- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Antibiotics were derived originally from wild organisms and therefore understanding how these compounds evolve among different lineages might help with the design of new
antimicrobial drugs. We report the draft genome sequence of Alexander Fleming’s original fungal isolate behind the discovery of penicillin, now classified as _Penicillium rubens_ Biourge
(1923) (IMI 15378). We compare the structure of the genome and genes involved in penicillin synthesis with those in two ‘high producing’ industrial strains of _P. rubens_ and the closely
related species _P. nalgiovense_. The main effector genes for producing penicillin G (_pcbAB_, _pcbC_ and _penDE_) show amino acid divergence between the Fleming strain and both industrial
strains, whereas a suite of regulatory genes are conserved. Homologs of penicillin N effector genes _cefD1_ and _cefD2_ were also found and the latter displayed amino acid divergence between
the Fleming strain and industrial strains. The draft assemblies contain several partial duplications of penicillin-pathway genes in all three _P. rubens_ strains, to differing degrees,
which we hypothesise might be involved in regulation of the pathway. The two industrial strains are identical in sequence across all effector and regulatory genes but differ in duplication
of the _pcbAB_–_pcbC_–_penDE_ complex and partial duplication of fragments of regulatory genes. We conclude that evolution in the wild encompassed both sequence changes of the effector genes
and gene duplication, whereas human-mediated changes through mutagenesis and artificial selection led to duplication of the penicillin pathway genes. SIMILAR CONTENT BEING VIEWED BY OTHERS
UNRAVELING THE GENOME OF _BACILLUS VELEZENSIS_ MEP218, A STRAIN PRODUCING FENGYCIN HOMOLOGS WITH BROAD ANTIBACTERIAL ACTIVITY: COMPREHENSIVE COMPARATIVE GENOME ANALYSIS Article Open access
13 December 2023 HIGH INTRAGENOMIC, INTERGENOMIC, AND PHENOTYPIC DIVERSITY IN PULCHERRIMIN-PRODUCING _METSCHNIKOWIA_ YEASTS INDICATES A SPECIAL MODE OF GENOME EVOLUTION Article Open access
08 May 2024 WHOLE GENOME SEQUENCE CHARACTERIZATION OF _ASPERGILLUS TERREU_S ATCC 20541 AND GENOME COMPARISON OF THE FUNGI _A. TERREU_S Article Open access 05 January 2023 INTRODUCTION
Clinical use of antibiotics has revolutionised treatment of bacterial infection. The characterization of penicillin1 followed from Alexander Fleming’s discovery of lysis and inhibition of
the growth of _Staphylococcus_ on petri dishes colonized by the fungus _Penicillium rubens_2 (named at the time as _P. notatum,_ and until recently as _P. chrysogenum_3,4). There followed a
golden age in the use of antibiotics to combat bacterial disease5. It was soon realized, however, that this might be short-lived, as more and more pathogenic bacteria evolved resistance to
these compounds6,7,8. The result is an arms race between clinical use of antibiotics and the evolution of resistance in target bacteria, which requires new classes of antibiotics and new
methods of delivery if medicine is to retain the upper hand. One potentially useful way to improve antibiotic use and deployment is to draw inspiration from the evolution of antibiotic
production and resistance in nature. Micro-organisms produce antibiotics to suppress the growth of their antagonists and reduce competition for space and resources9,10. Accordingly,
organisms are under selection to evolve resistance to the antibiotics of others, yet producers are under reciprocal selection to increase the efficacy of their own antibiotics against their
antagonists. With such an arms race11, it is expected that as antibiotic resistance evolves, antibiotics themselves will evolve too12. Genome sequencing can reveal variation in antibiotic
production pathways among organisms with different antibiotic profiles. For instance, the ability to produce new compounds might evolve by modification of the synthesis genes or metabolic
pathways, or there might be changes to existing compounds to counter the degradation of the antibiotic by resistant bacteria. These adaptations could encompass changes in the amino acid
sequence of effector genes, changes in regulators of the antibiotic-production pathway, or duplication of effector genes. Gene duplication might lead to increased expression of the enzyme if
the copies are conserved, or to production of multiple variants of the antibiotic if paralogous copies diverge in function13. In recent decades, considerable effort has gone into the study
of the evolution of antibiotic resistance as a solution to the crisis7,14, but the evolution of antibiotics themselves remains largely neglected. Understanding how antibiotics coevolve in
natural arms races with resistant bacteria might help to design methods for countering resistance evolution in the parallel arms race in clinical settings. In order to gain insights into the
evolution of genes underlying the production of the classic antibiotic, penicillin, we here present a draft genome sequence for Fleming’s original isolate, _Penicillium rubens_ (IMI 15378).
Cryopreserved living samples of this isolate are kept in numerous global collections, and we revived the fungus from the CABI (IMI) living culture collection for DNA extraction and
whole-genome sequencing. We compare the overall genome structure and variation in a set of genes involved in the penicillin-production pathway with closely related _Penicillium_ isolates
with sequenced genomes. In particular, we compare Fleming’s isolate to two industrial strains of _P. rubens_ derived from a second isolation from the wild in the USA. These strains were
originally misnamed as _P. chrysogenum_ (and still are in some public sequence databases) but were subsequently shown by multigene analysis to belong to the _P. rubens_ clade3,4. The
original wild US isolate (NRRL1951), isolated from a mouldy cantaloupe, was subjected to multiple rounds of X-ray, chemical (chlormethine) and ultraviolet mutagenesis and artificial
selection15 in order to generate isolates with high production rates of penicillin for industry16, including P2niaD18 and Wisconsin 54-125517. The two lines split after an initial shared
phase of both UV and X-ray mutagenesis and selection (via a common ancestor strain of Wis Q-176, see Fig. 1 in16,18,19). Wisconsin 54-1255 was derived from further rounds of UV and nitrogen
mustard mutagenesis and selection, while P2niaD18 was derived from a separate round of undocumented improvements by Panlabs Inc, followed by deletion of the nitrate reductase gene
niaD16,18,19. Any differences from the Fleming isolate that are shared by these two strains resulted either from evolution in the wild progenitors, or from the first steps of artificial
selection and mutagenesis in the lab. In contrast, differences between the two industrial strains are solely the result of mutagenesis and artificial selection for high production: for
example, comparison of P2niaD18 to the original Wisconsin 54-1255 genome17 revealed evidence for structural rearrangements and tandem duplication of penicillin-producing genes caused by the
mutagenesis20. To provide a broader context for evolution in the wild, we also compare the Fleming genome to another penicillin-producing species from the _Penicillium_ section _Chrysogena_,
_P. nalgiovense_21,22, Our focus is primarily on short-term evolution among the closely related strains rather than the longer-term acquisition of penicillin production and so we do not
repeat previous comparisons among more distantly related organisms23,24. As well as comparing genome structure, we searched for genes involved in the penicillin pathway and compared copy
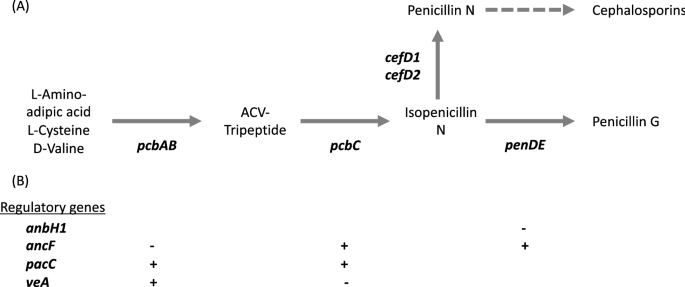
number and sequence divergence among strains. Penicillin is a beta-lactam and encompasses several natural variations of the compound. _P. rubens_ produces penicillin G via a 15 kb gene
cluster of 3 genes (_pcbAB_, _pcbC_ and _penDE_) present in the genomes of various filamentous fungi25,26,27 (Fig. 1). The first two genes _pcbAB_ and _pcbC_ encode the enzymes
delta-(l-alpha-aminoadipyl)-l-cysteinyl-d-valine synthetase (ACVS) and isopenicillin N synthase respectively23,28, which catalyse the formation of the first bioactive molecule in the
pathway, isopenicillin N26. All beta-lactams share this two-step pathway (including cephalosporins and cephamycins23,26,28). Evidence indicates that the genes _pcbAB_ and _pcbC_ were
horizontally acquired from bacteria by the beta-lactam producing fungi24. The third gene _penDE_ encodes the enzyme isopenicillin N acyltransferase, which catalyses the final step of the
biosynthetic pathway that synthesizes penicillin G27,29. This gene is hypothesized to have evolved within the beta-lactam producing fungi rather than being horizontally acquired from
bacteria24. In the beta-lactam producing fungi _Acremonium chrysogenum_, the genes _cefD1_ and _cefD2_ encode the isopenicillin N epimerase system and provide an alternative biosynthetic
pathway to produce penicillin N from isopenicillin N, and cephalosporin C from penicillin N30,31,32. Several genes have been identified that play a role in regulating the pathway leading to
penicillin G production, and evolved within beta-lactam producing fungi, described in more detail below24,33. We hypothesized that selection on antibiotic production should most likely
result in changes in the coding sequence or copy number of effector genes at later stages of the pathway (i.e._ penDE_, which is unique to the production of penicillin G as opposed to other
beta-lactams), or of the regulatory genes, rather than the upstream effector genes that generate pre-cursors used by multiple antibiotics. MATERIALS AND METHODS CULTURING, DNA EXTRACTION AND
SEQUENCING The fungus _Penicillium rubens_ Biourge (1923)34 IMI 15378 (= ATCC 8537; NRRL 824; CBS 205.57) was obtained from the CABI IMI culture collection. As part of a separate experiment
(not reported here), replicates of fungus were grown for 11 weeks at 20 °C on petri dishes with LB Lennox agar media with addition of 20 g/L of sucrose. Fungus from each of 6 treatments was
then cultured in LB Lennox Broth with 20 g/L of sucrose at room temperature for a week prior to the DNA extraction. For each of the six treatments, around 100 mg of washed mycelium was
ground under liquid nitrogen and DNA was extracted using the DNeasy Plant Mini Kit (Qiagen). DNA libraries were prepared with an Illumina TruSeq PCR-free kit at the Department of
Biochemistry, University of Cambridge, and sequenced with Illumina MiSeq v2 technology with 2 × 150 paired-end sequencing and 350 bp insert size. Separate library preparations and sequencing
were performed for the 6 separate extractions, but subsequent results indicated no changes had accrued among the treatments, so reads were pooled for the assembly and analyses presented
here. GENOME ASSEMBLY AND ANALYSIS Raw reads were filtered for low-quality bases and adapter sequences using BBTools ‘bbduk’ v38.22 (available at https://sourceforge.net/projects/bbmap/)
with the parameters ‘ktrim = r k = 23 mink = 11 hdist = 2 maq = 10 minlen = 100 tpe tbo’. An initial assembly was constructed using SPAdes v3.13.035 with default parameters and potential
contamination was assessed using BlobTools v1.036. Genome completeness was assessed using Benchmarking Universal Single-Copy Ortholog (BUSCO) gene sets v3.0.2 for Eukaryota (_n_ = 303) and
Fungi (_n_ = 290)37. Assembled genomes for the two industrial strains, P2niaD18 and Wisconsin 54-1255, and _P. nalgiovense_ (IBT 13039) were downloaded from NCBI GenBank (Table 1). The
P2niaD18 genome was scaffolded into whole chromosomes in the source paper by comparing alignments to the Wisconsin 54-1255 genome. Genomes were aligned using nucmer in the mummer package
4.0beta, and structural changes visualized with dotplots using the DNANexus Dot browser (available at https://dnanexus.github.io/dot/). All raw sequence data have been deposited in the
relevant International Nucleotide Sequence Database Collaboration (INSDC) databases under the Study ID PRJEB35151 (see Table S1 for run accessions). PENICILLIN PATHWAY GENES We searched for
the _pcbAB_, _pcbC_ and _penDE_ genes in each genome using BLAST, and for a paralog of _penDE_ that was first discovered in the Wisconsin 54-1255 genome17,38 and functionally
characterized39. Query sequences are listed in Table S2. We also searched for _cefD1_ and _cefD2_ genes, which catalyse an alternative pathway for converting isopenicillin N to penicillin N
rather than penicillin G, and were previously discovered in the Wisconsin 54-1255 genome17 and shown to be expressed. In addition, we searched for a suite of genes identified as playing a
regulatory role in penicillin production: _anBH1_, the three subunits of the transcription factor _ancF_ (_hapB_, _hapC_, and _hapE_), _pacC_, and _veA_. Functions of these genes are
summarised in Fig. 1. In brief, PacC is a wide domain pH and carbon source dependent regulator_,_ which upregulates the _pcbAB_ and _pcbC_ genes in _P. chrysogenum_ in an alkaline
environment and/or when the fungus is grown on a depleted carbon source40,41. VeA is a wide domain light dependent regulator in _P. chrysogenum_, _A. chrysogenum_ and _Aspergillus nidulans_.
It is involved in upregulation of _pcbAB_ and downregulation of _pcbC_26,42,43. The transcription factor ancF consists of 3 subunits hapB, hapC and hapE and is responsible for the
downregulation of the gene _pcbAB_ and upregulation of the genes _pcbC_ and _penDE_ in _P. chrysogenum_44,45,46. The _anBH1_ gene produces the basic-region helix loop helix protein (bHLH)
that binds to the promoter region upstream of _penDE_ and downregulates the transcription of _penDE_26,47. For each gene, we generated an alignment (including multiple copies where present)
using MAFFT 1.3.648 and reconstructed a phylogenetic tree by maximum likelihood using the GTR + invgamma model in PHYML 2.2.349, implemented in Geneious 9.1.8 (Biomatters Ltd, Auckland, New
Zealand, https://www.geneious.com). We tested for evidence of positive selection among strains by running codon models in PAML 450 for genes displaying variation: the null model of a single
dN/dS ratio across codons, referred to as ω; the neutral model with a fraction _p_1 of codons that are under purifying selection (dN/dS < 1; _ω_1) and a fraction _p_2 evolving neutrally
(dN/dS = 1; _ω_2); and the positive selection model including an additional fraction _p_3 codons evolving positively (dN/dS > 1; _ω_3). We used the Akaike Information Criterion to select
the best model while penalizing for differences in the number of free parameters (null = 1, neutral = 2, positive = 4). The test is conservative because it requires substantial changes in
amino acids to detect positive selection, whereas in reality a single amino acid change could underlie functional divergence. In addition to comparing best models, we plotted the average
dN/dS ratio across the genome (ω) as a measure of degree of amino acid conservation among strains, with low values indicating stronger overall purifying selection. RESULTS GENOME ASSEMBLY OF
_P. RUBENS_ (IMI 15378) A total of 2.86 Gb trimmed data (82.3% raw; Table S1) were used to generate an initial assembly comprising 274 scaffolds spanning 30.51 Mb. Screens for potential
contamination resulted in the removal of 6 scaffolds (15.7 kb) that were not marked as from the genus _Penicillium_ based on sequence similarity to NCBI ‘nt’ and UniRef90 public repositories
(Fig S1). Scaffolds less than 500 bp in length were also discarded, resulting in a final draft assembly of 101 scaffolds spanning 30.46 Mb total length (~ 94X coverage), with an N50
scaffold length of 1.62 Mb. Assembly quality, based on the presence of core eukaryotic and fungal genes (BUSCO), indicated a high level of gene completeness (99.0% and 99.3% respectively)
and a low level of duplication (0.3% and 1.0% respectively), suggesting a haploid genome assembly in common with the other strains (Table 2). The assembly is marginally smaller than the
assemblies of the two industrial strains (32.4 and 32.2 Mb respectively) and has similar GC content (48.9% vs 49% for both industrial strains). The final genome assembly for _P. rubens_ IMI
15378 (nPRUBv1) has been deposited at DDBJ/ENA/GenBank under the accession GCA_902636305.1 (CACPRF010000001–CACPRF010000101). STRUCTURAL COMPARISON AMONG PENICILLIUM GENOMES The genome of
Fleming’s _P. rubens_ (IMI 15378) is broadly colinear with the P2niaD18 genome that was assembled to whole chromosome level, with relatively few cases of translocation or transversions (Fig.
2). More rearrangements are apparent between the Wisconsin 54-1255 strain and the P2niaD18 genome, perhaps indicative of structural mutations caused by mutagenesis during the improvement
process as previously reported20. The _P. nalgiovense_ IBT 13039 genome was broadly colinear with P2niaD18, although the greater fragmentation of the assembly makes it harder determine any
large-scale rearrangements. All three genomes of _P. rubens_ are highly similar at the sequence level: the Fleming genome is 0.106% divergent from the P2niaD18 genome across aligned regions
(31,547 SNPs from 29.7 Mb alignment) whereas the Wisconsin 54-1255 is only 0.0038% divergent (1231 SNPs from 32.2 Mb alignment). _P. nalgiovense_ IBT 13039 is 5.8% divergent (1,484,129 SNPs
from 24.8 Mb aligned regions). The structure of the penicillin effector genes is conserved across species and always falls into the well characterized cluster of _pcbAB_, _pcbC_ and _penDE_
genes (Fig. 3). The P2niaD18 genome alone has a single tandem duplication of the whole cluster, rather than multiple complete or partial tandem duplications of the cluster present in other
industrial penicillium strains20,51,52. In addition to the main loci, a partial duplicate exhibiting a match to the final 123 bp of _pcbAB_ but with three amino acid substitutions is found
in a non-coding region in the two industrial strains, 2704 bp downstream of _pcbAB_ (Fig. 3, Table S3). This fragment, labelled B1 in the previous analysis of Wisconsin 54-1255 by Fierro et
al_._52 is found in both tandem duplicates in P2niaD18, but absent from the Fleming genome (as confirmed by mapping raw reads of Fleming strain onto the Wisconsin 54-1255 genome, Fig. S2).
We speculate that this might play a functional role in the region, for example in regulating expression of _pcbAB_, but it might simply be a neutral or deleterious side-effect of the
mutagenesis during improvement of those strains. Other putative beta-lactam effector genes were found in the genomes of all the four strains compared. All four strains contained the paralog
of _penDE_ first identified in the Wisconsin 54-1255 genome17. The _cefD1_ region contains additional 36 to 57 bp long fragments that blast to genome regions outside the main coding region.
BLAST confirmed that these are not repeated domains or regions found elsewhere in the genome but represent single partial duplications similar to those observed in _pcbAB_. Two of these
duplicate fragments were found only in the three _P. rubens_ strains and two only in _P. nalgiovense_. The _cefD2_ region was not duplicated but recovered as two long sections and one short
section in all three genomes, indicating the absence of match across the full-length region found in the _P. arizonense_ gene used for the query. The genes involved in regulation of
penicillin production were scattered across the genome of each strain (Table S3). The _hapB_ gene in the _ancF_ transcription factor complex displays a partial duplicated fragment of 95 bp
in the two industrial strains, which is lacking in the other two genomes. A clear _hapE_ match was missing for _P. nalgiovense_. All other regulatory genes are present in single copy in all
four genomes. SEQUENCE DIVERGENCE OF PENICILLIN EFFECTOR AND REGULATORY GENES The two industrial strains, P2niaD18 and Wisconsin 54-1255, were identical at the sequence level for all the
focal genes and therefore for subsequent analyses only sequences from Wisconsin 54-1255 were used to represent the American isolate of _P. rubens_. In contrast, penicillin-pathway genes have
diverged in amino acid sequence between the Fleming strain and the US strains. All three effector genes encoding enzymes in the penicillin G pathway have diverged, but _pcbAB_ and _penDE_
showed the highest rates of amino acid divergence relative to silent changes whereas _pcbC_ was strongly conserved (Fig. 4, Table S4). The level of divergence in _pcbAB_ is unexpected since
this gene functions to produce the initial precursor in the pathway, which is shared in the production of other beta-lactams. The homologs of _cefD1_ and _cefD2_ genes were found in all
genomes of _P. rubens_ strains. This was unexpected as these genes are involved in the synthesis of the cephalosporin intermediate penicillin N in _A. chrysogenum_ and are not known to have
a functional role in _P. rubens_17,53,54. The penicillin N effector gene _cefD2_ also showed a high level of amino acid divergence whereas _cefD1_ was more strongly conserved than other
effector genes. The best sequence model for the effector genes plus _hapB_ was a model with most codons being under constraint (dN/dS < < 1) but with a significant proportion of codons
being unconstrained (dN/dS = 1). There was no sequence divergence between American and British _P. rubens_ isolates in the _penDE_ paralog, _pacC_, _ancF_ (_hapB_, _C_ and _E_) or _veA_. To
further investigate possible regulatory changes, we looked for sequence variation within transcription factor binding sites within the intergenic region between _pcbAB_ and _pcbC_, which is
a bidirectional promotor region for these genes. Among 28 binding sites previously identified in _P. chrysogenum_55, all were found in the Fleming genome, and just one site was lost in both
industrial strains (GATA to GGTA mutation, Table S5). Thus, the divergence of known binding sites is low, similar to that seen for regulatory proteins. DISCUSSION Nearly a century since
Alexander Fleming discovered the action of penicillin in bacterial cultures contaminated by _P. rubens_, we report the first draft genome sequence of his original strain. Very soon after the
original discovery and isolation of penicillin, a second wild isolate of _P. rubens_ from the USA was employed for future industrial manufacture owing to its greater rate of penicillin
production15. Consequently, two of the strains derived from this isolate have been the focus of previous whole genome sequencing within the _P. rubens_ clade16,17,20. We compared these
genomes with each other and a third, more distantly related genome of _P. nalgiovense_56. Comparison of the two USA strains provides insights into the industrial mutagenesis and artificial
selection process16, which was originally performed by selecting phenotypically useful mutants without knowledge of the underlying genomic basis15. There were no amino acid differences at
any genes encoding the enzymes in the penicillin pathway and regulatory genes. Instead, there was evidence for structural rearrangement across the genome, including tandem duplication of the
_pcbAB_–_pcbC_–_penDE_ cluster in P2niaD18, which has previously been studied in these and other industrial strains51,52. This fits with the type of mutagenesis and artificial selection
used for this process. Experimental work showed that tandem duplication of the _pcbAB_–_pcbC_–_penDE_ cluster does not directly increase penicillin production over short time periods—a
strain of P2niaD18 that was modified to lose one copy did not produce significantly less penicillin over a 96-h assay period57. Substantial copy number multiplication of the region among
industrial strains still seems to implicate gene duplication in penicillin production, but perhaps only under specific growth conditions or over longer periods18,51. Another plausible source
of variation would be changes in regulatory regions, but experimental evidence indicates that such variation is unlikely to contribute to increased penicillin production18. Comparison
between the UK and US genomes sheds light on both evolved differences between the wild progenitors of the strains, and potential initial changes in the domestication steps prior to the
divergence of P2niaD18 and Wisconsin 54-1255. One structural difference shared by the US genomes was the partial duplication of the final portion of the _pcbAB_ gene. Read mapping confirmed
that this region is missing from the Fleming genome and not just absent due to assembly artefacts (Fig. S2). Partial duplication and inversion have been documented previously at the ends of
the amplified region containing the penicillin synthesis genes for Wisconsin 54-125552. Furthermore, partial duplication has been found to play a role in generating novel diversity
previously, e.g., in the case of pathogen resistance in barley58, and could play a role in gene regulation. Without further sequencing, we cannot be certain whether this change occurred in
the wild progenitor of the US strains or during initial stages of domestication. Because of the nature of these changes in relation to the predicted effects of mutagenesis, however, and the
fact that further such differences arose between P2niaD18 and Wisconsin 54-1255, it seems plausible that shared structural differences of the two industrial strains from the Fleming genome
occurred during their initial shared history of mutagenesis prior to their separation. No sequence divergence was observed between the two US strains in any of the genes involved in
penicillin G production and regulation of the pathway: mutagenesis and selection for improved function resulted in major structural changes but no substitutions at these loci. In contrast,
penicillin-pathway enzymes have diverged in amino acid sequence between the Fleming strain and the US strains, especially _pcbAB_, _penDE_ and _cefD2_. While it is possible in principle that
these changes were caused by mutagenesis during domestication of the US strains, we think that this is unlikely: subsequent rounds of the same process led to no sequence divergence between
the US strains, and the numbers of substitutions involved would seem more commensurate with longer periods of time elapsing. Instead, these differences are likely to have accrued during
evolutionary divergence of the UK and US strains of _P. rubens_ in the wild. Although the level of divergence did not meet the statistical criteria for detecting significant evidence of
positive selection, a low level of constraint on protein sequence of these genes could still indicate a history of divergent selection at a subset of codons. Alternatively, it could indicate
that the function of these proteins is less dependent on amino acid identity at several sites than is the case for the other genes. In _A. nidulans_, the _aatA_ gene (an ortholog of
_penDE_) encodes the enzyme isopenicillin N acyltransferase26,59. It has been found that disruption of this gene does not disrupt penicillin production in _A. nidulans_. A paralog of _aatA_,
_aatB_ compensates for this as it encodes a homolog of isopenicillin N acyltransferase59. It should be noted that the isopenicillin N acyltransferase encoded by _aatA_ is only 55.2% similar
to its homolog encoded by _aatB_ and the two genes themselves are only 58% similar59. Additionally, the liquid chromatography–mass spectrometry (LC–MS) data for penicillin compounds
synthesized by either of the genes indicate unexplained significant peaks in proximity to the peaks representing standard penicillin V or penicillin G compounds synthesized by these genes.
These unexplained peaks could represent penicillin analogues synthesized by _aatB_ and _aatA_. It would be worthwhile to investigate further how the differences in penicillin effector genes
translate into altered function of the enzymes encoded, such as variation in the substrate specificity or efficiency of the enzymes60. Such variation in specificity of the enzymes could
result in synthesis of penicillin G analogues. Furthermore, presence of a _penDE_ paralog, and _cefD1_ and _cefD2_ homologs in all the genomes compared in this study suggest the possibility
that these genes encode homologs of isopenicillin N acyltransferase and isopenicillin N epimerase respectively17,39,53,61. These enzymes could potentially synthesize analogues of penicillin
G and penicillin N. Other beta-lactam gene variants such as homologs of the gene encoding 7-alpha-cephemmethoxylase subunit, _cmcJ_, have also been identified in the genome of _P.
chysogenum_17. Studies suggest that many of these gene variants are expressed but further work is needed to elucidate the functional importance of these genes, which is currently
unclear17,39,54. The biosynthesis of penicillin G in _P. chrysogenum_ and _P. rubens_ consists of a simple three gene pathway, but in certain bacteria such as _S. clavuligerus_, as many as
twelve genes can be involved in the synthesis of beta-lactams such as cephamycin C26,62. Much of what is known regarding the evolution of diversity of natural antibiotics stems from the
concept of rearrangement of genes in an existing biosynthetic gene cluster, or by addition of novel genes to existing clusters via processes such as horizontal gene transfer12,63. Our
analyses indicate that individual genes of beta-lactam biosynthetic pathways can themselves vary between species. Evidence indicates that many penicillin producing species such as _P.
chrysogenum_ are genetically diverse, and allelic variation within wild _P. chrysogenum_ populations can impact penicillin production within these populations64,65. Thus, it is plausible
that sequence variation in the genomes that we describe could account for the production of novel penicillin analogues. Subtle variation in chemical structure of antibiotics has been
identified for other antibiotics such as antimycins produced by _Streptomyces_63,66,67. Future work to sample variation more widely in _P. rubens_ and measure the impacts of variation on
chemical structure of penicillin compounds is needed to distinguish these alternatives. In conclusion, our results provide preliminary evidence that genes involved in the production of
penicillin display relatively high rates of amino acid divergence between populations, as predicted if antibiotics evolve in an arms race with antagonistic microbes. Moreover, the results
indicate that natural changes involving point mutation and amino acid substitutions were not fully explored by the classical industrial mutagenesis approach, which instead produced larger
structural rearrangements. Thus, the mutagenesis approach employed previously may have missed some solutions for optimizing penicillin design compared to natural selection in the wild,
especially in the context of robustness to evolving antibiotic resistance. Future approaches could use solutions explored by nature as a template for the development of novel antibiotic
varieties. REFERENCES * Chain, E. _et al._ Penicillin as a chemotherapeutic agent. _The Lancet_ 236, 226–228. https://doi.org/10.1016/S0140-6736(01)08728-1 (1940). Article Google Scholar *
Fleming, A. On the antibacterial action of cultures of a _Penicillium_, with special reference to their use in the isolation of _B. influenzae_. _Br. J. Exp. Pathol._ 10, 226–236 (1929).
CAS PubMed Central Google Scholar * Houbraken, J., Frisvad, J. C. & Samson, R. A. Fleming’s penicillin producing strain is not _Penicillium chrysogenum_ but _P. rubens_. _IMA Fungus_
2, 87–95. https://doi.org/10.5598/imafungus.2011.02.01.12 (2011). Article PubMed PubMed Central Google Scholar * Houbraken, J. _et al._ New penicillin-producing _Penicillium_ species and
an overview of section _Chrysogena_. _Persoonia_ 29, 78–100. https://doi.org/10.3767/003158512X660571 (2012). Article CAS PubMed PubMed Central Google Scholar * Gaynes, R. The
discovery of Penicillin—new insights after more than 75 years of clinical use. _Emerg. Infect. Dis._ 23, 849–853. https://doi.org/10.3201/eid2305.161556 (2017). Article PubMed Central
Google Scholar * Fair, R. J. & Tor, Y. Antibiotics and bacterial resistance in the 21st century. _Perspect. Med. Chem._ 6, 25–64. https://doi.org/10.4137/PMC.S14459 (2014). Article
Google Scholar * Ventola, C. L. The antibiotic resistance crisis: part 2: management strategies and new agents. _P&T Peer-Rev. J. Formul. Manag._ 40, 344–352 (2015). Google Scholar *
Ventola, C. L. The antibiotic resistance crisis: part 1: causes and threats. _P&T Peer-Rev. J. Formul. Manag._ 40, 277–283 (2015). Google Scholar * Abrudan, M. I. _et al._ Socially
mediated induction and suppression of antibiosis during bacterial coexistence. _Proc. Natl. Acad. Sci._ 112, 11054–11059. https://doi.org/10.1073/pnas.1504076112 (2015). Article ADS CAS
PubMed PubMed Central Google Scholar * Granato, E. T., Meiller-Legrand, T. A. & Foster, K. R. The evolution and ecology of bacterial warfare. _Curr. Biol._ 29, R521–R537.
https://doi.org/10.1016/j.cub.2019.04.024 (2019). Article CAS PubMed Google Scholar * Brockhurst, M. _et al._ Running with the Red Queen: the role of biotic conflicts in evolution.
_Proc. R. Soc. Lond. B_ https://doi.org/10.1098/rspb.2014.1382 (2014). Article Google Scholar * Pathak, A., Kett, S. & Marvasi, M. Resisting antimicrobial resistance: lessons from
fungus farming ants. _Trends Ecol. Evol._ 34, 974–976. https://doi.org/10.1016/j.tree.2019.08.007 (2019). Article PubMed Google Scholar * Jia, N., Ding, M.-Z., Luo, H., Gao, F. &
Yuan, Y.-J. Complete genome sequencing and antibiotics biosynthesis pathways analysis of _Streptomyces lydicus_ 103. _Sci. Rep._ 7, 44786–44786. https://doi.org/10.1038/srep44786 (2017).
Article ADS CAS PubMed PubMed Central Google Scholar * Blair, J. M. A., Webber, M. A., Baylay, A. J., Ogbolu, D. O. & Piddock, L. J. V. Molecular mechanisms of antibiotic
resistance. _Nat. Rev. Microbiol._ 13, 42. https://doi.org/10.1038/nrmicro3380 (2014). Article CAS PubMed Google Scholar * Backus, M. P. & Stauffer, J. F. The production and
selection of a family of strains in _Penicillium chrysogenum_. _Mycologia_ 47, 429–463. https://doi.org/10.1080/00275514.1955.12024468 (1955). Article Google Scholar * Salo, O. V. _et al._
Genomic mutational analysis of the impact of the classical strain improvement program on β-lactam producing _Penicillium chrysogenum_. _BMC Genomics_ 16, 937–937.
https://doi.org/10.1186/s12864-015-2154-4 (2015). Article CAS PubMed PubMed Central Google Scholar * van den Berg, M. A. _et al._ Genome sequencing and analysis of the filamentous
fungus _Penicillium chrysogenum_. _Nat. Biotechnol._ 26, 1161 (2008). Article CAS PubMed Google Scholar * Newbert, R. W., Barton, B., Greaves, P., Harper, J. & Turner, G. Analysis of
a commercially improved _Penicillium chrysogenum_ strain series: involvement of recombinogenic regions in amplification and deletion of the penicillin biosynthesis gene cluster. _J. Ind.
Microbiol. Biotechnol._ 19, 18–27. https://doi.org/10.1038/sj.jim.2900411 (1997). Article CAS PubMed Google Scholar * Barreiro, C., Martín, J. F. & García-Estrada, C. Proteomics
shows new faces for the old Penicillin producer _Penicillium chrysogenum_. _J. Biomed. Biotechnol._ https://doi.org/10.1155/2012/105109 (2012). Article PubMed PubMed Central Google
Scholar * Specht, T., Dahlmann, T. A., Zadra, I., Kürnsteiner, H. & Kück, U. Complete sequencing and chromosome-Scale Genome Assembly of the Industrial Progenitor Strain P2niaD18 from
the Penicillin Producer _Penicillium chrysogenum_. _Genome Announc._ 2, e00577-e1514. https://doi.org/10.1128/genomeA.00577-14 (2014). Article PubMed PubMed Central Google Scholar *
Laich, F., Fierro, F., Cardoza, R. E. & Martin, J. F. Organization of the gene cluster for biosynthesis of penicillin in _Penicillium nalgiovense_ and antibiotic production in cured dry
sausages. _Appl. Environ. Microbiol._ 65, 1236–1240 (1999). Article CAS PubMed PubMed Central Google Scholar * Andersen, S. J. & Frisvad, J. C. Penicillin production by _Penicillium
nalgiovense_. _Lett. Appl. Microbiol._ 19, 486–488. https://doi.org/10.1111/j.1472-765X.1994.tb00988.x (1994). Article CAS PubMed Google Scholar * Aharonowitz, Y., Cohen, G. &
Martin, J. F. Penicillin and cephalosporin biosynthetic genes: structure, organization, regulation, and evolution. _Annu. Rev. Microbiol._ 46, 461–495.
https://doi.org/10.1146/annurev.mi.46.100192.002333 (1992). Article CAS PubMed Google Scholar * Brakhage, A. A., Al-Abdallah, Q., Tüncher, A. & Spröte, P. Evolution of β-lactam
biosynthesis genes and recruitment of trans-acting factors. _Phytochemistry_ 66, 1200–1210. https://doi.org/10.1016/j.phytochem.2005.02.030 (2005). Article CAS PubMed Google Scholar *
Díez, B. _et al._ The cluster of penicillin biosynthetic genes. Identification and characterization of the pcbAB gene encoding the alpha-aminoadipyl-cysteinyl-valine synthetase and linkage
to the pcbC and penDE genes. _J. Biol. Chem._ 265, 16358–16365 (1990). PubMed Google Scholar * Brakhage, A. A. _et al._ Aspects on evolution of fungal β-lactam biosynthesis gene clusters
and recruitment of trans-acting factors. _Phytochemistry_ 70, 1801–1811. https://doi.org/10.1016/j.phytochem.2009.09.011 (2009). Article CAS PubMed Google Scholar * Weber, S. S., Polli,
F., Boer, R., Bovenberg, R. A. L. & Driessen, A. J. M. Increased penicillin production in _Penicillium chrysogenum_ production strains via balanced overexpression of isopenicillin N
acyltransferase. _Appl. Environ. Microbiol._ 78, 7107–7113. https://doi.org/10.1128/AEM.01529-12 (2012). Article CAS PubMed PubMed Central Google Scholar * Cooper, R. D. G. The enzymes
involved in biosynthesis of penicillin and cephalosporin; their structure and function. _Bioorg. Med. Chem._ 1, 1–17. https://doi.org/10.1016/S0968-0896(00)82098-2 (1993). Article CAS
PubMed Google Scholar * Tobin, M. B., Fleming, M. D., Skatrud, P. L. & Miller, J. R. Molecular characterization of the acyl-coenzyme A: isopenicillin N acyltransferase gene (penDE)
from _Penicillium chrysogenum_ and _Aspergillus nidulans_ and activity of recombinant enzyme in _Escherichia coli_. _J. Bacteriol._ 172, 5908–5914.
https://doi.org/10.1128/jb.172.10.5908-5914.1990 (1990). Article CAS PubMed PubMed Central Google Scholar * Martín, J. F., Ullán, R. V. & García-Estrada, C. Regulation and
compartmentalization of β-lactam biosynthesis. _Microb. Biotechnol._ 3, 285–299. https://doi.org/10.1111/j.1751-7915.2009.00123.x (2010). Article CAS PubMed PubMed Central Google Scholar
* Ullán, R. V. _et al._ A novel epimerization system in fungal secondary metabolism involved in the conversion of Isopenicillin N into Penicillin N in _Acremonium chrysogenum_. _J. Biol.
Chem._ 277, 46216–46225 (2002). Article PubMed Google Scholar * Ullán, R. V., Casqueiro, J., Naranjo, L., Vaca, I. & Martín, J. F. Expression of cefD2 and the conversion of
isopenicillin N into penicillin N by the two-component epimerase system are rate-limiting steps in cephalosporin biosynthesis. _Mol. Genet. Genomics_ 272, 562–570.
https://doi.org/10.1007/s00438-004-1087-4 (2004). Article CAS PubMed Google Scholar * García-Estrada, C., Domínguez-Santos, R., Kosalková, K. & Martín, J.-F. Transcription factors
controlling primary and secondary metabolism in filamentous fungi: the β-lactam paradigm. _Fermentation_ 4, 47. https://doi.org/10.3390/fermentation4020047 (2018). Article CAS Google
Scholar * Biourge, P. Les moissisures du groupe _Penicillium_ Link. _La Cellule_ 33, 7–331 (1923). Google Scholar * Bankevich, A. _et al._ SPAdes: a new genome assembly algorithm and its
applications to single-cell sequencing. _J. Comput. Biol._ 19, 455–477. https://doi.org/10.1089/cmb.2012.0021 (2012). Article MathSciNet CAS PubMed PubMed Central Google Scholar *
Laetsch, D. R. & Blaxter, M. L. BlobTools: interrogation of genome assemblies. _F1000Research_ 6, 1287 (2017). Article Google Scholar * Simao, F. A., Waterhouse, R. M., Ioannidis, P.,
Kriventseva, E. V. & Zdobnov, E. M. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. _Bioinformatics_ 31, 3210–3212.
https://doi.org/10.1093/bioinformatics/btv351 (2015). Article CAS PubMed Google Scholar * Van Den Berg, M. A. _Penicillium chrysogenum_: genomics of an antibiotics producer. In _Genomics
of Soil- and Plant-Associated Fungi Soil Biology, Ch. 10_ (eds Horwitz, B. A. _et al._) 229–254 (Springer, Berlin, 2013). Chapter Google Scholar * García-Estrada, C. _et al._ Molecular
characterization of a fungal gene paralogue of the penicillin penDE gene of _Penicillium chrysogenum_. _BMC Microbiol._ 9, 104. https://doi.org/10.1186/1471-2180-9-104 (2009). Article CAS
PubMed PubMed Central Google Scholar * Suárez, T. & Peñalva, M. A. Characterization of a _Penicillium chrysogenum_ gene encoding a PacC transcription factor and its binding sites in
the divergent pcbAB–pcbC promoter of the penicillin biosynthetic cluster. _Mol. Microbiol._ 20, 529–540. https://doi.org/10.1046/j.1365-2958.1996.5421065.x (1996). Article PubMed Google
Scholar * Bergh, K. T. & Brakhage, A. A. Regulation of the _Aspergillus nidulans_ penicillin biosynthesis gene _acvA_ (_pcbAB_) by amino acids: implication for involvement of
transcription factor PACC. _Appl. Environ. Microbiol._ 64, 843 (1998). Article CAS PubMed Central Google Scholar * Kato, N., Brooks, W. & Calvo, A. M. The expression of
sterigmatocystin and penicillin genes in _Aspergillus nidulans_ is controlled by _veA_, a gene required for sexual development. _Eukaryot. Cell_ 2, 1178–1186.
https://doi.org/10.1128/EC.2.6.1178-1186.2003 (2003). Article CAS PubMed PubMed Central Google Scholar * Martín, J. F. Key role of LaeA and velvet complex proteins on expression of
β-lactam and PR-toxin genes in _Penicillium chrysogenum_: cross-talk regulation of secondary metabolite pathways. _J. Ind. Microbiol. Biotechnol._ 44, 525–535.
https://doi.org/10.1007/s10295-016-1830-y (2017). Article CAS PubMed Google Scholar * Bergh, K. T., Litzka, O. & Brakhage, A. A. Identification of a major cis-acting DNA element
controlling the bidirectionally transcribed penicillin biosynthesis genes acvA (pcbAB) and ipnA (pcbC) of _Aspergillus nidulans_. _J. Bacteriol._ 178, 3908–3916.
https://doi.org/10.1128/jb.178.13.3908-3916.1996 (1996). Article CAS PubMed PubMed Central Google Scholar * Brakhage, A. A. _et al._ HAP-Like CCAAT-binding complexes in filamentous
fungi: implications for biotechnology. _Fungal Genet. Biol._ 27, 243–252. https://doi.org/10.1006/fgbi.1999.1136 (1999). Article CAS PubMed Google Scholar * Steidl, S. _et al._ _AnCF_,
the CCAAT binding complex of _Aspergillus nidulans_, contains products of the _hapB_, _hapC_, and _hapE_ genes and is required for activation by the pathway-specific regulatory gene _amdR_.
_Mol. Cell. Biol._ 19, 99–106. https://doi.org/10.1128/MCB.19.1.99 (1999). Article CAS PubMed PubMed Central Google Scholar * Caruso, M. L., Litzka, O., Martic, G., Lottspeich, F. &
Brakhage, A. A. Novel Basic-region helix–loop–helix transcription factor (AnBH1) of _Aspergillus nidulans_ counteracts the CCAAT-binding complex AnCF in the promoter of a Penicillin
biosynthesis gene. _J. Mol. Biol._ 323, 425–439. https://doi.org/10.1016/S0022-2836(02)00965-8 (2002). Article CAS PubMed Google Scholar * Katoh, K., Misawa, K., Kuma, K.-I. &
Miyata, T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. _Nucleic Acids Res._ 30, 3059–3066. https://doi.org/10.1093/nar/gkf436 (2002). Article
CAS PubMed PubMed Central Google Scholar * Guindon, S. _et al._ New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. _Syst.
Biol._ 59, 307–321. https://doi.org/10.1093/sysbio/syq010 (2010). Article CAS PubMed Google Scholar * Yang, Z. PAML 4: phylogenetic analysis by maximum likelihood. _Mol. Biol. Evol._ 24,
1586–1591. https://doi.org/10.1093/molbev/msm088 (2007). Article CAS PubMed Google Scholar * van den Berg, M. A., Westerlaken, I., Leeflang, C., Kerkman, R. & Bovenberg, R. A. L.
Functional characterization of the penicillin biosynthetic gene cluster of _Penicillium chrysogenum_ Wisconsin 54-1255. _Fungal Genet. Biol._ 44, 830–844.
https://doi.org/10.1016/j.fgb.2007.03.008 (2007). Article CAS PubMed Google Scholar * Fierro, F. _et al._ The penicillin gene cluster is amplified in tandem repeats linked by conserved
hexanucleotide sequences. _Proc. Natl. Acad. Sci. U. S. A._ 92, 6200–6204. https://doi.org/10.1073/pnas.92.13.6200 (1995). Article ADS CAS PubMed PubMed Central Google Scholar * Kiel,
J. A. K. W. _et al._ Matching the proteome to the genome: the microbody of penicillin-producing _Penicillium chrysogenum_ cells. _Funct. Integr. Genomics_ 9, 167–184.
https://doi.org/10.1007/s10142-009-0110-6 (2009). Article CAS PubMed Google Scholar * Jami, S., Barreiro, C., García-Estrada, C. & Martin, J. Proteome analysis of the penicillin
producer _Penicillium chrysogenum_: characterization of protein changes during the industrial strain improvement. _Mol. Cell. Proteom._ 9, 1182–1198.
https://doi.org/10.1074/mcp.M900327-MCP200 (2010). Article CAS Google Scholar * Martín, J. F. Molecular control of expression of penicillin biosynthesis genes in fungi: regulatory
proteins interact with a bidirectional promoter region. _J. Bacteriol._ 182, 2355–2362. https://doi.org/10.1128/jb.182.9.2355-2362.2000 (2000). Article PubMed PubMed Central Google
Scholar * Nielsen, J. C. _et al._ Global analysis of biosynthetic gene clusters reveals vast potential of secondary metabolite production in Penicillium species. _Nat. Microbiol._ 2, 17044.
https://doi.org/10.1038/nmicrobiol.2017.44 (2017). Article CAS PubMed Google Scholar * Ziemons, S., Koutsantas, K., Becker, K., Dahlmann, T. & Kück, U. Penicillin production in
industrial strain _Penicillium chrysogenum_ P2niaD18 is not dependent on the copy number of biosynthesis genes. _BMC Biotechnol._ 17, 16–16. https://doi.org/10.1186/s12896-017-0335-8 (2017).
Article CAS PubMed PubMed Central Google Scholar * Rajaraman, J. _et al._ Evolutionarily conserved partial gene duplication in the Triticeae tribe of grasses confers pathogen
resistance. _Genome Biol._ 19, 116. https://doi.org/10.1186/s13059-018-1472-7 (2018). Article CAS PubMed PubMed Central Google Scholar * Spröte, P. _et al._ Identification of the novel
penicillin biosynthesis gene aatB of _Aspergillus nidulans_ and its putative evolutionary relationship to this fungal secondary metabolism gene cluster. _Mol. Microbiol._ 70, 445–461.
https://doi.org/10.1111/j.1365-2958.2008.06422.x (2008). Article CAS PubMed Google Scholar * Pavela-Vrancic, M., Dieckmann, R. & von Döhren, H. ATPase activity of non-ribosomal
peptide synthetases. _Biochim. Biophys. Acta (BBA) Proteins Proteom._ 1696, 83–91. https://doi.org/10.1016/j.bbapap.2003.09.012 (2004). Article CAS Google Scholar * Ullán, R. V., Campoy,
S., Casqueiro, J., Fernández, F. J. & Martín, J. F. Deacetylcephalosporin C production in _Penicillium chrysogenum_ by expression of the isopenicillin N epimerization, ring expansion,
and acetylation genes. _Chem. Biol._ 14, 329–339. https://doi.org/10.1016/j.chembiol.2007.01.012 (2007). Article CAS PubMed Google Scholar * Alexander, D. C. & Jensen, S. E.
Investigation of the _Streptomyces clavuligerus_ Cephamycin C gene cluster and its regulation by the CcaR protein. _J. Bacteriol._ 180, 4068–4079 (1998). Article CAS PubMed PubMed Central
Google Scholar * Seipke, R. F. & Hutchings, M. I. The regulation and biosynthesis of antimycins. _Beilstein J. Org. Chem._ 9, 2556–2563. https://doi.org/10.3762/bjoc.9.290 (2013).
Article PubMed PubMed Central Google Scholar * Wong, V. L., Ellison, C. E., Eisen, M. B., Pachter, L. & Brem, R. B. Structural variation among wild and industrial strains of
_Penicillium chrysogenum_. _PLoS ONE_ 9, e96784. https://doi.org/10.1371/journal.pone.0096784 (2014). Article ADS CAS PubMed PubMed Central Google Scholar * Henk, D. A. _et al._
Speciation despite globally overlapping distributions in _Penicillium chrysogenum_: the population genetics of Alexander Fleming’s lucky fungus. _Mol. Ecol._ 20, 4288–4301.
https://doi.org/10.1111/j.1365-294X.2011.05244.x (2011). Article CAS PubMed Google Scholar * Seipke, R. F. _et al._ A single _Streptomyces_ symbiont makes multiple antifungals to support
the fungus farming ant _Acromyrmex octospinosus_. _PLoS ONE_ 6, e22028. https://doi.org/10.1371/journal.pone.0022028 (2011). Article ADS CAS PubMed PubMed Central Google Scholar *
Joynt, R. & Seipke, R. F. A phylogenetic and evolutionary analysis of antimycin biosynthesis. _Microbiology_ 164, 28–39. https://doi.org/10.1099/mic.0.000572 (2018). Article CAS PubMed
Google Scholar Download references ACKNOWLEDGEMENTS This work was funded in part by a Natural Environment Research Council Grant NE/S010866/1 and a project bursary from the Masters by
Research in Computational Methods in Ecology and Evolution at Imperial College London. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Life Sciences, Imperial College London,
Silwood Park Campus, Ascot, Berkshire, SL5 7PY, UK Ayush Pathak, Reuben W. Nowell, Christopher G. Wilson & Timothy G. Barraclough * Department of Zoology, University of Oxford, 11a
Mansfield Rd, Oxford, OX1 3SZ, UK Reuben W. Nowell, Christopher G. Wilson & Timothy G. Barraclough * CABI, Bakeham Lane, Egham, Surrey, TW20 9TY, UK Matthew J. Ryan Authors * Ayush
Pathak View author publications You can also search for this author inPubMed Google Scholar * Reuben W. Nowell View author publications You can also search for this author inPubMed Google
Scholar * Christopher G. Wilson View author publications You can also search for this author inPubMed Google Scholar * Matthew J. Ryan View author publications You can also search for this
author inPubMed Google Scholar * Timothy G. Barraclough View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.P. and T.G.B. conceived the
study. M.R. provided materials. A.P. and C.G.W. grew cultures and extracted DNA. A.P., R.W.N. and T.G.B. analysed the data. A.P. and T.G.B. led writing the manuscript with further inputs
from R.W.N., M.R. and C.G.W. We thank 3 anonymous reviewers for extremely helpful comments on earlier drafts. CORRESPONDING AUTHOR Correspondence to Timothy G. Barraclough. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims
in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the
article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your
intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence,
visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Pathak, A., Nowell, R.W., Wilson, C.G. _et al._ Comparative genomics of
Alexander Fleming’s original _Penicillium_ isolate (IMI 15378) reveals sequence divergence of penicillin synthesis genes. _Sci Rep_ 10, 15705 (2020).
https://doi.org/10.1038/s41598-020-72584-5 Download citation * Received: 13 November 2019 * Accepted: 03 September 2020 * Published: 24 September 2020 * DOI:
https://doi.org/10.1038/s41598-020-72584-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative








