- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Prediction of complex epidemiological systems such as COVID-19 is challenging on many grounds. Commonly used compartmental models struggle to handle an epidemiological process that
evolves rapidly and is spatially heterogeneous. On the other hand, machine learning methods are limited at the beginning of the pandemics due to small data size for training. We propose a
deep learning approach to predict future COVID-19 infection cases and deaths 1 to 4 weeks ahead at the fine granularity of US counties. The multi-variate Long Short-term Memory (LSTM)
recurrent neural network is trained on multiple time series samples at the same time, including a mobility series. Results show that adding mobility as a variable and using multiple samples
to train the network improve predictive performance both in terms of bias and of variance of the forecasts. We also show that the predicted results have similar accuracy and spatial patterns
with a standard ensemble model used as benchmark. The model is attractive in many respects, including the fine geographic granularity of predictions and great predictive performance several
weeks ahead. Furthermore, data requirement and computational intensity are reduced by substituting a single model to multiple models folded in an ensemble model. SIMILAR CONTENT BEING
VIEWED BY OTHERS A NOVEL BIDIRECTIONAL LSTM DEEP LEARNING APPROACH FOR COVID-19 FORECASTING Article Open access 20 October 2023 SPATIO-TEMPORAL EPIDEMIC FORECASTING USING MOBILITY DATA WITH
LSTM NETWORKS AND ATTENTION MECHANISM Article Open access 20 March 2025 FINE-GRAINED FORECASTING OF COVID-19 TRENDS AT THE COUNTY LEVEL IN THE UNITED STATES Article Open access 11 April 2025
INTRODUCTION The highly infectious COVID-19 pandemic that has been with us since the early months of 2020 has been adversely affecting public health worldwide with about 155 million
reported cases of infection and 32 million deaths as of May 5, 20211,2,3. Despite adopting non-pharmaceutical social distancing measures (e.g., travel ban, cancellation of flights,
restrictions on gatherings, closure of schools and public transport) and pharmaceutical measures (i.e., vaccination), the number of coronavirus cases increased alarmingly fast in the United
States and elsewhere throughout large periods of the pandemic4,5,6,7,8. Since March 2021, the COVID-19 pandemic has jumped globally towards a new peak exceeding the previous peak of January
2021 due to uncontrolled outbursts in India, Europe, and South America3,5. Considering the catastrophic consequences of this pandemic, this study envisioned to develop a purely data-driven
model to forecast the number of infection cases and deaths 4 weeks ahead using a multi-variate deep long short-term memory (LSTM) network to guide policymakers to make timely appropriate
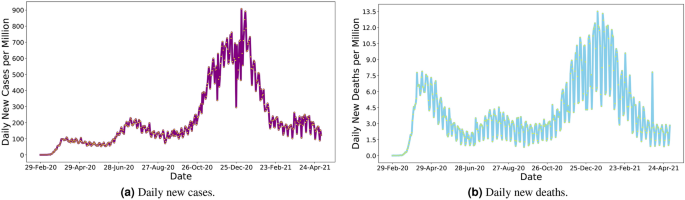
decisions. Although COVID-19 related infection cases and deaths have decreased by the time of this study, US citizens experienced the highest peak of the pandemic from November 2020 to
February 2021 (Fig. 1). Despite strong pharmaceutical and non-pharmaceutical control measures, large cohorts of people have been affected daily in all states (Fig. 2). Well into this
pandemic, the US has remained the most affected country in the world, with 21.39% and 18.23% of global confirmed cases and deaths, as of May 5, 20212. However, the severity of the pandemic
has started to taper off nationally since late February thanks to the successes of recent vaccine administration (i.e., 32% of people was fully vaccinated and 45% had at least one dose as of
May 5, 2021)3. Moreover, multiple new variants of the COVID-19 virus that transmit more readily from person to person and change the effectiveness of the vaccines are emerging and
circulating in the US and around the world10. Additionally, people are hesitant to receive vaccine against COVID-19 infection in the US11. Thus, a combination of evidence-based
pharmaceutical and non-pharmaceutical public health mitigation measures remains important to curtail viral transmission locally and beyond12,13,14,15,16,17,18. LITERATURE REVIEW The extant
literature shows that, from the inception of the pandemic, researchers from around the world have been developing and implementing COVID-19 prediction models to understand the severity of
the pandemic, delineate associated factors of virus infection, recovery, and death, and support the design of effective policies and operational measures to manage this unprecedented public
health crisis1,15,20,21,22. A wide range of methods and tools has been used in predictive models. Many studies have used machine learning and deep learning (e.g., random forest, support
vector machine, decision tree, artificial neural network, ridge and lasso regression, nearest neighbor methods) to predict the transmission of the COVID-19 virus as well as individual and
group responses to it23,24,25,26. A voluminous body of research has also been conducted with traditional epidemiological models to estimate viral diffusion and forecast the impact of policy
interventions on the rate of infection (e.g., the Susceptible-exposed-infectious-removed (SEIR) model and variants of it)27,28,29,30,31. However, compartmental models share several critical
limitations. Most importantly, they are dependent on a considerable number of hypothesized input parameters, including the probabilities for transitions of population between the S, E, I and
R states. Because of the strong sensitivity of SEIR models to changes in these input parameters, predictive accuracy can be substantially downgraded. In addition, SEIR models are based on
oversimplifying assumptions, one of which being that the probability values or transition rates are homogeneous over population32 and constant over time. Instead, the rates of transition
between the S, E, I and R population compartments for COVID-19 change over time and are very sensitive to socio-demographic conditions and mitigation policies. To alleviate some of these
drawbacks, other classes of models have been advanced for estimating the probability of COVID-19 transmission and identifying compliance with social distancing measures. These include
various hybrid models integrating neural networks and SEIR modeling33,34, simulation systems (e.g., agent-based models)35,36,37 and video processing for object detection (e.g., You Only Look
Once (YOLO))38,39. Some other studies have used econometric models (e.g., linear regression, structural equation model) to identify the factors that influence the diffusion of COVID-19 and
associated mitigation measures16,40. The convergence of recent developments in Information and Communication Technologies and in data analytics (i.e., production, access and storage of data,
and analysis of information, cloud computing) has empowered people to apply Artificial Intelligence (AI) (e.g., machine and deep learning, text mining) to model and control complex issues
in health care systems41,42,43. Hence, AI-based techniques are widely used to monitor social distancing patterns of people and to assess scenarios on the transition of COVID-19 accurately15.
They can solve multi-scalar, endogenous, non-linear, and ambiguous problems and extract insights from complex, unstructured and large data sets using their computational ability with
reliable prediction compared to traditional approaches. Additionally, hybrid models (i.e., combining of multiple machine learning techniques) may enhance the robustness and generalization
ability of machine learning models to handle reputedly wicked problems, such as a viral pandemic, quickly and effectively compared to single machine learning techniques and simulation
models44,45. One of the main motivations to predict the COVID-19 pandemic with machine learning is that it can more effectively estimate the effects of social distancing measures on the
transmission of virus considering socio-economic control factors, which conventional econometric methods or compartmental models pain to capture15. However, high-quality data devoid of
collection biases, robust methodologies, and validation with external data sets and models are necessary to develop a ubiquitous, reliable, and trustworthy model to provide consistent
predictions across diverse systems (e.g., data, settlement contexts)15,43,46. Considering the rapid transmission of COVID-19 and the time lapse for the implementation of control measures,
many studies have conducted spatio-temporal disease modeling to understand the geographic exposure and evolution patterns of associated risks of the pandemic4,47,48,49,50,51. Spatio-temporal
models are widely used by researchers to explain the temporal progress of disease over geographic regions and patterns of infection and mortality rates52. Recent studies have demonstrated
that space and time are two critical factors for determining health risks during the COVID-19 pandemic53,54. Spatio-temporal models consider infection rates of the surrounding neighbors to
reduce the variability in the estimation that may exist locally52. These models also consider retrospective infection rates for short- and long-term prediction, controlling the irregular
reporting of daily confirmed cases and deaths. Thus, spatio-temporal models yield better prediction, with a higher prediction accuracy and a lower tendency to over- or under-fit compared to
traditional epidemiological and machine learning-based models47. In this study, we used a deep learning-based space-time LSTM network to predict future COVID-19 cases and deaths, which can
guide policymakers for timely interventions to reduce the severity of the pandemic and for more effective management of health care resources (e.g., nursing and medical staff, ICU beds,
supplies, etc) under emergency conditions. A wide range of studies have developed models for predicting COVID-19 cases and deaths and have investigated the factors that influence virus
diffusion using machine learning and deep learning15,21,55. They mentioned that lockdown and confinement measures, and socioeconomic factors significantly influence the outbreak of the
pandemic and its dynamics. Specifically, the family of deep recurrent neural networks have proven to be an attractive approach for epidemic forecast56 due to their acute capability to learn
time series. Among these methods, uni-variate57 and multi-variate58,59 LSTM models have been successful at predicting influenza and COVID-19, mainly due to their capability to memorize
long-term dependencies. DeepGLEAM60 uses a stochastic Diffusion Convolutional RNN (DCRNN) model, which considers short (commuting) and long range (air flight) mobility network connections,
to forecast COVID-19 deaths at county and state granularities. Other versions of recurrent neural networks, such as attention networks61 and bidirectional LSTM62 networks, have also been
reported to be successful. Taking stock of best practices in the extant literature, we propose a multi-variate and multi-time series long short-term memory (MTS-LSTM) network to
simultaneously forecast confirmed cases, deaths, and mobility of all sub-populations at the county level using a single model. A study predicting the COVID-19 pandemic at this local level
would provide unique insights for the policymakers to make targeted interventions to cope with the pandemic, given that many critical operational decisions and actions are made locally.
Previous models of epidemics have used a variety of covariates including mobility, underlying health, socio-demographic and socio-economic variables for predicting COVID-19 confirmed cases
and death counts. In line with this precedence, we started with the implementation of models with various sets of independent variables. These variables included population density and
measures at the sub-population level of race, educational attainment, age, family size and status, poverty, income, employment, housing, being in metropolitan or micropolitan area, health
insurance status, percentage of deaths from other health conditions, percentage change in the time spent at work from Google mobility reports63, and number of visits to Points of Interest
from SafeGraph Places Schema data sets64. After training a whole suite of model specifications and investigating the impact of these variables on pandemic severity by comparison of
respective Root Mean Square Error (RMSE) statistics, it was found that COVID-19 is predicted with the highest accuracy with the SafeGraph mobility data as the sole covariate feature. The
capability of the proposed deep learning model to predict COVID-19 dynamics with high performance with a single covariate is one of its distinctive strengths. DATA PRE-PROCESSING In this
research, we proposed a multi-variate recurrent neural network with LSTM layers to predict the dynamics of a pandemic, which learns from multiple sample time-series simultaneously. To assess
the performance of the proposed multi-time series long short-term memory (MTS-LSTM) method, we collected data on COVID-19 confirmed cases and deaths and foot traffic at the county level for
a period of 33 weeks between January 26th and September 12th, 2020. First, the data for deaths and confirmed cases of COVID-19 infection were downloaded from the Center for Systems Science
and Engineering (CSSE) at Johns Hopkins University65 and pre-processed to correct inaccuracies. During data cleaning, for example, instances of decrease in cumulative counts were identified
and replaced by the value of the previous day. We calculated the weekly sum of confirmed cases and deaths corresponding to the Morbidity and Mortality Weekly Report (MMWR) weeks used by the
Centers for Disease Control and Prevention (CDC) for all counties in lower 48 states of the US. MMWR weeks are based on the epidemiological calendar, with start on Sunday and end on
Saturday. Daily reported cases and deaths were not selected since the new cases data are typically noisy. Instead, one may use the daily moving average. However, it is important to ensure no
data leakage occurs between training, validation, and test data sets due to temporal dependency. Next, the daily foot traffic patterns and points of interest (POI) for the top 5500 national
brands were obtained from SafeGraph’s Places Schema data set64. SafeGraph provides visit data from around 18 million unique devices nationwide, which translates to 5–6% of the US
population. These data are provided freely upon request for research purposes through the SafeGraph COVID-19 Data Consortium. We pre-processed and aggregated the foot traffic of the POIs
into the county geographies and calculated their trailing average for each week. Finally, to evaluate our model, we obtained predictions of an ensemble model implemented by COVID-19 Forecast
Hub in collaboration with CDC for the period of 4 weeks ending on September 12th, 2020. Every week, the ensemble model accepts forecasts of COVID-19 confirmed cases and deaths from eligible
models with a variety of assumptions and methodologies and takes the arithmetic mean and median of the forecasts for each geographic location. Evaluating the performance of the ensemble
model and comparing it with the performance of the series of input models, the COVID-19 Forecast Hub recommends using the ensemble instead of individual models for policy considerations66.
In addition, since the ensemble model is an average of several input models, it has less variability in performance over time than a single input model. As a result, the ensemble model is a
good criterion for assessing the performance of our model. However, only predictions of new cases are available for the ensemble model. MODEL IMPLEMENTATION The codes were written in Python
using the TensorFlow 2.5.0 package for deep learning. The implementation was conducted in the Google Colaboratory environment with GPU accelerators and high-RAM run-time shape. The model
predicts all variables at the same time with a 4-week horizon (T = 4) and a time step of 1 week. We had confirmed cases, deaths, and mobility data for 33 weeks. The time series were divided
into three sets. The last 4 weeks of the time series for all counties were kept aside for the test. We did not use data from these 4 weeks in any instances of the training process.
Predictions of the Ensemble Model for the same 4 weeks were downloaded for evaluating the MTS-LSTM. Then, we used an out-of-sample validation (cross-validation) for training our model on the
remaining 29 weeks (n = 33 − 4 = 29). After pre-processing of the time series, as explained in the model section, 70% of the feature vectors were used for training, and the rest (30%) for
validation. This process was repeated ten times, each with a new set of randomly selected training and validation sets. All models in our experiments were trained using a batch size of 1024,
an Adam optimizer with an initial learning rate of \(10^{-3}\) and Huber loss function for 100 epochs. After a set of experiments, the window size was optimized with a length of three time
steps (l = 3). This agrees with findings from other research that show the number of daily new cases of COVID-19 is related to population mobility of 3 weeks prior67. RESULTS AND DISCUSSION
Our research had three specific objectives. First, we aimed to study the impact of adding foot traffic (mobility) on prediction performance. Then, we wanted to find out how using multiple
sample time series affects the performance of the predictions. Finally, we compared our predictions with the ensemble model. To address the first question, we implemented the model with two
specifications. The first model includes time series of two variables, deaths and new cases (m = 2), while the second model also includes the foot traffic as the third variable (m = 3). For
the second objective, we trained the two models with different fractions of counties (1%–5%–10%–25%–50%–100%), henceforth called “experiments”, randomly selected among all counties in the
data set and involved in the training and validation processes. We trained the model with ten “replications” to estimate the variation of predictions, each with a different set of randomly
selected counties using a Monte Carlo sampling method. Results are presented in Fig. 3, where the two panels on the left show the \({\text{RMSE}}_{\text{week}}\) of new infection cases for
all counties, and the two panels on the right show the same measure for deaths in all counties. Colors represent the portion of counties that have been used in an experiment (1 to 100%). The
darker the color, the larger the fraction of counties that has been used in the experiment. Each observation point represents prediction of a single replication from the Monte Carlo
process. In other words, points with the same color show all replications of a specific experiment. Notice that the Box-and-Whisker diagrams are plotted based on the forecasts from the
experiment with 100% of counties. What stands out in this figure is that Model 2 (with mobility as a predictor) predicts the new cases with lower RMSE median and variance than Model 1
(without mobility as a predictor) when 100% of counties are used in the training processes, and the same can also be said for experiments with smaller fractions of counties. Interestingly,
Model 1 shows smaller RMSE values for the deaths compared to Model 2, while the differences are not significant. One possible explanation for this is that the window size is optimized with
respect to the relationship between mobility and new cases. At the same time, the number of deaths has an extra delay in response to the number of new cases. That is, training the second
model with a larger window size (possibly five or six for COVID-19) may lead to smaller death RMSE values for the second model compared to the first. From the figure, we can also find out
that involving population mobility in the model improves the performance for predicting over a longer horizon window. Predictions of new cases for the third and fourth weeks have RMSEs less
than the first-week prediction RMSE in the second model and their variance remains low. Median performances in the prediction of deaths are rather similar with the two model over a longer
horizon, but Model 2 exhibits lower variances. The color-coded RMSE values under different experiments show how increasing the number of counties in the training process affects the median
and variance of the predictions (in Fig. 3). The pattern is more consistent with the death predictions mainly because the data on new infection cases are more subject to noise in comparison
to the death data, which is more reliable. The MTS-LSTM network may result in small negative predictions for some counties. For the confirmed cases for example the minimum value for 10 Monte
Carlo repetitions is − 9.076, which is very small. The other two variables always remain positive in predictions. All negative values are replaced by zero before calculating RMSE values for
evaluation. Alternatively, one could add an activation function to the last dense layer to make sure all predictions are non-negative. Finally, we compared the prediction results of models
1 and 2 with the ensemble model from CDC. The ensemble model predictions for the 4 weeks between August 17th and September 12th were obtained68. Figure 4 shows the dynamics of the new cases
(left), deaths (middle), and foot traffic (right) over 33 weeks for several indicative counties. The predictions for the last 4 weeks from all three models are also reported in the panels of
this figure. Models 1 and 2 are represented with 95% confidence intervals. Predictions based on the ensemble model are point predictions. Most counties have experienced the same mobility
pattern over the study period, while their deaths and new cases series are shown to follow different trends and to be in different stages across the study period, including during the
prediction horizon window. Additional results for counties with large, medium or small population size are available in the supplementary materials (see additional information section).
These illustrative cases suggest that predictions from our MTS-LSTM model are overall acceptable, but that great variability exists across different population communities in the US. A
deeper comparison between predictions from Model 2 and the ensemble model is available in Fig. 5, where predicted values for each county are plotted against their actual values for each
model by week of the prediction horizon. Rows one and two represent the new infection cases from Model 1 and new cases from the ensemble model, respectively. The
\({\text{RMSE}}_{\text{week}}\) values for the two models are in the same range, indicating very similar performance between the two models. This can also be seen in the two maps of Fig. 6,
where the \({\text{RMSE}}_{\text{county}}\) measure is depicted for both models. The third row in Fig. 5 shows the death prediction results against their actually reported values. As we
observed in Fig. 3, the model’s performance to predict deaths is higher than for new cases, which is an indication that the deaths data are more reliable than data on new cases.
\({\text{RMSE}}_{\text{total}}^{\text{new cases}}\)is equal to 224.28, 169.84, and 159.92 for Model 1, Model 2, and the ensemble model, respectively.
\({\text{RMSE}}_{\text{total}}^{\text{deaths}}\) for Model 1 and Model 2 equals to 3.44 and 4.09, respectively. MULTI-TIME SERIES LONG SHORT-TERM MEMORY MODEL We designed a multi-variate
recurrent neural network with LSTM layers to predict three variables of COVID-19 confirmed cases and deaths, as well as foot traffic for multiple sub-populations using an iterated multi-step
(IMS) estimation. LSTM is a gradient-based method developed by69 to efficiently learn to store information over extended time intervals by recurrent back-propagation. This information is
the long-term dependencies in a time series and can be transferred using a structure called memory cell. Memory cells have three gate structures named, input, forget, and output gates. These
gates control the usage of historical information70. Several versions of LSTM have been developed for applications in disease spread59,60. To aid the presentation of model results, we may
refer to a uni-variate time series as a “feature”, and to a multi-variate time series as “features” from time-to-time. Also, a “feature vector” represents a sub-sequence of a multi-variate
time series. Batches of feature vectors are the inputs to the network. DATA MODEL PREPARATION Figure 7 demonstrates the data preparation and architecture of the network.
\(\text{x}_\text{i}\) is a multi-variate time series for sub-population \(\text{i}\) with \(\text{n}\) time steps and \(\text{m}\) features. A window \(\text{L}\) of length \(\text{l}\) is
selected to create the feature vectors and labels from each time series. \(\text{x}_\text{i}^{(\text{j})<\text{t}>}\) represents the time-step \(\text{t}\) of feature vector
\(\text{j}\) from sub-population \(\text{i}\). The sequence of feature values in each window (feature vector) is used to predict the next time step, where the actual values of the time
series for those steps are used as the labels. In the process of creating feature vectors from each time series, the last sub-sequences with lengths less than \(\text{l}\) are dropped. This
means that each time series has \(\text{n}-\text{l}\) feature vectors of the same length. We shuffled feature vectors from time series of all sub-populations to create a bag of feature
vectors. Such an approach has three advantages compared to the single time series forecasting with LSTM layers. First and foremost, it provides more data for training compared to using the
time series of each sub-population separately. Data are very limited at the early stages of the outbreaks, both in frequency and in quality. Therefore, data-driven approaches, especially
deep learning methods with many parameters, are not applicable or end up with inaccurate predictions. Second, the spread of infectious diseases such as COVID-19 has complex diffusion and
relocation processes following mobility patterns across space and time71. That is, disease spread is not uniform, and outbreaks are in different stages in sub-populations across space. Such
a method of creating a bag of feature vectors from all sub-populations provides an opportunity for the network to learn from later stages of an outbreak in sub-population A and uses this to
predict outbreak dynamics in sub-population B. Third, training the machine learning model on feature vectors of multiple sub-populations simultaneously is expected to have more robust
predictions when predicting disease dynamics spread in a new sub-population C. At the same time, intrinsic characteristics of different sub-populations such as mobility can affect the
dynamics of disease in sub-populations. Thus, it is useful to have a multi-variate model that takes into account the mobility of sub-populations. In the next step, batches (bags) of feature
vectors are created as inputs to the network. The size of each batch is klm, where k is the number of feature vectors within each batch. MTS-LSTM ARCHITECTURE The network has two LSTM layers
with 128 neurons each, followed by a fully connected layer of 32 neurons, a drop out layer, and a fully connected regression layer with m neurons representing m features to be predicted. In
our MTS-LSTM model, we have three features: new cases, deaths, and foot traffic. The structure of an LSTM memory cell is represented by the following equations:
$${\tilde{\text{h}}}^{<\text{t}>} = {\tanh}(\text{W}_\text{h}[\text{a}^{<\text{t}-\text{1}>},\text{x}_\text{i}^{(\text{j})<\text{t}>}]+\text{b}_\text{h})$$ (1)
$$\begin{aligned} \Gamma _\text{u}= \sigma
(\mathrm{W}_\text{u}[\mathrm{a}^{<\mathrm{t}-\mathrm{1}>},\mathrm{x}_\text{i}^{(\mathrm{j})<\mathrm{t}>}]+\mathrm{b}_\text{u})\end{aligned}$$ (2) $$\begin{aligned} \Gamma
_\text{f}= \sigma (\mathrm{W}_\text{f}[\mathrm{a}^{<\mathrm{t}-\mathrm{1}>},\mathrm{x}_\text{i}^{(\mathrm{j})<\mathrm{t}>}]+\mathrm{b}_\text{f})\end{aligned}$$ (3)
$$\begin{aligned} \Gamma _\text{o}= \sigma
(\mathrm{W}_\text{o}[\mathrm{a}^{<\mathrm{t}-\mathrm{1}>},\mathrm{x}_\text{i}^{(\mathrm{j})<\mathrm{t}>}]+\mathrm{b}_\text{o})\end{aligned}$$ (4) $$\begin{aligned}
\text{h}^{<\text{t}>}= \Gamma _\text{u}*{{\tilde {\text{h}}}}^{<\text{t}>}+\Gamma _\text{f}*\text{h}^{<\text{t}-\text{1}>}\end{aligned}$$ (5) $$\begin{aligned}
\mathrm{a}^{<\mathrm{t}>}= \Gamma _\text{o}*\mathrm{tanh}(\mathrm{h}^{<\mathrm{t}>}) \end{aligned}$$ (6) where \({\tilde{\mathrm{h}}}^{<\mathrm{t}>}\) is the candidate
value for updating memory cell parameter \(\mathrm{h}^{<\mathrm{t}>}\). \(\Gamma _\text{u}\), \(\Gamma _\text{f}\), and \(\Gamma _\text{o}\) are the update, forget, and the output
gates. \(\mathrm{a}^{<\mathrm{t}-\mathrm{1}>}\) is the activation value from time step \(\mathrm{t}-\mathrm{1}\) of the feature vector. \(\mathrm{W}_\text{h}\),
\(\mathrm{W}_\text{u}\), \(\mathrm{W}_\text{f}\), and \(\mathrm{W}_\text{o}\) are the network parameters and \(\mathrm{b}_\text{h}\), \(\mathrm{b}_\text{u}\), \(\mathrm{b}_\text{f}\), and
\(\mathrm{b}_\text{o}\) are the biases. The output features from the last LSTM layer, which provide representation for cases, deaths, and mobility, are concatenated before entering to the
dense layer to provide a new feature representation. This new feature has information from previous time steps (time lags) of the feature being predicted as well as previous time steps (time
lags) of secondary features. This way, all temporal dependencies, including various time lags between features, implicitly contributes to the predicted value of a feature in the
back-propagation process. This is the simplest way to exploit temporal dependencies of various features. More explicit ways, with an added layer of complexity, exist to exploit dependencies
of various features. For example, the cross-modal (cross-feature) LSTM72 separates the input features into a single time series and passes each of those through a separate three-layer LSTM
stream and can be used for this purpose. A mechanism for information flow between the LSTM streams is proposed by authors to explicitly exploit multi-feature dependencies. The outputs from
these LSTM layers are then concatenated into a new feature representation. This approach is more explicit because new hyper-parameters are added to the model that can be controlled in a
fine-tuning process. In this research study, we use the former approach. However, it is of interest to compare these two approaches of modeling between feature temporal dependencies in the
future research. Finally, one may use either of two approaches to forecast variables for multi-steps73. The first approach is to optimize the multi-step forecasting objective function
directly. This method is called direct multi-step (DMS) estimation, which is usually computationally expensive. Furthermore, the optimization of the objective function may fail if the model
is highly non-linear or when the parameters are not well initialized74. The other approach is to predict step by step in an iterative way. This method is called iterated multi-step (IMS)
approach. The choice of method depends on the bias and variance of the prediction, on the number of prediction steps, and on the non-linearity of the model75. IMS is easy to train, and
forecasts of arbitrary length are possible. However, it is prone to accumulative errors74. In this research, we used an IMS method since the length of the prediction horizon is not long.
EVALUATION We use three RMSE based metrics to evaluate predictions: $$\begin{aligned} \mathrm{RMSE}_\text{county}= \sqrt{\frac{\sum _{\mathrm{t}=1}^{\mathrm{T}}(\mathrm{P}_\text{t} -
\mathrm{O}_\text{t})^2}{\mathrm{T}}} \end{aligned}$$ (7) $$\begin{aligned} \mathrm{RMSE}_\text{week}= \sqrt{\frac{\sum _{\mathrm{j}=1}^{\mathrm{C}}(\mathrm{P}_\text{j} -
\mathrm{O}_\text{j})^2}{\mathrm{C}}} \end{aligned}$$ (8) $$\begin{aligned} \mathrm{RMSE}_\text{total}= & {} \sqrt{\frac{\sum _{\mathrm{j}=1}^{\mathrm{C}}\sum
_{\mathrm{t}=1}^{\mathrm{T}}(\mathrm{P}_{\mathrm{jt}} - \mathrm{O}_{\mathrm{jt}})^2}{\mathrm{CT}}} \end{aligned}$$ (9) \(\mathrm{RMSE}_{\mathrm{county}}\) calculates the RMSE of multi-step
predictions for a single sample (county). T is the length of the prediction horizon and \(\mathrm{P}_\text{t}\) and \(\mathrm{O}_\text{t}\) are the prediction and observation for time step
t. \(\mathrm{RMSE}_{\mathrm{week}}\) represents the RMSE of predictions for a single time step (week) and all counties, where j is the county index and C is the number of counties. Finally,
\(\mathrm{RMSE}_{\mathrm{total}}\) presents a single-value metric based on RMSE with all predictions in different counties and weeks. CONCLUSIONS AND FUTURE WORK We proposed a multi-variate
LSTM-based recurrent neural network with mobility trained on multiple time series samples at the same time to predict the spatio-temporal spread of a pandemic. Our results show that adding
mobility as a variable and using multiple samples to train the network improve the performance of the predictions both in terms of the bias and of the variance of forecasts. We also showed
that the predicted results have similar accuracy and spatial patterns with the ensemble model used for benchmarking. While this may not add a new capability in modeling performance, our
MTS-LSTM model has several attractive advantages. Compared to the single time-series LSTM, our model predicts the dynamics of disease within different sub-populations simultaneously. For a
study area with n sub-populations, we used a single model instead of n different models. Given our experimental results, it may also be possible to replace the ensemble model which is based
on several other models with a single model exhibiting the same level of performance, which would reduce data inputs and computational resources. From the point of view of training data
size, this approach takes advantage of disease spread data from a sub-population to predict disease dynamics in other sub-populations. This is a very attractive feature, especially at the
beginning of pandemics. At such crucial times, data availability is very limited, and machine learning and deep learning methods can be challenging to implement and use. Recurrent neural
networks are capable of being updated with new sets of observations as they become available. From this point of view, they are comparable with Bayesian learning. Besides, our model predicts
all variables at the same time. This is important because of the causal relationship between variables that may impact the predictions. For example, staying at home when volunteering may be
affected by the current number of new cases. Also, the model is highly flexible to add new variables such as hospital capacity or vaccination rates, as reliable data become available. There
is room to further improve our MTS-LSTM model. First, we optimized the size of the moving window based on the RMSE of new cases. It is better to optimize this hyperparameter by considering
all variables (in our research, new infection cases and deaths). Second, we predicted the dynamics for a single period of time. For the full evaluation of the model, it is useful to use the
current model to predict dynamics for other periods as well. Third, after using a specific amount of data (about 10%), the model starts to improve slowly. This means, on the one hand that,
if we have data from a portion of counties, we can get to an acceptable level of accuracy to predict dynamics of disease for all counties. On the other hand, this means there is room to use
more complex models. In this respect, experimenting with different architectures such as adding bidirectional LSTM layers, 1D convolutional layers, encoder-decoder layers, and attention
layers, presents a frontier for future work with opportunity to further enhance the predictive power of the MTS-LSTM model. Fourth, efficacy may also be improved by explicitly modeling
temporal dependencies between features using a Cross-modal (cross-feature) LSTM72. Fifth, we did not account for spatial dependencies between counties in this research. All counties that
participate in predictions of dynamics for a county have the same weights. However, we expect the counties closer to each other to have more similar values and, as a result, higher weights.
Thus, it is useful to apply methods to include the spatial dependence. This can be conducted in a preliminary process using geo-statistical models or using the integration of LSTM with
convolutional neural networks. The integration of LSTM with convolutional layers in graph based neural networks is also an interesting line of future research for disease spread
prediction60,76,77 . Along a somewhat related line of thought, our analysis did not support using a series of covariates as predictors, which would better differentiate between local
conditions. We would conjecture, however, that this requires further study, especially in the context of a model structure that captures spatial relationships more comprehensively. Finally,
the model can also be extended to series at different scales and granularities (e.g., state level and county level) to establish how performance changes or to experiment with policy-related
scenarios (e.g., statewide lockdowns) that operate at various jurisdictional levels. Our MTS-LSTM model was trained with COVID-19 cases and deaths series in the United States and for a
specific time period. It will be important to use this pre-trained model to predict disease dynamics in other geographic contexts, time periods, or even similar infectious diseases such as
influenza in order to demonstrate the reproducibility of our methodology and results. DATA AVAILABILITY Supplementary Information (e.g., codes, data, results) are available in GitHub at
https://github.com/behnamnkp/Covid-19-Prediction.git. REFERENCES * Gallo Marin, B. _et al._ Predictors of COVID-19 severity: A literature review. _Rev. Med. Virol._ 31, 1–10 (2021). Article
CAS PubMed Google Scholar * Worldometers. COVID-19 Coronavirus Pandemic. https://www.worldometers.info/coronavirus/ (Accessed 5 May 2021) (2021). * The New York Times. Coronavirus World
Map: Tracking the Global Outbreak. https://www.nytimes.com/interactive/2020/world/coronavirus-maps.html (Accessed 5 May) (2021). * Sartorius, B., Lawson, A. & Pullan, R. Modelling and
predicting the spatio-temporal spread of COVID-19, associated deaths and impact of key risk factors in England. _Sci. Rep._ 11, 5378 (2021). Article ADS CAS PubMed PubMed Central Google
Scholar * Gamio, L. & Symonds, A. Global Virus Cases Reach New Peak, Driven by India and South America. https://nyti.ms/3xYVO94 (Accessed on 5 May 2021) (2021). * Samuel, J. _et al._
COVID-19 public sentiment insights and machine learning for tweets classification. _Information_ 11, 314 (2020). Article Google Scholar * Borriello, A., Master, D., Pellegrini, A. &
Rose, J. M. Preferences for a COVID-19 vaccine in Australia. _Vaccine_ 39, 473–479 (2021). Article CAS PubMed Google Scholar * Samuel, J. _et al._ Feeling positive about reopening? New
normal scenarios from COVID-19 US reopen sentiment analytics. _IEEE Access_ 8, 142173–142190 (2020). Article PubMed Google Scholar * Max Roser, E. O.-O., Ritchie, H. & Hasell, J.
Coronavirus pandemic (COVID-19). https://ourworldindata.org/coronavirus (Accessed on 5 June 2021) (2020). * Centers for Disease Control and Prevention. About variants of the virus that
causes COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/transmission/variant.html (Accessed on 5 June 2021) (2021). * Ali, G. G. M. N. _et al._ Public perceptions of COVID-19 vaccines:
Policy implications from US spatiotemporal sentiment analytics. _Healthcare_ 9, 1110 (2021). Article PubMed PubMed Central Google Scholar * Al Zobbi, M., Alsinglawi, B., Mubin, O. &
Alnajjar, F. Measurement method for evaluating the lockdown policies during the COVID-19 pandemic. _Int. J. Environ. Res. Public Health_ 17, 5574 (2020). Article CAS PubMed Central Google
Scholar * Lu, X., Yuan, D., Chen, W. & Fung, J. A machine learning based forecast model for the COVID-19 pandemic and investigation of the impact of government intervention on COVID-19
transmission in China (2020). Preprint on webpage at www.researchsquare.com/article/rs-73671/v1. * Vinceti, M. _et al._ Lockdown timing and efficacy in controlling COVID-19 using mobile
phone tracking. _EClinicalMedicine_ 25, 100457 (2020). Article PubMed PubMed Central Google Scholar * Rahman, M. _et al._ Machine learning on the COVID-19 pandemic, human mobility and
air quality: A review. _IEEE Access_ 9, 72420–72450 (2021). Article PubMed Google Scholar * Rahman, M. _et al._ COVID-19 pandemic severity, lockdown regimes, and people’s mobility: Early
evidence from 88 countries. _Sustainability_ 12, 9101 (2020). Article Google Scholar * Silva, P. C. _et al._ COVID-ABS: An agent-based model of COVID-19 epidemic to simulate health and
economic effects of social distancing interventions. _Chaos Solitons Fractals_ 139, 110088 (2020). Article MathSciNet PubMed PubMed Central Google Scholar * Rahman, M. M. _et al._
Socioeconomic factors analysis for COVID-19 US reopening sentiment with Twitter and census data. _Heliyon_ 7, e06200 (2021). Article PubMed PubMed Central Google Scholar * USAFacts. US
COVID-19 cases and deaths by state. https://usafacts.org/visualizations/coronavirus-covid-19-spread-map/ (Accessed 5 June 2021) (2020). * Wynants, L. _et al._ Prediction models for diagnosis
and prognosis of covid-19: Systematic review and critical appraisal. _BMJ_. 369(8242), m1328 (2020). * Swapnarekha, H., Behera, H. S., Nayak, J. & Naik, B. Role of intelligent computing
in COVID-19 prognosis: A state-of-the-art review. _Chaos Solitons Fractals_ 138, 109947 (2020). Article CAS PubMed PubMed Central Google Scholar * Xiang, Y. _et al._ COVID-19 epidemic
prediction and the impact of public health interventions: A review of COVID-19 epidemic models. _Infect. Dis. Model._ 6, 324–342 (2021). * Roy, A. & Kar, B. Characterizing the spread of
COVID-19 from human mobility patterns and SocioDemographic indicators. In _Proceedings of the 3rd ACM SIGSPATIAL International Workshop on Advances in Resilient and Intelligent Cities_,
39–48 (2020). * Scarpone, C. _et al._ A multimethod approach for county-scale geospatial analysis of emerging infectious diseases: A cross-sectional case study of COVID-19 incidence in
Germany. _Int. J. Health Geogr._ 19, 32 (2020). Article PubMed PubMed Central Google Scholar * Polyzos, S., Samitas, A. & Spyridou, A. E. Tourism demand and the COVID-19 pandemic: An
LSTM approach. _Tour. Recreat. Res._ 46(2), 1777053 (2020). * Iwendi, C. _et al._ COVID-19 patient health prediction using boosted random forest algorithm. _Front. Public Health_ 8, 357
(2020). Article PubMed PubMed Central Google Scholar * Hou, X. _et al._ Intra-county modeling of COVID-19 infection with human mobility: assessing spatial heterogeneity with business
traffic, age and race (2020). Preprint on webpage at https://doi.org/10.1101/2020.10.04.20206763v1. * Tang, B. _et al._ An updated estimation of the risk of transmission of the novel
coronavirus (2019-nCov). _Infect. Dis. Model._ 5, 248–255 (2020). PubMed PubMed Central Google Scholar * Zhao, S. _et al._ Preliminary estimation of the basic reproduction number of novel
coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreak. _Int. J. Infect. Dis._ 92, 214–217 (2020). Article CAS PubMed PubMed
Central Google Scholar * Fanelli, D. & Piazza, F. Analysis and forecast of COVID-19 spreading in China, Italy and France. _Chaos Solitons Fractals_ 134, 109761 (2020). Article
MathSciNet PubMed PubMed Central Google Scholar * Choi, S. & Ki, M. Estimating the reproductive number and the outbreak size of COVID-19 in Korea. _Epidemiol. Health_ 42, e2020011
(2020). * Tolles, J. & Luong, T. Modeling epidemics with compartmental models. _JAMA_ 323, 2515–2516 (2020). Article PubMed Google Scholar * Soures, N. _et al._ SIRNet: Understanding
social distancing measures with hybrid neural network model for COVID-19 infectious spread. _arXiv_ (2020). preprint on webpage at arXiv:2004.10376. * Chimmula, V. K. R. & Zhang, L. Time
series forecasting of COVID-19 transmission in Canada using LSTM networks. _Chaos Solitons Fractals_ 135, 109864 (2020). Article PubMed PubMed Central Google Scholar * Wang, D. _et al._
Agent-based Simulation Model and Deep Learning Techniques to Evaluate and Predict Transportation Trends around COVID-19. _arXiv_ (2020). Preprint on webpage at arXiv:2010.09648. * Kai, D.,
Goldstein, G.-P., Morgunov, A., Nangalia, V. & Rotkirch, A. Universal masking is urgent in the covid-19 pandemic: Seir and agent based models, empirical validation, policy
recommendations. _arXiv_ (2020). Preprint on webpage at arXiv:2004.13553. * Panovska-Griffiths, J. _et al._ Modelling the potential impact of mask use in schools and society on COVID-19
control in the UK. _Sci. Rep._ 11, 8747 (2021). Article ADS CAS PubMed PubMed Central Google Scholar * Szczepanek, R. Analysis of pedestrian activity before and during COVID-19
lockdown, using webcam time-lapse from cracow and machine learning. _PeerJ_ 8, e10132 (2020). Article PubMed PubMed Central Google Scholar * Ahmed, I., Ahmad, M., Rodrigues, J. J., Jeon,
G. & Din, S. A deep learning-based social distance monitoring framework for COVID-19. _Sustain. Cities Soc._ 65, 102571 (2020). Article PubMed PubMed Central Google Scholar * Spada,
A. _et al._ Structural equation modeling to shed light on the controversial role of climate on the spread of SARS-CoV-2. _Sci. Rep._ 11, 8358 (2021). Article CAS PubMed PubMed Central
Google Scholar * Jiang, F. _et al._ Artificial intelligence in healthcare: Past, present and future. _Stroke Vasc. Neurol._ 2, e000101 (2017). Article Google Scholar * Khalifa, N. E. M.,
Taha, M. H. N., Ali, D. E., Slowik, A. & Hassanien, A. E. Artificial intelligence technique for gene expression by tumor RNA-Seq data: A novel optimized deep learning approach. _IEEE
Access_ 8, 22874–22883 (2020). Article Google Scholar * Dexter, G. P., Grannis, S. J., Dixon, B. E. & Kasthurirathne, S. N. Generalization of machine learning approaches to identify
notifiable conditions from a statewide health information exchange. _AMIA Summits Transl. Sci. Proc._ 2020, 152–161 (2020). PubMed PubMed Central Google Scholar * Wang, Y., Liao, Z.,
Mathieu, S., Bin, F. & Tu, X. Prediction and evaluation of plasma arc reforming of naphthalene using a hybrid machine learning model. _J. Hazard. Mater._ 404, 123965 (2021). Article CAS
PubMed Google Scholar * Shao, Y. E., Hou, C.-D. & Chiu, C.-C. Hybrid intelligent modeling schemes for heart disease classification. _Appl. Soft Comput._ 14, 47–52 (2014). Article
Google Scholar * Roberts, M. _et al._ Common pitfalls and recommendations for using machine learning to detect and prognosticate for COVID-19 using chest radiographs and CT scans. _Nat.
Mach. Intell._ 3, 199–217 (2021). Article Google Scholar * Sun, J. _et al._ Forecasting the long-term trend of COVID-19 epidemic using a dynamic model. _Sci. Rep._ 10, 1–10 (2020). Article
ADS CAS Google Scholar * Chen, Y., Qin, R., Zhang, G. & Albanwan, H. Spatial temporal analysis of traffic patterns during the COVID-19 epidemic by vehicle detection using planet
remote-sensing satellite images. _Remote Sens._ 13, 208 (2021). Article ADS Google Scholar * Briz-Redón, Á. & Serrano-Aroca, Á. A spatio-temporal analysis for exploring the effect of
temperature on COVID-19 early evolution in Spain. _Sci. Total Environ._ 728, 138811 (2020). Article ADS PubMed PubMed Central CAS Google Scholar * Liu, Q. _et al._ Spatiotemporal
patterns of COVID-19 impact on human activities and environment in mainland China using nighttime light and air quality data. _Remote Sens._ 12, 1576 (2020). Article ADS Google Scholar *
Jarvis, K. F. & Kelley, J. B. Temporal dynamics of viral load and false negative rate influence the levels of testing necessary to combat COVID-19 spread. _Sci. Rep._ 11, 1–12 (2021).
Article CAS Google Scholar * Ugarte, M. D., Adin, A., Goicoa, T. & Militino, A. F. On fitting spatio-temporal disease mapping models using approximate Bayesian inference. _Stat.
Methods Med. Res._ 23, 507–530 (2014). Article MathSciNet PubMed Google Scholar * Zhang, C. H. & Schwartz, G. G. Spatial disparities in coronavirus incidence and mortality in the
United States: An ecological analysis as of May 2020. _J. Rural Health_ 36, 433–445 (2020). Article PubMed PubMed Central Google Scholar * Fitzpatrick, K. M., Harris, C. & Drawve, G.
Fear of COVID-19 and the mental health consequences in America. _Psychol. Trauma Theory Res. Pract. Policy_ 12, S17–S21 (2020). Article Google Scholar * Lalmuanawma, S., Hussain, J. &
Chhakchhuak, L. Applications of machine learning and artificial intelligence for covid-19 (SARS-CoV-2) pandemic: A review. _Chaos Solitons Fractals_ 139, 110059 (2020). Article MathSciNet
PubMed PubMed Central Google Scholar * Adhikari, B., Xu, X., Ramakrishnan, N. & Prakash, B. A. Epideep: Exploiting embeddings for epidemic forecasting. In _Proceedings of the 25th
ACM SIGKDD International Conference on Knowledge Discovery & Data Mining_, 577–586 (2019). * Gautam, Y. Transfer learning for COVID-19 cases and deaths forecast using LSTM network. _ISA
Transactions_ (2021). * Chen, S. _et al._ Exploring feasibility of multivariate deep learning models in predicting covid-19 epidemic. _Front. Public Health_ 9, 661615 (2021). Article PubMed
PubMed Central Google Scholar * Venna, S. R. _et al._ A novel data-driven model for real-time influenza forecasting. _IEEE Access_ 7, 7691–7701 (2018). Article Google Scholar * Wu, D.
_et al._ Deepgleam: a hybrid mechanistic and deep learning model for covid-19 forecasting. _arXiv_ (2021). Preprint on webpage at arXiv:2102.06684. * Zhu, X. _et al._ Attention-based
recurrent neural network for influenza epidemic prediction. _BMC Bioinform._ 20, 575 (2019). Article Google Scholar * Ben Said, A., Erradi, A., Aly, H. & Mohamed, A. Predicting
covid-19 cases using bidirectional lstm on multivariate time series. _arXiv_ (2020). Preprint on webpage at arXiv:2009.12325. * Aktay A., A. _et al._ Google COVID-19 Community Mobility
Reports: anonymization process description (version 1.1). _arXiv_ (2020). Preprint on webpage at arXiv:2004.04145. * SafeGraph. SafeGraph Places Schema. https://docs.safegraph.com/docs
(Accessed 15 Sept 2020) (2020). * Johns Hopkins University. COVID-19 dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins University (JHU).
https://coronavirus.jhu.edu/us-map (Accessed 15 Sept 2020) (2020). * Ray, E. L. _et al._ Ensemble forecasts of coronavirus disease 2019 (covid-19) in the us. _MedRXiv_ (2020). Preprint on
webpage at https://www.medrxiv.org/content/10.1101/2020.08.19.20177493v1. * Cartenì, A., Di Francesco, L. & Martino, M. How mobility habits influenced the spread of the COVID-19
pandemic: Results from the Italian case study. _Sci. Total Environ._ 741, 140489 (2020). Article ADS PubMed PubMed Central CAS Google Scholar * Reich, N. _et al._ har96, x. _Zhang,
jinghuichen, G. Espana, X. Xinyue, H. Biegel, L. Castro, Y. Wang, qjhong, E. Lee, A. Baxter, S. Bhatia, E. Ray, and abrennen, and ERDC CV19 Modeling Team_ (2020). Preprint on webpage at
https://github.com/reichlab/covid19-forecast-hub/tree/master/data-processed/COVIDhub-ensemble. * Hochreiter, S. & Schmidhuber, J. Long short-term memory. _Neural Comput._ 9, 1735–1780
(1997). Article CAS PubMed Google Scholar * Yao, W., Huang, P. & Jia, Z. Multidimensional lstm networks to predict wind speed. In _2018 37th Chinese Control Conference (CCC)_,
7493–7497 (IEEE, 2018). * Cromley, E. K. & McLafferty, S. L. _GIS and Public Health_ (Guilford Press, XXX, 2011). Google Scholar * Veličković, P. _et al._ Cross-modal recurrent models
for weight objective prediction from multimodal time-series data. In _Proceedings of the 12th EAI International Conference on Pervasive Computing Technologies for Healthcare_, 178–186
(2018). * Shi, X. & Yeung, D.-Y. Machine learning for spatiotemporal sequence forecasting: A survey. _arXiv_ (2018). Preprint on webpage at arXiv:1808.06865. * Bengio, S., Vinyals, O.,
Jaitly, N. & Shazeer, N. Scheduled sampling for sequence prediction with recurrent neural networks. _arXiv_ (2015). Preprint on webpage at arXiv:1506.03099. * Taieb, S. B. & Hyndman,
R. Boosting multi-step autoregressive forecasts. In _International Conference on Machine Learning_, Vol. 32, 109–117 (PMLR, 2014). http://proceedings.mlr.press/v32/taieb14.html. *
Veličković, P. _et al._ Graph attention networks. _arXiv_ (2017). Preprint on webpage at arXiv:1710.10903. * Hamilton, W. L., Ying, R. & Leskovec, J. Inductive representation learning on
large graphs. _arXiv_ (2017). Preprint on webpage at arXiv:1706.02216. Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * The William States Lee College of Engineering,
University of North Carolina at Charlotte, 9201 University City Blvd, Charlotte, NC, 28223, USA Behnam Nikparvar & Md. Mokhlesur Rahman * Department of Urban and Regional Planning,
Khulna University of Engineering & Technology (KUET), Khulna, 9203, Bangladesh Md. Mokhlesur Rahman * Department of Geography and Earth Sciences, University of North Carolina at
Charlotte, 9201 University City Blvd, Charlotte, NC, 28223, USA Faizeh Hatami & Jean-Claude Thill * School of Data Science, University of North Carolina at Charlotte, 9201 University
City Blvd, Charlotte, NC, 28223, USA Jean-Claude Thill Authors * Behnam Nikparvar View author publications You can also search for this author inPubMed Google Scholar * Md. Mokhlesur Rahman
View author publications You can also search for this author inPubMed Google Scholar * Faizeh Hatami View author publications You can also search for this author inPubMed Google Scholar *
Jean-Claude Thill View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS B.N., M.M.R., F.H., and J.C.T. conceived the study. B.N., M.M.R., F.H.,
and J.C.T. identified the deep learning modeling framework and designed the specific features of the final model. B.N. coded the model for implementation and ran all the experiments. B.N.,
M.M.R., F.H., and J.C.T. identified the extant literature. B.N., M.M.R., F.H. compiled the data used in the study. B.N., M.M.R., F.H., and J.C.T. analyzed the results. B.N., M.M.R., F.H.,
and J.C.T. contributed to the writing of the manuscript. J.C.T. supervised the study. CORRESPONDING AUTHOR Correspondence to Jean-Claude Thill. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution
and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if
changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the
material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Nikparvar, B., Rahman, M.M., Hatami, F. _et al._ Spatio-temporal prediction of the COVID-19 pandemic in US counties: modeling with a deep LSTM neural network. _Sci Rep_ 11,
21715 (2021). https://doi.org/10.1038/s41598-021-01119-3 Download citation * Received: 07 July 2021 * Accepted: 20 October 2021 * Published: 05 November 2021 * DOI:
https://doi.org/10.1038/s41598-021-01119-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative







