- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The _SRY_ gene induces testis development even in XX individuals. However, XX/_Sry_ testes fail to produce mature sperm, due to the absence of Y chromosome carrying genes essential
for spermatogenesis. XX/_Sry_ Sertoli cells show abnormalities in the production of lactate and cholesterol required for germ cell development. Leydig cells are essential for male functions
through testosterone production. However, whether XX/_Sry_ adult Leydig cells (XX/_Sry_ ALCs) function normally remains unclear. In this study, the transcriptomes from XY and XX/_Sry_ ALCs
demonstrated that immediate early and cholesterogenic gene expressions differed between these cells. Interestingly, cholesterogenic genes were upregulated in XX/_Sry_ ALCs, although
downregulated in XX/_Sry_ Sertoli cells. Among the steroidogenic enzymes, CYP17A1 mediates steroid 17α-hydroxylation and 17,20-lyase reaction, necessary for testosterone production. In
XX/_Sry_ ALCs, the latter reaction was selectively decreased. The defects in XX/_Sry_ ALCs, together with those in the germ and Sertoli cells, might explain the infertility of XX/_Sry_
testes. SIMILAR CONTENT BEING VIEWED BY OTHERS IMPAIRED KETOGENESIS IN LEYDIG CELLS DRIVES TESTICULAR AGING Article Open access 07 May 2025 DAPL1 IS A NOVEL REGULATOR OF TESTOSTERONE
PRODUCTION IN LEYDIG CELLS OF MOUSE TESTIS Article Open access 17 September 2021 MOLECULAR CHARACTERISTICS AND REGULATORY ROLE OF INSULIN-LIKE GROWTH FACTOR 1 GENE IN TESTICULAR LEYDIG CELLS
OF TIBETAN SHEEP Article Open access 22 October 2024 INTRODUCTION It has been established that the _SRY_ (sex-determining region on the Y chromosome) gene is responsible for the
differentiation of the testes in mammals1,2. Indeed, injection of the _Sry_ gene into fertilized XX mouse eggs leads to testis development in XX fetuses. However, XX mice carrying the _Sry_
transgene (XX/_Sry_ mice) suffer from spermatogenic failure3,4. Although the developmental defects of germ cells have been thought to be caused by the lack of Y-chromosome genes essential
for spermatogenesis5, the reason for this infertility in XX/_Sry_ mice is still under discussion. In fact, our previous study identified disfunction of XX/_Sry_ Sertoli cells6. In general,
Sertoli cells support the differentiation of germ cells by providing them with nutrients including lactate7 and cholesterol8. XX/_Sry_ Sertoli cells were found to synthesize these substances
less than XY Sertoli cells, due to lower expression levels of the genes required for their synthesis6. In addition to Sertoli cells, testes contain Leydig cells, which are developmentally
divided into two types, fetal-type (FLCs) and adult-type (ALCs). During the fetal stage, FLCs emerge within the interstitial space of the fetal testes and increase in number during embryonic
development. After birth, FLCs are gradually substituted with ALCs9. Finally, in the adult stage, the testicular interstitial space is predominantly occupied by ALCs, although a small
population of FLCs remains9,10,11. With respect to the Leydig cells in XX/_Sry_ testes, it remains largely unclear whether the ALCs in XX/_Sry_ mice exhibit functions equivalent to XY ALCs.
In general, ALCs are characterized by the functional capacity to produce testosterone. Four enzymes have been implicated in the synthesis of testosterone from cholesterol: cytochrome P450
family members cholesterol side-chain cleavage enzyme (CYP11A1) and 17α-hydroxylase/17,20-lyase (CYP17A1); 3β-hydroxysteroid dehydrogenase (HSD3B1 and HSD3B6); and 17β-hydroxysteroid
dehydrogenase (HSD17B3)12,13. Of these enzymes, CYP17A1 uniquely mediates two distinct reactions: 17α-hydroxylation and C17,20-cleavage of steroids14. Both reactions are successively
mediated by CYP17A1 in the Leydig cells of all mammalian species. Ad4BP/SF-1 (adrenal-4 binding protein/steroidogenic factor 1/NR5A115) was initially identified as a nuclear receptor-type
transcription factor that regulates the gene transcription of _CYP11A1_ and _CYP11B1_ (steroid 11β-hydroxylase)16,17,18. Thereafter, many studies have investigated whether other
steroidogenic genes are also regulated by Ad4BP/SF-1. These studies identified _HSD3B2_19,20, _CYP17A1_21,22,23, and _CYP19A1_24 as target genes of this factor. Thus, it has been widely
accepted that Ad4BP/SF-1 plays a central role in the regulation of steroidogenic genes25,26. All steroid hormones are synthesized from cholesterol. In addition to special usage for
steroidogenesis, cholesterol is known to be an essential component of various cellular membranes27. In accordance with this broad range of requirements for cholesterol, cholesterogenic genes
are expressed in a variety of cell types. Extensive investigation of cholesterogenic gene regulation in the liver has led to the identification of sterol regulatory element binding protein
2 (SREBP2, encoded by _SREBF2_) as the key transcription factor regulating all cholesterogenic genes28. In addition to this key molecule, Ad4BP/SF-1 has recently been shown to be involved in
cholesterogenic gene regulation in steroidogenic cells29. In this study, we investigated whether XX/_Sry_ ALCs are functionally different from XY ALCs. Comparison of the transcriptomes
obtained from these two types of cells revealed that the expression of immediate early genes and cholesterogenic genes was altered in the XX/_Sry_ ALCs. In addition, we found that the
17,20-lyase reaction mediated by CYP17A1 was specifically affected in XX/_Sry_ ALCs. RESULTS INCREASE OF ALCS IN XX/SRY TESTES It was previously believed that FLCs are completely replaced by
ALCs after birth. However, our previous studies have demonstrated that FLCs persist in adult mouse testes11,30. Therefore, to selectively investigate ALCs, we established a mouse line
carrying _Ad4BP-BAC-EGFP_ and _mFLE-mCherry_ as transgenes. In the mouse testes, FLCs were labeled with both EGFP and mCherry, whereas ALCs were labeled with EGFP alone. This double
transgenic mouse line thus enabled us to isolate ALCs and FLCs with no mutual contamination. We transferred these two transgenes into XY and XX/_Sry_ mice to obtain XY and XX/_Sry_ ALCs as
