author
publications You can also search for this author inPubMed Google Scholar * Daiju Ueda View author publications You can also search for this author inPubMed Google Scholar * Antoine Choppin
View author publications You can also search for this author inPubMed Google Scholar * Akira Yamamoto View author publications You can also search for this author inPubMed Google Scholar *
Takashi Honjo View author publications You can also search for this author inPubMed Google Scholar * Yuki Shimahara View author publications You can also search for this author inPubMed
Google Scholar * Yukio Miki View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors contributed to the study conception and design.
Material preparation, data collection and analysis were performed by A.S., D.U., A.Y. and T.H. Model development was performed by A.C. and Y.S. The first draft of the manuscript was written
by A.S. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. CORRESPONDING AUTHOR Correspondence to Daiju Ueda. ETHICS
DECLARATIONS COMPETING INTERESTS Akitoshi Shimazaki has no relevant relationships to disclose. Daiju Ueda has no relevant relationships to disclose. Antoine Choppin is an employee of LPIXEL
Inc. Akira Yamamoto has no relevant relationships to disclose. Takashi Honjo has no relevant relationships to disclose. Yuki Shimahara is the CEO of LPIXEL Inc. Yukio Miki has no relevant
relationships to disclose. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which
permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to
the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless
indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory
regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Shimazaki, A., Ueda, D., Choppin, A. _et al._ Deep learning-based algorithm for
lung cancer detection on chest radiographs using the segmentation method. _Sci Rep_ 12, 727 (2022). https://doi.org/10.1038/s41598-021-04667-w Download citation * Received: 04 August 2021 *
Accepted: 29 December 2021 * Published: 14 January 2022 * DOI: https://doi.org/10.1038/s41598-021-04667-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative







