- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT This research study was conducted to determine if bird depredation in feedlots is associated with the prevalence of ciprofloxacin-resistant _Escherichia coli_ in cattle and to
determine if removal of invasive bird species could be an effective management strategy to help reduce ciprofloxacin-resistant _E. coli_ in cattle within the United States. European
starlings (_Sturnus vulgaris_) were collected from feedlots within multiple geographic regions within the United States and European starlings within all regions tested positive for
ciprofloxacin-resistant _E. coli_, but prevalence differed by region. Total number of birds on feedlots were positively associated with increased cattle fecal shedding of
ciprofloxacin-resistant _E. coli_. Targeted control of invasive European starlings reduced bird numbers on feedlots by 70.4%, but decreasing populations of European starlings was not
associated with corresponding reductions in bovine fecal prevalence of ciprofloxacin-resistant _E. coli_. These data provide evidence for the role of wild bird depredation in feedlots
contributing to fecal shedding of ciprofloxacin-resistant _E. coli_, but a single month of European starling control in feedlots was not sufficient to impact the fecal carriage of this
organism in cattle. SIMILAR CONTENT BEING VIEWED BY OTHERS INTENSIFIED LIVESTOCK FARMING INCREASES ANTIBIOTIC RESISTANCE GENOTYPES AND PHENOTYPES IN ANIMAL FECES Article Open access 18 April
2023 ANTIMICROBIALS USE AND RESISTANCE ON INTEGRATED POULTRY-FISH FARMING SYSTEMS IN THE AYEYARWADY DELTA OF MYANMAR Article Open access 30 September 2020 LOW-COST BIOSECURITY MEASURES ARE
ASSOCIATED WITH REDUCED DETECTION OF NON-TYPHOIDAL SALMONELLA IN NIGERIAN POULTRY WHILE INAPPROPRIATE ANTIBIOTIC USE IS WIDESPREAD Article Open access 09 September 2024 INTRODUCTION
Antimicrobial resistant (AMR) bacteria constitute a significant portion of the emerging infectious disease (EID) pathogens that have been reported since 1940, and analyses of EID events
suggest socioeconomic drivers (e.g., human population density, antibiotic drug use, and agricultural practices) are major determinants of their temporal and spatial patterns1,2,3.
Domestication of animals appears to be a driver of EID events4, especially within concentrated animal feeding operations where livestock production is associated with the emergence of AMR
bacteria5,6,7. Currently, there are gaps in our understanding of how AMR bacteria are maintained and moved through the food chain to human populations8. One potential gap in our
understanding is the ecological interactions between wildlife, livestock, and people. The presence of AMR bacteria is also an economic concern for cattle producers because AMR pathogens can
contribute to increased morbidity and mortality that will increase production expenses9,10. Additionally, multiple antibiotics have been used as growth promotants in cattle production
systems and concern over the emergence of AMR bacteria has led the Food and Drug Administration (FDA) to amend guidelines on use of antibiotics7. When fully implemented, these new guidelines
will limit the use of medically important antibiotics for growth promotion11. Thus, efforts designed to reduce public health risk associated with AMR pathogens may also reduce profitability
for feedlot operators. The objective of this study was twofold: (1) assess the relationship between bird abundance and ciprofloxacin-resistant _Escherichia coli_ within cattle feedlots in
the United States; (2) determine the efficacy of targeted invasive species management (removal of European starlings; _Sturnus vulgaris_) as a potential pre-harvest intervention strategy to
reduce cattle fecal shedding of ciprofloxacin-resistant _E. coli_. Specifically, we wanted to determine if higher total bird numbers were associated with increased cattle fecal shedding of
ciprofloxacin-resistant _E. coli_ and if the removal of invasive European starlings would reduce cattle fecal shedding of ciprofloxacin-resistant _E. coli_, relative to comparable reference
facilities not subjected to starling control operations. METHODS We conducted this study from December 4, 2012 through March 12, 2013 with the cooperation of 35 feedlots. Feedlots were
located within 4 regions; Eastern Colorado (n = 8), Kansas (n = 8), Texas panhandle (n = 8), Southern Iowa/Northern Missouri (n = 11). Feedlots experiencing bird damage (large foraging
flocks of birds) were identified with the help of local cattlemen’s associations. Bird damaged feedlots were randomly selected from a pool of commercial facilities, within each region, that
had reported severe bird damage the previous year. Comparable reference feedlots were selected within each geographical region. A total of 18 treated and 17 reference facilities were
included in the analysis. All participating facilities group housed animals in pens and produced feeder cattle as their primary commodity. Dairies, calving, or non-cattle livestock
facilities were not included in the study. This experimental protocol was approved by the USDA/APHIS/Wildlife Services, National Wildlife Research Center prior to data collection (Study
Director James C Carlson; NWRC Protocol number QA-1945). Starling control operations were conducted by biologists from the United States Department of Agriculture/APHIS/Wildlife Services.
Starling control was conducted following agency policy as stated in USDA/APHIS/WS Directive 2.505. All methods were carried out in accordance with relevant guidelines and regulations.
Wildlife Services biologists initially established starling feeding sites within the 18 treatment feedlots using a bait preferentially selected by European starlings. Once starlings were
observed to be consistently feeding on pre-bait, biologists used a 2% solution of DRC-1339 (3-chloro-p-toluidine hydrochloride) to reduce the number of depredating starlings. Technical
DRC-1339 powder was mixed with water to create a 2% solution. Starling feed was soaked in the 2% solution and screen dried. The bait was applied at a concentration of 1:10 treated to
untreated starling feed particles. All DRC-1339 applications were implemented consistent with directions “Compound DRC-1339 Concentrate – Feedlots”; EPA registration 56228–10. Each feedlot
was sampled twice, once before and once after starling control operations. During each sampling period we collected European starlings (n = 30) and cattle feces (n = 50) from within
feedlots. Within each feedlot up to 10 pens were selected. These same pens were sampled before and after starling control operations. Within each pen we collected a minimum of five cattle
fecal samples per visit. If a feedlot had fewer than 10 pens the total number of samples was distributed, as evenly as possible, among the available pens. For example, one facility housed
animals in 2 large pens. Within this feedlot we collected 25 fecal samples per pen per visit. Within some feedlots fewer than 30 starlings were collected if birds could not be found.
Collection of cattle fecal samples followed methods that have been described previously12. Cattle fecal samples were collected from the floor of animal pens and only freshly voided fecal
pats were sampled. In other words, the sample was collected from a fecal pat only after an animal was observed defecating. This procedure allowed us to standardize environmental exposure
time among fecal samples and estimate herd prevalence of ciprofloxacin-resistant _E. coli_ without confining animals for collection of rectal samples. Ten gram samples were scraped from the
top of the fecal pat with disposable plastic spoons and stored in sterile Whirl-Paks (Nasco, Fort Atkinson, WI). We only collected fecal samples if we could reasonably determine, by visual
inspection, that the sample was fresh and free of external environmental contaminants. All fecal samples were stored in coolers until they were shipped to the laboratory. Estimates of number
of birds in animal pens were collected at the same time as fecal sample collection. Number of birds on feedlots were estimated using counts of bird numbers on each pen’s floor, feed
bunkers, water troughs and feed lanes in front of the sampled pen. Estimates from these four locations were summed to calculate the total number of birds within pens. We averaged the total
number of birds within pens among all the sampled pens within a feedlot. This mean bird estimate was multiplied by the total number of pens within the facility to produce a facility level
bird estimate. All starlings were collected with shotguns and no birds were collected off feedlots. All starlings were collected from within the animal pens and pen lanes. Starling samples
were collected opportunistically and only done when it was safe to discharge firearms in feedlots. All specimens were individually bagged in sterile Whirl-Paks and stored in coolers until
shipping. Within each facility, diagnostic samples (starlings and cattle fecal samples) were collected on the same day and samples were shipped priority overnight to testing laboratories in
Iowa and Colorado. All samples were shipped, in insulated boxes packed with Ice-Brix (Polar Tech Industries, Genoa, IL), to laboratories for isolation of ciprofloxacin-resistant _E. coli_.
Only samples received by the laboratories within 24 hours of the date of collection were screened for ciprofloxacin-resistant _E. coli_. European starlings were shipped to the United States
Department of Agriculture, National Wildlife Research Center (NWRC) in Fort Collins, Colorado, USA. Cattle fecal samples were shipped to Ohio State University, Food Animal Health Research
Program in Wooster, OH, USA. All European starling dissections occurred at the NWRC and were conducted using published methods13. Starling lower gastrointestinal tracts (GI, duodenum to the
cloaca) were removed and placed in a sterile Whirl-Paks. To reduce risk of cross-contamination, we saturated the starling carcass, scissors, scalpels, and lab stations with 70% ethanol
before removal of each starling GI tract. Lab mats and gloves were replaced after processing each starling. The starling GI samples were macerated for 120 sec at 200 rpm using a Stomacher 80
Biomaster (Seward Laboratory Systems, Bohemia, NY) paddle blender. Fecal material from the macerated starling GI tracts was squeezed by hand to one corner of the bag and an aliquot was
extracted using sterile cotton swabs, making sure to completely saturate the tip of the swab. Starling fecal material, on the saturated cotton tipped swab, was then used for direct plating
onto selective media. Starling GI and cattle fecal samples were inoculated onto MacConkey agar (HiMedia, Mumbai, India) supplemented with 1 µg/ml ciprofloxacin (Sigma-Aldrich, St. Louis, MO)
using sterile cotton-tipped applicators and incubated at 37 °C for 18–24 hr. Colonies displaying typical _E. coli_ morphology were transferred to 10 ml of tryptic soy broth (TSB) and
incubated overnight at 37 °C for 18–24 hours. Species confirmation for starling GI samples was achieved using the API 20E system (bioMérieux, Marcy-l'Étoile, France). _E. coli_
susceptibility to ciprofloxacin was confirmed using the disk diffusion method following Clinical and Laboratory Standards Institute protocols and guidelines (CLSI, 2008). Species
confirmation for cattle fecal samples was conducted using lactose and indole tests. All lactose and indole positive isolates were cultured on MacConkey agar supplemented with 2 µg/ml
ciprofloxacin. Colonies growing on the agar were isolated and tested for both gyrA and parC chromosomal mutations by PCR using previously reported primers14. PCR products were
bi-directionally Sanger sequenced and the resulting data were aligned to the corresponding reference gene sequences available in NCBI Genbank (_gyrA_ gene ID: 946614; _parC_ gene ID:
947499). The _gyrA_ and _parC_ sequences were screened for combinations of chromosomal mutations expected to confer fluoroquinolone resistance15,16 and if they were detected the _E. coli_
isolate was classified as ciprofloxacin-resistant. We tested efficacy of DRC-1339 as a control tool for invasive birds on feedlots using a Poisson model of count data in PROC GLIMMIX in SAS
version 9.2 (SAS Institute, Cary, NC). The response variable was the estimated number of birds on feedlots. Fixed effects included treatment status (starling controlled feedlot/reference
feedlot), sampling period (before/after starling control) and the interaction between treatment status and sampling period. Feedlots nested within treatment status were included as a random
effect. Separate mixed effects logistic regression models were created to test the association between total bird number and ciprofloxacin-resistant _E. coli_ fecal shedding by cattle and to
test the efficacy of starling control as a pre-harvest intervention strategy to reduce ciprofloxacin-resistant _E. coli_ fecal shedding by cattle. Models were constructed using PROC GLIMMIX
in SAS version 9.2. Both models, were fitted using a binomial distribution and the response variable was the number of positive ciprofloxacin-resistant _E. coli_ samples divided by the
total number of samples collected per pen. Model parameters were estimated using the maximum likelihood method and degrees of freedom were estimated using the between within option. Within
both models, feedlots nested within treatment status, pens nested within feedlots, and the sampling period by feedlot interaction were all included as random effects. To test for an
association between total bird numbers and cattle fecal shedding of ciprofloxacin-resistant _E. coli_, we included region and the estimated number of birds on feedlots as fixed effects. To
test the efficacy of starling control as a pre-harvest intervention strategy to reduce cattle fecal shedding of ciprofloxacin-resistant _E. coli_, we included region, treatment status
(starling controlled feedlot/reference feedlot), sampling period (before/after starling control operations), and the interaction between treatment status and sampling period as fixed
effects. Additional explanatory variables for ciprofloxacin-resistant _E. coli_ in feedlots were assessed in univariable analyses using PROC GLIMMIX in SAS version 9.2. The model was fitted
using a binomial distribution and the response variable was the number of positive ciprofloxacin-resistant _E. coli_ samples divided by the total number of samples collected per pen. Model
parameters were estimated using the maximum likelihood method and degrees of freedom were estimated using the between within option. Feedlots nested within treatment status, pens nested
within feedlots, and the sampling period by feedlot interaction were all included as random effects. The additional explanatory variables were assessed to identify any potential wild bird,
facility management, or environmental variables that may potentially be associated with cattle fecal shedding of ciprofloxacin-resistant _E. coli_ in feedlots. The explanatory variables
assessed in the analyses were selected because they have been identified as or suspected of contributing to bacterial contamination in feedlots13,17,18,19,20. The variables assessed in these
analyses occurred at two spatial scales (feedlots and pens within feedlots). The variables include birds (birds in feed bunkers, birds on water troughs, total number of birds in pens),
cattle stocking (herd size, number of cattle within pen), environmental factors (temperature, time, and sampling period), and feedlot management factors (water troughs split pens, recycled
water used in water troughs, cattle days in pen, cattle days on finishing ration, entry weight, exit weight and weight gained by cattle). Most variables assessed within the univariable
analyses are intuitively obvious, but some variables may need additional clarification. For example, weight gain was calculated by subtracting the pen averaged entry weight from the pen
averaged exit weight data. Water troughs accessed by multiple pens identifies split-pen watering troughs that allow cattle from adjoining pens to drink from the same trough. Recycled water
identifies facilities that recirculate the water provided to cattle within troughs. Total number of birds per pen reflects the sum of the estimates of birds from water troughs, pen floor,
feed bunkers and pen lanes for each sampled pen. A total of 15 additional univariable models were analyzed (_m_ = 15). Because multiple tests were being conducted, we decided to control for
false discoveries using the Benjamini Hochberg procedure21. For all univariable analyses the false discovery rate was set at α = 0.05. Models were ranked by p-values from smallest (1) to
largest (_m_). Cutoff values for rejection of null hypotheses were calculated as (rank/_m_)*α. Reported odd ratios and their 95% confidence intervals were not adjusted for multiple testing.
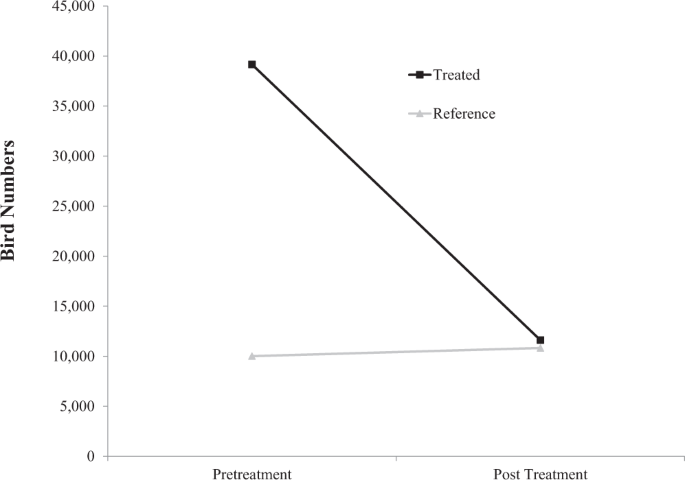
RESULTS Targeted control of invasive European starlings using DRC-1339 was effective at reducing bird numbers on feedlots. Total number of birds on treatment facilities relative to the
reference facilities not subjected to control operations decreased following DRC-1339 control operations (F1,33 = 95,598, P = < 0.0001). Bird count data suggests targeted starling control
operations reduced bird numbers by 70.4% on feedlots following DRC-1339 applications (Fig. 1). A total of 1,477 European starling specimens were collected for laboratory analysis. A total
of 10.2% of starling GI tracts tested positive for ciprofloxacin-resistant _E. coli_ and the probability of detection within starling GI tract samples appears to differ by geographical
region (Fig. 2). The odds of cattle shedding ciprofloxacin-resistant _E. coli_ significantly varied with region (F3,286 = 5.69, P = 0.0009) and the number of birds on feedlots (F1,286 =
4.46, P = 0.0355, Table 1). The probability of detecting ciprofloxacin-resistant _E. coli_ in cattle fecal samples increased as bird numbers increased on feedlots (odds ratio per 100 birds =
1.001, 95% CI = 1.000, 1.002) and effectively doubled when 65,000 birds were observed foraging on feedlots (odds ratio = 2.003, 95% CI = 1.048, 3.827, Fig. 3). Targeted control of invasive
European starlings was not an effective pre-harvest intervention strategy to reduce cattle fecal shedding of ciprofloxacin-resistant _E. coli_ (F1,33 = 0.60, P = 0.4454, Table 2). Based on
LS-Means estimates of ciprofloxacin-resistant cattle fecal samples there does not appear to be any reduction in cattle fecal shedding of ciprofloxacin-resistant _E. coli_ within starling
controlled feedlots relative to reference feedlots (Fig. 4). The analysis of the 15 univariable models of potential explanatory variables for cattle fecal shedding of ciprofloxacin-resistant
_E. coli_ did not reveal any statistically significant associations after Benjamini Hochberg adjustments were made (Table 3). DISCUSSION Wildlife incursions into animal agricultural
operations have long been suspected as sources for diseases of concern to veterinary and human health22,23,24. For example, indistinguishable AMR _S. enterica_ isolates were recovered from
starlings, cattle, and the feed and water sources they share13,20. Additionally, cattle fecal pats showing reduced susceptibility to cefotaxime and ciprofloxacin were spatially correlated to
starling night roosts in Ohio25. Proximity of starling night roosts was also shown to be spatially correlated with increased _E. coli_ O157:H7 cattle fecal shedding in dairies26. These data
are important because they provide indirect evidence that bird-livestock interactions may contribute to rates of cattle fecal shedding of _E. coli_ 0157:H7 as well as _S. enterica_ and _E.
coli_ with reduced susceptibilities to multiple antibiotics, including ciprofloxacin and cefotaxime. The data we present in this manuscript are the first to provide direct evidence to
support the hypothesis that large foraging flocks of birds can contribute to increased cattle fecal shedding of ciprofloxacin-resistant _E. coli_. Starling control operations reduced bird
numbers on our treatment feedlots by an average of 70.4%. Yet, the time between pre treatment and post treatment sampling did not result in any significant change in cattle fecal shedding of
ciprofloxacin-resistant _E. coli_. One would intuitively assume that significant reductions in bird numbers on feedlots should translate to cattle harboring fewer organisms with reduced
susceptibilities to ciprofloxacin. It is unclear why we did not see a significant reduction in the amount of ciprofloxacin-resistant _E. coli_ isolated from cattle fecal pats, while seeing a
positive correlation between bird numbers and cattle fecal shedding of ciprofloxacin-resistant _E. coli_. We suspect the time between starling control operations and post-treatment sampling
may not have been long enough for these management actions to produce meaningful results. If so, starling control may have to occur year round or the moment starlings arrive on feedlots in
the fall for it to be effective at reducing the amplification and spread of AMR organisms in animal agricultural operations. It is important to note that other studies have shown that bird
control was not an effective pre-harvest intervention strategy for reducing cattle fecal shedding of bacteria of concern to public health. Starling numbers were one of the strongest
predictors for _S. enterica_ contamination of cattle feed and water supplies, but starling numbers were not shown to be a good predictor for herd level prevalence of _S. enterica_27.
Controlling starlings was associated with reduced _S. enterica_ loads within cattle feed and water supplies, but starling control was not effective at reducing cattle fecal shedding of _S.
enterica_ over the time period of the study. Additionally, starling control programs were not an effective intervention strategy to reduce the overall prevalence of _Campylobacter jejuni_
within feedlot cattle despite starlings harboring diverse _C. jejuni_ strains including hypervirulent clone SA28. The totality of this information is discouraging. Bird numbers and bird
depredation in feedlots and dairies is associated with higher herd level prevalence for multiple zoonotic and AMR organisms, but temporarily or transiently reducing bird numbers, after they
have become established in animal agricultural operations, does not translate to quick reductions of herd level prevalence of those same organisms. In other words, once AMR organisms have
been introduced by starlings, they may persist within cattle herds for considerable periods of time. After population control programs were completed, approximately 30% of the pretreatment
birds remained on feedlots. It is conceivable that the microbiological impact of birds is not additive and that only a few birds, moving between feedlots and dairies, are necessary for
maintenance and amplification of ciprofloxacin-resistant _E. coli_ in concentrated animal feeding operations. Additional studies are needed to better assess interactions between birds,
cattle and the occurrence of AMR _E. coli_. For example, there is very little information related to antibiotic usage in agriculture, wildlife interactions and selective pressure on the
maintenance of ciprofloxacin-resistance _E. coli_ in livestock. It is conceivable that wildlife are contributing to these problems in complex and unforeseen ways. To adequately address
public and environmental health concerns created through wildlife-livestock interactions we need to understand the specific risks created by wildlife so we can develop targeted and cost
effective management strategies. Bird-livestock interactions in animal agricultural operations may create an ecologically important link for the spread of ciprofloxacin-resistant _E. coli_
to human populations. Synanthropic birds, especially European starlings, use feedlots in winter for food resources. Starlings typically quit using feedlots in spring when insects become
abundant29. During the spring and summer, starlings are commonly found breeding in suburban and urban environments30,31. The ecological interactions of starlings suggest they could
potentially move ciprofloxacin-resistant _E. coli_ and other AMR organisms to environments dominated by people; human-bird transfer of _E. coli_ has been documented before32. Birds seem to
act as transporters, or as reservoirs, of resistant bacteria and could therefore have an important epidemiological role in the dissemination of resistance33. Thus, because of the unique
ecology of invasive starlings in North America, they are a high risk species for the environmental dissemination of AMR organisms to environments and locations of concern to people. DATA
AVAILABILITY All raw data are archived at the National Wildlife Research Center (Study Director James C Carlson; NWRC Protocol number QA-1945) and are publicly available. Names and addresses
of cooperating feedlots have been redacted from the raw data. All facilities were referenced by an alpha-numeric code and names and addresses of cooperating facilities will not be provided
upon request as per the cooperator agreement established prior to data collection. REFERENCES * McEwen, S. A. & Fedorka-Cray, P. J. Antimicrobial use and resistance in animals. _Clinical
Infectious Disease_ 34, S93–S106 (2002). Article CAS Google Scholar * McEwen, S. A. Antibiotic use in animal agriculture: what have we learned and where are we going? _Animal
Biotechnology_ 17, 239–250 (2006). Article Google Scholar * Jones, K. E. _et al_. Global trends in emerging infectious disease. _Nature_ 451, 990–993 (2008). Article ADS CAS Google
Scholar * Wolfe, N. D., Dunavan, C. P. & Diamond, J. Origins of major human infectious diseases. _Nature_ 447, 279–283 (2007). Article ADS CAS Google Scholar * Gilchrist, M. J. _et
al_. The potential role of concentrated animal feeding operations in infectious disease epidemics and antibiotic resistance. _Environmental Health Perspectives_ 11(2), 313–316 (2007).
Article Google Scholar * Mirzaagha, P. _et al_. Distribution and characterization of ampicillin- and tetracycline-resistant _Escherichia coli_ from feedlot cattle fed subtherapeutic
antimicrobials. _BMC Microbiology_ 11, 78 (2011). Article CAS Google Scholar * FDA. Guidance for Industry #213: New animal drugs and new animal drug combination products administered in
or on medicated feed or drinking water of food-producing animals: recommendations for drug sponsors for voluntarily aligning product use conditions with GFI #209;
https://www.fda.gov/media/83488/download (2013). * World health Organization. Antimicrobial resistance: global report on surveillance. National Library of Medicine: QV 250 (2014). * Mathew,
A. G., Cissell, R. & Liamthong, S. Antibiotic resistance in bacteria associated with food animals: a United States perspective of livestock production. _Food-borne Pathogens and Disease_
4, 115–133 (2007). Article CAS Google Scholar * Call, D. R., Davis, M. A. & Sawant, A. A. Antimicrobial resistance in beef and dairy cattle production. _Animal Health Research
Reviews_ 9(2), 159–167 (2008). Article Google Scholar * FDA. Veterinary Feed Directive: 80 Federal Register 31707; https://federalregister.gov/a/2015-13393 (2015). * Carlson, J. C.,
Franklin, A. B., Hyatt, D. R., Pettit, S. E. & Linz, G. M. The role of starlings in the spread of Salmonella within concentrated animal feeding operations. _Journal of Applied Ecology_
2, 479–486 (2011a). Article Google Scholar * Carlson, J. C. _et al_. Mechanisms of antimicrobial resistant _Salmonella enterica_ transmission associated with starling-livestock
interactions. _Veterinary Microbiology_ 179, 60–68 (2015a). Article Google Scholar * Everett, M. J., Jin, Y. F., Ricci, V. & Piddock, L. J. V. Contributions of individual mechanisms to
fluoroquinolone resistance in 36 Escherichia coli strains isolated from humans and animals. _Antimicrobial Agents and Chemotherapy_ 40(10), 2380–2386 (1996). Article CAS Google Scholar *
Heisig, P. Genetic evidence for a role of _parC_ mutations in development of high-level fluoroquinolone resistance in _Escherichia coli_. _Antimicrobial Agents and Chemotherapy_ 40(4),
879–885 (1996). Article CAS Google Scholar * Kato, J. _et al_. New topoisomerase essential for chromosome segregation in _E. coli_. _Cell_ 63(2), 393–404 (1990). Article CAS Google
Scholar * Fedorka-Cray, P. J., Dargatz, D. A., Thomas, L. A. & Gray, J. T. Survey of Salmonella serotypes in feedlot cattle. _Journal of Food Protection_ 61, 525–530 (1998). Article
CAS Google Scholar * LeJeune, J. T., Besser, T. E., Merrill, N. L., Rice, D. H. & Hancock, D. D. Livestock drinking water microbiology and the factors influencing the quality of
drinking water offered to cattle. _Journal of Dairy Science_ 84, 1856–1862 (2001). Article CAS Google Scholar * LeJeune, J. T. & Wetzel, A. N. Preharvest control of Escherichia coli
O157 in cattle. _Journal of Animal Science_ 85(E. Suppl.), E73–E80 (2006). PubMed Google Scholar * Carlson, J. C. _et al_. Molecular characterization of Salmonella enterica isolates
associated with starling-livestock interactions. _Veterinary Microbiology_ 179, 109–118 (2015b). Article CAS Google Scholar * Benjamini, Y. & Hochberg, Y. Controlling for false
discovery rate: a practical and powerful approach to multiple testing. _Journal of the Royal Statistical Society_ 57, 289–300 (1995). MathSciNet MATH Google Scholar * Feare, C. J. The
Starling. Oxford University Press, New York (1984). * Daszak, P., Cunningham, A. A. & Hyatt, A. D. Emerging infectious diseases of wildlife–threats to biodiversity and human health.
_Science_ 287, 443–449 (2000). Article ADS CAS Google Scholar * Kirk, J. H., Holmberg, C. A. & Jeffrey, J. S. Prevalence of Salmonella spp. in selected birds captured on California
dairies. _Journal of the American Veterinary Medical Association_ 220, 359–362 (2002). Article Google Scholar * Medhanie, G. A. _et al_. Spatial Clustering of _Escherichia coli_ with
reduced susceptibility to cefotaxime and ciprofloxacin among dairy cattle farms relative to European starling night roosts. _Zoonoses and Public Health_ 64, 204–212,
https://doi.org/10.1111/zph.12296 (2017). Article CAS PubMed Google Scholar * Swirski, A. L. _et al_. Spatial Epidemiology of _Escherichia coli_ O157:H7 in Dairy Cattle in Relation to
night roosts of _Sturnus vulgaris_ (European Starlings) in Ohio, USA. _Zoonoses and Public Health_ 61, 427–435, https://doi.org/10.1111/zph.12092 (2013). Article PubMed Google Scholar *
Carlson, J. C. _et al_. Efficacy of European starling control to reduce _Salmonella enterica_ contamination in a concentrated animal feeding operation in the Texas panhandle. _BMC Veterinary
Research_ 7, 9 (2011b). Article Google Scholar * Tang, Y. _et al_. Wide but variable distribution of a hypervirulent _Campylobacter jejuni_ clone in beef and dairy cattle in the United
States. _Applied Environmantal Microbiology_ 83(24), e01425–17 (2017). Google Scholar * Linz G. M., Homan, H. J., Gaukler, S. M., Penry, L. B., & Bleier, W. J. European Starlings: A
review of an invasive species with far-reaching impacts in Managing vertebrate invasive species: Proceedings of an international symposium. (ed. G.W., Witmer, W.C., Pitt, K.A., Fagerstone).
USDA/APHIS/WS, National Wildlife Research Center, Fort Collins, pp. 378–386 (2007). * Blair, R. B. Land use and avian species diversity along an urban gradient. _Ecological Applications_ 6,
506–519 (1996). Article Google Scholar * Melles, S., Glenn, S. & Martin, K. Urban bird diversity and landscape complexity: species-environment association along a multiscale habitat
gradient. _Conservation Ecology_ 7, 5 (2003). Article Google Scholar * Bonnedahl, J. _et al_. Dissemination of _Escherichia coli_ with CTX-M type ESBL between humans and yellow-legged
gulls in the south of France. _PLoS ONE_ 4(6), e5958 (2009). Article ADS Google Scholar * Gordon, D. M. & Cowling, A. The distribution and genetic structure of _Escherichia coli_ in
Australian vertebrates: host and geographic effects. _Microbiology_ 149, 3575–3586 (2003). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This project was supported
through the USDA National Institute of Food and Agriculture Grant No. 2013-67015-20368. Guidance or livestock producer contact assistance was provided by National Cattlemen’s Beef
Association, Colorado Livestock Association, Texas Cattle Feeders Association, Kansas Livestock Association, Texas Wildlife Services and the USDA, APHIS, National Wildlife Disease Program.
AUTHOR INFORMATION Author notes * Jeffrey T. LeJeune Present address: Food and Agriculture Organization of the United Nations (FAO), Viale delle Terme di Caracalla, 00153, Rome, Italy
AUTHORS AND AFFILIATIONS * U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, National Wildlife Research Center, Fort Collins, CO, USA James C.
Carlson & Jeffrey C. Chandler * Department of Animal Science, College of Agriculture and Natural Resources, University of Wyoming, Laramie, WY, USA Jeffrey C. Chandler & Bledar Bisha
* The Ohio State University, Wooster, OH, USA Jeffrey T. LeJeune * Department of Veterinary Preventive Medicine, College of Veterinary Medicine, The Ohio State University, Columbus, OH, USA
Thomas E. Wittum Authors * James C. Carlson View author publications You can also search for this author inPubMed Google Scholar * Jeffrey C. Chandler View author publications You can also
search for this author inPubMed Google Scholar * Bledar Bisha View author publications You can also search for this author inPubMed Google Scholar * Jeffrey T. LeJeune View author
publications You can also search for this author inPubMed Google Scholar * Thomas E. Wittum View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
James C. Carlson (JCC): Experimental design, project management, Colorado field and laboratory data collection, statistical analyses, manuscript preparation and submission. Jeffrey C.
Chandler (JCC): Colorado laboratory data collection, manuscript preparation. Bledar Bisha (BB): Colorado laboratory data collection, manuscript preparation. Jeffrey T. LeJeune (JTL):
Funding, grant administration, manuscript preparation. Thomas Wittum (TW): Ohio laboratory data analysis, manuscript preparation. CORRESPONDING AUTHOR Correspondence to James C. Carlson.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which
permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to
the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless
indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Carlson, J.C., Chandler, J.C., Bisha, B. _et al._ Bird-livestock interactions associated with increased cattle fecal shedding of
ciprofloxacin-resistant _Escherichia coli_ within feedlots in the United States. _Sci Rep_ 10, 10174 (2020). https://doi.org/10.1038/s41598-020-66782-4 Download citation * Received: 09
February 2020 * Accepted: 22 May 2020 * Published: 23 June 2020 * DOI: https://doi.org/10.1038/s41598-020-66782-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative