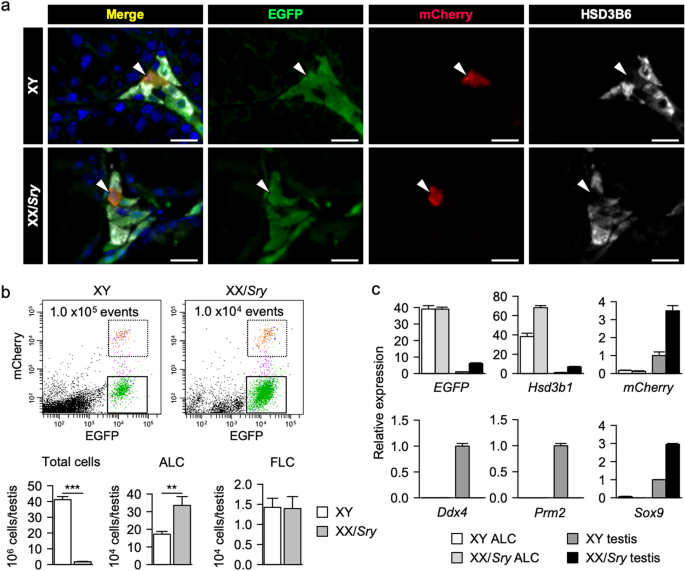
EGFP single-positive cells. As shown in Fig. 1a, we found both EGFP single-positive and EGFP/mCherry double-positive Leydig cells in both XY and XX/_Sry_ testes. HSD3B6, an ALC marker, was
colocalized with the EGFP in the single-positive Leydig cells, indicating that these cells were ALCs. Fluorescence-activated cell sorting (FACS) of the testicular cells enabled us to isolate
two distinct Leydig cell populations, EGFP single-positive ALCs and EGFP/mCherry double-positive FLCs, from both XY and XX/_Sry_ testes (Fig. 1b). Since the XX/_Sry_ adult testes were
hypoplastic and lacked all germ cells (Supplemental Fig. 1), the total number of cells in a single XX/_Sry_ testis was substantially lower than that in a single XY testis. Surprisingly,
however, the number of ALCs in the XX/_Sry_ testis was close to double that in the XY testis (Fig. 1b). The purity of the ALC fraction prepared by FACS was examined using qRT-PCR for
testicular cell marker genes. _EGFP_ and _Hsd3b1_ were highly enriched in ALCs from both XY and XX/_Sry_ testes, whereas _mCherry_ was barely detectable in either group (Fig. 1c). Germ cell
markers _Ddx4_ and _Prm2_ were undetectable in the ALCs, as was Sertoli cell marker _Sox9_. These results indicate that the ALC fraction used in this study was unlikely to have been
contaminated with FLCs, germ cells, or Sertoli cells. DIFFERENTIAL GENE EXPRESSION BETWEEN XY AND XX/SRY ALCS To investigate whether XX/_Sry_ ALCs differ from XY ALCs, transcriptomes were
obtained from three biologically independent sets of ALC samples each from XY and XX/_Sry_ testes. Considering the high unique mapping rate of the sequence reads (approximately 90%) and the
high reproducibility between the biological triplicates (correlation coefficient > = 0.992; Supplemental Fig. 2a and 2b), the quality of the transcriptome datasets was considered
sufficient for further examination. Comparison of the transcriptomes revealed that the expression levels of 302 and 285 genes were more than 1.5-fold higher and lower, respectively, in the
XX/_Sry_ ALCs compared to the XY ALCs (Fig. 2a, Supplemental Tables 1 and 2). These differentially expressed genes were subjected to GO pathway analysis to investigate which biological
processes are associated with the genes up- and downregulated in the XX/_Sry_ ALCs. As listed in Fig. 2b (left panel), ‘sterol biosynthetic process’, ‘cholesterol biosynthetic process’,
‘steroid biosynthetic process’, and ‘steroid metabolic process’ were strongly related to the genes upregulated in the XX/_Sry_ ALCs. Of these genes, the ones commonly associated with these
processes were predominantly cholesterogenic. The next most strongly represented process was ‘lipid metabolic process’. Although the gene list for this process includes cholesterogenic
genes, it also includes genes specifically required for lipid synthesis. In accordance with the sharing of cholesterogenic genes, REVIGO plot analysis suggested that these processes
involving cholesterogenesis seemed to form a cluster at the top left (right panel in Fig. 2b). Multiple terms related to blood vessels were listed, and these formed another cluster (Fig.
2b). This suggests that although we could not find any clear defect, the blood vessels of the XX/_Sry_ testes may be affected by the differential expression of these genes. In addition to
the genes included in the terms above, we noticed that the expression of extracellular matrix genes (such as those associated with several types of collagen, laminin, and biglycan) was
higher in the XX/_Sry_ ALCs, suggesting that the extracellular matrix surrounding XX/_Sry_ ALCs is different from that surrounding XY ALCs. A few biological processes were related to the
genes downregulated in the XX/_Sry_ ALCs, and their _p_-values were relatively large compared with those related to the upregulated genes (left panel in Fig. 2c). REVIGO plot analysis
suggested that these biological terms were not closely related (right panel in Fig. 2c). Although any close correlations between the listed terms and Leydig cell functions were unlikely, we
noticed that the term ‘response to cytokine’ includes the _Fos_, _Junb_, and _Jund_ genes. These gene products, leucine zipper-type transcription factors, have been studied extensively and
found to be activated in response to a variety of stimuli, such as serum, growth factors, and cytokines31. Since these genes have been classified into immediate early genes, we examined
whether the expression levels of other genes in this group were affected in XX/_Sry_ ALCs. Interestingly, many other immediate early genes, such as _Atf3_, _Egr1_, and _Myc_, were also
downregulated in XX/_Sry_ ALCs compared to XY ALCs (Fig. 2d). CHOLESTEROGENIC GENE EXPRESSION INCREASED IN XX/SRY ALCS Since cholesterogenic pathway is involved in the biological functions
activated in the XX/_Sry_ ALCs, we examined the expression of cholesterogenic genes in the XY and XX/_Sry_ ALCs. The transcriptome data indicated that almost all the cholesterogenic genes
were upregulated more than 1.5-fold in the XX/_Sry_ ALCs (Fig. 3a). This increased expression was confirmed by qRT-PCR (Fig. 3b). Numerous studies have demonstrated that SREBP2, encoded by
_Srebf2_, plays a crucial role in cholesterogenic gene regulation28. In fact, it has been demonstrated that SREBP2 accumulates in the regions upstream of cholesterogenic genes32. In
addition, we recently demonstrated that Ad4BP/SF-1 also accumulates at cholesterogenic gene loci in steroidogenic cells, including ALCs29. Therefore, we expected that at least one of these
two transcription factors would also be upregulated in the XX/_Sry_ ALCs. Although the expression of _Ad4BP/SF-1_ was unaltered in these cells, _Srebf2_ expression was slightly higher in the
XX/_Sry_ ALCs. This altered expression of _Srebf2_ could be responsible, at least in part, for the observed enhanced expression of cholesterogenic genes in the XX/_Sry_ ALCs. DIFFERENTIAL
EFFECTS ON GENE EXPRESSION BETWEEN XX/SRY ALCS AND SERTOLI CELLS We previously compared gene expression between XY and XX/_Sry_ Sertoli cells and found that cholesterogenic genes were
downregulated in the latter6. Accordingly, the present study showed that cholesterogenic gene expression was affected in opposite ways between the XX/_Sry_ ALCs and Sertoli cells. We
graphically compared whole-gene expression changes in the two types of cells. Fold changes in gene expression levels (XX/_Sry_ vs. XY) for Sertoli cells were plotted on the vertical axis and
for ALCs on the horizontal axis (Fig. 4). If genes were up- or downregulated in both types of XX/_Sry_ cells, they would be lie on or near the red broken line in Fig. 4a. However, there was
no particular pattern of distribution along this line. Instead, a considerable number of genes were aligned along the lines x = 0 or y = 0, suggesting that the alteration of gene expression
was probably cell-type specific. Cholesterogenic genes are indicated with red dots in the plot shown in Fig. 4b. As expected, many of these genes are localized in the lower right quadrant,
which is consistent with our finding that cholesterogenic genes were upregulated in the XX/_Sry_ ALCs but downregulated in the XX/_Sry_ Sertoli cells. As mentioned above, immediate early
genes were downregulated in the XX/_Sry_ ALCs. However, no biased expression of this group was detected in the XX/_Sry_ Sertoli cells. Consistent with this, the immediate early genes are
distributed within the left half of the plot area (Fig. 4c). ALTERED EXPRESSION OF GENES NORMALLY ENRICHED IN ALCS It has been established that the expression levels of _Insl3_,
_Ad4BP/SF-1_, and _Lhcgr_ (Luteinizing hormone/choriogonadotropin receptor) are enriched in ALCs33,34. In addition, we previously found several candidate genes that are probably enriched in
ALCs by comparing the transcriptomes of ALCs and FLCs30. In the present study, we examined the expression of these genes via in situ hybridization. As shown in Fig. 5a, _Agt_
(angiotensinogen) was expressed in ALCs but not in Sertoli or germ cells in adult testes. Enriched expression in ALCs has previously been observed for _Hmgcs2_
(3-hydroxy-3-methylglutaryl-CoA synthase 2)35,36, _Lcn2_ (lipocalin-2)37, and _Sepp1_ (selenoprotein P, plasma, 1)38. A high level of _Ptgds_ (prostaglandin D2 synthase) expression was
detected in ALCs, although the expression was also detected in Sertoli cells from some, but not all, testicular tubules39. Interestingly, the transcriptomes obtained in the present study
revealed that many of these genes were downregulated in the XX/_Sry_ ALCs (Fig. 5b). STEROIDOGENESIS POSSIBLY AFFECTED BY DECREASED STEROID 17,20-LYASE ACTIVITY We previously demonstrated
that the amount of testosterone synthesized in XX/_Sry_ testes at postnatal day 21 was smaller than in XY testes6. We therefore extracted the expression data for steroidogenic genes from our
transcriptome datasets. The expression of _Star_, _Cyp11a1_, _Cyp17a1_, and _Hsd17b3_ was decreased to approximately 70% of XY ALC levels in the XX/_Sry_ ALCs (Fig. 6a). Similar expression
profiles for these genes were obtained using qRT-PCR (Fig. 6b). The expression of _Ad4BP/SF-1_, a key regulator of steroidogenic gene expression, was not significantly affected in the
XX/_Sry_ ALCs, while that of _Lhcgr_ was more than doubled. To examine whether these changes affected steroidogenesis, the quantities of steroidal molecules were determined for both XY and
XX/_Sry_ testes. Testosterone synthesis from cholesterol is mediated by multiple enzymes (Fig. 7a). As indicated in Fig. 7b, the quantities of P5 (pregnenolone), P4 (progesterone), 17αOH-P5
(17α-hydroxypregnenolone), and 17αOH-P4 (17α-hydroxyprogesterone) in the XX/_Sry_ testes were greater than those in the XY testes. Interestingly, however, the quantities of DHEA
(dehydroepiandrosterone), A-dione (androstenedione), A-diol (androstenediol), and T (testosterone) in the XX/_Sry_ testes were smaller than those in the XY testes. Based on these steroid
quantities, the enzymatic activities were evaluated by calculating metabolic ratios. While 17α-hydroxylation, 3β-dehydrogenation, and 17β-hydroxylation were not significantly altered, the
17,20-lyase reaction was substantially reduced in the XX/_Sry_ testes (Fig. 7c). Interestingly, 17α-hydroxylation and 17,20-lyase reaction are mediated by a single enzyme, CYP17A1. Electrons
from NADPH/NADH required for these reactions are transferred to CYP17A1 from POR (P450 oxidoreductase) and/or CYB5A (cytochrome b5a). The expression of _Cyb5a_ was increased in the XX/_Sry_
ALCs (Fig. 7d), but that of _Por_ was not significantly altered. DISCUSSION In the present study, we aimed to determine whether XX/_Sry_ ALCs are functionally equivalent to XY ALCs. To
investigate it, transcriptomes obtained from XY and XX/_Sry_ ALCs were compared. As the consequence, the expression of 302 and 285 genes was found to be up- and downregulated, respectively,
in the XX/_Sry_ ALCs compared to XY ALCs. These gene sets suggested that several biological activities and processes are affected in XX/_Sry_ ALCs. There are potential reasons why the number
of ALCs was increased in XX/_Sry_ testes. LH has been established to be one of the key molecules for differentiation of ALCs. In fact, ALCs were decreased in the testis of _Lhcgr_ KO
mice40. Moreover, transgenic overexpression of human chorionic gonadotropin (_HCG_), which potentially binds and activates LHCGR, resulted in an increase of ALCs41,42. Based on these
findings, the increase of ALCs in the XX/_Sry_ testes might be due to the increased expression of _Lhcgr_. In addition to the endocrine factor above, there are several paracrine factors
regulating differentiation of ALCs. Desert hedgehog (DHH), secreted by Sertoli cells, stimulates proliferation of stem Leydig cells and their differentiation into ALCs43. However, our
previous study showed that the expression of _Dhh_ was not altered in the XX/_Sry_ Sertoli cells compared to XY cells6 (Supplemental Fig. 3a). Likewise, the expression of the hedgehog
signaling components such as _Ptch1/2_ and _Smo_ was not affected in the XX/_Sry_ ALCs (Supplemental Fig. 3b). PDGF is another factor to activate proliferation of stem Leydig cells43.
Although it has been unclear which cells synthesize PDGFs in adult testes, the expression of _Pdgfc_ was increased in the XX/_Sry_ Sertoli cells. Interestingly, the expression of the
receptor gene, _Pdgfra_, was increased in the XX/_Sry_ ALCs. Taken together, it was suggested that the increase of ALCs in the XX/_Sry_ testes might be attributable to the augmentation of
PDGF together with LH signaling. ALCs actively synthesize testosterone through abundant expression of steroidogenic genes. Our transcriptomic analysis revealed that the expression of all
steroidogenic genes except _Hsd3b1_ and _Hsd3b6_ was lower in the XX/_Sry_ ALCs compared to XY ALCs. Similarly, we found that the expression of genes normally enriched in ALCs was
suppressed, suggesting that the characteristic features of ALCs were affected in the XX/_Sry_ ALCs. With respect to the reason for the suppressed expression of these genes, it is interesting
to note the downregulation of immediate early genes, whose expression is activated by multiple stimuli31, in the XX/_Sry_ ALCs. Indeed, the immediate early genes such as _Fos_, _Jun_,
_Junb_, and _Jund_ (AP1 family members) are activated in ALCs by hCG44. It could therefore be assumed that the gene products above activate cellular functions by enhancing the transcription
of certain sets of target genes. In fact, steroidogenic gene transcription is regulated by the AP1 family members45,46. In addition to the steroidogenic genes, it has been demonstrated that
_LCN2_ displaying ALC-enriched expression is regulated by EGR147. In the present study, we demonstrated that the expression levels of immediate early genes were decreased in the XX/_Sry_
ALCs. The decreased expression of steroidogenic and ALC-enriched genes might therefore be caused by the downregulation of immediate early genes. Based on this scenario, the concentration of
LH secreted by the pituitary and the expression of its receptor, LHCGR, in ALCs should be considered. Our previous study showed that the serum LH concentration in the XX/_Sry_ mice was
comparable to that of the XY mice6, but the present study showed that the expression of _Lhcgr_ was higher in the XX/_Sry_ ALCs than in the XY ALCs. Therefore, the XX/_Sry_ ALCs might
receive more effectively the LH signal than the XY ALCs. If it is the case, gene transcription downstream of LH signal such as _Fos_ and _Jun_ could be activated. Nevertheless, the
expression of the immediate early genes was found to be downregulated. Therefore, this inconsistent outcome suggests that intracellular signal transduction might be abnormally regulated in
XX/_Sry_ ALCs, although it remains unknown which components and/or steps may be affected. Many transcription factors have been shown to regulate steroidogenic genes. Our transcriptome
datasets revealed that the expression of _Cebpb_ (_C/EBPβ_) and _Fos_ was decreased less than 0.67-fold while that of _Nr3c1_ was increased more than 1.5-fold in the XX/_Sry_ ALCs
(Supplemental Fig. 4). C/EBPβ and FOS were reported to regulate positively mouse _Star_ and human _CYP11A1_ genes46,48,49. Therefore, the decreased expression of _Cyp11a1_ and _Star_ genes
might be due to the downregulated expression of _Cebpb_ and _Fos_ in the XX/_Sry_ ALCs. NR3C1 (GR) was reported to act as a suppressor of mouse _Star_ gene transcription50. Thus, the
upregulated expression of _Nr3c1_ might be responsible for the decreased expression of _Star_ gene in the XX/_Sry_ ALCs. Steroidogenesis from cholesterol takes place via multiple enzymatic
reactions. Based on our analyses of the metabolic ratios, we realized that the 17,20-lyase reaction mediated by CYP17A1 was selectively affected in the XX/_Sry_ ALCs. CYP17A1 catalyzes two
reactions: 17α-hydroxylation and 17,20-lyase reaction14. In many mammalian species, cortisol (glucocorticoid) is synthesized in the zona fasciculata of the adrenal cortex, while testosterone
is synthesized in ALCs. In the former process, CYP17A1 mediates only 17α-hydroxylation, whereas in the latter process it mediates both 17α-hydroxylation and 17,20-lyase reaction. Many
studies have been performed to improve our understanding of the mechanism for selective regulation of these two reactions by CYP17A151. Some of them have focused on the two components, POR
and CYB5A, which transport electrons to CYP17A1. One study reported that POR preferentially activates the 17,20-lyase reaction52, while another reported that CYB5A is responsible for this
activation53. Concordantly, a KO study has shown that _Cyb5a_ is necessary for 17,20-lyase activity in ALCs54. Unexpectedly, however, the expression of _Por_ and _Cyb5a_ was not decreased in
the XX/_Sry_ ALCs_._ Another possible regulatory mechanism of the two reactions, phosphorylation of CYP17A1 by cAMP-dependent protein kinase, p38α, and an unknown kinase activated under
serum-free condition has been shown to selectively increase 17,20-lyase activity55,56,57. Unfortunately, however, our preliminary study failed to detect the phosphorylated CYP17A1 in the XY
and XX/_Sry_ testes. Although we could not unveil the mechanism for the selective regulation of the CYP17A1-mediated reactions, our study revealed that XX/_Sry_ ALCs could be an excellent
cellular tool for future investigation of it. In our previous study, we examined histone modifications and showed that accumulation of H3K4me3 around the upstream regions of cholesterogenic
genes was reduced in XX/_Sry_ Sertoli cells6. Considering that H3K4me3 is a mark for an active promoter, we concluded that this reduction may have led to the decreased expression of
cholesterogenic genes in the XX/_Sry_ Sertoli cells. Interestingly, our present study demonstrated that immediate early genes and cholesterogenic genes were differentially altered in
XX/_Sry_ ALCs and XX/_Sry_ Sertoli cells. Comparison of whole-genome histone modifications could contribute to a deeper understanding of the mechanisms underlying cell-type-specific
alteration of gene expression in XX/_Sry_ mice. MATERIALS AND METHODS ANIMALS Wild-type XY C57BL/6 and XX sex-reversed transgenic mice carrying the _Hsp-Sry_ transgene were used58. The
presence of the transgene and genetic sex were confirmed via PCR with primers for _Hsp-Sry_ and SX59 (Supplemental Table 3). SX is a single set of primers to amplify _Xlr_ and _Sly_ on the
X- and Y-chromosome, respectively, giving distinct banding patterns after electrophoresis. We also used _Ad4BP-BAC-EGFP_ mice and _mFLE-mCherry_ mice60, in which Leydig cells and FLCs are
labeled with EGFP and mCherry, respectively. _Sry_ transgenic mice were crossed with _Ad4BP-BAC-EGFP;mFLE-mCherry_ mice to obtain EGFP single-positive ALCs from the testes of XX/_Sry_ mice.
All protocols for the animal experiments were approved by the Animal Care and Use Committee of Kyushu University. All experiments were performed in accordance with the institutional
guidelines. CELL COUNTING AND SORTING Testes were collected from eight-week-old _Ad4BP-BAC-EGFP;mFLE-mCherry_ double-transgenic mice and dispersed with collagenase30. Total numbers of cells
from XY and XX/_Sry_ testes were counted using a Countess II FL (Thermo Fisher Scientific, Waltham, MA, USA). The dispersed cells were subjected to FACS using a BD FACS Aria SORP (BD
Biosciences, San Jose, CA, USA) and FACS Diva software (BD Biosciences) to sort the cells into two populations based on EGFP and mCherry fluorescence (ALCs: EGFP single-positive; FLCs:
EGFP/mCherry double-positive). 1,000,000 cells were analyzed to obtain the percentages of ALCs and FLCs, which were converted to the absolute numbers per testis by multiplying the total
numbers of testicular cells. The EGFP single-positive ALCs were purified by performing two FACS cycles. IMMUNOFLUORESCENCE ANALYSES Eight-week-old mice were perfused with 4% paraformaldehyde
(PFA) and their testes were collected and then immersed in 4% PFA at 4 °C for 48 h. The samples were subsequently cryoprotected in 20% sucrose at 4 °C and embedded in OCT Compound (Sakura
Finetek, Torrance, CA, USA). Immunofluorescence analyses were performed as described previously11. A rabbit antibody against HSD3B661 (1:500), a chicken antibody against EGFP (ab13970,
1:1000; Abcam, Cambridge, UK), and a mouse antibody against mCherry (ab125096, 1:200; Abcam) were used as the primary antibodies. Alexa Fluor 488-labeled goat anti-chicken IgY antibody
(ab150169, 1:500; Abcam), Alexa Fluor 555-labeled goat anti-mouse IgG antibody (A28180, 1:500; Life Technologies, Carlsbad, CA, USA), and Alexa Fluor 647-labeled goat anti-rabbit IgG
antibody (A27040, 1:500; Life Technologies) were used as the secondary antibodies. Nuclei were stained with DAPI (4′6-diamidino-2-phenylindole; Sigma–Aldrich, St. Louis, MO, USA).
Immunofluorescence was observed under a BZ-X700 microscope (Keyence, Osaka, Japan). IN SITU HYBRIDIZATION AND IMMUNOHISTOCHEMISTRY In situ hybridization was performed as previously
described62. RIKEN FANTOM cDNA clones for _Agt_ (angiotensinogen, A730059G17), _Hmgcs2_ (3-hydroxy-3-methylglutaryl-coenzyme A synthase 2, 1300002P16), _Ptgds_ (prostaglandin D2 synthase,
2010004I02), _Lcn2_ (lipocalin-2, 2G530015N18), and _Sepp1_ (selenoprotein P, plasma, 1; I920052L16) were purchased (DNAFORM, Yokohama, Japan). Digoxigenin-labeled riboprobes for these genes
were used (Roche, Basel, Switzerland). QRT-PCR qRT-PCR was performed as previously described63 and conducted following the MIQE guidelines64. In brief, total RNA was isolated from the
sorted cells or tissues using RNeasy Micro Kit or RNeasy Mini Kit (Qiagen, Hilden, Germany) and 50 ng of total RNA was reverse-transcribed to cDNA using random hexamers and M-MLV Reverse
Transcriptase (Thermo Fisher Scientific). RNA integrity numbers (RINs) of all samples were confirmed to be higher than 7.5 using a Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA).
qRT-PCR was performed using a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) with the SYBR Select Master Mix (Applied Biosystems, Foster City, CA, USA). Gene expression
was determined using the standard curve method. The correlation coefficients (R2) for the standard curves were higher than 0.99. Gene expression levels were normalized to those of _Actb_
(β-actin). The primers used for the PCR are listed in Supplemental Table 3. MRNA SEQUENCING mRNA sequencing was performed as described previously30. Briefly, poly(A) RNA content was isolated
from total RNA (10 ng per sample) prepared from sorted XY and XX/_Sry_ ALCs using the NEBNext Poly(A) mRNA Magnetic Isolation Module (New England Biolabs, Ipswich, MA, USA). Sequence
libraries were constructed using NEBNext Ultra II Directional RNA Library Prep Kit for Illumina (New England Biolabs) and NEBNext Multiplex Oligo for Illumina (Dual Index Primers Set 1; New
England Biolabs). cDNA libraries were sequenced using a NovaSeq 6000 (51-bp pair-end; Illumina, San Diego, CA, USA). DATA PROCESSING The FastQ files were mapped using STAR software65
(version 2.7.0a; standard option) to the mouse reference genome (UCSC mm10) and the genome annotation (modified to integrate the EGFP and mCherry transgenes) was downloaded from the UCSC
Genome Browser. Bam files were generated using SamTools66 (version 0.3.3). Quality control, mapping, read count, and CPM (counts per million mapped reads) were computed using featureCounts67
(version 1.6.4; option ‘-O -p’), edgeR68 (version 3.20.9), and an in-house pipeline. MicroRNA and small nucleolar RNA genes were excluded from the analyses. Gene expression data are
presented as CPM. Mean values for biological replicates (n = 3) were calculated, and genes with CPM values < 20 in both XY and XX/_Sry_ ALCs were removed. Differentially expressed genes
were identified based on fold change and subjected to Gene Ontology (GO) analyses using DAVID. The significantly enriched biological process GO terms with _p_-values < 0.001 were
visualized in two-dimensional plots using REVIGO69. Fold changes in gene expression levels (XX/_Sry_ vs. XY) for Sertoli cells were also calculated using the transcriptome data in our
previous study6 (accession number: DRA004090). When comparing whole gene expression changes in the two types of cells, a pseudo-count of 10 was added to the CPM values before the fold
changes were calculated. MEASUREMENT OF INTRATESTICULAR SEX STEROIDS Testes obtained from eight-week-old XY and XX/_Sry_ mice were lyophilized using a Vacuum Centrifugal Evaporator
(CVE-2000; EYELA, Tokyo, Japan) and stored at − 80 °C until later use. Gas chromatography–mass spectrometry steroid profiling was performed using a Shimadzu GC 2010 Plus gas chromatograph
coupled to a triple-quadrupole GCMS-TQ8050 (Shimadzu Corporation, Kyoto, Japan) as previously described70. Quantitative results were based on absolute quantities of steroid molecules per
testis, and their metabolic ratios were also calculated to express their corresponding enzymatic activities. STATISTICAL ANALYSIS At least three biologically independent samples were used in
all experiments. Data are presented as mean ± SEM. Differences between XY and XX/_Sry_ cells or testes were examined using two-tailed Student’s _t_-tests or Mann–Whitney _U_ tests, and
statistical significance was inferred at _p_ < 0.05. All statistical analyses were performed using R software version 3.4.3 (https://www.r-project.org). DATA AVAILABILITY The
transcriptome data have been deposited in DDBJ under the accession number DRA009797 and DRA010792. REFERENCES * Koopman, P., Münsterberg, A., Capel, B., Vivian, N. & Lovell-Badge, R.
Expression of a candidate sex-determining gene during mouse testis differentiation. _Nature_ 348, 450–452. https://doi.org/10.1038/348450a0 (1990). Article ADS CAS PubMed Google Scholar
* Kashimada, K. & Koopman, P. Sry: the master switch in mammalian sex determination. _Development_ 137, 3921–3930. https://doi.org/10.1242/dev.048983 (2010). Article CAS PubMed
Google Scholar * Koopman, P., Gubbay, J., Vivian, N., Goodfellow, P. & Lovell-Badge, R. Male development of chromosomally female mice transgenic for Sry. _Nature_ 351, 117–121.
https://doi.org/10.1038/351117a0 (1991). Article ADS CAS PubMed Google Scholar * Ishii, M. _et al._ Potency of testicular somatic environment to support spermatogenesis in XX/Sry
transgenic male mice. _Development_ 134, 449–454. https://doi.org/10.1242/dev.02751 (2006). Article CAS PubMed Google Scholar * Burgoyne, P. S. The role of the mammalian Y chromosome in
spermatogenesis. _Development_ 101(Suppl), 133–141 (1987). PubMed Google Scholar * Shishido, Y. _et al._ Differential lactate and cholesterol synthetic activities in XY and XX Sertoli
cells. _Sci. Rep._ 7, 41912. https://doi.org/10.1038/srep41912 (2017). Article ADS CAS PubMed PubMed Central Google Scholar * Boussouar, F. & Benahmed, M. Lactate and energy
metabolism in male germ cells. _Trends Endocrinol. Metab._ 15, 345–350. https://doi.org/10.1016/j.tem.2004.07.003 (2004). Article CAS PubMed Google Scholar * Keber, R., Rozman, D. &
Horvat, S. Sterols in spermatogenesis and sperm maturation. _J. Lipid Res._ 54, 20–33. https://doi.org/10.1194/jlr.r032326 (2012). Article PubMed Google Scholar * Ariyaratne, H. B. &
Mendis-Handagama, S. M. L. Changes in the testis interstitium of sprague dawley rats from birth to sexual maturity. _Biol. Reprod._ 62, 680–690. https://doi.org/10.1095/biolreprod62.3.680
(2000). Article CAS PubMed Google Scholar * Kerr, J. B. & Knell, C. M. The fate of fetal Leydig cells during the development of the fetal and postnatal rat testis. _Development_ 103,
535–544 (1988). CAS PubMed Google Scholar * Shima, Y. _et al._ Fetal leydig cells persist as an androgen-independent subpopulation in the postnatal testis. _Mol. Endocrinol._ 29,
1581–1593. https://doi.org/10.1210/me.2015-1200 (2015). Article CAS PubMed PubMed Central Google Scholar * Morohashi, K., Baba, T. & Tanaka, M. Steroid hormones and the development
of reproductive organs. _Sex. Dev._ 7, 61–79. https://doi.org/10.1159/000342272 (2013). Article CAS PubMed Google Scholar * Teerds, K. J. & Huhtaniemi, I. T. Morphological and
functional maturation of Leydig cells: From rodent models to primates. _Hum. Reprod. Update_ 21, 310–328. https://doi.org/10.1093/humupd/dmv008 (2015). Article CAS PubMed Google Scholar
* Zuber, M., Simpson, E. & Waterman, M. Expression of bovine 17 alpha-hydroxylase cytochrome P-450 cDNA in nonsteroidogenic (COS 1) cells. _Science_ 234, 1258–1261.
https://doi.org/10.1126/science.3535074 (1986). Article ADS CAS PubMed Google Scholar * Committee, N. R. N. A unified nomenclature system for the nuclear receptor superfamily. _Cell_
97, 161–163. https://doi.org/10.1016/s0092-8674(00)80726-6 (1999). Article Google Scholar * Lala, D. S., Rice, D. A. & Parker, K. L. Steroidogenic factor I, a key regulator of
steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. _Mol. Endocrinol._ 6, 1249–1258. https://doi.org/10.1210/mend.6.8.1406703 (1992). Article CAS PubMed Google
Scholar * Morohashi, K., Honda, S., Inomata, Y., Handa, H. & Omura, T. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. _J. Biol. Chem._
267, 17913–17919 (1992). CAS PubMed Google Scholar * Honda, S. _et al._ Ad4BP regulating steroidogenic P-450 gene is a member of steroid hormone receptor superfamily. _J. Biol. Chem._
268, 7494–7502 (1993). Article CAS PubMed Google Scholar * Leers-Sucheta, S., Morohashi, K.-I., Mason, I. J. & Melner, M. H. Synergistic activation of the human type II
3β-hydroxysteroid dehydrogenase/Δ 5 -Δ 4 isomerase promoter by the transcription factor steroidogenic factor-1/adrenal 4-binding protein and phorbol ester. _J. Biol. Chem._ 272, 7960–7967.
https://doi.org/10.1074/jbc.272.12.7960 (1997). Article CAS PubMed Google Scholar * Martin, L. J. _et al._ GATA factors and the nuclear receptors, steroidogenic factor 1/liver receptor
homolog 1, are key mutual partners in the regulation of the human 3β-hydroxysteroid dehydrogenase type 2 promoter. _Mol. Endocrinol._ 19, 2358–2370. https://doi.org/10.1210/me.2004-0257
(2005). Article CAS PubMed Google Scholar * Bakke, M. & Lund, J. Mutually exclusive interactions of two nuclear orphan receptors determine activity of a cyclic adenosine
3’,5’-monophosphate-responsive sequence in the bovine CYP17 gene. _Mol. Endocrinol._ 9, 327–339. https://doi.org/10.1210/mend.9.3.7776979 (1995). Article CAS PubMed Google Scholar *
Zhang, P. & Mellon, S. H. The orphan nuclear receptor steroidogenic factor-1 regulates the cyclic adenosine 3’,5’-monophosphate-mediated transcriptional activation of rat cytochrome
P450c17 (17 alpha-hydroxylase/c17-20 lyase). _Mol. Endocrinol._ 10, 147–158. https://doi.org/10.1210/mend.10.2.8825555 (1996). Article CAS PubMed Google Scholar * Sewer, M. B. _et al._
Transcriptional activation of human CYP17 in H295R adrenocortical cells depends on complex formation among p54 nrb /NonO, protein-associated splicing factor, and SF-1, a complex that also
participates in repression of transcription. _Endocrinology_ 143, 1280–1290. https://doi.org/10.1210/endo.143.4.8748 (2002). Article CAS PubMed Google Scholar * Michael, D. M., Kilgore,
M. W., Morohashi, K.-I. & Simpson, E. R. Ad4BP/SF-1 regulates cyclic AMP-induced transcription from the proximal promoter (PII) of the human aromatase P450 ( CYP19) gene in the ovary.
_J. Biol. Chem._ 270, 13561–13566. https://doi.org/10.1074/jbc.270.22.13561 (1995). Article CAS PubMed Google Scholar * Morohashi, K. I. & Omura, T. Ad4BP/SF-1, a transcription
factor essential for the transcription of steroidogenic cytochrome P450 genes and for the establishment of the reproductive function. _FASEB J._ 10, 1569–1577.
https://doi.org/10.1096/fasebj.10.14.9002548 (1996). Article CAS PubMed Google Scholar * Parker, K. L. & Schimmer, B. P. Steroidogenic factor 1: A key determinant of endocrine
development and function. _Endocr. Rev._ 18, 361–377. https://doi.org/10.1210/edrv.18.3.0301 (1997). Article CAS PubMed Google Scholar * Ikonen, E. Cellular cholesterol trafficking and
compartmentalization. _Nat. Rev. Mol. Cell. Biol._ 9, 125–138. https://doi.org/10.1038/nrm2336 (2008). Article CAS PubMed Google Scholar * Horton, J. D., Goldstein, J. L. & Brown, M.
S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. _J. Clin. Invest._ 109, 1125–1131. https://doi.org/10.1172/jci15593 (2002). Article CAS
PubMed PubMed Central Google Scholar * Baba, T. _et al._ Ad4BP/SF-1 regulates cholesterol synthesis to boost the production of steroids. _Commun. Biol._ 1, 18.
https://doi.org/10.1038/s42003-018-0020-z (2018). Article PubMed PubMed Central Google Scholar * Miyabayashi, K. _et al._ Alterations in fetal leydig cell gene expression during fetal
and adult development. _Sex. Dev._ 11, 53–63. https://doi.org/10.1159/000453323 (2017). Article CAS PubMed Google Scholar * Pérez-Cadahía, B., Drobic, B. & Davie, J. R. Activation
and function of immediate-early genes in the nervous system. _Biochem. Cell Biol._ 89, 61–73. https://doi.org/10.1139/o10-138 (2011). Article PubMed Google Scholar * Seo, Y.-K. _et al._
Genome-wide localization of SREBP-2 in hepatic chromatin predicts a role in autophagy. _Cell Metab._ 13, 367–375. https://doi.org/10.1016/j.cmet.2011.03.005 (2011). Article CAS PubMed
PubMed Central Google Scholar * Morohashi, K. _et al._ Functional difference between Ad4BP and ELP, and their distributions in steroidogenic tissues. _Mol. Endocrinol._ 8, 643–653.
https://doi.org/10.1210/mend.8.5.8058072 (1994). Article CAS PubMed Google Scholar * O’Shaughnessy, P. J., Willerton, L. & Baker, P. J. Changes in leydig cell gene expression during
development in the mouse. _Biol. Reprod._ 66, 966–975. https://doi.org/10.1095/biolreprod66.4.966 (2002). Article PubMed Google Scholar * Royo, T. _et al._ Testis and ovary express the
gene for the ketogenic mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase. _J. Lipid Res._ 34, 867–874 (1993). CAS PubMed Google Scholar * Bagheri-Fam, S. _et al._ The gene encoding
the ketogenic enzyme HMGCS2 displays a unique expression during gonad development in mice. _PLoS ONE_. https://doi.org/10.1371/journal.pone.0227411 (2020). Article PubMed PubMed Central
Google Scholar * Kang, Z., Qiao, N., Tan, Z., Tang, Z. & Li, Y. Expression patterns and changes of the LCN2 gene in the testes of induced cryptorchidism and busulfan-treated mice.
_Syst. Biol. Reprod. Med._ 63, 364–369. https://doi.org/10.1080/19396368.2017.1355416 (2017). Article CAS PubMed Google Scholar * Koga, M. _et al._ Expression of selenoprotein-p
messenger ribonucleic acid in the rat testis. _Biol. Reprod._ 58, 261–265. https://doi.org/10.1095/biolreprod58.1.261 (1998). Article CAS PubMed Google Scholar * Gerena, R. L., Eguchi,
N., Urade, Y. & Killian, G. J. Stage and region-specific localization of lipocalin-type prostaglandin D synthase in the adult murine testis and epididymis. _J. Androl._ 21, 848–854.
https://doi.org/10.1002/j.1939-4640.2000.tb03415.x (2000). Article CAS PubMed Google Scholar * Huhtaniemi, I. _et al._ Genetically modified mouse models in studies of luteinising hormone
action. _Mol. Cell. Endocrinol._ 252, 126–135. https://doi.org/10.1016/j.mce.2006.03.026 (2006). Article CAS PubMed Google Scholar * Rulli, S. B. _et al._ Elevated steroidogenesis,
defective reproductive organs, and infertility in transgenic male mice overexpressing human chorionic gonadotropin. _Endocrinology_ 144, 4980–4990. https://doi.org/10.1210/en.2003-0403
(2003). Article CAS PubMed Google Scholar * Matzuk, M. M., DeMayo, F. J., Hadsell, L. A. & Kumar, T. R. Overexpression of human chorionic gonadotropin causes multiple reproductive
defects in transgenic mice. _Biol. Reprod._ 69, 338–346. https://doi.org/10.1095/biolreprod.102.013953 (2003). Article CAS PubMed Google Scholar * Li, X. _et al._ Regulation of
seminiferous tubule-associated stem Leydig cells in adult rat testes. _Proc. Natl. Acad. Sci. U.S.A._ 113, 2666–2671. https://doi.org/10.1073/pnas.1519395113 (2016). Article ADS CAS
PubMed PubMed Central Google Scholar * Schultz, R., Kononen, J. & Pelto-Huikko, M. Induction of immediate early gene mRNAs and proteins by hCG in interstitial cells of rat testis. _J.
Endocrinol._ 144, 417–424. https://doi.org/10.1677/joe.0.1440417 (1995). Article CAS PubMed Google Scholar * Manna, P. R. & Stocco, D. M. The role of JUN in the regulation of
PRKCC-mediated STAR expression and steroidogenesis in mouse Leydig cells. _J. Mol. Endocrinol._ 41, 329–341. https://doi.org/10.1677/jme-08-0077 (2008). Article CAS PubMed Google Scholar
* Guo, I.-C., Huang, C.-Y., Wang, C.-K. & Chung, B.-C. Activating protein-1 cooperates with steroidogenic factor-1 to regulate 3′,5′-cyclic adenosine 5′-monophosphate-dependent human
CYP11A1 transcription in vitro and in vivo. _Endocrinology_ 148, 1804–1812. https://doi.org/10.1210/en.2006-0938 (2007). Article CAS PubMed Google Scholar * Zhao, Y. _et al._ TCF7L2 and
EGR1 synergistic activation of transcription of LCN2 via an ERK1/2-dependent pathway in esophageal squamous cell carcinoma cells. _Cell. Signal._ 55, 8–16.
https://doi.org/10.1016/j.cellsig.2018.12.007 (2019). Article CAS PubMed Google Scholar * Manna, P. R., Wang, X.-J. & Stocco, D. M. Involvement of multiple transcription factors in
the regulation of steroidogenic acute regulatory protein gene expression. _Steroids_ 68, 1125–1134. https://doi.org/10.1016/j.steroids.2003.07.009 (2003). Article CAS PubMed Google
Scholar * Mizutani, T. _et al._ C/EBPβ (CCAAT/enhancer-binding protein β) mediates progesterone production through transcriptional regulation in co-operation with SF-1 (steroidogenic
factor-1). _Biochem. J._ 460, 459–471. https://doi.org/10.1042/BJ20131522 (2014). Article CAS PubMed Google Scholar * Martin, L. J. & Tremblay, J. J. Glucocorticoids antagonize
cAMP-induced Star transcription in Leydig cells through the orphan nuclear receptor NR4A1. _J. Mol. Endocrinol._ 41, 165–175. https://doi.org/10.1677/JME-07-0145 (2008). Article CAS PubMed
Google Scholar * Miller, W. L. & Tee, M. The post-translational regulation of 17,20 lyase activity. _Mol. Cell. Endocrinol._ 408, 99–106. https://doi.org/10.1016/j.mce.2014.09.010
(2015). Article CAS PubMed Google Scholar * Yanagibashi, K. & Hall, P. F. Role of electron transport in the regulation of the lyase activity of C21 side-chain cleavage P-450 from
porcine adrenal and testicular microsomes. _J. Biol. Chem._ 261, 8429–8433 (1986). CAS PubMed Google Scholar * Onoda, M. & Hall, P. F. Cytochrome b5 stimulates purified testicular
microsomal cytochrome P-450 (C21 side-chain cleavage). _Biochem. Biophys. Res. Commun._ 108, 454–460. https://doi.org/10.1016/0006-291x(82)90850-6 (1982). Article CAS PubMed Google
Scholar * Sondhi, V. _et al._ Impaired 17,20-Lyase activity in male mice lacking cytochrome b5 in leydig cells. _Mol. Endocrinol._ 30, 469–478. https://doi.org/10.1210/me.2015-1282 (2016).
Article CAS PubMed PubMed Central Google Scholar * Zhang, L. H., Rodriguez, H., Ohno, S. & Miller, W. L. Serine phosphorylation of human P450c17 increases 17,20-lyase activity:
Implications for adrenarche and the polycystic ovary syndrome. _Proc. Natl. Acad. Sci. U.S.A._ 92, 10619–10623. https://doi.org/10.1073/pnas.92.23.10619 (1995). Article ADS CAS PubMed
PubMed Central Google Scholar * Tee, M. K. & Miller, W. L. Phosphorylation of human cytochrome P450c17 by p38α selectively increases 17,20 lyase activity and androgen biosynthesis. _J.
Biol. Chem._ 288, 23903–23913. https://doi.org/10.1074/jbc.M113.460048 (2013). Article CAS PubMed PubMed Central Google Scholar * Kempná, P., Hirsch, A., Hofer, G., Mullis, P. E. &
Flück, C. E. Impact of differential P450c17 phosphorylation by cAMP stimulation and by starvation conditions on enzyme activities and androgen production in NCI-H295R cells. _Endocrinology_
151, 3686–3696. https://doi.org/10.1210/en.2010-0093 (2010). Article CAS PubMed Google Scholar * Kidokoro, T. _et al._ Influence on spatiotemporal patterns of a male-specific Sox9
activation by ectopic Sry expression during early phases of testis differentiation in mice. _Dev. Biol._ 278, 511–525. https://doi.org/10.1016/j.ydbio.2004.11.006 (2005). Article CAS
PubMed Google Scholar * McFarlane, L., Truong, V., Palmer, J. S. & Wilhelm, D. Novel PCR assay for determining the genetic sex of mice. _Sex. Dev._ 7, 207–211.
https://doi.org/10.1159/000348677 (2013). Article CAS PubMed Google Scholar * Miyabayashi, K. _et al._ Heterogeneity of ovarian theca and interstitial gland cells in mice. _PLoS ONE_.
https://doi.org/10.1371/journal.pone.0128352 (2015). Article PubMed PubMed Central Google Scholar * Yamamura, K. _et al._ Immunolocalization of murine type VI 3β-hydroxysteroid
dehydrogenase in the adrenal gland, testis, skin, and placenta. _Mol. Cell. Endocrinol._ 382, 131–138. https://doi.org/10.1016/j.mce.2013.09.014 (2014). Article CAS PubMed Google Scholar
* Sato, Y. _et al._ Importance of forkhead transcription factor Fkhl18 for development of testicular vasculature. _Mol. Reprod. Dev._ 75, 1361–1371. https://doi.org/10.1002/mrd.20888
(2008). Article CAS PubMed Google Scholar * Shima, Y. _et al._ Contribution of Leydig and Sertoli cells to testosterone production in mouse fetal testes. _Mol. Endocrinol._ 27, 63–73.
https://doi.org/10.1210/me.2012-1256 (2012). Article CAS PubMed PubMed Central Google Scholar * Bustin, S. A. _et al._ The MIQE guidelines: Minimum information for publication of
quantitative real-time PCR experiments. _Clin. Chem._ 55, 611–622. https://doi.org/10.1373/clinchem.2008.112797 (2009). Article CAS PubMed Google Scholar * Dobin, A. _et al._ STAR:
Ultrafast universal RNA-seq aligner. _Bioinformatics_ 29, 15–21. https://doi.org/10.1093/bioinformatics/bts635 (2012). Article CAS PubMed PubMed Central Google Scholar * Li, H. _et al._
The sequence alignment/map format and SAMtools. _Bioinformatics_ 25, 2078–2079. https://doi.org/10.1093/bioinformatics/btp352 (2009). Article CAS PubMed PubMed Central Google Scholar *
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. _Bioinformatics_ 30, 923–930.
https://doi.org/10.1093/bioinformatics/btt656 (2013). Article CAS PubMed Google Scholar * Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: A bioconductor package for
differential expression analysis of digital gene expression data. _Bioinformatics_ 26, 139–140. https://doi.org/10.1093/bioinformatics/btp616 (2009). Article CAS PubMed PubMed Central
Google Scholar * Supek, F., Bošnjak, M., Škunca, N. & Šmuc, T. REVIGO summarizes and visualizes long lists of gene ontology terms. _PLoS ONE_
https://doi.org/10.1371/journal.pone.0021800 (2011). Article PubMed PubMed Central Google Scholar * Moon, J.-Y., Lee, H., Kim, J., Lee, J. & Choi, M. Supported liquid extraction
coupled to gas chromatography-selective mass spectrometric scan modes for serum steroid profiling. _Anal. Chim. Acta_ 1037, 281–292. https://doi.org/10.1016/j.aca.2018.02.059 (2018). Article
CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers JP17H06427
(K.-i.M.), JP16H05142 (K.-i.M.), JP16K08593 (T.B.), and JP19J12133 (S.Y.), Takeda Science Foundation (T.B.), and The Shin-Nihon Foundation of Advanced Medical Research (T.B.). This work was
technically supported by the Research Support Center, Graduate School of Medical Sciences, Kyushu University. We are profoundly thankful to Prof. Mikita Suyama (Kyushu University, Japan) for
technical support in the NGS data processing, and Profs Hitoshi Okamura and Masao Doi (Kyoto University, Japan) for kindly providing the HSD3B6 antibody. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Systems Life Sciences, Graduate School of Systems Life Sciences, Kyushu University, Maidashi 3-1-1, Higashi-ku, Fukuoka, 812-8582, Japan Shogo Yanai, Takashi
Baba, Kai Inui, Fumiya Takahashi, Yasuyuki Ohkawa & Ken-ichirou Morohashi * Department of Molecular Biology, Graduate School of Medical Sciences, Kyushu University, Maidashi 3-1-1,
Higashi-ku, Fukuoka, 812-8582, Japan Takashi Baba, Kanako Miyabayashi, Miki Inoue & Ken-ichirou Morohashi * Molecular Recognition Research Center, Korea Institute of Science and
Technology, Seoul, 02792, Korea Soyun Han & Man Ho Choi * Department of Veterinary Anatomy, The University of Tokyo, Yayoi 1-1-1, Bunkyo-ku, Tokyo, 113-8657, Japan Yoshiakira Kanai *
Division of Transcriptomics, Medical Institute of Bioregulation, Kyushu University, Maidashi 3-1-1, Higashi-ku, Fukuoka, 812-8582, Japan Yasuyuki Ohkawa * AMED-CREST, Japan Agency for
Medical Research and Development, Maidashi 3-1-1, Higashi-ku, Fukuoka, 812-8582, Japan Yasuyuki Ohkawa Authors * Shogo Yanai View author publications You can also search for this author
inPubMed Google Scholar * Takashi Baba View author publications You can also search for this author inPubMed Google Scholar * Kai Inui View author publications You can also search for this
author inPubMed Google Scholar * Kanako Miyabayashi View author publications You can also search for this author inPubMed Google Scholar * Soyun Han View author publications You can also
search for this author inPubMed Google Scholar * Miki Inoue View author publications You can also search for this author inPubMed Google Scholar * Fumiya Takahashi View author publications
You can also search for this author inPubMed Google Scholar * Yoshiakira Kanai View author publications You can also search for this author inPubMed Google Scholar * Yasuyuki Ohkawa View
author publications You can also search for this author inPubMed Google Scholar * Man Ho Choi View author publications You can also search for this author inPubMed Google Scholar *
Ken-ichirou Morohashi View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.Y., T.B. and K.-i.M. conceived and designed the experimental
approach and performed experiments. S.Y. and K.-i.M. prepared the manuscript. K.I. contributed to the computational analyses for mRNA-seq. M.I. and K.Mi. performed in situ hybridization.
S.H. and M.H.C. measured intratesticular sex steroids. F.T. constructed the mRNA-seq libraries. Y.K. provided the transgenic mice. Y.O. performed deep sequencing of the mRNA-seq libraries.
CORRESPONDING AUTHOR Correspondence to Ken-ichirou Morohashi. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION 1. SUPPLEMENTARY
INFORMATION 2. SUPPLEMENTARY INFORMATION 3. SUPPLEMENTARY INFORMATION 4. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative
Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yanai, S., Baba, T., Inui, K. _et al._ Gene expression and functional abnormalities
in XX/_Sry_ Leydig cells. _Sci Rep_ 11, 719 (2021). https://doi.org/10.1038/s41598-020-80741-z Download citation * Received: 15 April 2020 * Accepted: 02 December 2020 * Published: 12
January 2021 * DOI: https://doi.org/10.1038/s41598-020-80741-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative